Introduction
Management of endometrial cancer according to
ESGO-ESMO-ESTRO guidelines is based on the assessment of risk for
loco-regional or distant recurrence (1). Classification of a patient as low-risk
necessitates only total hysterectomy with bilateral
salpingo-oophorectomy, while high-intermediate or higher risk may
necessitate staging with pelvic and para-aortic lymphadenectomy. In
this context, histological subtype, grade, clinical stage and depth
of myometrial invasion, which are the main parameters affecting
assessment of risk, should and actually can be assessed
preoperatively, permitting the adequate establishment of surgical
plan.
Lymphovascular space invasion (LVSI), defined as the
presence of tumoral cells in the lymphatic and blood vessels, has
been recently considered as another decisive prognostic factor for
endometrial cancer (2). This may be
attributed to studies correlating LVSI with increased risk for
disseminated disease (3), lymph
node metastasis (4,5) and impaired overall survival (6–9) As a
result, ESGO guidelines have also enrolled LVSI status in the
decisional algorithm of risk assessment. Specifically, even
patients with endometrioid, grade 1–2 endometrial cancer are
considered of high-intermediate risk for recurrence, therefore
necessitating pelvic and para-aortic lymphadenectomy, apart from
standard surgical treatment.
In contrary with other prognostic factors affecting
risk for recurrence according to decisional algorithm (histological
subtype, grade and depth of myometrial invasion), LVSI may not be
assessed easily on preoperative level. This is due to the
reasonable absence of myometrium from preoperative biopsy specimen,
which is actually the most frequent scenario (10). The issue acquires higher clinical
significance since new origins of endometrial cancer, as those
derived from adenomyosis in which involvement of LVSI is rather
possible, are increasingly reported. (11,12)
Therefore, an early-stage, endometrioid, grade 1–2 endometrial
cancer, necessitating only a total hysterectomy with bilateral
salpingo-oophorectomy, may be upgraded to high-intermediate risk
for recurrence in case of positive LVSI in final specimen. This
should actually necessitate a restaging with pelvic and para-aortic
lymphadenectomy based strictly on ESGO-ESMO-ESTRO guidelines with
the alternative of sentinel node being also acceptable in these
cases based on newly launched ESGO guidelines (1,13).
However, management of under-staged patients with
these characteristics is still not well-established in the
literature. There are no studies assessing the added value of such
restaging in the overall prognosis of patients, taking into
consideration both the increased operative risk as well as the
effectiveness of complementary therapies. Therefore, the dilemma of
restaging or not early-stage, initially low-risk patients that have
been upgraded to high-intermediate risk only based on positive
LVSI, has not yet been adequately assessed.
Main objective of the present study is to assess
whether restaging with lymphadenectomy of such patients
significantly affects prognosis and therefore should be always
performed.
Materials and methods
Study character
A retrospective cohort study based on prospectively
collected data was performed in Gynaeocologic Oncology Unit of
Institut Bergonie, Bordeaux, France. Study concerned the period
January 2006-May 2019. A hand-made search was performed in the
electronical records of oncological patients treated in our
Institution. All elements of patients are recorded prospectively on
our computerized database, including all epidemiological, clinical,
histopathological characteristics as well as survival and
oncological outcomes from the moment of patient's admission
onwards. All patients included in the present study have provided
written approval for the inclusion of their elements in this
retrospective cohort. As this is a retrospective study,
Institutional Review Board approval was not necessitated based on
the academic policy and requirements of Institution.
Inclusion and exclusion criteria
Included patients were all women with preoperative
diagnosis of endometrioid, grade 1–2, clinically early-stage
endometrial cancer patients that were initially treated with total
hysterectomy and bilateral salpingo-oophorectomy and were
postoperatively diagnosed as LVSI positive according to final
histopathological specimen. Preoperative radiotherapy or
chemotherapy, imaging diagnosis of nodal metastases as well as past
history of any other malignancy were considered as exclusion
criteria. Type of surgical approach (laparotomy or laparoscopy) was
not considered as selection criterion.
Included patients were divided into two groups, the
first group enrolling patients that were re-operated in order to
perform pelvic and para-aortic lymphadenectomy for staging (group
1) and another group including patients with identical
characteristics that were given postoperative radiotherapy without
performing restaging (group 2).
Epidemiological data, primary and
secondary outcomes
Age, BMI, cardiovascular risks factors, tamoxifen
usage, cardiovascular risk factors were the main epidemiological
aspects retrieved. Depth of myometrial invasion, grade, number of
resected lymph nodes, number of invaded nodes as well as adjuvant
radiation or chemotherapy treatment was recorded for all
patients.
Primary study outcomes were defined to be overall
survival and progression-free survival. Overall survival was
defined as the time from admission to death from any cause.
Progression-free survival was defined as the time from end of
therapy until recurrence or death from any cause. Secondary outcome
was defined as the absolute number of recurrence and death.
Statistical analysis
Frequency distributions between categorical
variables were assessed by using Fisher's exact test. Survival
curves between the group with lymphadenectomy and without
lymphadenectomy were estimated by Kaplan-Meier curves. The log-rank
test was utilized to compare the curves, while survival analysis
was performed with the Cox proportional hazards model. Level of
significance was defined at P<.05. All statistical tests were
two-sided. Analysis was conducted using STATA 14.2 version.
Results
Patients and characteristics
There were overall 30 patients retrieved that
fulfilled inclusion criteria and were therefore included in the
present analysis, of which 21 for group 1 and 9 for group 2. The
two study groups of patients were comparable in terms of age, BMI,
tamoxifen use, cardiovascular risk factors, depth of invasion and
grade. All patients of group 1 had more than 10 lymph nodes
resected, while rate of lymph node metastasis was 23.81% (n=5).
CT after hysterectomy was performed in 9.5% of women
of group 1 (n=2) and in 77.7% of patients in group 2 (n=7).
Decision for further diagnostic/therapeutic approach was obtained
in a Multidisciplinary Tumour Board, taking into account various
epidemiological and histopathological criteria of cases on an
individualized basis. Notably, all imaging results of CT were
negative for the presence of nodal metastases. Furthermore, all
patients enrolled in this study had preoperatively performed a CT,
which was negative for suspicious nodes.
No significant difference was also observed between
study groups regarding adjuvant radiation and chemotherapy
administered. Indeed, 90.5% of patients of group 1 received
external beam radiotherapy and brachytherapy and 9.5% only
brachytherapy vs. 77.8 and 11.1% respectively of group 2 patients,
which did not indicate significant difference (P=.291).
Furthermore, adjuvant chemotherapy was given to 14.3% of group 1
patients, while all group 2 patients did not receive adjuvant
chemotherapy.
Table I presents the
epidemiological, clinical and histopathological characteristics of
the two study groups.
 | Table I.Epidemiological, clinical and
histopathological characteristics of the two study groups. |
Table I.
Epidemiological, clinical and
histopathological characteristics of the two study groups.
| Variables | Restaging group, n
(%) (N=21) | No restaging group, n
(%) (N=9) | P-value |
|---|
| Age, years |
|
|
|
|
<55 | 4 (19.05) | 0 (0.00) | 0.15 |
|
55-70 | 12 (57.14) | 4 (44.44) |
|
|
>70 | 5 (23.81) | 5 (55.56) |
|
| BMI |
|
|
|
| Normal
weight | 8 (42.11) | 2 (28.57) | 0.33 |
|
Overweight | 6 (31.58) | 1 (14.29) |
|
|
Obesity | 5 (26.32) | 4 (57.14) |
|
| Tamoxifen use |
|
|
|
| Yes | 1 (4.76) | 2 (22.22) | 0.14 |
| No | 20 (95.24) | 7 (77.78) |
|
| Cardiovascular risk
factors |
|
|
|
| Yes | 7 (33.33) | 6 (66.67) | 0.09 |
| No | 14 (66.67) | 3 (33.33) |
|
| Depth of
invasion |
|
|
|
| IA | 12 (57.14) | 4 (44.44) | 0.52 |
| IB | 9 (42.86) | 5 (55.56) |
|
| Grade |
|
|
|
| 1 | 10 (47.62) | 2 (22.22) | 0.19 |
| 2 | 11 (52.38) | 2 (52.38) |
|
| Lymph nodes
removed |
|
|
|
|
<10 | 0 (0.00) | - | - |
| ≥10 | 21 (100.00) | - |
|
| Lymph node
metastases |
|
|
|
| Yes | 5 (23.81) | - | - |
| No | 16 (79.19) | - |
|
| CT performed after
hysterectomy |
|
|
|
| Yes | 2 (9.52) | 7 (77.78) | <0.001 |
| No | 19 (90.48) | 2 (22.22) |
|
| Adjuvant
radiation |
|
|
|
| None | 0 (0.00) | 1 (11.11) | 0.29 |
| External
beam | 0 (0.00) | 0 (0.00) |
|
|
Brachytherapy | 2 (9.52) | 1 (11.11) |
|
| External
beam+brachytherapy | 19 (90.48) | 7 (77.78) |
|
| Adjuvant
chemotherapy |
|
|
|
| Yes | 3 (14.29) | 0 (0.00) | 0.23 |
| No | 18 (85.71) | 9 (100.00) |
|
Primary and secondary outcomes
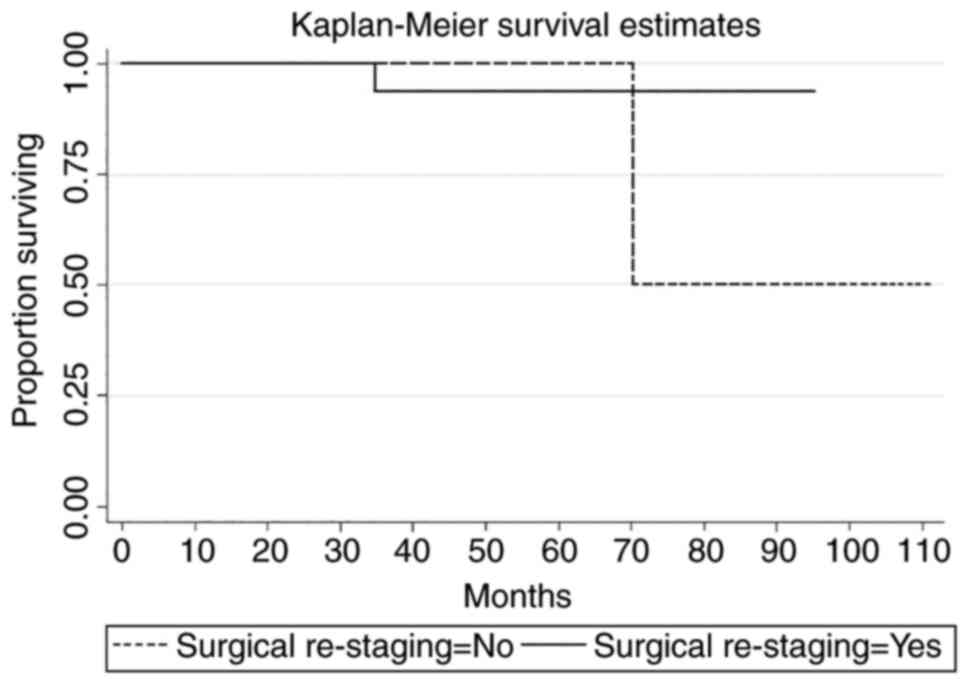
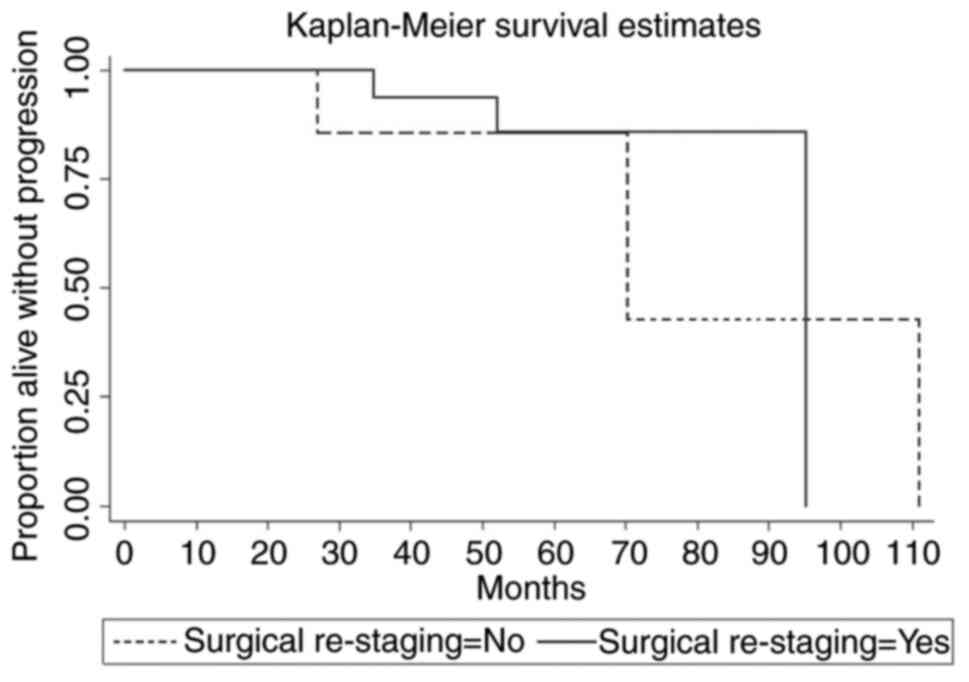
No significant difference was observed in terms of
overall survival and progression-free survival between two groups
of patients based on log-rank test and Cox proportional hazards
model.
Median follow-up was 49.86 months in the group 1 and
43.21 months in group 2 (P=.49). Median OS in group 1 was 91.31 and
90.61 in group 2 (HR:0.71, 95% CI: 0.03-16.58, P=0.829). Median PFS
was 87.95 and 81.52 respectively for two groups (HR:0.85, 95% CI:
0.12-5.91, P=0.869).
Furthermore, no significant difference was observed
between two study groups regarding percentages of recurrence and
death. Specifically, survival rate was 95.2% for patients of group
1 (n=20) vs. 88.9% of patients for group 2 (n=8), (P=.523).
Recurrence rate was 14.3% for group 1 (n=3) vs. 22.2% for group 2
(n=22), (P=.593).
Finally, regarding site and kind of recurrence,
there was one cerebral and two pelvic recurrences in group 1 vs.
one cerebral and one pulmonary recurrence in group 2.
Table II presents
the primary and secondary outcomes within study groups. Figs. 1 and 2 present the Kaplan-Meir overall survival
and progression-free survival curves for study groups.
 | Table II.Primary and secondary outcomes within
study groups. |
Table II.
Primary and secondary outcomes within
study groups.
| Variables | Restaging group
(N=21) | No restaging group
(N=9) | P-value |
|---|
| Follow up in months,
mean (range) | 49.86
(7.49-95.08) | 43.21 (0.68-110) | 0.49 |
| Primary
outcomesa |
|
|
|
| 5-year OS
(proportion surviving) | 0.9375 | 1 | 0.83 |
| 5-year
PFS (proportion alive without progression) | 0.8594 | 0.8571 | 0.87 |
| Secondary outcomes, n
(%) |
|
|
|
| Vital
status, n (%)b |
|
|
|
|
Death | 1 (4.76) | 1 (11.11) | 0.52 |
|
Alive | 20 (95.24) | 8 (88.89) |
|
| Recurrence, n
(%)b |
|
|
|
|
Yes | 3 (14.29) | 2 (22.22) | 0.59 |
|
No | 18 (85.71) | 7 (77.78) |
|
| Site of recurrence,
n (%)b |
|
|
|
|
Distance | 1 (33.33) | 2 (100.00) | 0.14 |
|
Local
(pelvis) | 2 (66.67) | 0 (0.00) |
|
Discussion
The present study demonstrated that restaging with
pelvic and para-aortic lymphadenectomy has no additional benefit on
the prognosis of early-stage, endometrioid-type, grade 1–2 patients
with positive LVSI in the final histopathological specimen. All
examined survival parameters were comparable between these patients
and patients not restaged and given directly postoperative
radiotherapy. Therefore, it is assumed that the performance of
lymphadenectomy based on a solely indication of positive LVSI in
such patients did not improve overall survival and progression-free
survival compared to no lymphadenectomy.
This is potentially one amongst very few
publications examining the necessity of additional restaging with
lymphadenectomy in patients otherwise classified as low-risk but
finally characterised as high-intermediate risk because of only
positive LVSI. There has only been a relative review publication by
Harris et al (14), in which
authors discuss the additional management required in such
patients. Authors underline the lack of relative studies, therefore
recommending usage of an LVSI quantification system that could
actually alter decision in order to distinguish between substantial
or focal LVSI. Specifically, they recommend only adjuvant
radiotherapy and not lymphadenectomy in case of substantial LVSI,
but in cases with focal LVSI they recommend lymphadenectomy. Even
if these recommendations have not yet been applicable to clinical
practice, it is a parameter of importance actually to consider and
evaluate not the presence of LVSI itself, but mainly the degree and
kind of expression this may have to tumoral cells.
Our results may pose a new suggestion to consider
for guidelines of treatment for endometrial cancer. Indeed, ESGO
guidelines either in the old or in the new version do not give any
treatment options in early, LVSI unstaged patients (1,13). In
contrary, NCCN guidelines provide multiple options for such
patients, as they give the option of imaging and thereafter
lymphadenectomy in patients with suspicion of nodal metastases in
imaging (15). Such a strategy
could actually have two main advantages. The first one would be the
avoidance of unnecessary pelvic and para-aortic lymphadenectomy,
which on their own pose severe operative danger and increased risk
for lymphedema, lymphocele and neuralgia (16,17).
The second would be the avoidance of unnecessary complementary
treatments that otherwise should be kept as additional future
therapy in case of recurrence. Such a strategy is rather enhanced
by our observation that lymphadenectomy for restaging does not
alter prognosis of such patients and thereafter should be performed
only in patients with imaging suspicion of bulky nodes. In the
other hand, there should be always considered that LVSI poses a
negative prognostic factor and its presence should not be
underestimated in an effort to minimize complementary therapy
(18–20).
An additional remark could also be that enrolment of
both sentinel node and molecular profiling for endometrial cancer
patients, based on revised ESGO guidelines, could further help our
therapeutic strategy. First of all, as indicated by new ESGO
guidelines, sentinel node should rather be performed in all
intermediate risk cases, while it is highly recommended, even if
not mandatory, in low-risk patients. Performance of sentinel node
could rather help us in such cases as those discussed in the
present study, since the staging information would be available and
further treatment could be tailored based on sentinel node result
(13,21).
Furthermore, evaluation of molecular profiling of
endometrial cancer in the context of risk-of-disease stratification
could rather undermine the added value of postoperative LVSI.
However, our results have not taken into account this parameter for
two major reasons. First of all, implementation of new molecular
staging has not yet been globally achieved in current practice.
Secondly, our remarks refer to patients of a previous era in which
no molecular profiling for endometrial cancer patients was
performed as standard of care. Furthermore, even with the new
revised guidelines, the case might be a POLE case with positive
LVSI (+) where therapeutic questions may be raised. The current
study rather firmly indicated that no therapeutic and prognostic
value is associated with restaging based solely on LVSI positivity,
therefore, even in the era of molecular profiling, our results may
actually have a significant clinical impact.
Limitations of the present study mainly concern its
retrospective nature and the small number of patients. However,
this is potentially the first publication to provide a direct
comparison of survival parameters between two categories of such
patients, those restaged and not restaged. Accordingly, our initial
remark that restaging with lymphadenectomy has no beneficial impact
could actually trigger the development of a relatively large
multicentre RCT, enrolling sufficient sample size and with severe,
prospective enrolment of patients.
In conclusion, our study indicated no survival
benefit of restaging lymphadenectomy for patients with early-stage,
low-grade, endometrioid-type, LVSI-positive endometrial cancer.
Imaging could be an alternative to identify cases with suspicious
nodes and limit the performance of lymphadenectomies only to such
cases, in which full staging and resection of suspicious nodes
consists of the optimal diagnostic and therapeutic option. The
results of the present study rather pose a remarkable observation
to be actively enrolled in daily clinical practice. Further
prospective, multicentre RCTs are rather demanded in order to
assess definitively the beneficial impact of restaging
lymphadenectomy in such patients.
Acknowledgements
This abstract was presented at the ESGO 2022
Congress, 27–30 October, Berlin, Germany and was published as
Abstract no. 1047 (https://ijgc.bmj.com/content/32/Suppl_2/A132.1).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BN, AF, GB and FG have made substantial
contributions to the conception and design of the study, and the
acquisition of data. BN, CMS, GB and SP wrote the initial draft.
BN, CMS, SP, AF and FG confirm the authenticity of all the raw
data. BN, GB, CMS and SP performed the statistical analysis, and AF
and FG have significantly contributed to the interpretation of
results. GB, AF and FG have substantially reviewed the initial
draft. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Based on Institution's policy, no ethical approval
is needed in order to perform retrospective studies and therefore
this criterion is not applicable for the present manuscript.
Patients provided written informed consent for the inclusion of
their elements anonymously for potential analysis and inclusion in
future studies.
Patient consent for publication
Patients provided written informed consent for the
inclusion of their elements anonymously and for publication.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Int J Gynecol Cancer.
26:2–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jorge S, Hou JY, Tergas AI, Burke WM,
Huang Y, Hu JC, Ananth CV, Neugut AI, Hershman DL and Wright JD:
Magnitude of risk for nodal metastasis associated with
lymphvascular space invasion for endometrial cancer. Gynecol Oncol.
140:387–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakayama A, Kudaka W, Matsumoto H, Aoyama
H, Ooyama T, Taira Y, Arakaki Y, Shimoji Y, Nakasone T, Nishihira
K, et al: Lymphatic vessel involvement is predictive for lymph node
metastasis and an important prognostic factor in endometrial
cancer. Int J Clin Oncol. 23:532–538. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohn DE, Horowitz NS, Mutch DG, Kim SM,
Manolitsas T and Fowler JM: Should the presence of lymphvascular
space involvement be used to assign patients to adjuvant therapy
following hysterectomy for unstaged endometrial cancer? Gynecol
Oncol. 87:243–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang SJ, Kong TW, Kim WY, Yoo SC, Yoon
JH, Chang KH and Ryu HS: Lymph-vascular space invasion as a
significant risk factor for isolated para-aortic lymph node
metastasis in endometrial cancer: A study of 203 consecutive
patients. Ann Surg Oncol. 18:58–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Briët JM, Hollema H, Reesink N, Aalders
JG, Mourits MJE, Ten Hoor KA, Pras E, Boezen HM, van der Zee AG and
Nijman HW: Lymphvascular space involvement: An independent
prognostic factor in endometrial cancer. Gynecol Oncol. 96:799–804.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sari ME, Yalcin İ, Sahin H, Meydanli MM
and Gungor T: Risk factors for paraaortic lymph node metastasis in
endometrial cancer. Int J Clin Oncol. 22:937–944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaizoglu F, Yuce K, Salman MC, Basaran D,
Calis P, Ozgul N and Usubutun A: Lymphovascular space involvement
is the sole independent predictor of lymph node metastasis in
clinical early stage endometrial cancer. Arch Gynecol Obstet.
288:1391–1397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Wang C and Feng W:
Clinicopathological risk factors for pelvic lymph node metastasis
in clinical early-stage endometrioid endometrial adenocarcinoma.
Int J Gynecol Cancer. 22:1373–1377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borghesi Y, Narducci F, Bresson L, Tresch
E, Meurant JP, Cousin S, Cordoba A, Merlot B and Leblanc E:
Managing endometrial cancer: The role of pelvic lymphadenectomy and
secondary surgery. Ann Surg Oncol. 22 (Suppl 3):S936–S943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scioscia M, Noventa M and Laganà AS:
Abnormal uterine bleeding and the risk of endometrial cancer: Can
subendometrial vascular ultrasound be of help to discriminate
cancer from adenomyosis. Am J Obstet Gynecol. 223:605–606. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laganà AS and Scioscia M: Endometrial
cancer in women with adenomyosis: An underestimated risk? Int J
Fertil Steril. 14:260–261. 2020.PubMed/NCBI
|
|
13
|
Concin N, Matias-Guiu X, Vergote I, Cibula
D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Int J Gynecol Cancer. 31:12–39. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris KL, Maurer KA, Jarboe E, Werner TL
and Gaffney D: LVSI positive and NX in early endometrial cancer:
Surgical restaging (and no further treatment if N0), or adjuvant
ERT? Gynecol Oncol. 156:243–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Rustum NR, Yashar CM, Bradley K,
Campos SM, Chino J, Chon HS, Chu C, Cohn D, Crispens MA, Damast S,
et al: NCCN guidelines® insights: Uterine neoplasms,
version 3.2021. J Natl Compr Canc Netw. 19:888–895. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardosi RJ, Cox CS and Hoffman MS:
Postoperative neuropathies after major pelvic surgery. Obstet
Gynecol. 100:240–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volpi L, Sozzi G, Capozzi VA, Ricco M,
Merisio C, Di Serio M, Chiantera V and Berretta R: Long term
complications following pelvic and para-aortic lymphadenectomy for
endometrial cancer, incidence and potential risk factors: A single
institution experience. Int J Gynecol Cancer. 29:312–319. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cusano E, Myers V, Samant R, Sudai T,
Keller A, Le T, E C, Grimes S and Xu Y: Prognostic significance of
lymphovascular space invasion in the absence of lymph node
metastases in early-stage endometrial cancer. Int J Gynecol Cancer.
28:890–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loizzi V, Cormio G, Lorusso M, Latorre D,
Falagario M, Demitri P, Scardigno D and Selvaggi LE: The impact of
lymph vascular space invasion on recurrence and survival in
patients with early stage endometrial cancer. Eur J Cancer Care
(Engl). 23:380–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosse T, Peters EEM, Creutzberg CL,
Jürgenliemk-Schulz IM, Jobsen JJ, Mens JWM, Lutgens LCHW, van der
Steen-Banasik EM, Smit VTHBM and Nout RA: Substantial
lymph-vascular space invasion (LVSI) is a significant risk factor
for recurrence in endometrial cancer-a pooled analysis of PORTEC 1
and 2 trials. Eur J Cancer. 51:1742–1750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cignini P, Vitale SG, Laganà AS, Biondi A,
Valentina Lucia La Rosa VL and Cutillo G: Preoperative work-up for
definition of lymph node risk involvement in early stage
endometrial cancer: 5-year follow-up. Updates Surg. 69:75–82. 2017.
View Article : Google Scholar : PubMed/NCBI
|