Introduction
Small cell lung cancer (SCLC) accounts for ~14% of
all lung cancer cases (1). Until
recently, the standard first-line treatment for patients with SCLC
with extensive disease (ED-SCLC) was platinum and etoposide
combination chemotherapy. Despite a median patient survival time of
~10 months, there has been no significant improvement in overall
survival (OS) time for over 2 decades (2,3).
ED-SCLC is a malignant disease with an objective response rate
(ORR) of 44–78% for first-line treatment, a median progression-free
survival (PFS) time of 4.3-5.7 months, a median OS time of 7.5-10.9
months and a 5-year survival rate of only 2.8% (3,4).
Atezolizumab, a programmed death ligand 1 inhibitor,
was studied in the IMpower133 trial in combination with carboplatin
and etoposide therapies (5,6). The trial determined that median PFS
and OS times were significantly more favorable in patients who
received atezolizumab with carboplatin and etoposide than in
patients who received a placebo with carboplatin and etoposide (5.2
vs. 4.3 months and 12.3 vs. 10.3 months, respectively). However,
the IMpower133 trial was a study of a cohort of patients with a
favorable performance status (PS) of 0–1 (7), and there was no investigation for a PS
of 2. Furthermore, the trial was designed for patients with
preserved organ function and no autoimmune diseases or other
complications under favorable conditions. Thus, to the best of our
knowledge, there are currently no published studies on the addition
of immune checkpoint inhibitors (ICIs) in patients with poor PS,
organ dysfunction or complications.
There have been two phase III trials that included
patients with a PS of 3, and these patients may be eligible for
chemotherapy if the therapeutic effect on SCLC can improve PS
(8,9). A phase III trial (JCOG9702) of
carboplatin and etoposide versus split cisplatin (cisplatin
administered in divided doses) and etoposide in patients aged ≥70
(PS, 0–2) and <70 (PS, 3) years showed more grade 3/4
thrombocytopenia in patients given carboplatin and etoposide (56
vs. 16%, respectively; P<0.01), but ORR (73 vs. 73%,
respectively) and OS time (median 7.1 vs. 6.9 months, respectively,
in the subgroup analysis of patients with PS 3 <70 years) were
similar (10). Therefore, PS could
be improved with cytotoxic drug chemotherapy, and even if treatment
is initially started with carboplatin and etoposide for the first
cycle due to complications, ICIs can be intermittently added during
the sequential course of carboplatin and etoposide chemotherapy.
However, the efficacy and feasibility of starting carboplatin and
etoposide at the beginning of first-line chemotherapy and adding
atezolizumab during the treatment course in patients with ED-SCLC
with a poor PS or complications during the initial cycle of
carboplatin and etoposide have not been investigated. Furthermore,
to the best of our knowledge, no reports have examined the
significance of additional ICIs in malignancies other than lung
cancer. Therefore, the present investigation aimed to examine the
efficacy and feasibility of carboplatin and etoposide as treatment
options at the beginning of therapy with atezolizumab administered
during the treatment course in patients with ED-SCLC.
Materials and methods
Patients
The present retrospective study assessed the
clinical records of patients diagnosed with ED-SCLC who received
atezolizumab in addition to carboplatin and etoposide combination
therapy for SCLC between August 2019 and September 2020 in four
institutions (Toyama Prefectural Central Hospital, Toyama, Japan;
International Medical Center, Saitama Medical University, Saitama,
Japan; Jichi Medical University, Saitama Medical Center, Saitama,
Japan; and University of Fukui, Fukui, Japan). The inclusion
criteria were: i) Cytological or histopathological diagnosis of
stage III/IV SCLC without curative radiotherapy or postoperative
recurrence; ii) first-line chemotherapy with carboplatin and
etoposide; and iii) addition of atezolizumab during carboplatin and
etoposide combination therapy. Key exclusion criteria were: i) A
history of previous treatment with immune-checkpoint blockade
therapies; and ii) having no measurable lesions to assess tumor
shrinkage efficacy. The pathological stage was evaluated based on
the Tumor-Node-Metastasis (TNM) classification of the Union for
International Cancer Control, eighth edition (11). The Eastern Cooperative Oncology
Group (ECOG)-PS scale scores range from 0–4, where low scores
indicate a good general condition and high scores signal a poor
prognosis (7). A PS of 0 indicated
the best general condition and a PS of 4 indicated the poorest
general condition. Before therapy, all patients underwent
systematic evaluation and standardized staging procedures. The
clinical stage was assigned according to the results of physical
examination, chest X-ray, thoracic and abdominal computed
tomography (CT), brain CT or magnetic resonance imaging, and bone
scintigraphy or 18F-fluorodeoxyglucose positron emission
tomography to evaluate the TNM stage. Aspiration cytology and/or
biopsy as part of the clinical staging procedure was performed as
needed. Data were extracted from the medical records of eligible
patients. Data from some patients (7 patients) who received
atezolizumab plus carboplatin and etoposide were used in a
previously reported analysis (12).
The present study was approved by the Institutional Review Board of
the International Medical Center of Saitama Medical University
(Hidaka, Japan; approval no. 2021-113). All procedures complied
with the ethical standards of the institutional and/or national
research committee and the Declaration of Helsinki of 1964 and its
subsequent amendments or comparable ethical standards. As this was
a retrospective study, the requirement for informed consent was
waived.
Evaluation of treatment and
response
None of the patients in the cohort had previously
received ICIs, including atezolizumab, carboplatin or combination
etoposide chemotherapy. Each patient was administered up to six
cycles of carboplatin [area under the curve (AUC) of 3.5-5
min/mg/ml; intravenous injection on day 1 of each cycle] and
etoposide (60–100 mg/m2 body surface area; intravenous
injection on days 1–3 of each cycle), followed by atezolizumab
maintenance every 3 weeks. Atezolizumab (fixed dose of 1,200 mg,
intravenous injection on day 1 of each cycle) was added to the
carboplatin and etoposide therapy based on the attending
physician's decision. Granulocyte colony-stimulating factor was
administered as a prophylaxis against neutropenia at the discretion
of the attending physician. Treatment was terminated when disease
progression or irreversible toxicity was observed, or when the
patient withdrew consent to chemotherapy. When treatment failure
occurred with the combination of atezolizumab with carboplatin and
etoposide therapy, subsequent chemotherapy with a cytotoxic drug or
best supportive care alone was performed at the discretion of the
treating physician.
Radiographic tumor responses were evaluated in line
with the best overall response and maximum tumor shrinkage
according to the Response Evaluation Criteria for Solid Tumors,
version 1.1 (13).
Treatment-related toxicities were classified according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events (version 5.0) (14).
Statistical analysis
PFS time was calculated from the first day of
carboplatin and etoposide combination chemotherapy until
progressive disease (PD) or death for any reason. OS time was
calculated from the first day of carboplatin and etoposide
combination chemotherapy until death or censored on the day of the
last consultation. Post-progression survival (PPS) time was
calculated as the period from PD to death or censored on the date
of the last consultation or follow-up. PFS and OS times were
evaluated using the Kaplan-Meier method. All statistical analyses
were performed using JMP statistical software, version 11.0, for
Windows (SAS Institute, Inc.).
Results
Patient backgrounds
A total of 98 patients were screened who received
atezolizumab with carboplatin and etoposide combination
chemotherapy. Atezolizumab was added from the middle course of
carboplatin and etoposide therapy in 16 patients, who were
evaluated in the present analysis. The patient selection diagram is
shown in Fig. S1. The
characteristics of the 16 patients are listed in Table I and detailed clinical information
for each patient is presented in Table
II. The median age of all patients was 73.5 years (range, 62–79
years), and 14 patients (87.5%) were male. Stage III disease was
observed in 1 patient, and stage IV in 15 patients. ECOG-PS at the
first administration of carboplatin and etoposide was 0, 1, 2 and 3
in 3, 4, 5 and 4 patients, respectively. Meanwhile, ECOG-PS in
patients with the addition of atezolizumab was 0, 1 and 2 in 2, 13
and 1 patient, respectively. The number of cycles with atezolizumab
addition was 2, 3, 4, and 5 in 10, 3, 2 and 1 patient,
respectively. The median number of carboplatin and etoposide (plus
atezolizumab) administration cycles was 4 (range, 1–6). The most
common doses of carboplatin and etoposide were AUC 5 mg/min/ml and
100 mg/m2, respectively (n=10; 62.5%). Only 1 patient
received palliative radiotherapy before atezolizumab
administration. The most common reason for adding atezolizumab to
carboplatin and etoposide therapy was poor PS, followed by
complications from autoimmune diseases.
 | Table I.Baseline patient characteristics
(n=16). |
Table I.
Baseline patient characteristics
(n=16).
| Characteristic | Value |
|---|
| Sex, n |
|
| Male | 14 |
|
Female | 2 |
| Age, years |
|
|
Median | 73.5 |
|
Range | 62–79 |
| Disease stage, n |
|
| III | 1 |
| IV | 15 |
|
Postoperative recurrence | 0 |
| ECOG-PS at first
administration of carboplatin + etoposide, n |
|
| 0 | 3 |
| 1 | 4 |
| 2 | 5 |
| 3 | 4 |
| 4 | 0 |
| Smoking status,
n |
|
| Yes | 16 |
| No | 0 |
| Histology, n |
|
| Small
cell carcinoma | 16 |
| Combined
small cell carcinoma | 0 |
| History of
postoperative adjuvant chemotherapy, n |
|
| Yes | 0 |
| No | 16 |
| Intracranial
metastases at initial treatment, n |
|
| Yes | 6 |
| No | 10 |
| Liver metastases at
initial treatment, n |
|
| Yes | 5 |
| No | 11 |
| Bone metastases at
initial treatment, n |
|
| Yes | 6 |
| No | 10 |
| Cycle at addition of
atezolizumab, n |
|
| 2 | 10 |
| 3 | 3 |
| 4 | 2 |
| 5 | 1 |
| ECOG-PS at addition
of atezolizumab, n |
|
| 0 | 2 |
| 1 | 13 |
| 2 | 1 |
| 3 | 0 |
| 4 | 0 |
| Number of cycles
carboplatin + etoposide (+ atezolizumab) administered |
|
|
Median | 4 |
|
Range | 1–6 |
| Number of cycles of
atezolizumab maintenance therapy administered |
|
|
Median | 2.5 |
|
Range | 0–15 |
| Starting dose,
n |
|
| CBDCA
(AUC 5) + etoposide (100 mg/m2) | 10 |
| CBDCA
(AUC 5) + etoposide (80–99 mg/m2) | 1 |
| CBDCA
(AUC 4) + etoposide (80–99 mg/m2) | 3 |
| CBDCA
(AUC 5) + etoposide (<80 mg/m2) | 1 |
| CBDCA
(AUC 3.5) + etoposide (<80 mg/m2) | 1 |
| With or without
G-CSF prophylaxis, n |
|
|
Yes | 14 |
| No | 2 |
| Prior radiotherapy,
n |
|
|
Yes | 1 |
| No | 15 |
| Reason for addition
of atezolizumab to carboplatin + etoposidea, n |
|
| Poor
PS | 7 |
| Due to
complications of immune disease | 4 |
|
Concurrent with
radiotherapy | 2 |
| Due to
the extensive tumor | 1 |
|
Elderly | 1 |
| Due to
complications of pneumothorax | 1 |
| Steroid treatment
for adverse eventsb,
n |
|
|
Yes | 3 |
| No | 13 |
| Continuing
administration of atezolizumab at data cutoff, n |
|
|
Yes | 1 |
| No | 15 |
 | Table II.Detailed list of individual
patients. |
Table II.
Detailed list of individual
patients.
| Patient | Age, years | Sex | Smoking history, BI
index | Stage | PS at initiation of
CE | Cycle no. at
addition of atezolizumab | Reason for
addition | PS at addition of
atezolizumab | Total CE
administration cycles | Best overall
response | PFS, months | OS, months | Death event | irAE | No. of
subsequent-treatment lines |
|---|
| 1 | 78 | M | 1040 | IVB | 1 | 4 | Elderly | 1 | 4 | PR | 5.8 | 24.4 | Yes | - | 2 |
| 2 | 76 | F | 560 | IVB | 2 | 4 | Poor PS (PS 2) | 1 | 4 | PR | 5.4 | 8.7 | Yes | - | 1 |
| 3 | 66 | F | 860 | IVA | 1 | 2 | Concurrent with
palliative radiotherapy | 1 | 4 | PR | 10.4 | 22.3 | No | Hypothyroidism
grade 2 | 1 |
| 4 | 63 | M | 630 | IVB | 2 | 2 | Poor PS (PS 2) | 1 | 2 | PD | 0.8 | 2.3 | No | - | 1 |
| 5 | 75 | M | 1480 | IVA | 0 | 2 | Complications of
immune disease (untreated hyperthyroidism) | 0 | 4 | PR | 2.6 | 11.3 | Yes | - | 1 |
| 6 | 72 | M | 2400 | IVA | 1 | 3 | Complications of
immune disease (ILD) | 1 | 4 | PR | 4.9 | 7.0 | Yes | Pneumonitis grade
3 | 0 |
| 7 | 75 | M | 1080 | IIIA | 0 | 3 | Complications of
immune disease (ILD) | 0 | 4 | PR | 14.3 | 14.3 | No | Adrenal
insufficiency grade 2, skin rash grade 1 | On maintenance
therapy at data cut-off |
| 8 | 65 | M | 1800 | IVA | 3 | 2 | Poor PS (PS 3) | 2 | 6 | SD | 4.4 | 6.6 | Yes | - | 1 |
| 9 | 72 | M | 520 | IVB | 2 | 2 | Extensive
tumor | 1 | 4 | PR | 11.2 | 12.9 | No | - | 1 |
| 10 | 74 | M | 1120 | IVB | 2 | 2 | Poor PS (PS 2) | 1 | 2 | PR | 2.0 | 2.0 | Yes | - | 0 |
| 11 | 73 | M | 2120 | IVB | 2 | 5 | Poor PS (PS 2) | 1 | 4 | PR | 8.0 | 21.9 | Yes | - | 3 |
| 12 | 75 | M | 1560 | IVB | 3 | 2 | Poor PS (PS 3) | 1 | 3 | PR | 5.3 | 14.0 | Yes | - | 1 |
| 13 | 74 | M | 1650 | IVB | 3 | 3 | Poor PS (PS 3) | 1 | 4 | PR | 7.4 | 12.9 | No | - | 0 |
| 14 | 79 | M | 2400 | IVB | 3 | 2 | Concurrent with
palliative radiotherapy | 1 | 4 | SD | 7.8 | 10.0 | Yes | - | 0 |
| 15 | 70 | M | 960 | IVA | 0 | 3 | Complications of
pneumothorax | 1 | 4 | PR | 3.1 | 13.0 | Yes | - | 1 |
| 16 | 62 | M | 1260 | IVB | 1 | 2 | Complications of
immune disease (RA) | 1 | 4 | PD | 2.7 | 4.5 | Yes | - | 1 |
Treatment efficacy
Treatment response results are shown in Table III. The median follow-up period
was 12.1 months (range, 1.9-24.3 months). Although a complete
response was not achieved in any patient, a partial response (PR)
was observed in 12 patients, stable disease (SD) in 2 and PD in 2.
The response and disease control rates were 75.0% [95% confidence
interval (CI), 50.0-90.2] and 87.5% (95% CI, 62.7-97.7),
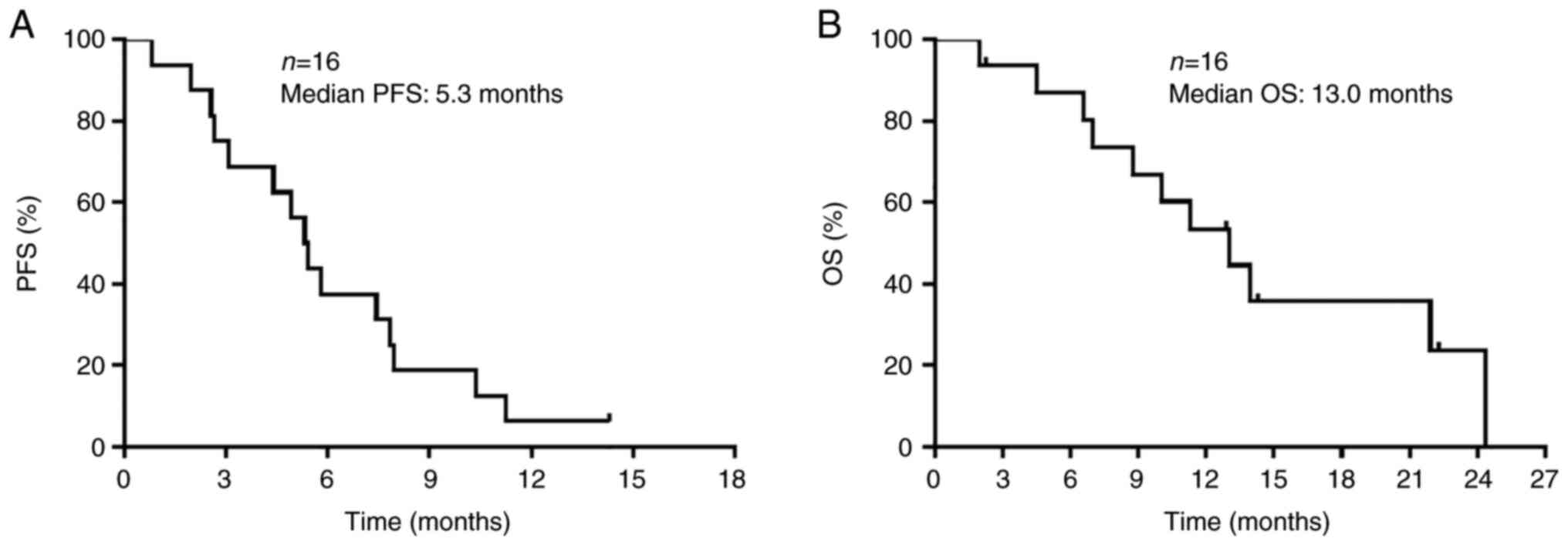
respectively. The median PFS and OS times were 5.3 months (95% CI,
2.6-7.8 months) and 13.0 months (95% CI, 6.9-24.3 months),
respectively (Fig. 1). All 11
deaths in the study were directly attributable to SCLC events. The
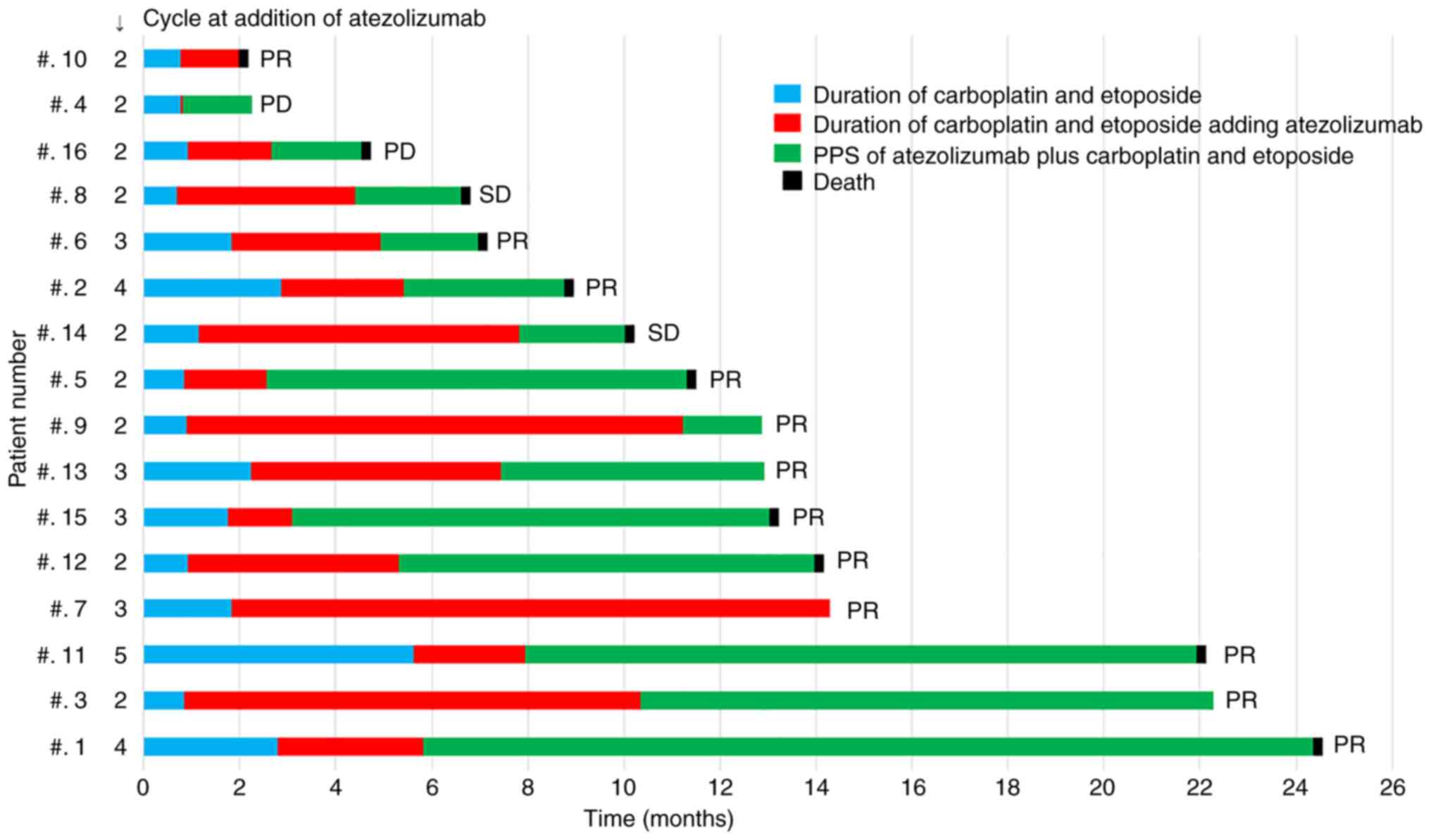
swimmer plot shown in Fig. 2 shows
the duration of carboplatin and etoposide treatment, the duration
of atezolizumab plus carboplatin and etoposide treatment, and the
duration of PPS of atezolizumab plus carboplatin and etoposide
after PD in all 16 patients. Long-term survivors with a median
survival time of ≥13.0 months tended to have a longer PPS after
adding atezolizumab to carboplatin and etoposide therapy, with PPS
accounting for more than one-half of the survival period, except
for 1 patient who remained on atezolizumab maintenance therapy. All
patients achieved a PR.
 | Table III.Treatment response. |
Table III.
Treatment response.
| Treatment
response | Value |
|---|
| Complete response,
n | 0 |
| Partial response,
n | 12 |
| Stable disease,
n | 2 |
| Progressive
disease, n | 2 |
| Not evaluated | 0 |
| Response rate, %
(95% CI) | 75.0
(50.0–90.2) |
| Disease control
rate, % (95% CI) | 87.5
(62.7–97.7) |
Toxicity
Treatment-related adverse events were assessed in
all 16 patients (Table IV).
Adverse events of any grade were observed in all 16 patients, and
an adverse event that led to discontinuation of treatment
(pneumonia grade 3) occurred in 1 patient. The most common
treatment-related adverse event was hematological toxicity. The
most frequent adverse events of any grade were decreased platelet
count (n=15; 93.8%), anemia (n=14; 87.5%), decreased white blood
cell count (n=11; 68.8%), and decreased neutrophil count (n=10;
62.5%). The incidence of immune-related adverse events was
generally low. In 1 patient with a grade ≥3 adverse event, a
diagnosis of grade 3 pneumonitis was seen as a serious enough
adverse event to discontinue treatment. The patient received
prednisolone (0.5 mg/kg) and died of the primary disease. No deaths
due to the combination of carboplatin and etoposide (plus
atezolizumab) were observed during the study period.
 | Table IV.Adverse events. |
Table IV.
Adverse events.
| Adverse event | Any grade, n
(%) | Grade ≥3, n
(%) |
|---|
| Led to
discontinuation | 1 (6.3) | 1 (6.3) |
| Led to death | 0 (0.0) | 0 (0.0) |
|
Treatment-relateda |
|
|
| White
blood cell decreased | 11 (68.8) | 8 (50.0) |
|
Neutrophil count
decreased | 10 (62.5) | 9 (56.3) |
|
Anemia | 14 (87.5) | 3 (18.8) |
|
Platelet count decreased | 15 (93.8) | 7 (43.8) |
| Febrile
neutropenia | 2 (12.5) | 2 (12.5) |
|
Immune-relatedb |
|
|
|
Pneumonitis | 1 (6.3) | 1 (6.3) |
| Skin
rash | 1 (6.3) | 0 (0.0) |
|
Hypothyroidism | 1 (6.3) | 0 (0.0) |
| Adrenal
insufficiency | 1 (6.3) | 0 (0.0) |
Subsequent treatments
Among the 15 patients who developed PD, 11 received
subsequent-line chemotherapy. The most common second-line
chemotherapy was amrubicin monotherapy. A total of 5 patients did
not receive subsequent treatment for PD after first-line treatment
and were treated with the best supportive care alone. The
subsequent treatments administered beyond PD are listed in Table SI.
Discussion
The efficacy and feasibility of the therapeutic
option of adding atezolizumab during carboplatin and etoposide
treatment in patients with SCLC has remained to be determined. In
the present study, atezolizumab was added to carboplatin and
etoposide therapy during treatment, and this revealed favorable
efficacy with no new safety concerns in patients with SCLC. This
outcome suggests its tolerability in patients with SCLC with
problems, such as a poor PS and immune disease complications, at
the start of the initial treatment. The efficacy of treatment in
the patients enrolled in the present analysis was comparable to
that of the atezolizumab plus carboplatin and etoposide arm of the
phase III IMpower133 trial (5).
Furthermore, the ORR was comparable, while median PFS and OS times
in the present study were slightly shorter than those from the
atezolizumab plus carboplatin and etoposide clinical practice data
in our previous study, which were 73.8%, 5.4 and 15.9 months,
respectively (15). The results of
the present study suggest that this treatment efficacy is
comparable to the conventional results of atezolizumab with
carboplatin and etoposide therapy from the first cycle of treatment
and may be a sufficient option for clinical practice. Thus, the
feasibility of these treatments is considered adequate. It should
be emphasized that the novelty of the present study is the clinical
significance of intermittent addition of atezolizumab to
carboplatin and etoposide therapy during treatment since some
concern prevented their initial combination.
Detailed data from the patients are presented in
Table II and Fig. 2. In the cohort, 7 of the 16 patients
received atezolizumab from an intermittent cycle of carboplatin and
etoposide due to a PS of 2–3. Of these patients, 6 improved to a PS
of 1, and 1 improved from a PS of 3 to a PS of 2. Therefore,
atezolizumab was added to the therapy. There were 4 patients with
autoimmune diseases who did not receive atezolizumab at the
beginning of carboplatin and etoposide therapy. However, their
autoimmune diseases varied widely, and 1 patient with interstitial
pneumonia (patient 6) developed drug-induced interstitial
pneumonitis after receiving atezolizumab. However, the treatment
choice of starting additional doses of atezolizumab after the
subsequent cycle is at the discretion of the treating physician and
is inconsistent, as shown in Table
II. Additionally, in a small number of patients, it is
difficult to determine whether there is a survival benefit from
adding atezolizumab to the combination of carboplatin and etoposide
for various reasons during drug therapy. However, all 6 long-term
survivors, with a median survival of ≥13 months, achieved a PR and
a PS of 0–1 at the beginning of atezolizumab administration. Of
these 6 patients, PPS after PD with atezolizumab plus carboplatin
and etoposide combination therapy represented more than one-half of
the OS time period. These patients received at least one line of
anticancer agent therapy as a subsequent treatment, except for 1
patient who continued atezolizumab maintenance therapy at the data
cut-off. Of the 6 patients, 4 started with additional atezolizumab
after the third cycle. Additionally, all patients with SD or PD had
a below median OS time, and 2 patients with PD had a poor OS time
of <5 months. A patient with PR (patient 10) died of cerebral
infarction and had a short OS time of 2.0 months. Based on the
aforementioned data, long-term OS time can be expected even in
patients who start combination therapy with carboplatin and
etoposide, if the addition of atezolizumab is considered even in
the latter half of the combination therapy and if PPS, which
accounts for more than half of the OS time period, is
controlled.
Regarding safety, the addition of atezolizumab
during carboplatin and etoposide treatment was well managed in the
current population. However, the percentage of patients who
exhibited hematological toxicities, such as decreased neutrophil,
white blood cell and platelet count, was higher in the patients in
the present study than in the IMpower133 study (5). Furthermore, hematological toxicity was
somewhat higher than that in our previously reported cohort,
starting with atezolizumab plus carboplatin and etoposide
chemotherapy (15). It is possible
that hematologic toxicity was slightly higher in this population
due to poor PS or other reasons that would have prevented the
administration of atezolizumab from the first cycle. These
perceptions suggest that the toxicity signal from the addition of
atezolizumab during the carboplatin and etoposide treatment period
may be feasible in patients with SCLC who have some problems at the
beginning of initial therapy.
Although the combination of atezolizumab plus
carboplatin and etoposide has recently become a standard treatment
choice for patients with ED-SCLC in good general condition, such as
a good PS, good organ function and no comorbidities, the efficacy
and feasibility of atezolizumab to carboplatin and etoposide
treatment for patients with SCLC with any problems at the beginning
of initial treatment have not been evaluated. The present analysis
demonstrated that treatment with atezolizumab in combination with
carboplatin and etoposide therapy has a favorable effect in any
untreated patients with ED-SCLC who have problems at the start of
first-line treatment. While previous phase III trials on ICIs, such
as atezolizumab and durvalumab in combination with platinum and
etoposide, have focused on patients with a good PS (PS, 0–1) and
enrolled patients with strict eligibility criteria, such as lack of
palliative radiotherapy or absence of complications of autoimmune
or interstitial lung diseases (5,16), the
patients in the present study not only had a poor PS, but were also
heterogeneous in their population characteristics (i.e., patients
included those with autoimmune diseases, elderly patients and
patients who had started concurrent radiotherapy). However, the
efficacy and safety results of this analysis suggest that the
addition of atezolizumab to carboplatin and etoposide therapy may
be a treatment option for such patients.
There are some limitations to the present analysis.
Firstly, the current study was retrospective with a small sample
size. Therefore, it is an exploratory study and cannot be
definitive. Although it does suggest a possible treatment option,
further validations are needed to evaluate the clinical efficacy
and feasibility of the findings. Secondly, the treatment strategy
of starting carboplatin and etoposide at the beginning of initial
treatment and adding atezolizumab during the treatment cycle is
largely at the discretion of the physician according to the policy
of each institution. Similarly, the physician decides whether to
reduce, delay or skip chemotherapeutic agents. These decisions of
individual physicians and institutions are undeniably subject to
selection bias, which is an inherent s of retrospective analyses.
It is necessary to interpret the present results cautiously, as
this bias may have affected the effectiveness of the treatment.
The results of the present study demonstrate that
adding atezolizumab to carboplatin and etoposide therapy during the
treatment cycle may be a tolerable therapeutic option with
favorable efficacy for patients with SCLC with any problems at the
start of initial treatment. The present study may therefore provide
a new direction in the treatment strategy for patients with any
problems at the beginning of the initial treatment cycle. However,
this is a small retrospective study, and further validation in
clinical practice is needed.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Kyoko Nakagawa
(International Medical Center, Saitama Medical University, Saitama,
Japan), Dr Kosuke Hashimoto (International Medical Center, Saitama
Medical University, Saitama, Japan), Dr Yu Miura (International
Medical Center, Saitama Medical University, Saitama, Japan), Dr
Fuyumi Nishihara (International Medical Center, Saitama Medical
University, Saitama, Japan) and Dr Kunihiko Kobayashi
(International Medical Center, Saitama Medical University, Saitama,
Japan) for their assistance in preparing the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT and HI designed the study. YN, YU, AS, JS, OY,
AM, HT, TI and HK were responsible for the acquisition of data, and
HI and KK performed the analysis and interpretation of data. TT, HI
and KK prepared and wrote the original draft of the manuscript. YN,
YU, AS, JS, OY, AM, HT, TI and HK reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript. TT, HI and KK confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All procedures complied with the ethical standards
of the institutional and/or national research committee and the
1964 Helsinki Declaration and its subsequent amendments or
comparable ethical standards. The study design was approved by the
Institutional Ethics Committee of The International Medical Center,
Saitama Medical University (Hidaka, Japan; approval no. 2021-113).
The requirement of written informed consent was waived by the
ethics committee of Saitama Medical University due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
CT
|
computed tomography
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
ED
|
extensive disease
|
|
ICI
|
immune checkpoint inhibitor
|
|
ORR
|
objective response rate
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PFS
|
progression-free survival
|
|
PPS
|
post-progression survival
|
|
PR
|
partial response
|
|
PS
|
performance status
|
|
SCLC
|
small cell lung cancer
|
|
SD
|
stable disease
|
|
TNM
|
Tumor-Node-Metastasis
|
References
|
1
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health. 85:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Byers LA and Rudin CM: Small cell lung
cancer: Where do we go from here? Cancer. 121:664–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farago AF and Keane FK: Current standards
for clinical management of small cell lung cancer. Transl Lung
Cancer Res. 7:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schabath MB, Nguyen A, Wilson P, Sommerer
KR, Thompson ZJ and Chiappori AA: Temporal trends from 1986 to 2008
in overall survival of small cell lung cancer patients. Lung
Cancer. 86:14–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu SV, Reck M, Mansfield AS, Mok T,
Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J,
Califano R, Nishio M, et al: Updated overall survival and PD-L1
subgroup analysis of patients with extensive-stage small-cell lung
cancer treated with atezolizumab, carboplatin, and etoposide
(IMpower133). J Clin Oncol. 39:619–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Girling DJ: Comparison of oral etoposide
and standard intravenous multidrug chemotherapy for small-cell lung
cancer: A stopped multicentre randomised trial. Medical research
council lung cancer working party. Lancet. 348:563–566. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souhami RL, Spiro SG, Rudd RM, de Elvira
MC, James LE, Gower NH, Lamont A and Harper PG: Five-day oral
etoposide treatment for advanced small-cell lung cancer: Randomized
comparison with intravenous chemotherapy. J Natl Cancer Inst.
89:577–580. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto H, Watanabe K, Kunikane H,
Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T and
Saijo N: Randomised phase III trial of carboplatin plus etoposide
vs split doses of cisplatin plus etoposide in elderly or poor-risk
patients with extensive disease small-cell lung cancer: JCOG 9702.
Br J Cancer. 97:162–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai H, Nagai Y, Minemura H, Tsuda T,
Yamada Y, Wasamoto S, Kishikawa T, Shiono A, Shiihara J, Yamaguchi
O, et al: Efficacy and safety of amrubicin monotherapy after
atezolizumab plus carboplatin and etoposide in patients with
relapsed small-cell lung cancer. Invest New Drugs. 40:1066–1079.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Cancer Institute, . CTCAEv 5.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40January
4–2023
|
|
15
|
Shiono A, Imai H, Wasamoto S, Tsuda T,
Nagai Y, Minemura H, Yamada Y, Kishikawa T, Umeda Y, Takechi H, et
al: Real-world data of atezolizumab plus carboplatin and etoposide
in elderly patients with extensive-disease small-cell lung cancer.
Cancer Med. 12:73–83. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small cell lung cancer
(Caspian): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|