Spandidos Publications style
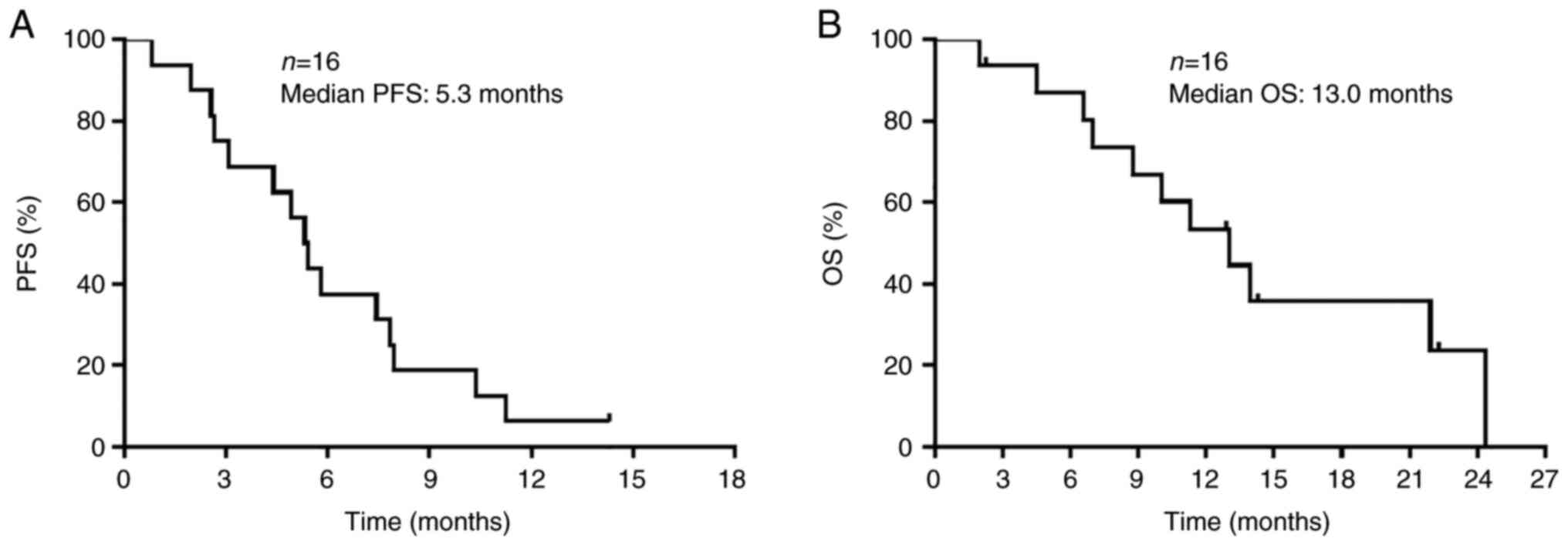
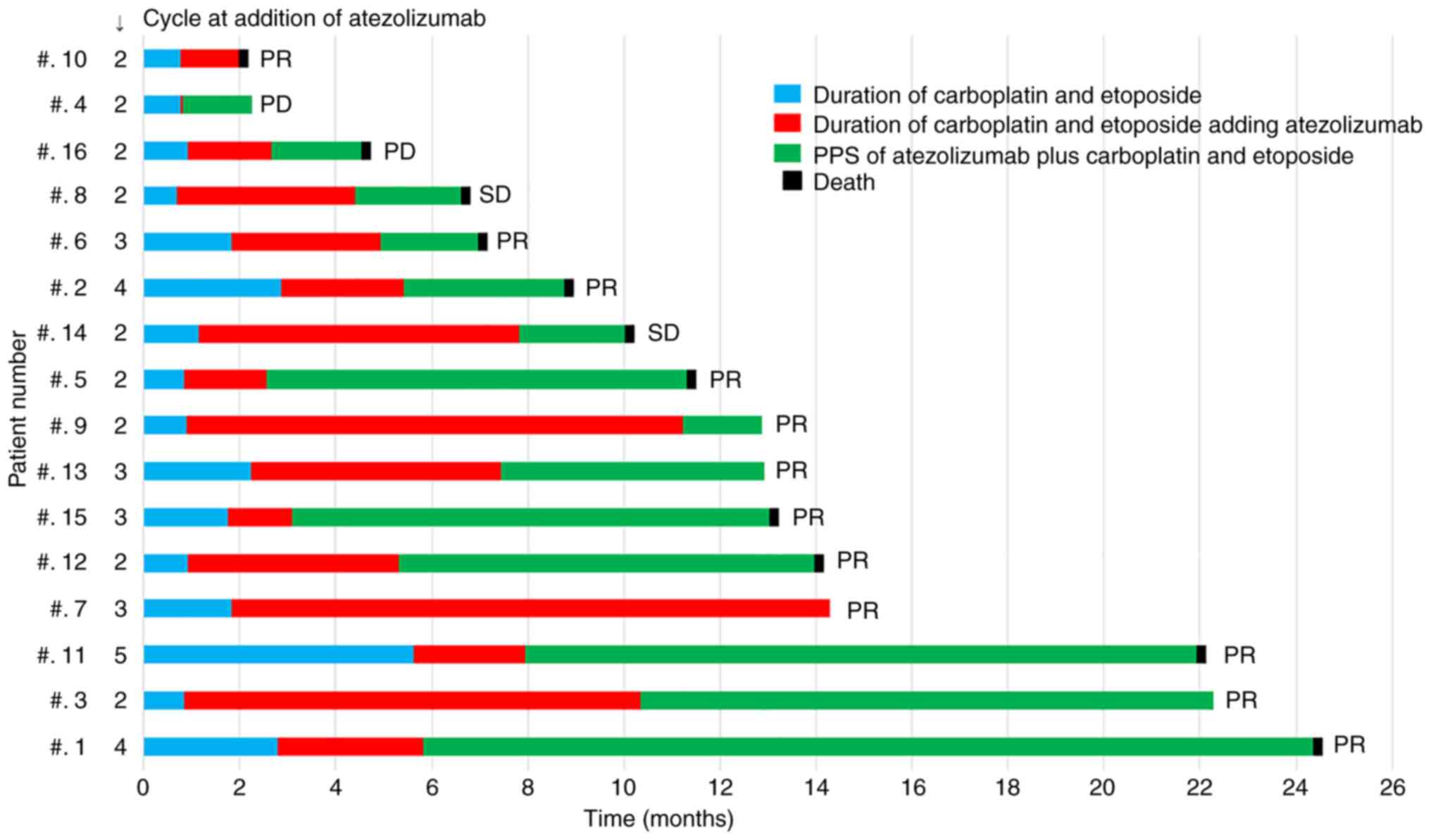
Tsuda T, Imai H, Nagai Y, Umeda Y, Shiono A, Shiihara J, Yamaguchi O, Mouri A, Kaira K, Ishizuka T, Ishizuka T, et al: Intermittent administration of atezolizumab with combined carboplatin and etoposide therapy for patients with extensive‑disease small cell lung cancer. Oncol Lett 25: 111, 2023.
APA
Tsuda, T., Imai, H., Nagai, Y., Umeda, Y., Shiono, A., Shiihara, J. ... Kagamu, H. (2023). Intermittent administration of atezolizumab with combined carboplatin and etoposide therapy for patients with extensive‑disease small cell lung cancer. Oncology Letters, 25, 111. https://doi.org/10.3892/ol.2023.13696
MLA
Tsuda, T., Imai, H., Nagai, Y., Umeda, Y., Shiono, A., Shiihara, J., Yamaguchi, O., Mouri, A., Kaira, K., Ishizuka, T., Taniguchi, H., Kagamu, H."Intermittent administration of atezolizumab with combined carboplatin and etoposide therapy for patients with extensive‑disease small cell lung cancer". Oncology Letters 25.3 (2023): 111.
Chicago
Tsuda, T., Imai, H., Nagai, Y., Umeda, Y., Shiono, A., Shiihara, J., Yamaguchi, O., Mouri, A., Kaira, K., Ishizuka, T., Taniguchi, H., Kagamu, H."Intermittent administration of atezolizumab with combined carboplatin and etoposide therapy for patients with extensive‑disease small cell lung cancer". Oncology Letters 25, no. 3 (2023): 111. https://doi.org/10.3892/ol.2023.13696