Introduction
Macamides are a distinct class of bioactive amide
alkaloids isolated from the plant Lepidium meyenii, also
known as maca, which has been extensively used as food or as a folk
medicine in Peru (1). In addition
to being safe for human consumption, macamides have been shown to
possess multiple biological activities in previous years (1,2). For
example, macamides have been shown to relieve exercise-induced
fatigue and regulate lipid metabolism by inhibiting the activity of
fatty acid amide hydrolase (3–5).
Macamides also display anti-inflammatory effects by reducing the
expression of proinflammatory factors and alleviating
inflammatory-induced pain, such as in colitis (6,7). In
addition, macamides can exert neuroprotective activity and
attenuate hypoxic-ischemic brain damage through the regulation of
apoptosis or autophagy (8,9). Fu et al (10) demonstrated that macamides possess
antitumor activity in multiple cancer cell lines, such as A549,
SW480 and SMMC-7721. However, to the best of our knowledge, there
have been no systematic experiments that reveal the role of
macamide B in specific types of cancer, especially in lung cancer,
which has a large number of patients worldwide.
Lung cancer is a serious malignancy with a very high
incidence and death rate worldwide (11). According to the latest statistical
report by the American Cancer Society, the estimated number of
newly diagnosed patients with lung cancer in the USA in 2021 will
be 119,100,whereas the number of deaths will be 131,880 (11). The situation is more serious in
China. Nearly 733,000 individuals suffer from lung cancer each year
and ~610,000 patients die from it (12,13).
However, the main therapies for lung cancer still consist of
surgical removal of tumor tissues followed by chemo/radiotherapy
(13,14). In addition to great pain and low
quality of life, these therapies provide little benefit for
patients with metastases and those with recurrence. As a result,
prognosis for this disease remains poor. The 5-year survival rate
for patients with lung cancer with distant metastasis is only 6%
and >55% of patients are diagnosed at a late stage (11). Therefore, the development of new
strategies to treat this disease is urgent.
In the present study, the activities of macamide B
on the proliferation, invasion and apoptosis of lung cancer cells
were evaluated. The mechanism of macamide B was also explored. In
addition, the combined effect of macamide B with olaparib, a poly
(ADP-ribose) polymerase (PARP) inhibitor, was determined. To the
best of our knowledge, for the first time, the present study
provides new evidence regarding the use of macamides in lung cancer
therapy.
Materials and methods
Cell culture and reagents
Human lung cancer cell lines,H460, H1299 and A549,
were purchased from Beyotime Institute of Biotechnology and were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin solution (Shanghai Yeasen Biotechnology
Co., Ltd.) under an atmosphere of 5% CO2 at 37°C. H460
and A549 cell lines contain the p53 gene, whereas the H1299 cell
line lacks the p53 gene. The two types of cell lines therefore may
better reflect the role of macamide B in lung cancer. Macamide B
(Chemical Abstracts Service no., 74058-71-2) was obtained from
Shanghai Yuanye Biotechnology Co., Ltd. and dissolved in DMSO
(Gibco; Thermo Fisher Scientific, Inc.). Small interfering RNA
(siRNA) oligonucleotides targeting the ataxia-telangiectasia
mutated (ATM) gene (siATM) were designed and chemically synthesized
by General Biosystems (Anhui) Co., Ltd. The siATM sequence was as
follows: 5'-AGGTGCTTATGAATCAACAAAAT-3'. The siRNA negative control
(siNC) sequence was as follows: 5'-AACACCGAACGAGACACGATT-3'.
Transfection of siRNA
siATM and siNC were transfected into lung cancer
cells. Briefly, 50 pmol siATM or siNC was mixed with 1 µl liposomal
transfection reagent (Shanghai Yeasen Biotechnology Co., Ltd.) for
20 min at room temperature. The mixture was added to lung cancer
cells (A549, H1299 and H460) that had been cultured for 12 h in
24-well plates at 50,000 cells/well, and the cells were then
cultured for 6 h at 37°C. Subsequently, fresh medium was added to
each well and the cells were cultured for another 48 h before
subsequent experiments were performed.
Cell proliferation and viability
assays
Cancer cells, including A549, H1299 and H460 cells,
were cultured in 96-well plates at 4×103 cells/well and
were treated with macamide B at concentrations of 1, 2, 4, 8, 16
and 32 µmol/l for 48 h at 37°C, or with macamide B at 3 µmol/l
(H1299 and H460 cells) and 4 µmol/l (A549 cells) for 24, 48 or 72 h
at 37°C. In the control group, PBS (Gibco; Thermo Fisher
Scientific, Inc.) was added. Subsequently, MTT reagent (5 mg/ml;
Shanghai Yeasen Biotechnology Co., Ltd.) was added to each well at
10% volume of the culture medium and cultured for another 4 h.
After incubation, the supernatants were gently removed, and
formazan was dissolved with 150 µl DMSO, followed by detection at
490 nm. The cell inhibition rate was calculated compared with the
PBS group, as follows: (OD value of macamide B group-e OD value of
PBS group)/OD value of PBS group.
To detect the IC50 value of olaparib
(Beyotime Institute of Biotechnology) in lung cancer cells,
5×103 cells/well were seeded in 96-well plates and were
treated with olaparib (5, 10, 20, 40, 80 and 160 nmol/l) for 48 h.
Subsequently, MTT reagent was added and OD490 was detected as
aforementioned. In addition, to assess the effects of a combination
of olaparib and macamide B on lung cancer cells, 20 nmol/l olaparib
was used alone or combined with macamide B to treat cancer cells
for 24, 48 and 72 h at 37°C. OD490 was detected as
aforementioned.
Cell invasion assay
Transwell inserts (pore size, 8 µm) were pretreated
with Matrigel (Corning, Inc.) at 37°C for 30 min before being
placed into 24-well plates. Cancer cells were then added into the
insert at 1×104 cells/well with 200 µl serum-free DMEM
and were treated with macamide B at 3 or 4 µmol/l, according to its
IC50 value for that cell line. DMEM containing 10% FBS
was added into the lower chamber. After culturing for 48 h, the
cells on the inner surface of the inserts were removed gently and
cells on the outer surface were fixed with 4% paraformaldehyde at
room temperature for 15 min followed by staining with 0.1% crystal
violet for 10 min at room temperature. Images of the positively
stained cells were captured and counted using a light microscope
(Olympus Corporation).
Cell apoptosis detection
Cancer cells were cultured in 6-well plates at
4×104 cells/well and treated with macamide B at 3 or 4
µmol/l for 48 h at 37°C. Cancer cells were then collected, washed
with PBS, stained with a dye solution containing 5 µl Annexin
V/FITC-A (Beyotime Institute of Biotechnology) for 15 min in the
dark at room temperature and analyzed with a
FACSCelesta™ flow cytometer (BD Biosciences, USA). The
data were analyzed with FlowJo software (FlowJo V10; FlowJo
LLC).
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using the RNAeasy
Kit (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions and was quantified on a OneDrop1000 UV
spectrophotometer (Nanjing Zihanmu Scientific Instrument Co.,
Ltd.). The first strand of cDNA was synthesized according to the
BeyoRT II cDNA kit (Beyotime Institute of Biotechnology) with
0.5-1.0 ng total RNA as the template. Subsequently, 1 µl cDNA
underwent qPCR to detect target genes using the BeyoFast SYBR Green
qPCR Mix (Beyotime Institute of Biotechnology) on the LineGene9600
(Hangzhou Bioer Co., Ltd.). The protocol for qPCR was as follows:
95°C for 5 min, followed by 95°C for 15 sec and 60°C for 20 sec for
40 cycles. GAPDH was used as the internal control. The relative
expression of target genes was calculated by the 2−ΔΔCq
method (15). The primers used were
as follows: ATM, forward5′-TGGAAGCTGCTTGGGAGAAG-3′, reverse
5′-AGGCCAGCATTGGATCTGTT-3′; GAPDH,
forward5′-GCACCGTCAAGGCTGAGAA-3′, reverse
5′-TAAGCAGTTGGTGGTGCAGG-3′.
Western blotting
Cancer cells were collected after treatment with
macamide B, PBS or siATM and total proteins were extracted using
the Protein Easy Extracting Kit (Beyotime Institute of
Biotechnology) followed by quantification using a OneDrop
spectrophotometer (OneDrop). A total of 10 µg protein was separated
by SDS-PAGE on a 12% gel, transferred onto PVDF membranes
(MilliporeSigma), blocked with 5% nonfat milk at room temperature
for 1 h, and incubated with antibodies against each target protein
for 12 h at 4°C. Subsequently, the membranes were washed with
TBS-Tween 20 (0.5%) (Tansoole) before being incubated with
HRP-conjugated secondary antibodies for 1 h at room temperature.
The membranes were then washed with TBS-Tween 20 buffer three times
and analyzed using an Enhanced ECL Chemiluminescent Substrate Kit
(Shanghai Yeasen Biotechnology Co., Ltd.) and Tanon 4600 Multi
detection equipment (Tanon Science and Technology Co., Ltd.,
Shanghai, China). GAPDH was used as the internal control. The
densitometry of each protein band was analyzed by ImageJ software
(Ver1.51j8; National Institutes of Health). The following
antibodies were used: ATM (cat. no. AF1399; dilution 1:5,000),
RAD51 (cat. no. AF7860; dilution 1:500), Bcl-2 (cat. no. AF0060;
dilution 1:1,000), p53 (cat. no. AF1162; dilution 1:5,000), cleaved
caspase-3 (cat. no. AC033; dilution 1:1,000) and HRP-conjugated
goat anti-rabbit IgG (cat. no. A0208; dilution 1:1,000) (all
purchased from Beyotime Institute of Biotechnology). Anti-GAPDH
(cat. no. ab181602; dilution 1:5,000) was purchased from Abcam.
Statistical analysis
The data in the present study were obtained from at
least three replicates, are displayed as the mean ± standard
deviation, and were analyzed using SPSS11.0 software (SPSS, Inc.).
Unpaired Student's t-test was employed to evaluate the difference
between two groups and one-way ANOVA followed by Tukey's post hoc
test was used for three groups or more. P<0.05 was considered to
indicate a statistically significant difference among groups.
Results
Macamide B inhibits the proliferation
of lung cancer cells
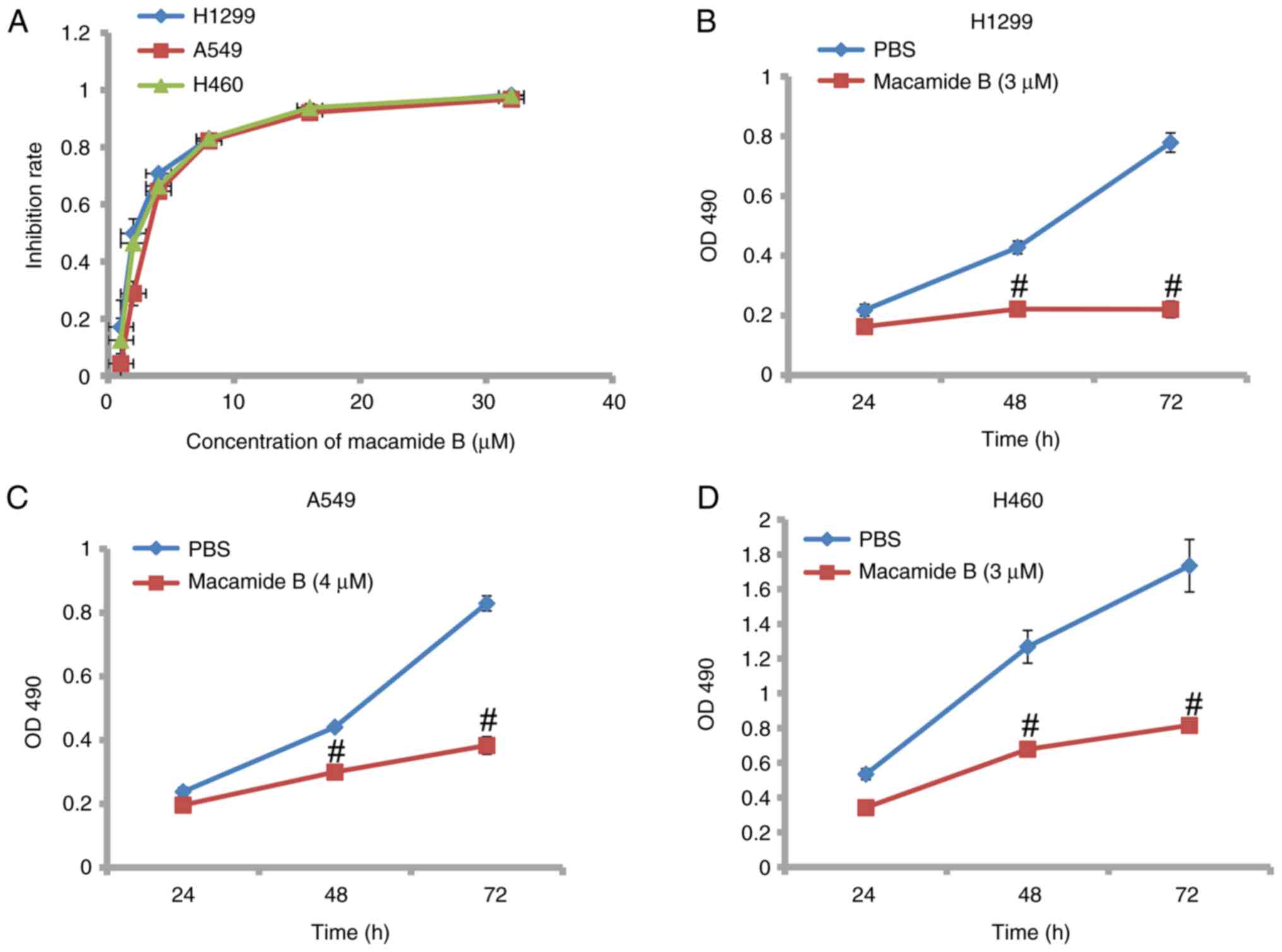
As shown in Fig. 1A,
macamide B exhibited potent dose-dependent inhibitory effects on
the proliferation of lung cancer cell lines. The IC50
value of macamide B was ~2.5, 3.7 and 2.8 µmol/l respectively in
H1299, A549 and H460 cells. The inhibitory effect of macamide B was
also time dependent. As shown in Fig.
1B-D, after 72 h of macamide B treatment, the difference in the
proliferation of H1299, A549 and H460 cells was much lower than at
48 h compared with the PBS control. These findings indicated that
macamide B inhibited the proliferation of lung cancer cell lines in
a dose- and time-dependent manner.
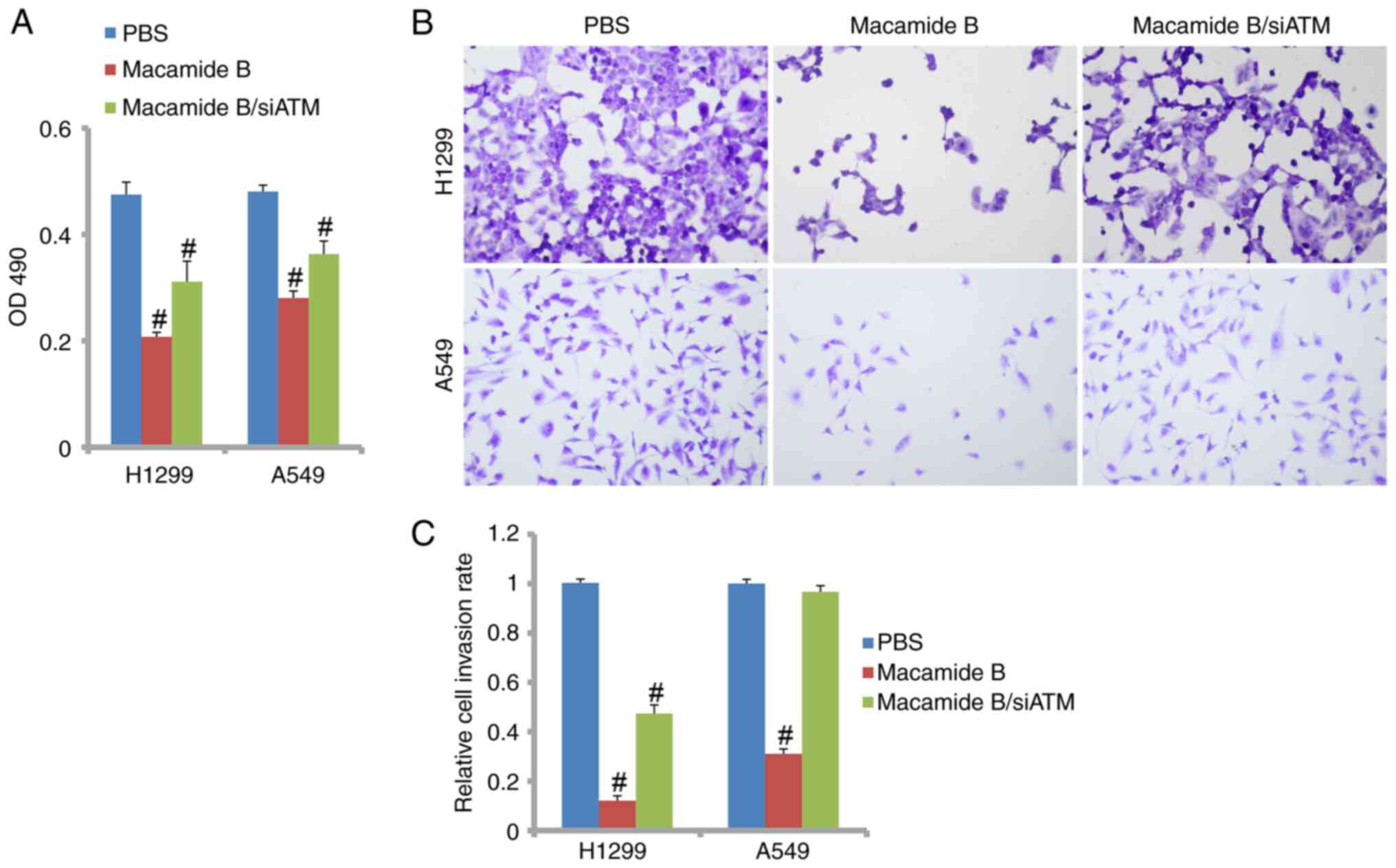
Macamide B inhibits the invasive
ability of lung cancer cells
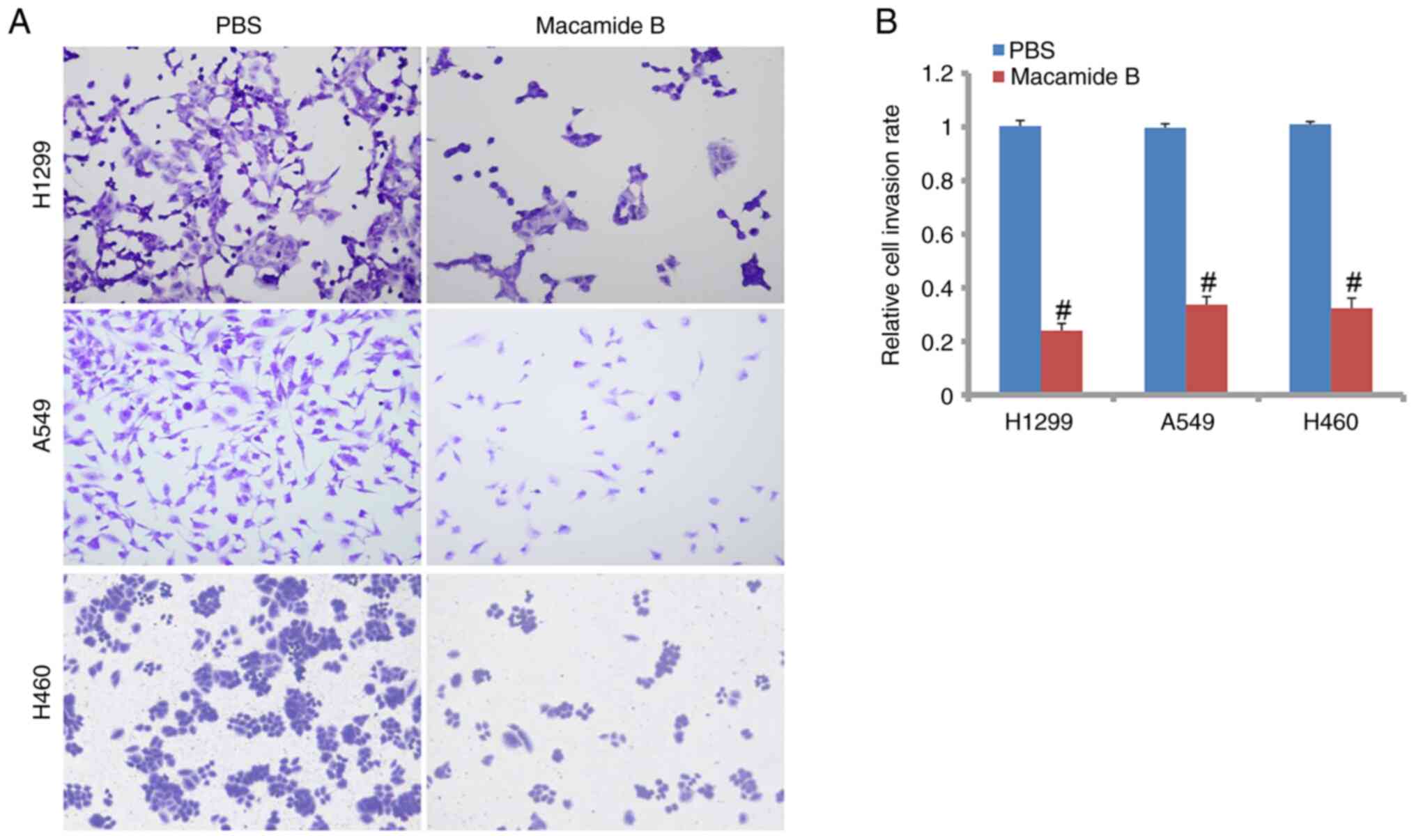
As shown in Fig. 2A and
B, cell invasive abilities were markedly suppressed by macamide
B compared with the PBS control in H1299, A549 and H460 cells. The
relative cell invasion rates of H1299, A549 and H460 cells were
24.1, 33.7 and 67.7% of that in the PBS group, respectively
(Fig. 2B). The differences between
the macamide B and PBS groups were significant.
Macamide B induces the apoptosis of
lung cancer cells
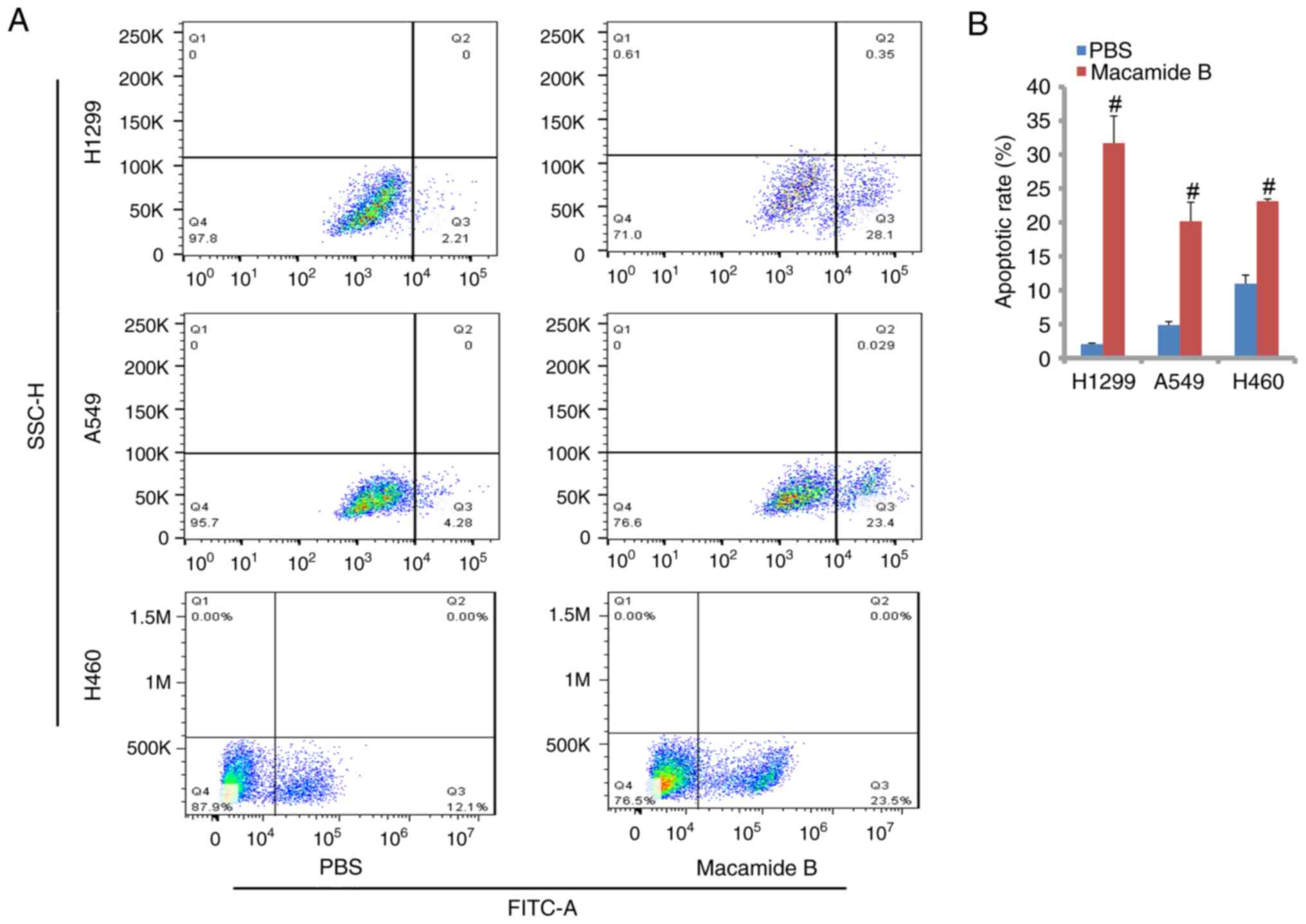
As shown in Fig. 3A and
B, macamide B exerted a potent apoptosis-promoting role in lung
cancer cell lines. The relative apoptotic rates in H1299, A549 and
H460 cells were 31.7, 20.2 and 23.1% in the macamide B group,
respectively. The differences between the macamide B and PBS groups
were significant.
Effects of a PARP inhibitor on lung
cancer cells are enhanced by macamide B
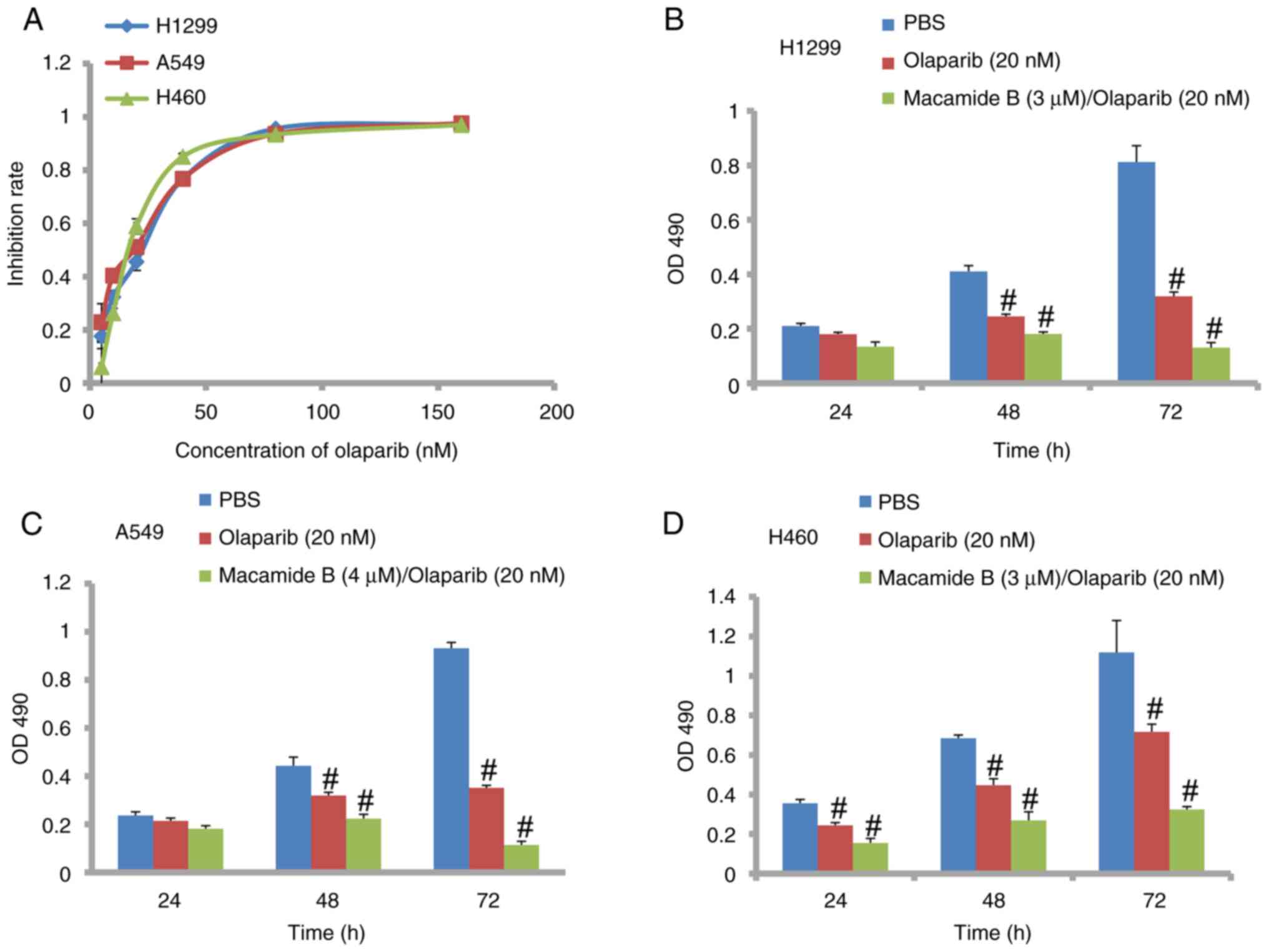
Fig. 4A shows that
olaparib, an inhibitor targeting PARP, displayed a
proliferation-suppressing role in lung cancer cells. The
IC50 values of olaparib in H1299, A549 and H460 cells
were 18.5, 15.6 and 19.1 nmol/l, respectively. It was also found
that the inhibitory effects of olaparib increased when combined
with macamide B. As shown in Fig.
4B-D, combined treatment with macamide B and olaparib exerted a
much greater inhibitory effect at both 48 and 72 h on H1299, A549
and H460 cells than olaparib alone. In H460 cells, the combined
group also showed a significant inhibitory effect at 24 h compared
with olaparib alone. This suggested that macamide B may increase
the sensitivity of cancer cells to olaparib.
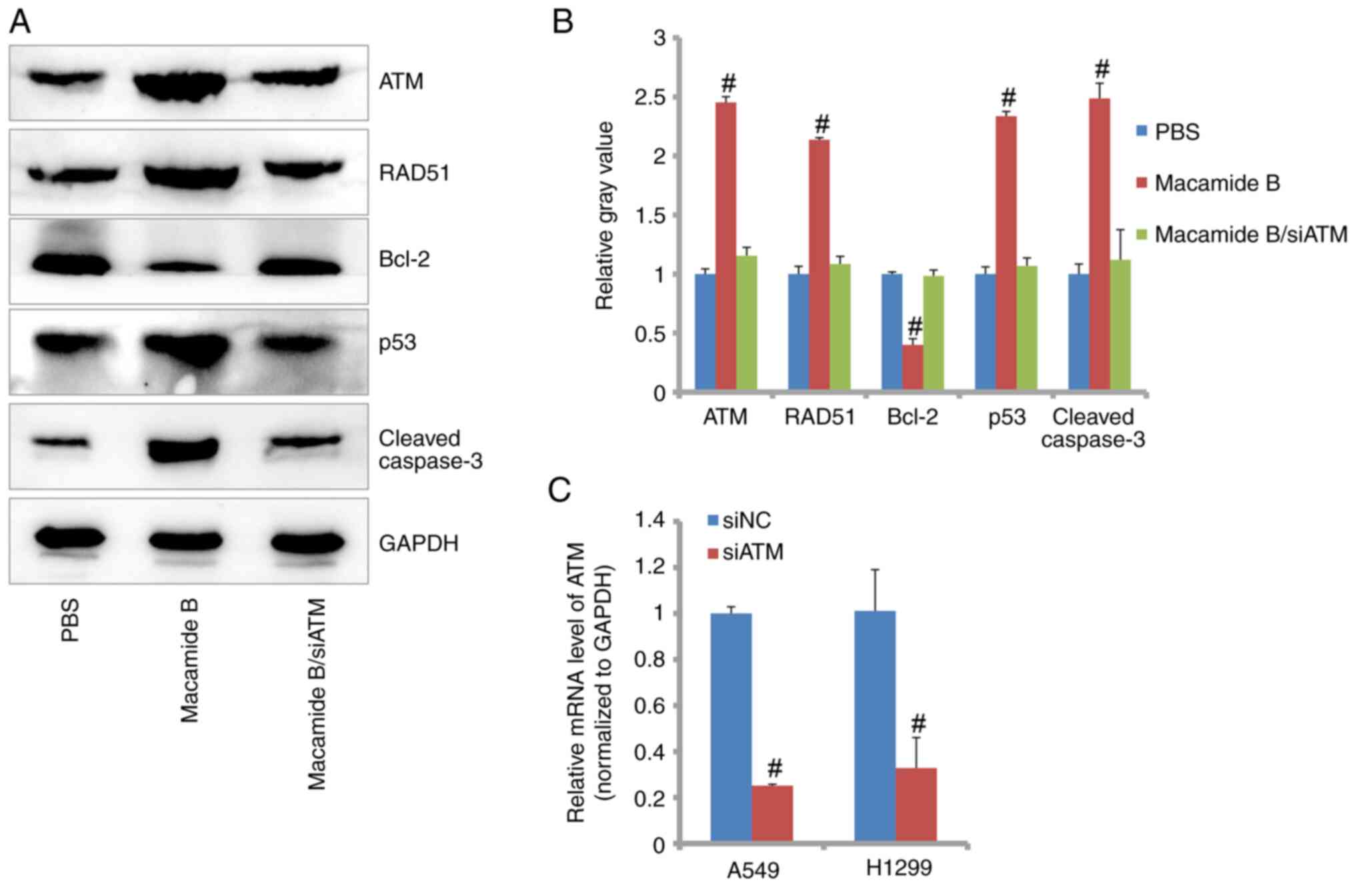
ATM signaling pathway is affected by
macamide B
Generally, the molecular signaling pathway is
explored in one cell line. In the present study, the A549 cell line
was used for this aim. As shown in Fig.
5A and B, using western blotting, it was revealed that the ATM
signaling pathway was activated by macamide B. The expression
levels of ATM were increased by ~2.5-fold compared with that in the
PBS control group. Consistently, the expression levels of
downstream proteins, including RAD51, p53 and cleaved caspase-3,
were also significantly increased by macamide B. However, Bcl-2
expression was reduced by macamide B. Furthermore, when ATM
expression was knocked down by siRNA targeting ATM (Fig. 5C), the expression patterns were
reversed (Fig. 5A and B). At the
cell level, the viability and invasiveness of H1299 and A549 cells
was affected when ATM was knocked down. H1299 cells lacks the p53
gene, whereas both A549 and H460 cells harbor the wildtype p53
gene; therefore, H1299 and A549 cells were used for further study.
As shown in Fig. 6A, cell viability
was suppressed by macamide B but ATM knockdown relieved this
inhibitory effect. As shown in Fig. 6B
and C, ATM knockdown also significantly recovered the invasive
ability of lung cancer cells when macamide B was administered.
Therefore, ATM may be a critical mediator of macamide B in lung
cancer.
Discussion
Lung cancer is a great threat to human life and
causes thousands of deaths yearly (11). However, due to the limited
information regarding this malignant disease, the main treatment of
surgery assisted by chemo/radiotherapy does not provide many
benefits to patient survival rate (11–14).
New therapeutics, such as targeted drugs and immunotherapy, may
slightly improve clinical outcomes (13–16).
Therefore, it is necessary to improve knowledge of this disease in
order to develop new drugs or therapies for treatment.
Traditional medicine is a large resource consisting
of thousands of active natural products. These products possess
multiple activities, such as anti-fatigue, anti-inflammation and
antitumor activities. Macamides are a class of active ingredients
that have been extracted from maca in recent years (1). Macamides were originally shown to have
neuroprotective effects on the nervous system and have anti-fatigue
activity (8,9). Multiple reports also indicated that
macamides exhibited anti-inflammatory effects (6,7). A
large number of experiments have demonstrated that inflammatory
responses often precede cancer transformation (17); therefore, it is reasonable to
consider that macamides may have antitumor activity. Notably,
macamides were recently reported to inhibit cell proliferation in
more than one cancer cell type, includingtheA549 lung cancer cell
line (10). Consistently, the
present study also revealed that macamide B inhibited the
proliferation of H1299, A549 and H460 lung cancer cells. This
inhibition was dose and time-dependent. Furthermore, macamide B was
also shown in the present study to suppress the invasive ability of
lung cancer cell lines. Uncontrolled expansion and aggressive
invasion are typical hallmarks in nearly all types of cancer
(18). Therefore, macamide B may be
considered a potential candidate for lung cancer therapy. In
addition, it was suggested that this inhibition was independent of
p53, as macamide B also exerted inhibitory effects on H1299 cells
lacking p53.
Programmed cell death is a strict process that
maintains homeostasis and occurs throughout the entire lifespan of
cells (19). In the present study,
macamide B was shown to induce the apoptosis of H1299, A549 and
H460 cells. To the best of our knowledge, this is the first report
exploring the role of macamides in cell apoptosis in cancer and the
first demonstration of the pro-apoptotic role of macamides. At the
molecular level, the data further support this suggestion. The
expression levels of Bcl-2 were greatly reduced by macamide B in
lung cancer cell lines. Bcl-2 is a typical anti-apoptotic protein
that negatively regulates apoptosis (19). p53 is a well-known tumor suppressor
and p53 mutations can inhibit cell apoptosis signaling, which is
frequently detected in the tumor genome (20). In the present study, macamide B
increased p53 expression in lung cancer cell lines, which further
confirmed the apoptosis-promoting role of macamide B. Consistently,
an increase in caspase-3 expression by macamide B was observed.
Caspase-3 is a critical effector in the caspase cascade response
and is cleaved when apoptosis is activated (19). In addition, in the present study, it
was found that ATM and RAD51 levels were significantly increased by
macamide B. ATM and RAD51 are important members of the ATM/ATR
signaling pathway, which is responsible for DNA damage repair
(21). Macamide B may therefore
participate in DNA damage repair in lung cancer.
Olaparib is a specific inhibitor against PARP, which
has been applied in the clinic to treat ovarian and prostate cancer
(22). In lung cancer, olaparib has
been reported to increase the efficacy of temozolomide in relapsed
lung cancer cases (23).
Temozolomide is a DNA-target drug that causes cell apoptosis after
DNA damage (23). In the present
study, it was revealed that a combination of macamide B and
olaparib further inhibited cell proliferation, suggesting that the
sensitivity of lung cancer cells to olaparib was increased by
macamide B. In addition, olaparib has shown a potent inhibitory
role in cancer bearing BRCA1/2 mutations (24). BRCA1/2 play important roles in the
DNA damage repair system (25).
Cancer cases with BRCA1/2 mutations are more sensitive to PARP
inhibitors, which is due to disruption of the ATM-mediated DNA
damage repair system (25). Based
on the increased expression levels of ATM and RAD51 observed in the
present study, macamide B may also participate in the DNA damage
repair process in lung cancer. When ATM was knocked down by siRNA
oligonucleotides, the expression patterns of RAD51, Bcl-2, p53 and
cleaved caspase-3 after macamide B treatment were reversed. Cell
proliferation and cell invasion abilities were also partially
recovered by siATM. These data suggested that macamide B may cause
DNA damage in lung cancer and that the ATM signaling pathway might
be involved in this process. However, additional experiments are
warranted to support this conclusion. For example, the role of
macamide B in an in vivo model will be a focus of future
studies. In addition, DNA damage assays, such as the comet assay,
may further confirm the conclusions of the present study. Our
future aims are to assess the mechanism in more cell lines.
In summary, in the present study, macamide B was
shown to inhibit the proliferation and invasion, and induce the
apoptosis of lung cancer cells. Macamide B also participated in
regulating the ATM signaling pathway. In addition, macamide B
increased the inhibitory effect of olaparib on lung cancer cells.
The present study therefore provides novel information regarding
the treatment of patients with lung cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by the Shandong Medical and Health
Science and Technology Development Fund (grant no.
202102080591).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX designed the whole study and reviewed the
manuscript. HT acquired the experimental data and wrote the
manuscript. HS and MW completed the data analysis and reviewed the
manuscript. HT and YX confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu H, Hu B, Hua H, Liu C, Cheng Y, Guo
YH, Yao W and Qian H: Macamides: A review of structures, isolation,
therapeutics and prospects. Food Res Int. 138:1098192020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzales-Arimborgo C, Yupanqui I, Montero
E, Alarcón-Yaquetto DE, Zevallos-Concha A, Caballero L, Gasco M,
Zhao J, Khan IA and Gonzales GF: Acceptability, safety, and
efficacy of oral administration of extracts of black or red maca
(Lepidium meyenii) in adult human subjects: A randomized,
double-blind, placebo-controlled study. Pharmaceuticals (Basel).
9:492016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Q, Jin W, Lv X, Dai P, Ao Y, Wu M,
Deng W and Yu L: Effects of macamides on endurance capacity and
anti-fatigue property in prolonged swimming mice. Pharm Biol.
54:827–834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alasmari M, Bӧhlke M, Kelley C, Maher T
and Pino-Figueroa A: Inhibition of fatty acid amide hydrolase
(FAAH) by macamides. Mol Neurobiol. 56:1770–1781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Wang R, Hua H, Cheng Y, Guo Y, Qian
H and Du P: The macamide relieves fatigue by acting as inhibitor of
inflammatory response in exercising mice: From central to
peripheral. Eur J Pharmacol. 917:1747582022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apaza Ticona L, Peña-Rojas G, Andía-Ayme
V, Durán García B and Rumbero Sánchez A: Anti-glycative and
anti-inflammatory effects of macamides isolated from Tropaeolum
tuberosum in skin cells. Nat Prod Res. 36:5803–5807. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zha R, Ge EH, Guo LR, Gao Q, Lin Q, Zhou
W, Jin X, Xie W, Yin H and Liu T: A newly identified
polyunsaturated macamide alleviates dextran sulfate sodium-induced
colitis in mice. Fitoterapia. 152:1049162021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Wang M, Zhou Q, Bai Y, Liu J, Yang
J, I L, Li G and Luo L: Macamide B pretreatment attenuates neonatal
hypoxic-ischemic brain damage of mice induced apoptosis and
regulates autophagy via the PI3K/AKT signaling pathway. Mol
Neurobiol. 59:2776–2798. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu Z, Li D, Zhai S, Xu H, Liu H, Ao M,
Zhao C, Jin W and Yu L: Neuroprotective effects of macamide from
maca (Lepidium meyenii Walp.) on corticosterone-induced
hippocampal impairments through its anti-inflammatory,
neurotrophic, and synaptic protection properties. Food Funct.
12:9211–9228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu L, Wei J, Gao Y and Chen R: Antioxidant
and antitumoral activities of isolated macamide and macaene
fractions from Lepidium meyenii (maca). Talanta.
221:1216352021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch ER, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khandia R and Munjal A: Interplay between
inflammation and cancer. Adv Protein Chem Struct Biol. 119:199–245.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hausman DM: What is cancer? Perspect Biol
Med. 62:778–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Obeng E: Apoptosis (programmed cell death)
and its signals-a review. Braz J Biol. 81:1133–1143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J, Cao J, Topatana W, Juengpanich S, Li
S, Zhang B, Shen J, Cai L, Cai X and Chen M: Targeting mutant p53
for cancer therapy: Direct and indirect strategies. J Hematol
Oncol. 14:1572021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fedak EA, Adler FR, Abegglen LM and
Schiffman JD: ATM and ATR activation through crosstalk between DNA
damage response pathways. Bull Math Biol. 83:382021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bochum S, Berger S and Martens UM:
Olaparib. Recent Results Cancer Res. 211:217–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farago AF, Yeap BY, Stanzione M, Huang YP,
Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, et
al: Combination olaparib and temozolomide in relapsed small-cell
lung cancer. Cancer Discov. 9:1372–1387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turan V and Okatay K: BRCA-related
ATM-mediated DNA double-strand break repair and ovarian aging. Hum
Reprod Update. 26:43–57. 2020. View Article : Google Scholar : PubMed/NCBI
|