Introduction
Head and neck squamous cell carcinoma (HNSCC) is one
of the most common malignant tumors, accounting for ~90% of the
total number of head and neck tumors (1), with >800,000 new cases globally
every year (2). Multiple therapies,
including surgery, radiotherapy, chemotherapy and systemic therapy,
can be applied for HNSCC treatment (3); however, the 5-year survival rate is
only 50% (4). HNSCC is an
immunosuppressive disease which demonstrates impaired function of
immune cells (5–7). The mechanisms of immune escape in
HNSCC include upregulation of programmed death-ligand 1 in human
papillomavirus (HPV)-positive tumors, downregulation of interferon
regulatory factors and activation of the STAT1 signaling pathway
(3), which contribute to the
development of HNSCC. Previous studies have reported that
Keytruda® and Opdivo® can efficiently prevent
the immune escape state and improve the prognosis of patients due
to their specific binding to PD-1 (8,9).
Therefore, it is important to explore immune checkpoints in the
development of targeted drugs to improve the prognosis of patients
with HNSCC.
Collagen triple helix repeat containing 1 (CTHRC1)
was first discovered in the injured arteries of rats (10). CTHRC1 serves an important role in
wound repair (11), hepatocyte
fibrosis (12), bone reconstruction
(13) and adipose tissue formation
(14). In previous studies, CTHRC1
has been reported to be abnormally expressed in colorectal and
gastric cancer (15–17). Furthermore, CTHRC1 inhibits collagen
deposition by mediating the Wnt/planar cell polarity (PCP),
TGF-β/bone morphogenetic protein and ERK signaling pathways, and
participates in the metastasis of tumors (18–20).
CTHRC1 promotes the proliferation of HeLa cells via activation of
the Wnt/PCP signaling pathway and promotion of the proliferation of
breast cancer cells via a Linc00707-mediated competing endogenous
RNA mechanism (21,22). Furthermore, upregulation of CTHRC1
results in a worse prognosis for patients with liver and epithelial
ovarian cancers (19,23). Therefore, CTHRC1 can be considered a
carcinogenic driving factor for the progression and metastasis of
esophageal squamous cell carcinoma, and as a potential biomarker
for prognosis and individualized treatment (20,24).
It has been reported that CTHRC1 is involved in immune cell
infiltration in the tumor microenvironment, has a pivotal role in
the regulation of M2 macrophage polarization in ovarian tumors and
is seen as a target for antitumor immunotherapy (25). However, the roles of CTHRC1 in HNSCC
and its tumor immune microenvironment remain unclear. Therefore, in
the present study, the Tumor Immune Estimation Resource (TIMER) and
ONCOMINE databases were used to analyze the transcriptional levels
of CTHRC1 in HNSCC. The University of ALabama at Birmingham CANcer
data analysis Portal (UALCAN) and CIBERSORT websites were used to
evaluate the association between CTHRC1 expression levels and
clinical features or the immune microenvironment, to provide solid
evidence for the significance of CTHRC1 in HNSCC.
Materials and methods
The cancer genome atlas (TCGA)
database analysis
The RNA-seq data (workflow type, HTSeq-FPKM) and
relevant clinical data for the ‘TCGA-HNSC’ cohort, including 502
tumor samples and 44 normal samples, were downloaded from the TCGA
database (https://tcga-data.nci.nih.gov/tcga/) (26).
ONCOMINE database analysis
Expression levels of CTHRC1 were analyzed in
multiple cancer types using the ONCOMINE database (https://www.oncomine.org/resource/main.html) (27), and the cut-off P-value used was
0.05, while the log fold change cut-off was equal to 1.
TIMER database analysis
The TIMER database (https://cistrome.shinyapps.io/timer/) (28) has incorporated 39 types of cancer in
the TCGA database. Gene expression levels were presented as log2
transcripts per million. The TIMER database was used to evaluate
the transcriptional level of CTHRC1 in multiple cancer types and
its correlation with immune cell infiltration.
Kaplan-Meier (KM) plotter
analysis
KM plotter contains data and clinical information
from the Gene Expression Omnibus, TCGA and European Genome-Phenome
Archive databases. The prognostic value of the mRNA expression
levels of CTHRC1 in 21 cancer types was evaluated using the KM
plotter (http://kmplot.com/analysis/).
P<0.05 was considered to indicate a statistically significant
difference.
UALCAN analysis
UALCAN (http://ualcan.path.uab.edu) (29) contains level 3 RNA-seq data and
clinical information from 31 cancer types from the TCGA database
and is an interactive and comprehensive web resource for analyzing
cancer omics data. In the present study, UALCAN was used to
investigate the relationship between the levels of CTHRC1 and
clinicopathologic parameters, including age, TP53 mutation, nodal
metastasis (N0, no regional lymph node metastasis; N1, metastases
in 1–3 axillary lymph nodes; N2, metastases in 4–9 axillary lymph
nodes; N3, metastases in ≥10 axillary lymph nodes), individual
cancer stages, tumor grades (grade 1, well differentiated; grade 2,
moderately grade; grade 3, poorly differentiated; grade 4,
undifferentiated) (30,31) and HPV status. Unpaired student's
t-test was used to assess transcriptional expression and P<0.05
was considered to indicate a statistically significant
difference.
Tumor infiltration cell (TIC) profile
analysis
Based on the validated leukocyte gene signature
matrix (LM22), the CIBERSORT (http://cibersort.stanford.edu/) computational method
was used to analyze the infiltration ratio of 22 TICs in each HNSCC
sample, and the ratios of all were equal to 1.
Barplot, corrplot, vioplot, Venn and
scatter plot
R 64.4.0.0 software (https://www.r-project.org) was used to plot
associations with TICs. The Barplot and corrplot were generated
using the corrplot package (version 0.84, http://cran.r-project.org/src/contrib/Archive/corrplot/),
while vioplot was produced using the BiocManager (version 3.12,
http://www.bioconductor.org/install)
and vioplot packages (version 0.3.4, http://cran.r-project.org/src/contrib/Archive/vioplot/).
The Venn plot was generated using the Venn Diagram package
(http://www.rdocumentation.org/packages/VennDiagram/versions/1.6.18).
The scatter plot was generated using the ggplot2 (version 3.2.1,
http://cran.r-project.org/src/contrib/Archive/ggplot2/),
ggpubr (version 0.2.4, http://cran.r-project.org/src/contrib/Archive/ggpubr/)
and ggExtra packages (version 0.9, http://cran.r-project.org/src/contrib/Archive/ggExtra/).
All packages were used with standard settings.
Gene sets for enrichment analysis
(GSEA)
GSEA were downloaded from the Broad Institute
Website (version 4.0.2, http://software.broadinstitute.org/gsea/index.jsp).
HALLMARK, Kyoto Encyclopedia of Genes and Genomes (KEGG) and IMMUNE
SIGNATURE gene sets were obtained from the Molecular Signatures
Database (http://www.gsea-msigdb.org/gsea/msigdb/index.jsp). The
transcriptome of patients with HNSCC were assessed using GSEA
software (version 4.0.2, http://software.broadinstitute.org/gsea/downloads.jsp)
and all the samples with nominal (NOM) P<0.01 and false
discovery rate (FDR) Q<0.06 were considered to indicate a
statistically significant difference.
Tissue microarray (TMA) and ethical
approval
From January 2020 to December 2020, 70 patients with
primary HNSCC who underwent radical resection of tumors, did not
receive preoperative radiotherapy or chemotherapy in the People's
Hospital of Tongxu County (Kaifeng, China) and provided written
informed consent were enrolled in the present study, and their
tumor tissue samples were collected. A total of 11 patients with
benign head and neck lesions were included, informed consent was
signed and normal tissue samples adjacent to benign lesions were
collected. Patients with HNSCC were between 32 and 79 years old,
with a mean age of 59.9 years. Human tissue specimens were obtained
from patients who had provided written informed consent and the
present study and tissue collection were approved by the Ethics
Supervision Committee of The People's Hospital of Tongxu County
(Kaifeng, China; approval no. TX20NP003) and the National Human
Genetic Resources Sharing Service Platform (2005DKA21300). The
study protocol was approved by the Ethics Committee of Union
Hospital, Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China; approval no. 2020IEC-J050).
Immunohistochemistry (IHC)
The tissue samples were fixed using 10% formalin
solution at room temperature for 24 h, dehydrated, embedded in
paraffin and sectioned into 4 µm sections. The expression of CTHRC1
protein was assessed using the immunohistochemistry ultrasensitive
TMS-P method. After the sections were deparaffinized, hydrated and
washed, they were placed in EDTA repair solution (pH 9.0), and
boiled using a microwave oven for 2 min, and then cooled to room
temperature naturally. Then sections were blocked using 3%
H2O2 solution for 15 min at room temperature
in the dark room. The sections were washed and incubated overnight
at 4°C with CTHRC1 antibodies (1:200; cat no. 16534-1-AP; Wuhan
Sanying Biotechnology). After washing three times with phosphate
buffered saline, the sections were stained with secondary
antibodies using the Ready-to-use Ultrasensitive™ SP kit
(cat. no. Kit-9720; Fuzhou Maxin Biotechnology Development Co.,
Ltd.) and incubated for 30 min in a wet box at 37°C. DAB
horseradish peroxidase color development kit (cat no. P0202;
Beyotime Institute of Biotechnology) was used for color
development. The sections were re-stained using hematoxylin
staining solution (cat no. C0107; Beyotime Institute of
Biotechnology) for 2 min at room temperature, dehydrated using
ethanol, sealed and imaged using a Pannoramic MIDI scanner
(3DHISTECH, Ltd.) and ImageJ software (version 1.8.0, National
Institutes of Health) was used for image analysis. The cells on the
microarrays were scored according to the staining intensity of the
marker, as follows: 0, no coloring; 1, light yellow; 2,
brown-yellow; and 3, tan. The proportion of cells under each score
was then recorded, and the H-score value of each specimen was
calculated. H-scores were defined as: (1× percentage of cells
staining at 1) + (2× percentage of cells staining at 2) + (3×
percentage of cells staining at 3).
Statistical analysis
SPSS 26.0 software (IBM Corp.) was used for
statistical analyses. Fisher's exact test was used to analyze the
relationship between clinical characteristics and CTHRC1 mRNA
expression levels. The Mann-Whitney U test was used for calculating
statistical differences in the H-scores in TMA and the ratio
differentiation level of 21 types of immune cells in high and low
CTHRC1 expression groups in HNSCC. Pearson correlation coefficient
analysis was used to assess the correlation value between two kinds
of cells. P<0.05 was considered to indicate a statistically
significant difference.
Results
CTHRC1 was upregulated in multiple
human cancer types
To evaluate the distinct prognostic and potential
therapeutic value of CTHRC1 in HNSCC, the mRNA expression level of
CTHRC1 was studied using the TCGA, ONCOMINE and TIMER databases.
Data from the TCGA database showed that CTHRC1 was highly expressed
in 16 cancer types, including HNSCC (Fig. 1A and B). Results from the ONCOMINE
database also showed that the mRNA expression levels of CTHRC1 were
significantly upregulated in multiple tumor tissues compared with
normal tissues (Fig. 1C).
Furthermore, the same result was demonstrated by HNSCC clinical
tissues samples when compared with normal tissues (Fig. 1D and E). Taken together, these data
indicated that CTHRC1 was enriched in multiple human cancer
types.
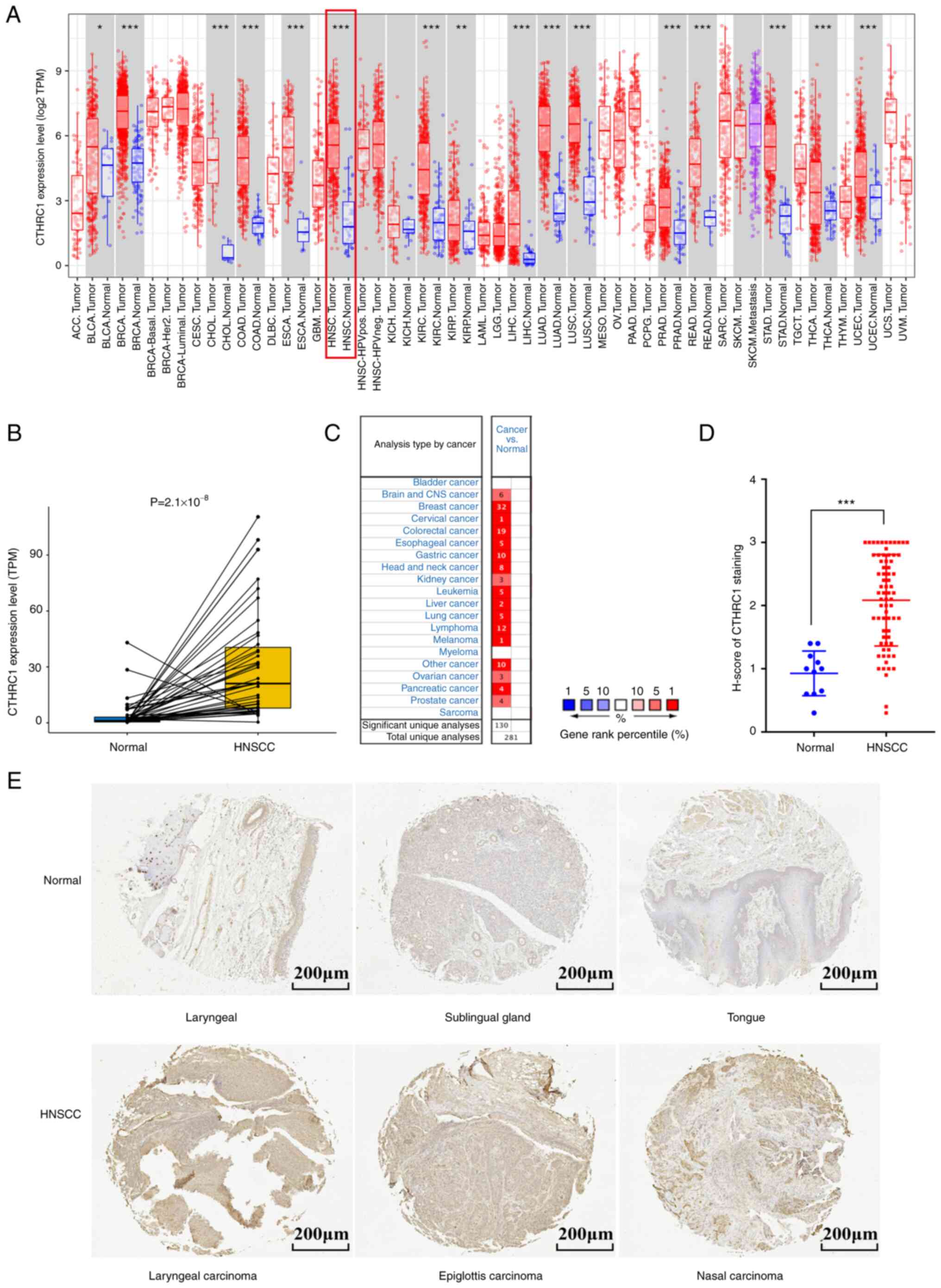 | Figure 1.CTHRC1 expression levels in different
types of human cancer. (A) The transcription levels of human CTHRC1
in different tumor types in the TCGA database were assessed using
TIMER. (B) CTHRC1 transcriptional levels in adjacent and tumor
tissues in patients with HNSCC from the TCGA database. (C)
Expression level of CTHRC1 in different cancer datasets compared
with normal tissues in the ONCOMINE database, the number indicated
the number of data sets included by The National Center for
Biotechnology Information that matched the conditions. (D) Scatter
plot of CTHRC1 expression (using H-score) in HNSCC tissues compared
with normal tissues. (E) Representative immunohistochemistry of
CTHRC1 in clinical specimens. *P<0.05, **P<0.01,
***P<0.001. TCGA, The Cancer Genome Atlas; ACC, adrenocortical
carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast
invasive carcinoma; CESC, cervical squamous cell carcinoma and
endocervical adenocarcinoma; CHOL, cholangio carcinoma; CNS,
central nervous system; COAD, colon adenocarcinoma; DLBC, lymphoid
neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma;
GBM, glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; HPV, human papillomavirus; neg, negative; pos, positive;
KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma;
KIRP, kidney renal papillary cell carcinoma; LGG, (brain) lower
grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO,
mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD,
pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; TGCT, testicular germ cell tumor; THCA,
thyroid carcinoma; THYM, thymoma, UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma;
CTHRC1, collagen triple helix repeat containing 1; TPM, transcripts
per million. |
Association of CTHRC1 expression with
clinicopathological parameters of patients with HNSCC
Since clinical pathology can determine the
progression and prognosis of diseases, the association of
transcriptional levels of CTHRC1 with clinicopathological
parameters in patients with HNSCC was investigated using UALCAN. As
presented in Fig. 2 and Table I, the transcriptional level of
CTHRC1 was significantly associated with age (Fig. 2A), TP53 mutation (Fig. 2B), HPV status (Fig. 2C), tumor grade (Fig. 2D), nodal metastasis status (Fig. 2E) and individual cancer stages
(Fig. 2F). The mRNA expression
level of CTHRC1 increased markedly with age. Patients with TP53
mutations and those who were HPV-negative also had higher CTHRC1
mRNA expression levels. CTHRC1 mRNA expression levels increased
with tumor progression and, in general, the more lymph node
metastases in patients, the higher the mRNA expression level of
CTHRC1. Among the patients, the transcriptional level of CTHRC1 in
the N2 group (4–9 axillary lymph node metastasis) was lower than
that of the N3 group (≥10 axillary lymph node metastasis), which
may be due to the limited sample size. Additionally, the mRNA
expression level of CTHRC1 in pathological stage IV patients was
significantly higher than those in stage III or II, but markedly
lower than that in patients with stage I, which may also be due to
the difference in sample size. In summary, these results suggested
that the mRNA expression level of CTHRC1 was closely related to the
clinicopathological parameters of patients with HNSCC.
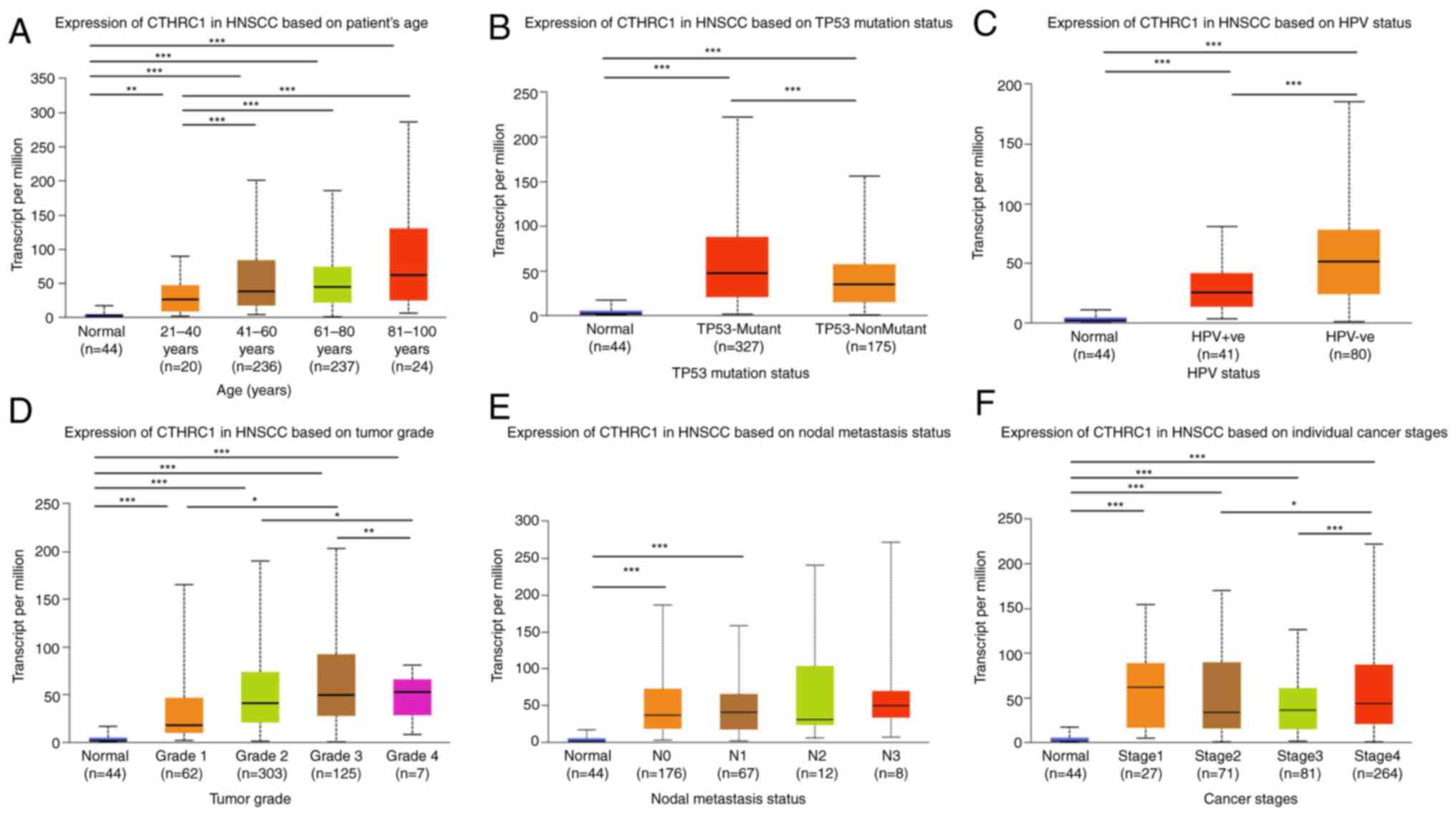 | Figure 2.Relationship between mRNA expression
levels of CTHRC1 and the clinical characteristics of patients with
HNSCC. Relationship between CTHRC1 mRNA expression level and (A)
age, (B) TP53 mutation, (C) HPV status, (D) tumor grade, (E) nodal
metastasis status and (F) cancer stages in HNSCC patients.
*P<0.05, **P<0.01, ***P<0.001. CTHRC1, collagen triple
helix repeat containing 1; HNSCC, head and neck squamous cell
carcinoma; HPV, human papillomavirus; TCGA, The Cancer Genome
Atlas; Yrs, years. |
 | Table I.Relationship between clinical
characteristics and CTHRC1 expression in patients with head and
neck squamous cell carcinoma. |
Table I.
Relationship between clinical
characteristics and CTHRC1 expression in patients with head and
neck squamous cell carcinoma.
| Characteristic | Low expression of
CTHRC1, n=251 (%) | High expression of
CTHRC1, n=250 (%) | P-value |
|---|
| Sex, n |
|
| 0.978 |
|
Female | 67 (26.7) | 67 (26.8) |
|
|
Male | 184 (73.3) | 183 (73.2) |
|
| Age, n |
|
| 0.072 |
| <60
years | 121 (48.4) | 100 (40.0) |
|
| ≥60
years | 129 (51.6) | 150 (60.0) |
|
| HPV |
|
| 0.009 |
| status, n |
|
|
|
|
Negative | 196 (81.3) | 216 (90.0) |
|
|
Positive | 45 (18.7) | 24 (10.0) |
|
| Tumor |
|
| 0.118 |
| stage, n |
|
|
|
| 1 | 20 (9.0) | 26 (11.7) |
|
| 2 | 73 (32.7) | 59 (26.6) |
|
| 3 | 54 (24.2) | 42 (18.9) |
|
| 4 | 76 (34.1) | 95 (42.8) |
|
| Node |
|
| 0.412 |
| stage, n |
|
|
|
| 0 | 93 (45.8) | 78 (38.0) |
|
| 1 | 32 (15.8) | 33 (16.1) |
|
| 2 | 75 (36.9) | 90 (43.9) |
|
| 3 | 3 (1.5) | 4 (2.0) |
|
| Metastasis |
|
| >0.999 |
| stage, n |
|
|
|
| 0 | 97 (99.0) | 89 (100.0) |
|
| 1 | 1 (1.0) | 0 (0) |
|
| Pathological |
|
| 0.070 |
| Stage, n |
|
|
|
| I | 10 (4.6) | 15 (6.9) |
|
| II | 44 (20.4) | 26 (12.0) |
|
|
III | 41 (19.0) | 37 (17.1) |
|
| IV | 121 (56.0) | 139 (64.1) |
|
| Grade, n |
|
| 0.016 |
| 1 | 39 (16.2) | 22 (9.1) |
|
| 2 | 150 (62.2) | 150 (62.2) |
|
| 3 | 50 (20.7) | 69 (28.6) |
|
| 4 | 2 (0.8) | 0 (0) |
|
| Radiation |
|
| 0.985 |
| therapy, n |
|
|
|
| No | 77 (34.8) | 73 (34.9) |
|
|
Yes | 144 (65.2) | 136 (65.1) |
|
Potential value of CTHRC1 mRNA
expression level in assessment of the survival time of patients
with HNSCC
Since CTHRC1 was differentially expressed in diverse
cancer types, the relationship between CTHRC1 expression levels and
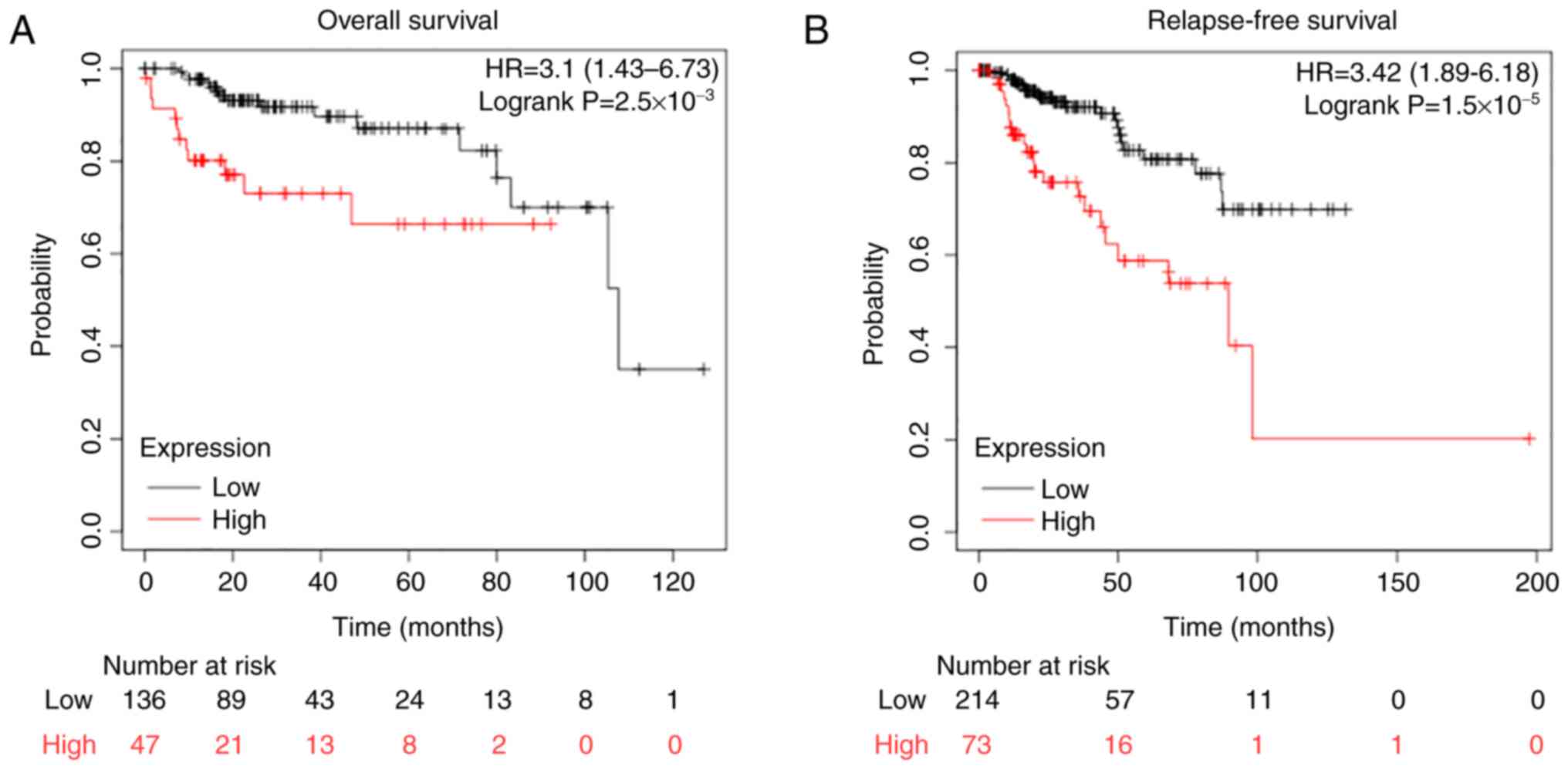
the survival time of patients with HNSCC was next evaluated using
the KM plotter. Patients were grouped, into CTHRC1 high and CTHRC1
low expression groups, using the auto select best cutoff function
on the KM plotter website (32).
The data indicated that the CTHRC1 high expression group had a
worse OS (Fig. 3A) and RFS time
(Fig. 3B). These results
demonstrated that CTHRC1 had the potential to be a prognostic
biomarker in HNSCC.
CTHRC1 has a potential role in
mediating tumor progression
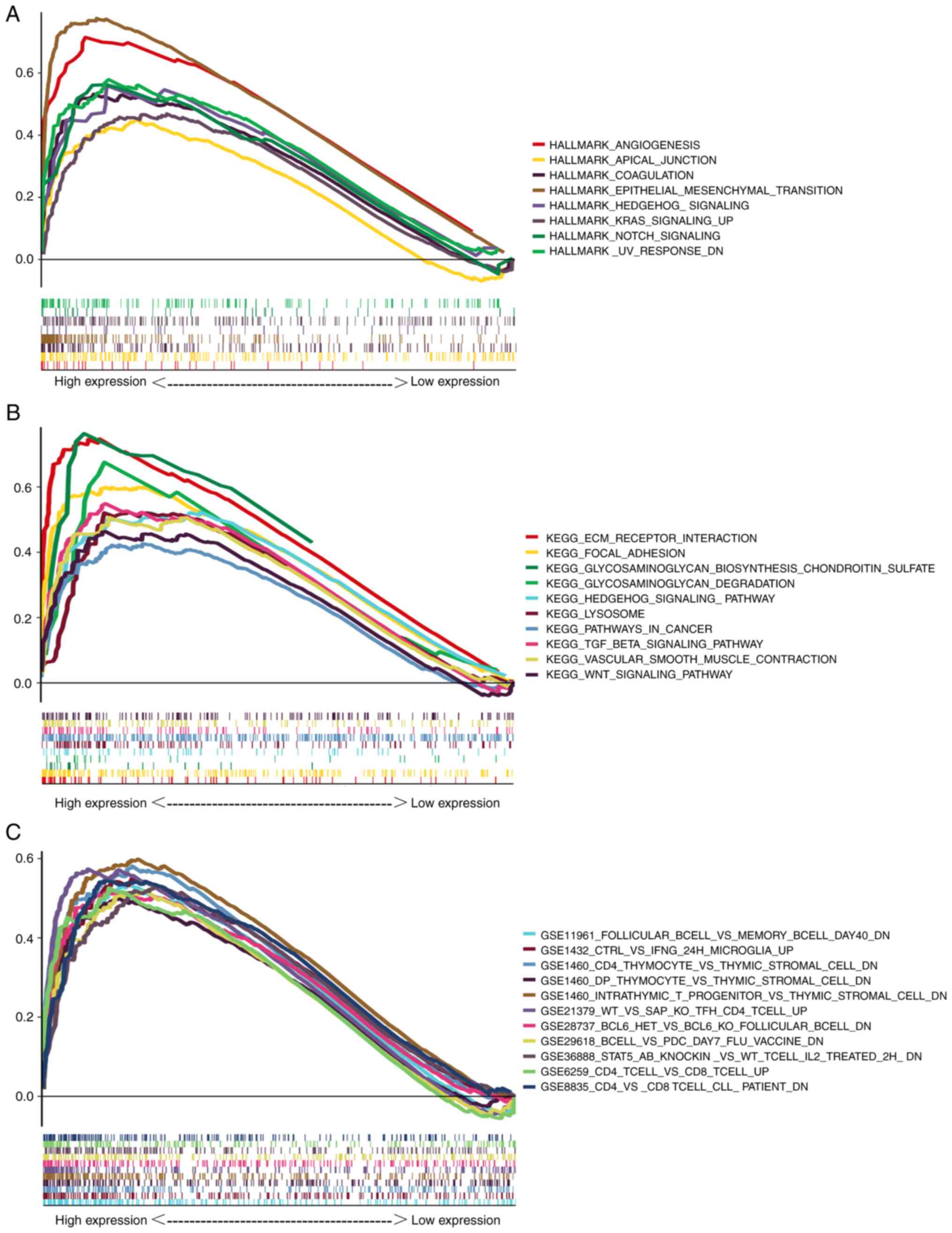
In order to further investigate the potential
function of CTHRC1, GSEA of the data from patients with HNSCC was
conducted. A total of eight HALLMARK gene sets (Fig. 4A), 14 KEGG gene sets (Fig. 4B) and 1,231 immune signature gene
sets (Fig. 4C) were significantly
enriched in the high CTHRC1 expression groups (NOM P<0.01; FDR
Q<0.06). These gene sets included angiogenesis, apical junction,
coagulation, epithelial mesenchymal transformation (EMT), the KRAS
signaling pathway, the notch signaling pathway, glycosaminoglycan
biosynthesis, chondroitin sulfate and the TGF-β signaling pathway.
Most of these gene sets serve pivotal roles in tumorigenesis.
Therefore, CTHRC1 may mediate immune cell infiltration during
cancer development, and it may be regarded as an important
indicator for cancer progression.
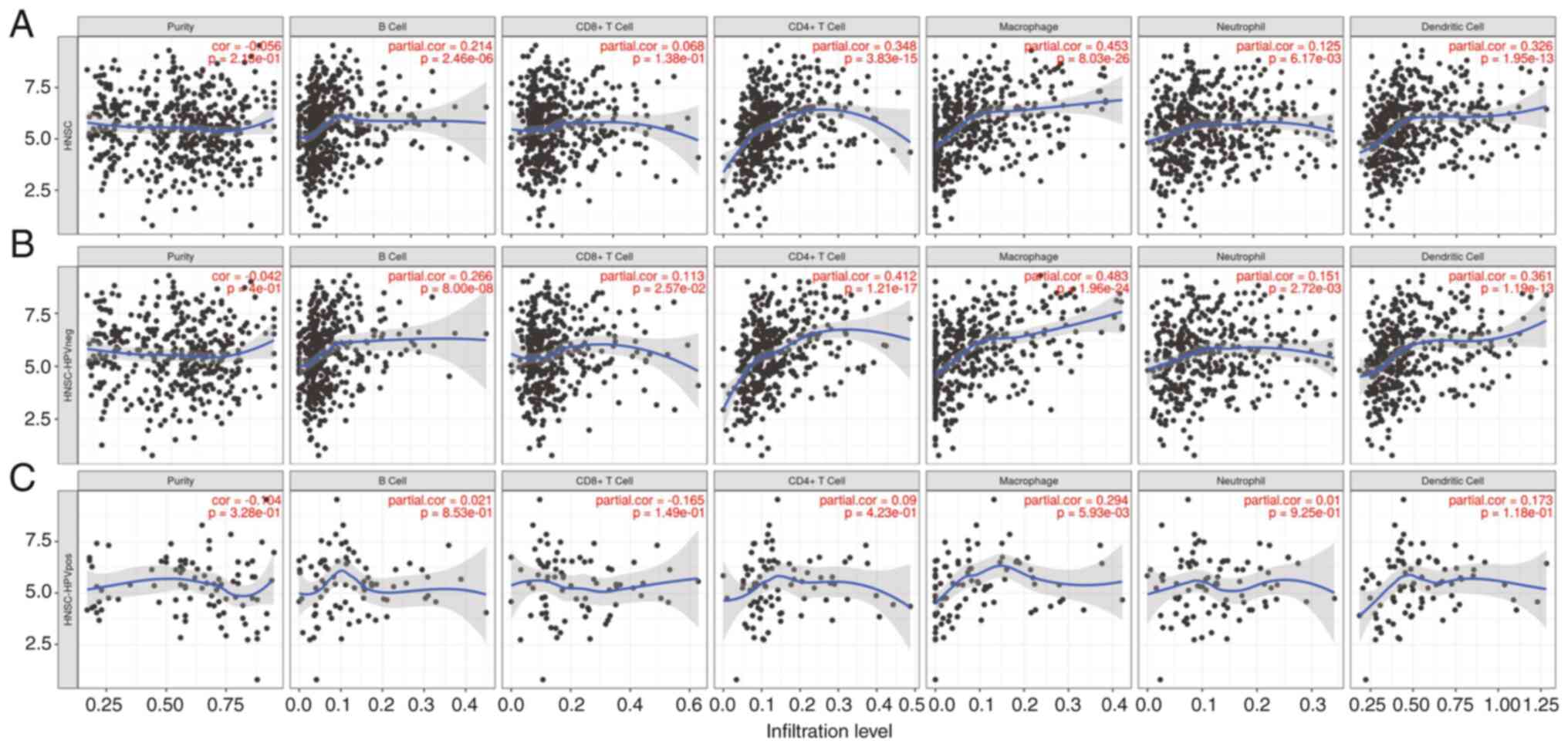
Association of CTHRC1 with immune cell
infiltration in HNSCC
The immune system serves pivotal roles in the
tumorigenesis of HNSCC (3). The
aforementioned data showed that CTHRC1 may be involved in immune
responses and tumor associated pathways. Therefore, the association
between CTHRC1 expression and immune cell invasion in patients with
HNSCC from the TIMER database was evaluated (Fig. 5A). The results indicated that CTHRC1
expression was significantly correlated with the degree of
infiltration of B cells, CD4+ T cells, neutrophils and
dendritic cells (DCs) in HPV negative patients with HNSCC (Fig. 5B). However, no significant
correlation was demonstrated for these groups in HPV positive
patients with HNSCC. The expression of CTHRC1 was significantly
associated with the infiltration of macrophages in both HPV
negative and positive patients with HNSCC (Fig. 5C). This suggested that the
relationship between CTHRC1 expression and macrophage infiltration
was not related to HPV infection. Collectively, these results
demonstrated that CTHRC1 expression was significantly associated
with immune cell infiltration in HNSCC.
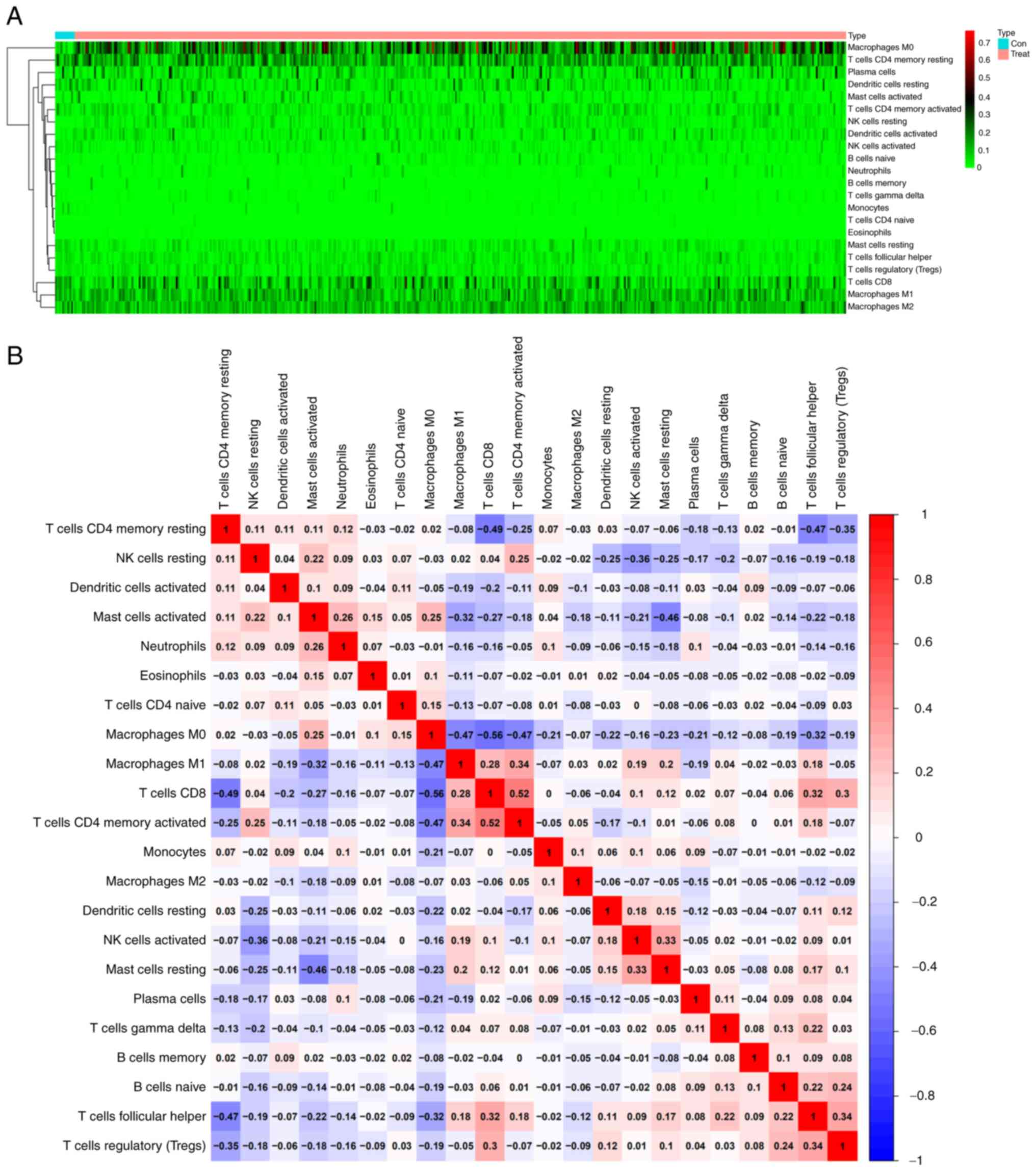
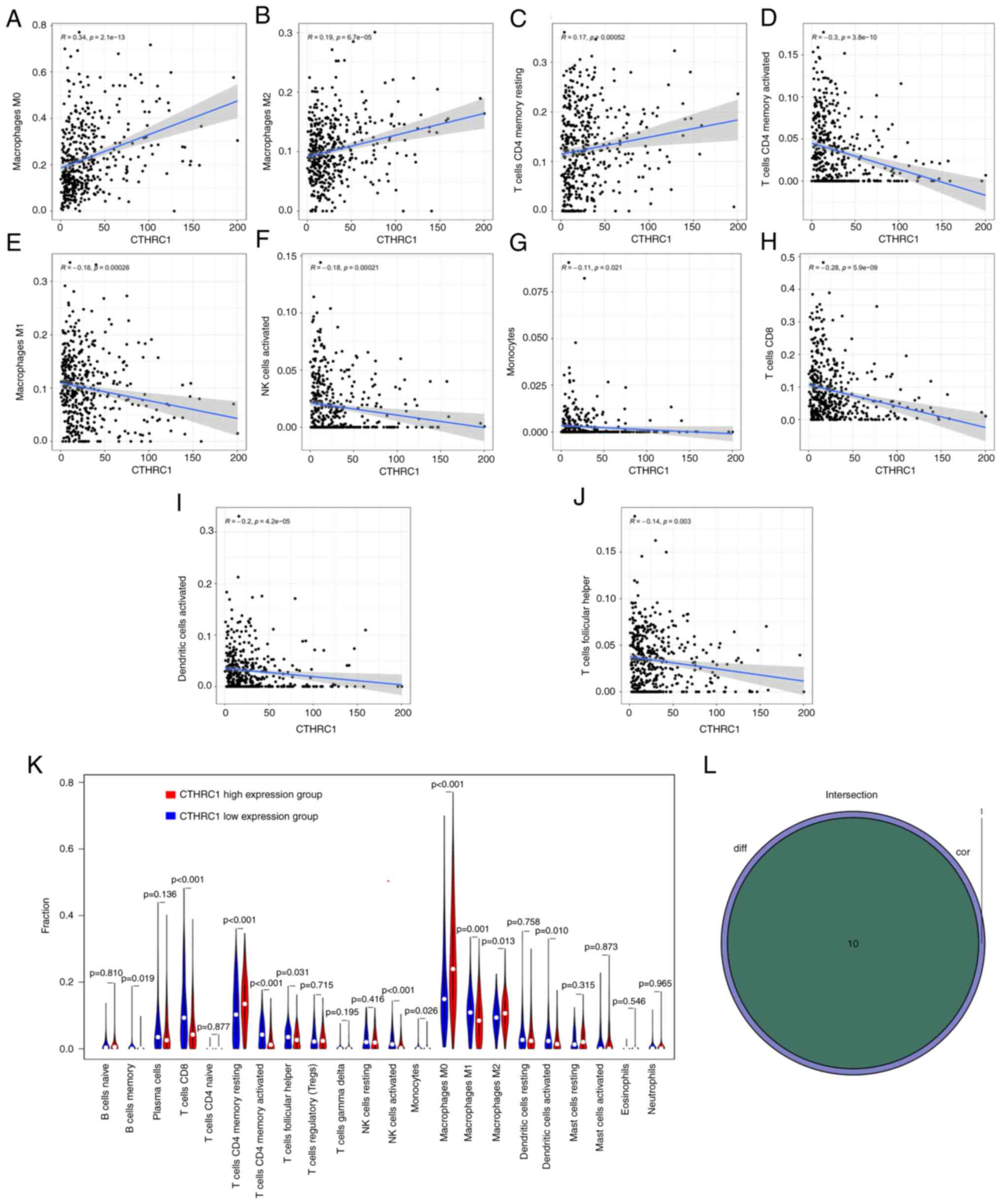
The correlation of CTHRC1 expression
with the proportion and distribution of TICs in HNSCC
The infiltration of 22 TICs in each HNSCC sample and
the CIBERSORT algorithm was used to further evaluate the
correlation between the transcriptional levels of CTHRC1 and the
immune microenvironment (Fig. 6A and
B). Correlation analysis indicated that the transcriptional
levels of CTHRC1 were significantly positively correlated with M0
macrophages (P=2.1×10−13; Fig. 7A), M2 macrophages
(P=6.7×10−5; Fig. 7B)
and resting CD4+ memory T cells (P=5.2×10−4;
Fig. 7C), and were significantly
negatively associated with the levels of activated CD4+
memory T cells (P=3.8×10−10; Fig. 7D), M1 macrophages
(P=2.6×10−4; Fig. 7E),
activated natural killer (NK) cells (P=2.1×10−4;
Fig. 7F), monocytes
(P=2.1×10−2; Fig. 7G),
CD8+ T cells (P=5.9×10−9; Fig. 7H), activated DCs
(P=4.2×10−5; Fig. 7I)
and follicular helper T (Tfh) cells (P=3.0×10−3;
Fig. 7J) (Table II). Analysis demonstrated that the
transcriptional levels of CTHRC1 were correlated with the
infiltration of 10 types of TICs, including resting CD4+
memory T cells, memory B cells, activated CD4+ memory T
cells, CD8+ T cells, Tfh cells, M0 macrophages,
activated NK cells, M1 macrophages, M2 macrophages and activated
DCs (Fig. 7K and L). The high
CTHRC1 expression group tended to have a larger proportion of
resting CD4+ memory T cells, M0 macrophages and M2
macrophages compared with the low CTHRC1 expression group, whereas
the low CTHRC1 expression group had a larger proportion of resting
CD4+ memory T cells, memory B cells, activated
CD4+ memory T cells, CD8+ T cells, Tfh cells,
activated NK cells, M1 macrophages and activated DCs compared with
the high CTHRC1 expression group.
 | Table II.Correlation analysis between collagen
triple helix repeat containing 1 and related gene markers of
tumor-infiltrating immune cells in Tumor Immune Estimation
Resource. |
Table II.
Correlation analysis between collagen
triple helix repeat containing 1 and related gene markers of
tumor-infiltrating immune cells in Tumor Immune Estimation
Resource.
|
|
| Nonea | Purityb |
|---|
|
|
|
|
|
|---|
| Cell type | Gene marker | Corc | P-value | Cor | P-value |
|---|
| CD8+ T
cell | CD8A | −0.005 |
9.19×10−1 | −0.277 |
4.03×10−10 |
|
| CD8B | 0.048 |
2.90×10−1 | −0.242 |
5.06×10−8 |
| T cell
(general) | CD3D | 0.029 |
5.22×10−1 | −0.298 |
1.35×10−11 |
|
| CD3E | 0.110 |
1.49×10−2 | −0.299 |
1.17×10−11 |
|
| CD2 | 0.116 |
9.77×10−3 | −0.285 |
1.15×10−10 |
| B cell | CD19 | 0.028 |
5.36×10−1 | −0.261 |
4.33×10−9 |
|
| CD79A | 0.053 |
2.43×10−1 | −0.228 |
3.05×10−7 |
| Monocyte | CD86 | 0.358 |
2.55×10−16 | −0.295 |
2.26×10−11 |
|
| CSF1R | 0.415 |
5.94×10−22 | −0.304 |
4.98×10−12 |
| TAM | CCL2 | 0.394 |
1.07×10−19 | −0.258 |
5.98×10−9 |
|
| CD68 | 0.272 |
9.12×10−10 | −0.172 |
1.24×10−4 |
|
| IL10 | 0.332 |
4.03×10−14 | −0.313 |
1.21×10−12 |
| M1 Macrophage | NOS2 | 0.122 |
6.55×10−3 | 0.071 |
1.16×10−1 |
|
| IRF5 | 0.103 |
2.26×10−2 | −0.001 |
9.75×10−1 |
|
| PTGS2 | −0.006 |
8.96×10−1 | 0.1 |
2.67×10−2 |
| M2 Macrophage | CD163 | 0.399 |
3.28×10−20 | −0.286 |
1.02×10−10 |
|
| VSIG4 | 0.425 |
5.06×10−23 | −0.257 |
7.42×10−9 |
|
| MS4A4A | 0.438 |
1.69×10−24 | −0.287 |
8.35×10−11 |
| Neutrophils | CEACAM8 | 0.001 |
9.90×10−1 | 0.034 |
4.48×10−1 |
|
| ITGAM | 0.416 |
5.06×10−23 | −0.137 |
2.27×10−3 |
|
| CCR7 | 0.194 |
1.53×10−5 | −0.322 |
2.23×10−13 |
| Natural killer
cell | KIR2DL1 | −0.006 |
8.93×10−1 | −0.093 |
3.81×10−2 |
|
| KIR2DL3 | −0.01 |
8.20×10−1 | −0.136 |
2.56×10−3 |
|
| KIR2DL4 | −0.134 |
2.89×10−3 | −0.183 |
4.30×10−5 |
|
| KIR3DL1 | −0.042 |
3.48×10−1 | −0.144 |
1.35×10−3 |
|
| KIR3DL2 | 0.047 |
2.97×10−1 | −0.147 |
1.05×10−3 |
|
| KIR3DL3 | −0.045 |
3.23×10−1 | −0.086 |
5.68×10−2 |
|
| KIR2DS4 | 0.017 |
7.12×10−1 | −0.148 |
9.93×10−4 |
| Dendritic cell | HLA-DPB1 | 0.223 |
6.05×10−7 | −0.302 |
7.78×10−12 |
|
| HLA-DQB1 | 0.178 |
7.19×10−5 | −0.228 |
3.28×10−7 |
|
| HLA-DRA | 0.194 |
1.41×10−5 | −0.299 |
1.16×10−11 |
|
| HLA-DPA1 | −0.209 |
7.58×10−2 | −0.52 |
2.00×10−6 |
|
| BDCA-1 (CD1C) | −0.01 |
9.34×10−1 | −0.58 |
5.92×10−8 |
|
| BDCA-4 (NRP1) | 0.455 |
5.15×10−5 | 0.065 |
5.80×10−1 |
|
| CD11c (ITGAX) | −0.064 |
5.88×10−1 | −0.387 |
6.55×10−4 |
| Th1 | T-bet (TBX21) | 0.166 |
1.59×10−1 | −0.617 |
4.68×10−9 |
|
| STAT4 | 0.133 |
2.64×10−1 | −0.496 |
7.06×10−6 |
|
| STAT1 | 0.291 |
1.26×10−2 | 0.05 |
6.69×10−1 |
|
| IFN-γ (IFNG) | 0.092 |
4.37×10−1 | −0.421 |
1.85×10−4 |
|
| TNF-α (TNF) | 0.112 |
1.03×10−1 | −0.467 |
2.7×10−5 |
| Th2 | GATA3 | 0.248 |
2.63×10−8 | −0.227 |
3.63×10−7 |
|
| STAT6 | 0.056 |
2.12×10−1 | 0.069 |
1.26×10−1 |
|
| STAT5A | 0.188 |
2.81×10−5 | −0.132 |
3.28×10−3 |
|
| IL13 | 0.071 |
1.15×10−1 | −0.154 |
5.78×10−4 |
| Tfh | BCL6 | 0.192 |
1.03×10−1 | 0.25 |
3.19×10−2 |
|
| IL21 | NA | NA | NA | NA |
| Th17 | STAT3 | 0.124 |
2.96×10−1 | 0.062 |
5.98×10−1 |
|
| IL17A | 0.236 |
4.42×10−2 | −0.238 |
4.09×10−2 |
| Treg | FOXP3 | 0.312 |
7.25×10−3 | 0.009 |
9.37×10−1 |
|
| CCR8 | 0.129 |
2.77×10−1 | −0.33 |
4.11×10−3 |
|
| STAT5B | 0.008 |
9.44×10−1 | −0.036 |
7.59×10−1 |
|
| TGFβ (TGFB1) | 0.49 |
1.08×10−5 | −0.263 |
2.35×10−2 |
| T cell
exhaustion | PD-1 (PDCD1) | 0.109 |
3.58×10−1 | −0.581 |
5.89×10−8 |
|
| CTLA4 | 0.206 |
8.04×10−2 | −0.504 |
4.78×10−6 |
|
| LAG3 | 0.195 |
9.89×10−2 | −0.259 |
2.61×10−2 |
|
| TIM-3 (HAVCR2) | −0.001 |
9.94×10−1 | −0.552 |
3.34×10−7 |
|
| GZMB | 0.062 |
6.05×10−1 | −0.406 |
3.33×10−4 |
Discussion
HNSCC is among the six most common types of human
tumor. Despite the development of medical technology the survival
rate of patients with HNSCC is still low (33). Abnormal gene expression or mutations
may be closely related to the occurrence, development and prognosis
of tumors. However, the molecular mechanisms of HNSCC still need to
be investigated. In the present study, it was found that CTHRC1 was
abnormally upregulated in HNSCC and was significantly associated
with age, TP53 mutation, nodal metastasis status, individual cancer
stages and HPV status. Furthermore, high expression levels of
CTHRC1 were significantly associated with shorter OS and RFS times
in patients with HNSCC. These results demonstrated that CTHRC1 may
function as a pro-oncogene in HNSCC.
CTHRC1 is a secretory glycoprotein which negatively
regulates the deposition of collagen matrix and is involved in
vascular remodeling and cell migration (10). In previous studies, the mRNA and
protein expression levels of CTHRC1 in oral squamous cell carcinoma
samples were found to be higher than those in normal specimens
(34), and were associated with
metastasis in tongue squamous cell carcinoma (35). Therefore, it could be hypothesized
that CTHRC1 may be involved in the development of head and neck
tumors.
In the present study, analysis indicated that
upregulation of CTHRC1 was mainly involved in tumor and
immune-related pathways, such as angiogenesis, apical junction, EMT
and the KRAS and TGF-β signaling pathways. The EMT signaling
pathway is associated with visibility, acquisition of mobility and
self-renewal (36). The abnormal
activation of the KRAS (37), notch
(38) and TGF-β (39) signaling pathways are closely related
to tumorigenesis. In the present study, upregulation of CTHRC1 was
indicated in 1,231 immune related pathways, which suggested a
potential regulatory role of CTHRC1 in the tumor immune
microenvironment. Furthermore, the results also showed that CTHRC1
was significantly positively associated with M0 macrophages, M2
macrophages and resting CD4+ memory T cells, and
significantly negatively associated with the levels of activated
CD4+ memory T cells, activated NK cells, M1 macrophages,
CD8+ T cells, monocytes, activated DCs and Tfh cells.
Moreover, the high CTHRC1 expression group tended to have a larger
proportion of M0 macrophages and M2 macrophages compared with the
low CTHRC1 expression group, while the low CTHRC1 expression group
had a larger proportion of M1 macrophages compared with the high
CTHRC1 expression group. A previous study reported that CTHRC1 can
activate the STAT6 signaling pathway, induce the M2-like macrophage
phenotype in a dose-dependent manner, and improve the migration and
invasion ability of ovarian cancer cells (40). Therefore, CTHRC1 may mediate the
occurrence and development of HNSCC by mediating macrophage
polarization, which leads to poor prognosis.
There are several limitations and challenges in the
present study. Firstly, the sample size is small. Larger studies in
HNSCC are needed to confirm the results. Secondly, CTHRC1
expression may not be a highly specific diagnostic and prognostic
biomarker of HNSCC in humans, but be a shared diagnostic and
prognostic biomarker of survival in different human cancers
(23,24). These may limit its use in clinical
diagnosis and prognosis.
In conclusion, the upregulation of CTHRC1 in
patients with HNSCC is related to the pathological grade,
Tumor-Node-Metastasis stage, lymphatic metastasis, HPV status, TP53
mutation and TICs, which may lead to poor prognosis in patients
with HNSCC. In addition, upregulation of CTHRC1 may activate tumor
and immune related pathways, leading to the infiltration of immune
cells and macrophage polarization in the tumor microenvironment in
HNSCC. The present study identified a potential role of CTHRC1 in
immunology and its adverse prognostic value in HNSCC. CTHRC1 should
therefore be considered as a prognostic marker and therapeutic
target for HNSCC.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Hubei Provincial
Natural Science Foundation of China (grant no. 2019CFB470) and
Hubei Key Laboratory of Biological Targeted Therapy, China (grant
no. 2020swbx014).
Availability of data and materials
The data used in the present study may be accessed
from the TCGA (https://tcga-data.nci.nih.gov/tcga/, head and neck
squamous cell carcinoma, the RNA-seq data ‘workflow type,
HTSeq-FPKM’ and relevant clinical data for the ‘TCGA-HNSC’ cohort),
oncomine (https://www.oncomine.org/resource/main.html, Gene,
CTHRC1; cut-off P-value, 0.05; cut-off fold change, 1), KM plotter
(http://kmplot.com/analysis/, head and
neck squamous cell carcinoma, KM plotter contains data and clinical
information from the Gene Expression Omnibus, TCGA and European
Genome-Phenome Archive databases), UALCAN (http://ualcan.path.uab.edu, TCGA-head and neck
squamous cell carcinoma, contains level 3 RNA-seq data and clinical
information from the TCGA database and is an interactive and
comprehensive web resource for analyzing cancer omics data) and
Timer (https://cistrome.shinyapps.io/timer/, head and neck
squamous cell carcinoma, gene expression levels were presented as
log2 transcripts per million) databases. The remaining datasets
used and/or analyzed during the current study are available from
the corresponding author on reasonable request.
Authors' contributions
LW and JHZ proposed the idea for and designed the
study. RLZ, MLY and RZ completed the data analysis work, RLZ and
MLY drew the figures, MLY and LW drafted the manuscript and JHZ
reviewed the manuscript. RLZ, MLY and LW confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Human tissue specimens were obtained from patients
who provided written informed consent and the present study and
tissue collection was approved by the Ethics Supervision Committee
of The People's Hospital of Tongxu County (Kaifeng, China; approval
no. TX20NP003) and the National Human Genetic Resources Sharing
Service Platform (2005DKA21300). The study protocol was approved by
the Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China;
approval no. 2020IEC-J050).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wyss A, Hashibe M, Chuang SC, Lee YC,
Zhang ZF, Yu GP, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N,
et al: Cigarette, cigar, and pipe smoking and the risk of head and
neck cancers: Pooled analysis in the international head and neck
cancer epidemiology consortium. Am J Epidemiol. 178:679–690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferris RL: Immunology and immunotherapy of
head and neck cancer. J Clin Oncol. 33:3293–3304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuss I, Hathaway B, Ferris RL, Gooding W
and Whiteside TL: Decreased absolute counts of T lymphocyte subsets
and their relation to disease in squamous cell carcinoma of the
head and neck. Clin Cancer Res. 10:3755–3762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bauernhofer T, Kuss I, Henderson B, Baum
AS and Whiteside TL: Preferential apoptosis of CD56dim natural
killer cell subset in patients with cancer. Eur J Immunol.
33:119–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferris RL: Progress in head and neck
cancer immunotherapy: Can tolerance and immune suppression be
reversed? ORL J Otorhinolaryngol Relat Spec. 66:332–340. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. New Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pyagay P, Heroult M, Wang Q, Lehnert W,
Belden J, Liaw L, Friesel RE and Lindner V: Collagen triple helix
repeat containing 1, a novel secreted protein in injured and
diseased arteries, inhibits collagen expression and promotes cell
migration. Circ Res. 96:261–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan X, Yuan X, Yao B, Song W, Li Z,
Enhejirigala, Kong Y, Wang Y, Fu X and Huang S: The role of CTHRC1
in promotion of cutaneous wound healing. Signal Transduct Target
Ther. 7:1832022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bian Z, Miao Q, Zhong W, Zhang H, Wang Q,
Peng Y, Chen X, Guo C, Shen L, Yang F, et al: Treatment of
cholestatic fibrosis by altering gene expression of Cthrc1:
Implications for autoimmune and non-autoimmune liver disease. J
Autoimmun. 63:76–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeshita S, Fumoto T, Matsuoka K, Park
KA, Aburatani H, Kato S, Ito M and Ikeda K: Osteoclast-secreted
CTHRC1 in the coupling of bone resorption to formation. J Clin
Invest. 123:3914–3924. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stohn JP, Wang Q, Siviski ME, Kennedy K,
Jin YR, Kacer D, DeMambro V, Liaw L, Vary CP, Rosen CJ, et al:
Cthrc1 controls adipose tissue formation, body composition, and
physical activity. Obesity (Silver Spring). 23:1633–1642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ni SJ, Ren F, Xu M, Tan C, Weng W, Huang
Z, Sheng W and Huang D: CTHRC1 overexpression predicts poor
survival and enhances epithelial-mesenchymal transition in
colorectal cancer. Cancer Med. 7:5643–5654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding X, Huang R, Zhong Y, Cui N, Wang Y,
Weng J, Chen L and Zang M: CTHRC1 promotes gastric cancer
metastasis via HIF-1α/CXCR4 signaling pathway. Biomed Pharmacother.
123:1097422020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan L, Yu J, Tan F, Ye GT, Shen ZY, Liu H,
Zhang Y, Wang JF, Zhu XJ and Li GX: SP1-mediated microRNA-520d-5p
suppresses tumor growth and metastasis in colorectal cancer by
targeting CTHRC1. Am J Cancer Res. 5:1447–1459. 2015.PubMed/NCBI
|
|
18
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278. 278e1–e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou MZ, Cheng ZQ, Shen HW, He S, Li Y, Pan
Y, Feng C, Chen X, Zhang Y, Lin M, et al: High expression of CTHRC1
promotes EMT of epithelial ovarian cancer (EOC) and is associated
with poor prognosis. Oncotarget. 6:35813–35829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Li Z, Shao F, Yang X, Feng X, Shi
S, Gao Y and He J: High expression of collagen triple helix repeat
containing 1 (CTHRC1) facilitates progression of oesophageal
squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. J
Exp Clin Canc Res. 36:842017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng M, Zhou Q, Liu X, Wang C and Liu G:
CTHRC1 overexpression promotes cervical carcinoma progression by
activating the Wnt/PCP signaling pathway. Oncol Rep. 41:1531–1538.
2019.PubMed/NCBI
|
|
22
|
Yuan RX, Bao D and Zhang Y: Linc00707
promotes cell proliferation, invasion, and migration via the
miR-30c/CTHRC1 regulatory loop in breast cancer. Eur Rev Med
Pharmaco. 24:4863–4872. 2020.PubMed/NCBI
|
|
23
|
Zhou H, Su L, Liu C, Li B, Li H, Xie Y and
Sun D: CTHRC1 may serve as a prognostic biomarker for
hepatocellular carcinoma. Onco Targets Ther. 12:7823–7831. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sial N, Ahmad M, Hussain MS, Iqbal MJ,
Hameed Y, Khan M, Abbas M, Asif R, Rehman JU, Atif M, et al: CTHRC1
expression is a novel shared diagnostic and prognostic biomarker of
survival in six different human cancer subtypes. Sci Rep.
11:198732021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai Y, Yin K, Su T, Ji F and Zhang S:
CTHRC1 in ovarian cancer promotes M2-like polarization of
tumor-associated macrophages via regulation of the STAT6 signaling
pathway. Onco Targets Ther. 13:5743–5753. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Jensen MA and Zenklusen JC: A
practical guide to the cancer genome atlas (TCGA). Methods Mol
Biol. 1418:111–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reis-Filho JS and Tutt ANJ: Triple
negative tumours: A critical review. Histopathology. 52:108–118.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shien T, Shimizu C, Seki K, Shibata T,
Hojo T, Ando M, Kohno T, Katsumata N, Akashi-Tanaka S, Kinoshita T
and Fujiwara Y: Comparison among different classification systems
regarding the pathological response of preoperative chemotherapy in
relation to the long-term outcome. Breast Cancer Res Treat.
113:307–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lánczky A and Győrffy B: Web-based
survival analysis tool tailored for medical research (KMplot):
Development and implementation. J Med Internet Res. 23:e276332021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee CE, Vincent-Chong VK, Ramanathan A,
Kallarakkal TG, Karen-Ng LP, Ghani WM, Rahman ZA, Ismail SM,
Abraham MT, Tay KK, et al: Collagen triple helix repeat
containing-1 (CTHRC1) expression in oral squamous cell carcinoma
(OSCC): Prognostic value and clinico-pathological implications. Int
J Med Sci. 12:937–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu GL, Sengupta PK, Jamal B, Yang HY,
Bouchie MP, Lindner V, Varelas X and Kukuruzinska MA:
N-glycosylation induces the CTHRC1 protein and drives oral cancer
cell migration. J Biol Chem. 288:20217–20227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scheel C, Eaton EN, Li SHJ, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weidhaas JB, Harris J, Schaue D, Chen AM,
Chin R, Axelrod R, El-Naggar AK, Singh AK, Galloway TJ, Raben D, et
al: The KRAS-variant and cetuximab response in head and neck
squamous cell cancer: A secondary analysis of a randomized clinical
trial. JAMA Oncol. 3:483–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Loganathan SK, Schleicher K, Malik A,
Quevedo R, Langille E, Teng K, Oh RH, Rathod B, Tsai R,
Samavarchi-Tehrani P, et al: Rare driver mutations in head and neck
squamous cell carcinomas converge on NOTCH signaling. Science.
367:1264–1269. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oshimori N, Oristian D and Fuchs E: TGF-β
promotes heterogeneity and drug resistance in squamous cell
carcinoma. Cell. 160:963–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo B, Yan H, Li L, Yin K, Ji F and Zhang
S: Collagen triple helix repeat containing 1 (CTHRC1) activates
Integrin β3/FAK signaling and promotes metastasis in ovarian
cancer. J Ovarian Res. 10:692017. View Article : Google Scholar : PubMed/NCBI
|