Introduction
The early diagnosis of cancer and the development of
novel treatments have led to increased cancer-free survival;
however, cancer therapy-related cardiac dysfunction (CTRCD) has
increasingly become a concern (1).
Chemotherapeutic agents that are associated with CTRCD include
anthracyclines (e.g., doxorubicin), human epidermal growth factor 2
(HER2) inhibitors (e.g., trastuzumab), alkylating agents (e.g.,
cyclophosphamide) and taxanes (e.g., paclitaxel) (1,2). The
incidence of CTRCD varies depending on the chemotherapeutic agent
that is used (3–5). In a cohort of 2,625 patients who were
receiving anthracycline-containing therapy, the overall incidence
of cardiotoxicity was 9% (3). Rossi
et al (4) reported severe
cardiac adverse events in 32 (4.7%) of 681 trastuzumab-treated
patients with metastatic breast cancer. In another study, the
incidence of cyclophosphamide-induced cardiotoxicity with treatment
at the therapeutic dose ranged from 7 to 28% (5).
Among the various definitions for CTRCD (1,2), the
most accepted definition originates from the expert consensus of
the American Society of Echocardiography and European Association
of Cardiovascular Imaging, which defines CTRCD as a decrease in the
left ventricular ejection fraction (LVEF) of >10%, or to a value
<53%, confirmed by repeat imaging (6). Although the standard drug therapy used
for heart failure (HF) may be helpful for diagnosing CTRCD, there
are no current recommendations for this treatment owing to limited
evidence (1,2,6).
Several studies have evaluated the effectiveness of
the HF therapy regimens, such as beta-blocker or
angiotensin-converting enzyme inhibitors/angiotensin-II receptor
blockers (ACEIs/ARBs), in HF prevention due to CTRCD; the results
indicated that these drugs moderately attenuate the frequency of
clinically overt cardiotoxicity and prevent ventricular remodeling
(7–9). Sacubitril/valsartan, a novel
angiotensin receptor neprilysin inhibitor, has been recommended for
patients with HF with reduced LVEF (HFrEF) to alleviate the
mortality and hospitalization risk (10,11).
However, it has remained elusive whether sacubitril/valsartan is
effective in patients with CTRCD, as these patients are usually
excluded from clinical trials (11). Therefore, the present review was
conducted to systematically evaluate the usefulness of
sacubitril/valsartan as a treatment for CTRCD.
Materials and methods
Search strategy
The search strategy, study selection, and data
extraction and analysis methods were pre-designed according to the
principles of the Preferred Reporting Items for Systematic Reviews
and Meta-Analyses statement (12),
although this review was not registered.
Data source and search strategy
A systematic search was conducted to identify
studies published in online databases, including PubMed (https://www.ncbi.nlm.nih.gov/pubmed/),
Embase (https://www.elsevier.com/solutions/embase-biomedical-research),
the Cochrane Library (https://www.cochranelibrary.com/advanced-search) and
ClinicalTrials.gov (https://www.clinicaltrial.gov/) from their
inception to May 13, 2021. The keywords included ‘LCZ696’,
‘sacubitril/valsartan’, ‘sacubitril valsartan’, ‘cancer’ and
‘tumor’. EndNote X6.0 (Thomson Reuters) was used to manage records
and exclude duplicates. To ensure comprehensiveness of the present
analysis, the reference lists of identified studies were manually
checked to identify other potentially eligible studies.
Study selection
The inclusion criteria were as follows: Studies of
i) patients with CTRCD; ii) including treatment with
sacubitril/valsartan; iii) including comparison or no comparison;
iv) including at least one heart remodeling parameter, such as the
New York Heart Association (NYHA) functional class (13), LVEF or N-terminal pro-B-type
natriuretic peptide (NT-proBNP), as the outcomes; and v) with an
experimental or observational study design. There were no
restrictions on the indication for treatment, dose or the clinical
setting in which sacubitril/valsartan was administered.
Review articles, systematic reviews, meta-analyses,
commentaries, editorials and meeting abstracts were excluded from
the analysis.
Data extraction and quality
assessment
Data extraction and evaluation of the quality of the
studies were performed independently by two researchers (YH and
LLM). Disagreements, if any, were resolved with a third
investigator (YYZ, CBF, WFX or HZ). The following general study
information was retrieved: Author, publication year, study design,
country, cancer type, chemotherapy, numbers of cases, age, LVEF
measurement and follow-up duration.
The 2011 Oxford Criteria were used for grading the
level of evidence of the included studies (14). The criteria developed by Murad et
al (15) in 2018 were used to
assess the quality of the included studies, wherein scores of
<3, 3–5 and 6–8 indicated low-quality, moderate-quality and
good-quality evidence, respectively (16).
Statistical analysis
A meta-analysis was not performed, as all of the
included studies were descriptive studies. A total of three outcome
variables were assessed, namely NYHA functional class, LVEF and
NT-proBNP. The assessments were performed at baseline and on
completion of the sacubitril/valsartan intervention. All variables
were qualitatively analyzed.
Results
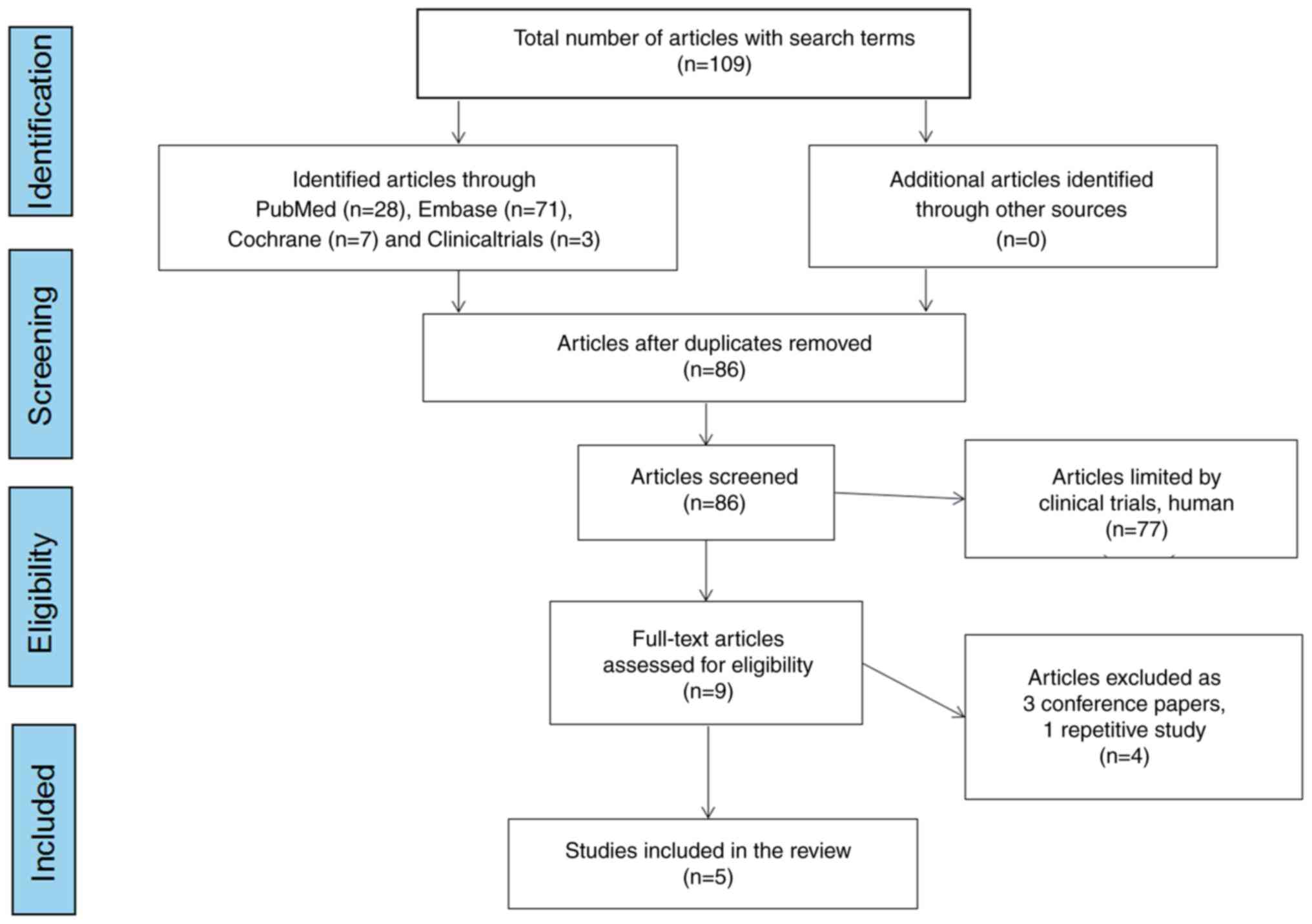
Search results and study characteristics. Following
the search strategy, a total of 109 articles were obtained from the
online databases. After excluding all duplicates, 86 articles were
retained. After checking the titles and abstracts and reviewing the
full texts of these articles, 5 studies (two from Spain and one
each from Canada, Argentina and Italy) (17–21)
eventually met the inclusion criteria and were included in the
review. Fig. 1 illustrates the
literature search protocol.
The five studies, including one letter to the editor
(which includes the original research data) and four case series,
were all descriptive studies that enrolled patients with CTRCD who
were treated with sacubitril/valsartan. Of the total of 109
patients, 52 had breast cancer, 34 had lymphoma and 23 had other
cancers. Depending on the type of the tumor, most of the patients
were treated with anthracyclines (e.g., doxorubicin and
epirubicin), anti-HER2 agents (e.g., trastuzumab) and other
chemotherapeutic agents. The follow-up period of the studies ranged
from 2 to 24 months. Table I
presents the detailed characteristics of the included studies.
 | Table I.Characteristics of the studies
evaluating sacubitril/valsartan treatment for patients with cancer
therapy-related cardiac dysfunction. |
Table I.
Characteristics of the studies
evaluating sacubitril/valsartan treatment for patients with cancer
therapy-related cardiac dysfunction.
| Author, year | Type of study | Country | Level of
evidence | Sample size
(male/female) | Patient age,
years | History of
cardiovascular disease | Cancer type | Chemotherapy | Measurement of
LVEF | Follow-up duration
(months) | Funding | (Refs.) |
|---|
| Sheppard and Anwar,
2019 | Case series
study | Canada | 4 | 1 female | 68 | No risk factors for
heart disease | Mantle cell
lymphoma | R-CHOP/R-DHAP
induction chemotherapy | Echocardiography,
MRI | 6 | No | (17) |
|
|
|
|
| 1 female | 76 | / | ER/PR-positive,
HER2-negative left-sided breast cancer | FEC and tamoxifen for
5 years followed by letrozole (discontinued due to
intolerance) | Echocardiography | / |
|
|
| Gregorietti,
2020 | Case series
study | Argentina | 4 | 3/25 | 56.2±13.4 | Hypertension, 50%;
smoking, 43%; hypercholesterolemia, 36%; diabetes, 29%; ischemic
cardiac disease, 7% | Left breast cancer
64.3% | Doxorubicin, 82.1%;
Cyclophosphamide, 82.1%; Docetaxel, 10.7%; Trastuzumab, 28.6%;
Pertuzumab, 14.3% | Echocardiography | 24 | No | (18) |
| Vecchis and Paccone,
2020 | Case series
study | Italy | 4 | 1 female | 55 | / | Hodgkin lymphoma | ABVD | Echocardio graphy,
MRI | 4 | Novartis Pharma | (19) |
|
|
|
|
| 1 female | 65 | / | Invasive ductal
carcinoma in the right breast with esophageal metastasis | Anastrozole, TAC and
cyclophosphamide | Echocardiography | 2 | S.p.A |
|
| Martín-García,
2020a | Letter to the
editor | Spain | 4 | 4/6 | 65-78 |
| 6 lymphoma, 2
breast cancer, 1 myeloma and 1 lung cancer | Anthracyclines,
80%; Alkylating agents,80%; Antimicrotubule agents, 60%; Rituximab,
60%; Anti metabolites, 20%; PD-1 in-hi bitors, 20%; Trastuzumab,
anti VGEF antibodies, lenalidomide or pomalidomide, 10% | MRI | >3 | European Regional
Development Fund (PIE14/00066), Spanish Cardiovascular Network
(CIBERCV) | (20) |
| Martín-García,
2020b | Case series
study | Spain | 4 | 24/43 | 63±14 | Hypertension, 43%;
dyslipidaemia, 54%; diabetes, 28% | 30 Breast cancer
and 26 lymphoma | Anthracyclines,
70%; Alkylating agents, 60%; Antimicrotubule agents, 50%;
Antimetabolites, 25%; Tyrosine kinase inhibitors,22%; Anti-HER2
humanized antibody, 12%; Topoisomerase inhibitors, 6%; PD-1
inhibitors, 3% |
Echocardiography | Median, 4.6 | European Regional
Development Fund (PIE14/00066), Spanish Cardiovascular Network
(CIBERCV) | (21) |
Level of evidence and quality
assessment
The level of evidence of the five studies was graded
as 4, as per the 2011 Oxford Criteria (14). With regard to quality assessment,
all studies were scored as 4–5, which is considered good-quality
evidence. Although a score of 6–8 indicates good quality as per
Murad et al (15), questions
4, 5 and 6 are mostly relevant to cases of adverse drug events;
therefore, this scoring was not included in the present article.
Table II presents the detailed
results of the quality assessment of the included studies.
 | Table II.Quality assessment of the studies
included in the review. |
Table II.
Quality assessment of the studies
included in the review.
| Domain | Questions | Sheppard, 2019
(17) | Gregorietti, 2020
(18) | Vecchis, 2020
(19) | Martín-García, 2020
(20) | Martín-García, 2020
(21) |
|---|
| Selection | 1. Does the
patient(s) represent(s) the whole experience of the investigator
(center) or is the selection method unclear to the extent that
other patients with similar presentation may not have been
reported? | 0 | 1 | 0 | 0 | 1 |
| Ascertainment | 2. Was the exposure
adequately ascertained? | 1 | 1 | 1 | 1 | 1 |
|
| 3. Was the outcome
adequately ascertained? | 1 | 1 | 1 | 1 | 1 |
| Causality | 4. Were other
alternative causes that may explain the observation ruled out? | / | / | / | / | / |
|
| 5. Was there a
challenge/rechallenge phenomenon? | / | / | / | / | / |
|
| 6. Was there a
dose-response effect? | / | / | / | / | / |
|
| 7. Was the
follow-up long enough for outcomes to occur? | 1 | 1 | 1 | 1 | 1 |
| Reporting | 8. Is/are the
case(s) described with sufficient details to allow other
investigators to replicate the research or to allow
practitionersmake inferences related to their own practice? | 1 | 1 | 1 | 1 | 1 |
| Score |
| 4 | 5 | 4 | 4 | 5 |
Treatment characteristics of
sacubitril/valsartan
Data from the present review of the selected studies
(17–21) revealed interindividual variations in
the time from anticancer therapy to HFrEF (5–141 months) or from
HFrEF to sacubitril/valsartan treatment (1–52 months). Prior to
undergoing sacubitril/valsartan treatment, patients received triple
HF therapy, including ACEI/ARB, beta-blockers, mineralocorticoid
receptor antagonists and diuretics, as relevant. The dose of
sacubitril/valsartan was titrated from a low dose [e.g., 50 mg
twice a day (b.i.d.)] gradually to the maximum tolerated target
dose (e.g., 100 or 200 mg b.i.d.).
Effect of sacubitril/valsartan on
patients with CTRCD
In terms of the changes from the basal
echocardiography, sacubitril/valsartan treatment improved the LVEF
of patients with CTRCD (Table
III). Furthermore, a reduction in NT-proBNP levels was evident
(Table III). In addition,
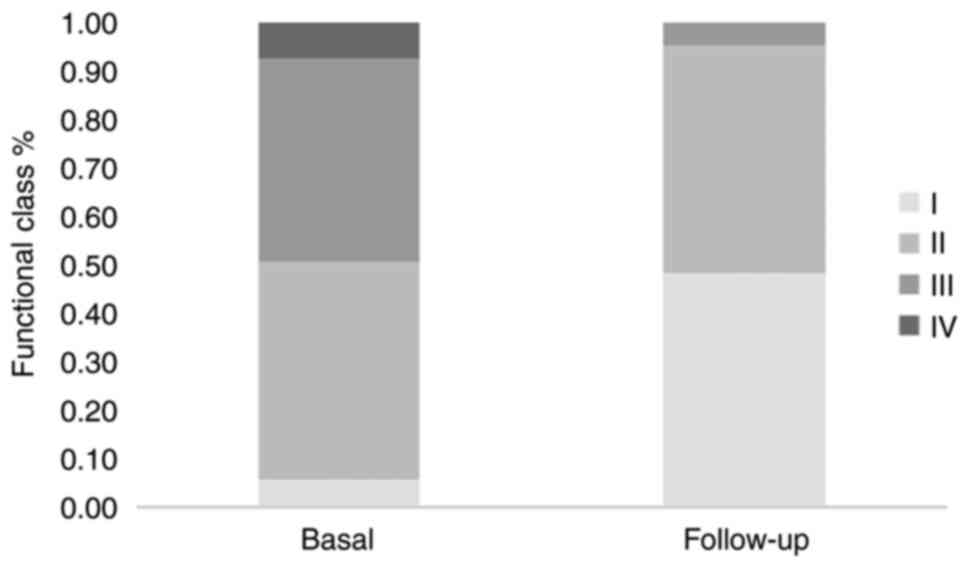
patients exhibited an improvement in exercise tolerance at
follow-up, as indicated by the change in the NYHA functional class
(at the end of follow-up, 48 and 47% of patients had NYHA class I
and II, respectively; Table III
and Fig. 2). Regardless of the
dosage or treatment duration of sacubitril/valsartan, these
clinical, echocardiographic and biochemical improvements were
observed.
 | Table III.Heart remodeling parameters before
and after sacubitril/valsartan treatment. |
Table III.
Heart remodeling parameters before
and after sacubitril/valsartan treatment.
| Author, year | Cardiovascular
pharmacological treatment | Time from HFrEF to
S/V initiation, months | Time from
anticancer therapy to HFrEF, months | Dose of
sacubitril/valsartan | NYHA functional
class | LVEF, % | NT-proBNP,
pg/ml |
|
|---|
|
|
|
|
|---|
| Before | After | Before | After | Before | After | (Refs.) |
|---|
| Sheppard and Anwar,
2019 | Bisoprolol,
Candesartan, Spironolactone, Furosemide as required,
Nitroglycerin | 12 | 1.5 | →200 mg b.i.d. | II | / | 33 | 59 | 68,281 | 2,783 | (17) |
|
| Enalapril,
Carvedilol, Eplerenone, Furosemide | 72 | 3 | 50 mg bid →100 mg
b.i.d | / | / | 25 | 28 | 4676 | / |
|
| Gregorietti,
2020 | Beta-blockers, 93%;
ACEI, 43%; ARB, 46%; Digitalis, 32%; Mineralocorticoid receptor
antagonist, 64%; Diuretics 71% | 6.1±3.2 | 1.6±0.4 | 100 mg in 12
patients; 200 mg in 16 patients | III, 78.6%; IV,
21.4% | I, 57.1%; II,
42.9% | 26.7±5.4 | 32.3±5.5 | 997.5
(663.8-2380.8) | 416.5
(192.0-798.2) | (18) |
| Vecchis, 2020 | Carvedilol,
Enalapril, Canrenone, Furosemide as required | 8 | 1 | 50 mg bid →100 mg
b.i.d | / | / | 22 | 55 | 1,5281 | 1,800 | (19) |
|
| Ramipril,
Carvedilol, Canrenone, Furosemide as required | 5 | >6 | 50 mg bid →100 mg
b.i.d | / | / | 26 | 45 | 1,2467 | / |
|
| Martín-García,
2020 | Triple HFrEF
therapy | 31 (9–113) | 11 (2–24) | 24/26 mg 70% →49/51
mg 50% →97/103 mg 10% | II, 60%; III, 30%;
IV, 10% | I, 40%; II,
60% | 35±8 | 47±9 | 1,063
(492–1,818) | 458 (217–648) | (20) |
| Martín-García,
2020 | ACEI/ARB, 80%;
Beta-blocker, 85%; Mineralocorticoid receptor antagonists, 76%;
Diuretics, 52% | 43 (10–141) | 13 (2–52) | 50 mg b.i.d. 78%
→50 mg b.i.d. 60% →100 mg b.i.d. 32% →200 mg b.i.d. 8% | II, 61%; III, 28%;
IV, 1% | I, 45%; II,
47% | 33 (27–37) | 42 (35–50) | 1,552 (692-3,
624) | 776 (339-1,
458) | (21) |
Furthermore, certain studies reported improvements
in other cardiac remodeling parameters, such as left ventricular
internal diameter in diastole, the ratio between early mitral
inflow velocity and mitral annular early diastolic velocity (E/e')
and mitral regurgitation (18);
left ventricle end-diastolic or systolic volume index, left
ventricle mass index, global right ventricle ejection fraction,
right ventricle end-diastolic or systolic volume index, and global
circumferential or longitudinal strain feature tracking (20); and left ventricle end-diastolic or
systolic volume, E/e' and global longitudinal strain (21).
Effect of sacubitril/valsartan on
creatinine or potassium
In 2020, Gregorietti et al (18) and Martín-García et al
(21) both reported a lack of a
difference between the baseline and follow-up creatinine and
potassium serum levels.
Discussion
A total of five studies, which comprised 109
patients, were included in the present review. The findings from
this review supported the hypothesis that sacubitril/valsartan was
effective and safe for patients with CTRCD, based on improved LVEF
and exercise tolerance, as indicated by the change in the NYHA
class and reduction in NT-proBNP levels; however, no change in the
serum creatinine or potassium level was identified. The evidence
was weak, as only descriptive studies were included.
Dexrazoxane remains the only clinically available
option to prevent anthracycline-induced cardiomyopathy. In
addition, current guidelines recommend pre-therapy, and once LVD
occurs, post-therapy LVEF evaluation and discontinuation of
chemotherapy, together with the initiation of standard HF therapy
comprising beta-blockers and ACEI/ARB (22,23).
Although the results of related clinical studies revealed the
effectiveness of ACEI and beta-blockers against anticancer
drug-induced cardiomyopathy, which was indicated by symptom relief,
improved LVEF and decreased NYHA class, these agents have only been
evaluated in a small number of small-sample studies in the clinical
setting (1–3). Thus, additional prospective
multicenter studies with a larger number of patients are warranted
to obtain a more definitive conclusion.
Furthermore, sacubitril/valsartan is recommended for
patients with HFrEF in the European Society of Cardiology
guidelines (10), but there is also
a lack of evidence of its efficacy and safety in CTRCD. Indeed,
outside of the descriptive studies included in the present review,
there are no exhaustive data on the subject. Based on current
evidence, the following is also revealed: i) Patients with a
cardiovascular disease history should be highly alert to the
occurrence of CTRCD (Table I); and
ii) there are interindividual variations in the time from
anticancer therapy to HF (5–141 months), and therefore, patients
should pay attention to the occurrence of CTRCD during the entire
treatment process (Table
III).
Current studies reveal a multifactorial pathogenesis
of CTRCD. A review indicated that anthracycline-induced
cardiomyopathy is related to the stabilization of topoisomerase II,
which causes DNA double-strand breaks, genomic instability and
impaired mitochondrial biogenesis capacity; furthermore, this
condition resulted in semiquinone cycling, which causes Complex I
impairment, loss of membrane potential, and activation of
mitophagy; non-canonical Wnt signaling, which upregulates
autophagosome formation; autophagolysosome accumulation due to
impaired lysosomal enzyme activity; and calcium mishandling, which
causes impaired contractile function (24). Furthermore, it was reported that
trastuzumab may block the myocardial HER-2 receptor pathway, which
mediates cardioprotective function. Mitochondrial impairment is
caused by HER-2 inhibition, which results in decreased antioxidant
reserve and reactive oxygen species (ROS) accumulation, as well as
the inhibition of the downstream signaling of the PI3K/Akt pathway
that causes upregulation of the proapoptotic Bcl-2 family protein
Bcl-xS and downregulation of the antiapoptotic protein Bcl-xL; the
abovementioned sequence of events is considered the primary
etiopathogenetic mechanism of HER2 inhibitor-induced cardiomyopathy
(25).
Experimental studies of the molecular mechanisms
mediating the potential benefits of sacubitril/valsartan in CTRCD
are scarce. In a study using a rabbit model of doxorubicin-induced
HF, sacubitril/valsartan treatment reduced inflammation and
oxidative stress, which resulted in improved cardiac function
(26). Another study demonstrated
that doxorubicin increased angiotensin-II receptor type I
expression through ERK1/2 activation by inducing mitochondrial
ROS-triggered heat-shock factors 2-related nuclear translocation
and activation; this indicates the potential mechanisms of action
of ACEI and ARB (27). Thus,
further investigations are required in this field.
There are certain limitations to this systematic
review. First, only five descriptive studies on
sacubitril/valsartan were included in this review, and it was thus
not feasible to perform a quantitative analysis; therefore, all
variables underwent qualitative analysis. Furthermore, only studies
published in English were included. In addition, publication bias
could not be assessed, as the level of evidence included grade 5 as
per the 2011 Oxford Criteria. Finally, methodological quality was
addressed through an appropriate method; thus, cautious
interpretation of the results is advised in clinical practice.
In conclusion, the evidence from the present review
limited to descriptive studies supports the effectiveness of
sacubitril/valsartan in the improvement of heart function following
CTRCD. Further studies are warranted to assess the long-term
benefit and safety of sacubitril/valsartan in patients with
CTRCD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Technology Plan of
Zhejiang Medical and Health Science (grant no. 2019KY604), 2021
Zhejiang Pharmaceutical Association Hospital Pharmacy Special
Scientific Research Funding Project (grant no. 2021ZYY41), the
Traditional Chinese Medicine Scientific Research Fund Project of
Zhejiang Province (grant no. 2021ZB263) and NINGBO Medical &
Health Leading Academic Discipline Project (grant no.
2022-F06).
Availability of data and materials
Not applicable.
Authors' contributions
YH, YZ, WX, CF, HZ and LM contributed to the study
conception and design. Material preparation and data collection and
analysis were performed by YH, LM and YZ. The first draft of the
manuscript was written by YH and it was revised by WX, CF and HZ.
All authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bloom MW, Hamo CE, Cardinale D, Ky B,
Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR
and Butler J: Cancer Therapy-Related cardiac dysfunction and heart
failure part 1: definitions, pathophysiology, risk factors, and
imaging. Circ Heart Fail. 9:e0026612016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez IE, Alam ST, Hernandez GA and
Sancassani R: Cancer therapy-related cardiac dysfunction: An
overview for the Clinician. Clin Med Insights Cardiol.
13:11795468198664452019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardinale D, Colombo A, Bacchiani G,
Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N,
Curigliano G, et al: Early detection of anthracycline
cardiotoxicity and improvement with heart failure therapy.
Circulation. 131:1981–1988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossi M, Carioli G, Bonifazi M, Zambelli
A, Franchi M, Moja L, Zambon A, Corrao G, La Vecchia C, Zocchetti C
and Negri E: Trastuzumab for HER2+ metastatic breast cancer in
clinical practice: Cardiotoxicity and overall survival. Eur J
Cancer. 52:41–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashif I, Mohammad KI, Sumit S, Ansari MA,
Najmi AK, Ali SM, Ali J and Haque SE: Molecular mechanism involved
in cyclophosphamide-induced cardiotoxicity: Old drug with a new
vision. Life Sci. 218:112–131. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Expert Consensus for Multimodality Imaging
Evaluation of Adult Patients during and after Cancer Therapy, . A
Report from the American Society of Echocardiography and the
European Association of Cardiovascular Imaging. J Am Soc
Echocardiog. 27:911–939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Avila MS, Ayub-Ferreira SM, de Barros
Wanderley MR Jr, das Dores Cruz F, Gonçalves Brandão SM, Rigaud
VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, et
al: Carvedilol for prevention of chemotherapy-related
cardiotoxicity: The CECCY trial. J Am Coll Cardiol. 71:2281–2290.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang S, Zhao Q, Yang ZG, Diao KY, He Y,
Shi K, Shen MT, Fu H and Guo YK: Protective role of beta-blockers
in chemotherapy-induced cardiotoxicity-a systematic review and
meta-analysis of carvedilol. Heart Fail Rev. 24:325–333. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang K, Zhang Y, Liu W and He C: Effects
of angiotensin-converting enzyme inhibitor/angiotensin receptor
blocker use on cancer therapy-related cardiac dysfunction: A
meta-analysis of randomized controlled trials. Heart Fail Rev.
26:101–109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task Force for
the Diagnosis and Treatment of Acute and Chronic Heart Failure of
the European Society of Cardiology (ESC). Developed with the
special contribution of the Heart Failure Association (HFA) of the
ESC. Eur Heart J. 37:2129–2200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. BMJ. 339:b25352009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White PD and Myers MM: The classification
of cardiac diagnosis. JAMA. 77:1414–1415. 1921. View Article : Google Scholar
|
|
14
|
Howick J, Chalmers I and Glasziou P: The
oxford 2011 levels of evidence. Oxford, UK: Oxford Centre for
Evidence-Based Medicine; 2011
|
|
15
|
Murad MH, Sultan S, Haffar S and
Bazerbachi F: Methodological quality and synthesis of case series
and case reports. BMJ Evid Med. 23:60–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rashid M, Rajan AK, Chhabra M and Kashyap
A: Levetiracetam and cutaneous adverse reactions: A systematic
review of descriptive studies. Seizure. 75:101–109. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheppard CE and Anwar M: The use of
sacubitril/valsartan in anthracycline-induced cardiomyopathy: A
mini case series. J Oncol Pharm Pract. 25:1231–1234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gregorietti V, Fernandez TL, Costa D,
Chahla EO and Daniele AJ: Use of Sacubitril/valsartan in patients
with cardio toxicity and heart failure due to chemotherapy.
Cardiooncology. 6:242020.PubMed/NCBI
|
|
19
|
Vecchis RD and Paccone A: A case series
about the favorable effects of sacubitril/valsartan on
anthracycline cardiomyopathy. SAGE Open Med Case Rep.
8:2050313X209521892020.PubMed/NCBI
|
|
20
|
Martín-García A, Díaz-Peláez E,
Martín-García AC, Sánchez-González J, Ibáñez B and Sánchez PL:
Myocardial function and structure improvement with
sacubitril/valsartan in cancer therapy-induced cardiomyopathy. Rev
Esp Cardiol (Engl Ed). 73:360–271. 2020.PubMed/NCBI
|
|
21
|
Martín-Garcia A, López-Fernández T, Mitroi
C, Chaparro-Muñoz M, Moliner P, Martin-Garcia AC, Martinez-Monzonis
A, Castro A, Lopez-Sendon JL and Sanchez PL: Effectiveness of
sacubitril-valsartan in cancer patients with heart failure. ESC
Heart Fail. 7:763–767. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure. Eur J Heart Fail.
18:891–975. 2016.(In Polish). View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamo CE, Bloom MW, Cardinale D, Ky B,
Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR
and Butler J: Cancer Therapy-related cardiac dysfunction and heart
failure part 2: Prevention, treatment, guidelines, and future
directions. Circ Heart Fail. 9:e0028432016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dadson K, Calvillo-Argüelles O,
Thavendiranathan P and Billia F: Anthracycline-induced
cardiomyopathy: Cellular and molecular mechanisms. Clin Sci (Lond).
134:1859–1885. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu QC, Bai BC, Tian C, Li D, Yu H, Song B,
Li B and Chu X: The molecular mechanisms of cardiotoxicity induced
by HER2, VEGF, and tyrosine kinase inhibitors: An updated review.
Cardiovasc Drug Ther. 36:511–524. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu C, Li D, Li Z and Zhai C: Effect of
sacubitril/valsartan on inflammation and oxidative stress in
doxorubicin-induced heart failure model in rabbits. Acta
Pharmaceutica. 71:473–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang CY, Chen JY, Kuo CH, Pai PY, Ho TJ,
Chen TS, Tsai FJ, Padma VV, Kuo WW and Huang CY: Mitochondrial
ROS-induced ERK1/2 activation and HSF2-mediated AT1 R upregulation
are required for doxorubicin-induced cardiotoxicity. J Cell
Physiol. 233:463–475. 2018. View Article : Google Scholar : PubMed/NCBI
|