Introduction
According to cancer statistics released in 2021
(1), breast cancer has surpassed
lung cancer as the most frequently occurring malignant tumor. Its
high prevalence in women makes it a leading cause of death in
women, and its incidence continues to increase. At present,
surgical resection, chemotherapy, endocrine therapy, targeted
therapy and radiation therapy are the mainstream treatments for
breast cancer (2,3). The connections between breast cancer
cells are loose and easily broken, and detached cells can migrate
via the blood or lymphatic system to develop metastases in other
regions of the body (4), which
accounts for >90% of breast cancer-associated fatalities
(5). Despite the success of
emerging immunotherapies in metastatic breast cancer (6), high costs and recurrence rates prevent
a large number of patients with advanced cancer from benefiting
from them. Paclitaxel (PTX) is a chemotherapeutic medication
commonly used in the treatment of breast cancer that blocks
microtubule dissociation, hinders cell cycle progression and stops
mitosis (7). Resistance to
chemotherapeutic drugs, however, is a pressing issue in cancer
treatment. Treatment with PTX may induce resistance in patients,
resulting in chemotherapy failure (8). Specific heredity, epigenetic
aberrations in cancer cells and altered drug transport, where the
drug is pumped out of the tumor cells, may all be associated with
the induction of drug resistance (9,10).
In a study of glioblastoma, lysophosphatidylcholine
acyltransferase 1 (LPCAT1) was suggested to play a role in
remodeling the structure of the plasma membrane by modifying the
phospholipid composition of the cytoplasmic membrane, increasing
the stabilization of epidermal growth factor receptor, and
transmitting and amplifying growth signals (11). In addition, LPCAT1 has been reported
to promote the advancement of cutaneous squamous cell carcinoma via
the protein kinase B and p38MAPK signaling pathways (12). The upregulation of LPCAT1 in
hepatocellular carcinoma tissue specimens is associated with a poor
prognosis and contributes to progression by encouraging cell growth
and metastasis (13). LPCAT1 is
also highly expressed in endometrial carcinoma samples, and the
silencing of LPCAT1 inhibits endometrial carcinoma cell
proliferation (14). To the best of
our knowledge, it has not yet been revealed whether LPCAT1 has
growth-promoting or pro-metastatic effects in breast cancer.
However, analyses performed using UALCAN (15) and Gene Expression Profiling
Interactive Analysis (GEPIA) (16)
based on data in The Cancer Genome Atlas (TCGA) indicate that
LPCAT1 is upregulated in breast cancer tissues and is associated
with a poor prognosis.
The aim of the present study was to investigate the
involvement of LPCAT1 in breast cancer and to explore its
mechanism. The Human Transcription factor Database (HumanTFDB)
(17) indicates that the
transcription factor forkhead box A1 (FOXA1) can bind to the LPCAT1
promoter and regulate its transcriptional regulation. As a
corollary, it was hypothesized that the FOXA1-mediated
transcriptional upregulation of LPCAT1 promotes the malignant
progression and drug resistance of breast cancer cells.
Materials and methods
Bioinformatics
The UALCAN database (ualcan.path.uab.edu) was used
to compare LPCAT1 and FOXA1 expression levels between tumor and
normal tissues using TCGA data. GEPIA (gepia.cancer-pku.cn) was
used to compare overall and disease-free survival between patients
with low and high LPCAT1 expression using TCGA data. The HumanTFDB
database (bioinfo.life.hust.edu.cn) predicted the binding sites for
FOXA1 and the LPCAT1 promoter.
Cell culture
MCF-10A mammary epithelial cells and MDA-MB-231,
BT-549, HCC1937, SK-BR-3 and MCF-7 breast cancer cell lines were
obtained from Shanghai EK-Bioscience Biotechnology Co., Ltd.
PTX-resistant MDA-MB-231 (MDA-MB-231/PTX) cells were generated by 3
months of continuous exposure to a stepwise steadily increasing
concentration of PTX (0–100 nM; MedChemExpress) at 37°C as
previously described (18).
MDA-MB-231 cells were cultured in Leibovitz's L-15 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin (P/S; all Gibco; Thermo Fisher Scientific,
Inc.) without CO2 at 37°C. The other cell lines were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1% P/S at 37°C in a 5% CO2
atmosphere.
Cell transfection
In order to reduce the expression of LPCAT1 and
overexpress FOXA1, MDA-MB-231 cells were transfected with short
hairpin (sh)RNAs targeting LPCAT1 (sh-LPCAT1-1 and −2) and
FOXA1-overexpression plasmids (oe-FOXA1), respectively. Cells
transfected with non-targeting shRNA and empty plasmid served as
the negative controls (sh-NC and oe-NC, respectively). These
pLVX-shRNAs and pcDNA3.1 plasmids were constructed by
VectorBuilder, Inc. Briefly, cells (1×104/well) were
seeded in 96-well plates 1 day before transfection, and
transfection with a final concentration of 50 nM shRNA and/or 15 nM
overexpression plasmids was then performed for 48 h at 37°C using
FuGENE® transfection reagents (Promega Corporation). The
interval between transfection and subsequent experiments was 48 h.
The target sequences were as follows: sh-LPCAT1-1,
5′-GGAACTCTGATCCAGTATATA-3′; sh-LPCAT1-2,
5′-GGGAACTCTGATCCAGTATAT-3′; and sh-NC,
5′-GCACTACCAGAGCTAACTCAG-3′.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was added to the cells, mixed and allowed
to stand at room temperature for 5 min. Chloroform was then added,
the lysate was centrifuged at 12,000 × g for 12 min at 4°C, the
upper aqueous phase was collected and the RNA was precipitated with
isopropanol. The isolated RNA was reverse transcribed to generate
cDNA using a PrimeScript™ RT Reagent Kit (Takara Bio,
Inc.). The reaction conditions for reverse transcription were as
follows: 30°C for 10 min, 42°C for 30 min and 70°C for 15 min. A
QuantiTect SYBR Green PCR kit (Qiagen, Inc.) was used for qPCR
according to the manufacturer's protocol. The qPCR was performed in
a 20-µl reaction system containing 10 µl Master Mix, 10 ng DNA
template and 500 nM specific forward and reverse primers. The
thermocycling reaction conditions were as follows: Predenaturation
at 95°C for 15 min and 40 cycles of denaturation at 94°C for 30
sec, annealing at 60°C for 30 sec and extension at 68°C for 30 sec.
The relative mRNA levels were measured using the 2−∆∆Cq
method (19) following
normalization against GAPDH. The primer sequences were as follows
(5′-3′): LPCAT1, forward, ATGAGGCTGCGGGGATG and reverse,
GATGGCCTTCAGCAGGAAGT; FOXA1, forward, CCCTCTGGCGCCTCTAAC and
reverse, TGGAGAACGGGTGGTTGAAG; GAPDH, forward,
GACTCATGACCACAGTCCATGC and reverse, AGAGGCAGGGATGATGTTCTG.
Western blotting
Protein was isolated from cells following treatment
with RIPA lysis buffer (Life-iLab Bio) and quantified using a Nano
300 protein detector (YPH-Bio). Protein separation (30 µg per lane)
was achieved using 10% SDS-polyacrylamide gel electrophoresis and
the separated proteins were transferred to PVDF membranes (Roche
Diagnostics). The membranes were incubated with 5% skimmed milk for
2 h at room temperature, with primary antibodies overnight at 4°C
and HRP-conjugated secondary antibodies for 2 h at room temperature
in sequence. The primary antibodies against LPCAT1 (cat. no.
ab214034; 1:2,000), FOXA1 (cat. no. ab170933; 1:1,000), Ki67 (cat.
no. ab92742; 1:5,000), proliferating cell nuclear antigen (PCNA;
cat. no. ab92552; 1:1,000), MMP2 (cat. no. ab92536; 1:1,000), MMP9
(cat. no. ab76003; 1:1,000), Bcl-2 (cat. no. ab32124; 1:2,000), Bax
(cat. no. ab32503; 1:1,000), cleaved caspase 3 (cat. no. ab32042;
1:500) and GAPDH (cat. no. ab9485; 1:2,500), and the secondary
antibodies (cat. no. ab6721; 1:4,000) were all from Abcam. Blots
were visualized after treatment with Immobilon ECL Ultra Western
HRP (Merck KGaA) and gray values were analyzed with ImageJ software
(v1.8.0; National Institutes of Health).
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was used to evaluate the proliferation
of transfected cells and the viability of resistant cells. In
brief, transfected cells (3×103/well) were seeded in
96-well plates and cultured for 24, 48 and 72 h. The resistant
cells were treated with PTX (0–100 nM) for 72 h at 37°C. CCK-8
solution (Absin Bioscience, Inc.) was added to each well and
incubation was continued for another 2 h. The optical density (OD)
was then determined at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Colony formation
Control and transfected MDA-MB-231 cells were seeded
into culture dishes at a density of 500 cells/dish. They were
cultured for 2 weeks and the medium was changed every 3 days.
Thereafter, cells were washed twice with PBS, fixed with 4%
paraformaldehyde (Merck KGaA) for 20 min at room temperature and
stained with 0.5% crystal violet (Shanghai Gefan Biotechnology Co.,
Ltd.) for 20 min at room temperature. The colonies were counted
manually. A cluster of >50 cells was considered a colony.
Wound healing and Transwell
The migration and invasion of the control and
transfected MDA-MB-231 cells were separately assessed using wound
healing and Transwell assays, respectively. In the wound healing
assay, cells were cultured until a confluent monolayer formed and a
sterile pipette tip was used to generate a wound in the middle of
the cells that were cultured in serum-free Leibovitz's L-15 medium.
Images were captured at 0 and 24 h. In the Transwell assay, cells
(1×104 cells/well) were cultivated in serum-free
Leibovitz's L-15 medium in the upper chamber, which was pre-coated
with Matrigel (Corning, Inc.) at 37°C for 1 h. Leibovitz's L-15
containing 20% FBS was loaded into the lower chamber. Following 24
h of incubation at 37°C, the invasive cells were fixed and stained
with 0.1% crystal violet solution at room temperature for 15 min.
Results for both assays were observed under a light microscope
(magnification ×100; Olympus Corporation).
Flow cytometry
The apoptosis of MDA-MB-231 and MDA-MB-231/PTX cells
with or without transfection was analyzed using Annexin V-FITC
Apoptosis Detection Kit (Beyotime Institute of Biotechnology) and
flow cytometry. Briefly, cells (1×105) were washed twice
with precooled PBS and suspended in 1 ml binding buffer. A 100-µl
sample of the cell suspension was transferred in a culture tube and
incubated with Annexin V-FITC and propidium iodide at room
temperature in the absence of light for 15 min. Results were
obtained using flow cytometry using a BD FACSCanto™
instrument (BD Biosciences) and FlowJo version 10 software (GlowJo
LLC).
Luciferase reporter
The promoter site of LPCAT1 and a mutated form
(CGCCCAGGC) of this site were cloned into a dual-luciferase
reporter vector (Promega Corporation). The reporter vector was
co-transfected along with oe-FOXA1 or oe-NC into MDA-MB-231 cells
using FuGENE® transfection reagents (Promega
Corporation). At 48 h post-transfection, the luciferase activity
was assessed using the Dual-luciferase Reporter Assay System
(Promega Corporation), according to the manufacturer's protocol,
and normalized to Renilla luciferase activity.
Chromatin immunoprecipitation
(ChIP)
The association between LPCAT1 and FOXA1 was
evaluated using a ChIP Detection Kit (cat. no. 17–295; EZ-ChIP;
MilliporeSigma). Briefly, the MDA-MB-231 cells were treated with 1%
formaldehyde, followed by lysis buffer and then sonicated. The
cells were subsequently incubated with an anti-FOXA1 (cat. no.
ab170933; 1:50; Abcam) or anti-IgG antibody (cat. no. ab172730;
1:50; Abcam) overnight at 4°C. Following the incubation, 60 µl
protein A agarose beads was added to harvest the protein-DNA
complex. The complex was washed in low-salt and high-salt washing
buffers at 4°C, for 5 min each time, 4 times in total. The liquid
was removed by centrifugation at 1,000 × g for 1 min at 4°C, and 5
mmol/l NaCl was added to retrieve the DNA. The enrichment of LPCAT1
was determined using RT-qPCR.
Statistical analysis
For statistical analysis, GraphPad Prism 8.0
(GraphPad Software, Inc.) was utilized. All data are presented as
the mean ± SD and all experiments were performed ≥3 times
independently. To compare differences between two and multiple
groups, the unpaired Student's t-test and one-way ANOVA followed by
Tukey's post hoc test were used, respectively. P<0.05 was
considered to indicate a statistically significant difference.
Results
Role of LPCAT1 in cell proliferation
and metastatic potential
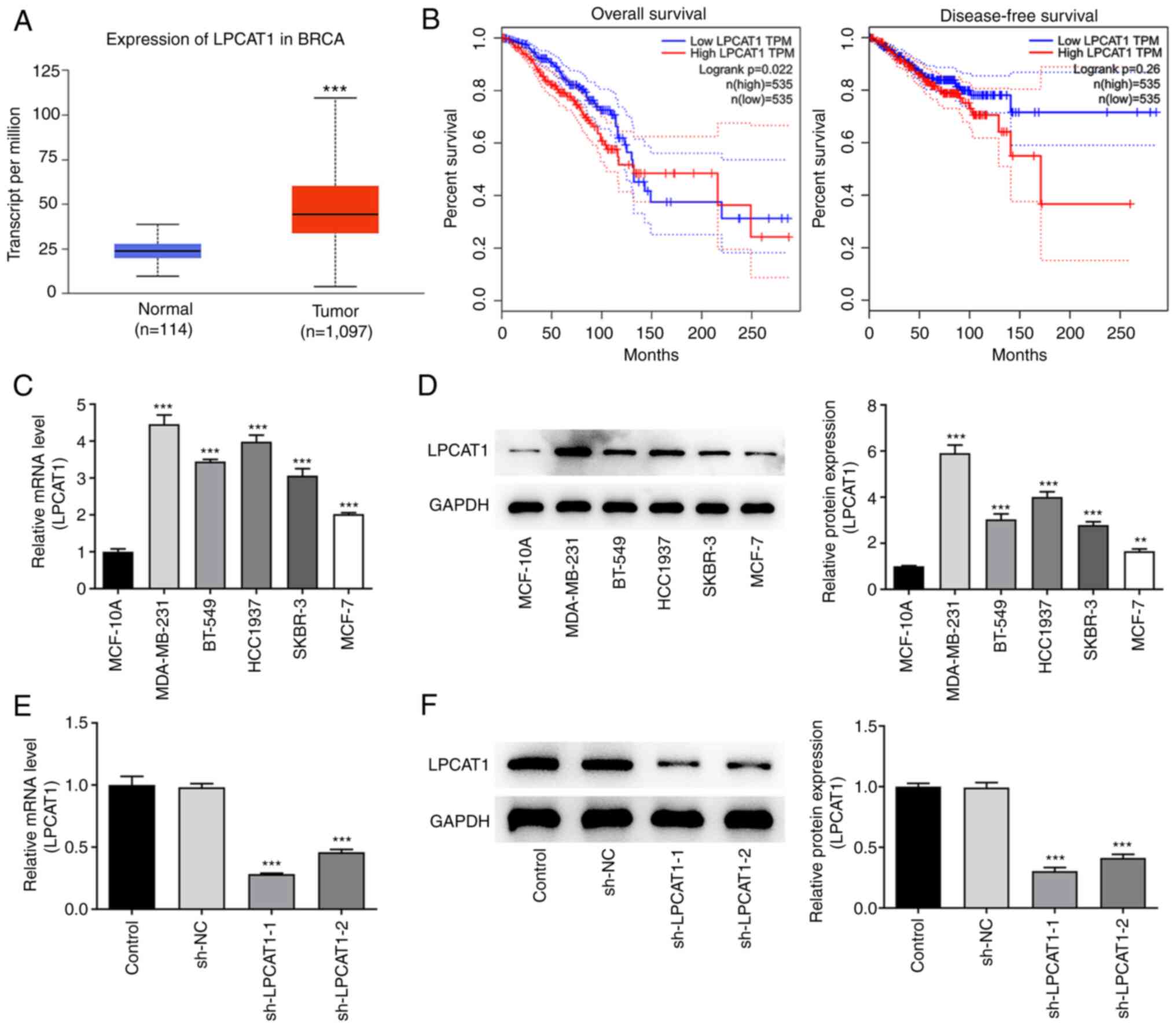
Analysis performed using the UALCAN database
revealed that LPCAT1 is expressed at significantly higher levels in
breast cancer tissues compared with normal tissues (Fig. 1A). Furthermore, survival analysis
performed using GEPIA indicated that patients with high LPCAT1
expression were more likely than those with low LPCAT1 expression
to have a poor prognosis in terms of overall survival and
disease-free survival within 10 years; LPCAT1 is significantly
associated with the poor overall survival of patients (Fig. 1B). Thereafter, the expression levels
of LPCAT1 in various cell lines were determined using RT-qPCR
(Fig. 1C) and western blotting
(Fig. 1D). The results revealed
that LPCAT1 expression was significantly elevated in breast cancer
cell lines compared with MCF-10A cells. To highlight the potential
role of LPCAT1 in breast cancer, the MDA-MB-231 cell line was
selected for further analysis. The expression of LPCAT1 in the
transfected MDA-MB-231 cell line was markedly reduced by
transfection with sh-LPCAT1 as shown by the results of RT-qPCR
(Fig. 1E) and western blotting
(Fig. 1F). Since the level of
knockdown was superior in cells in the sh-LPCAT1-1 group, the
proliferation of cells transfected with sh-LPCAT1-1 (henceforth
referred to as sh-LPCAT1) was further examined.
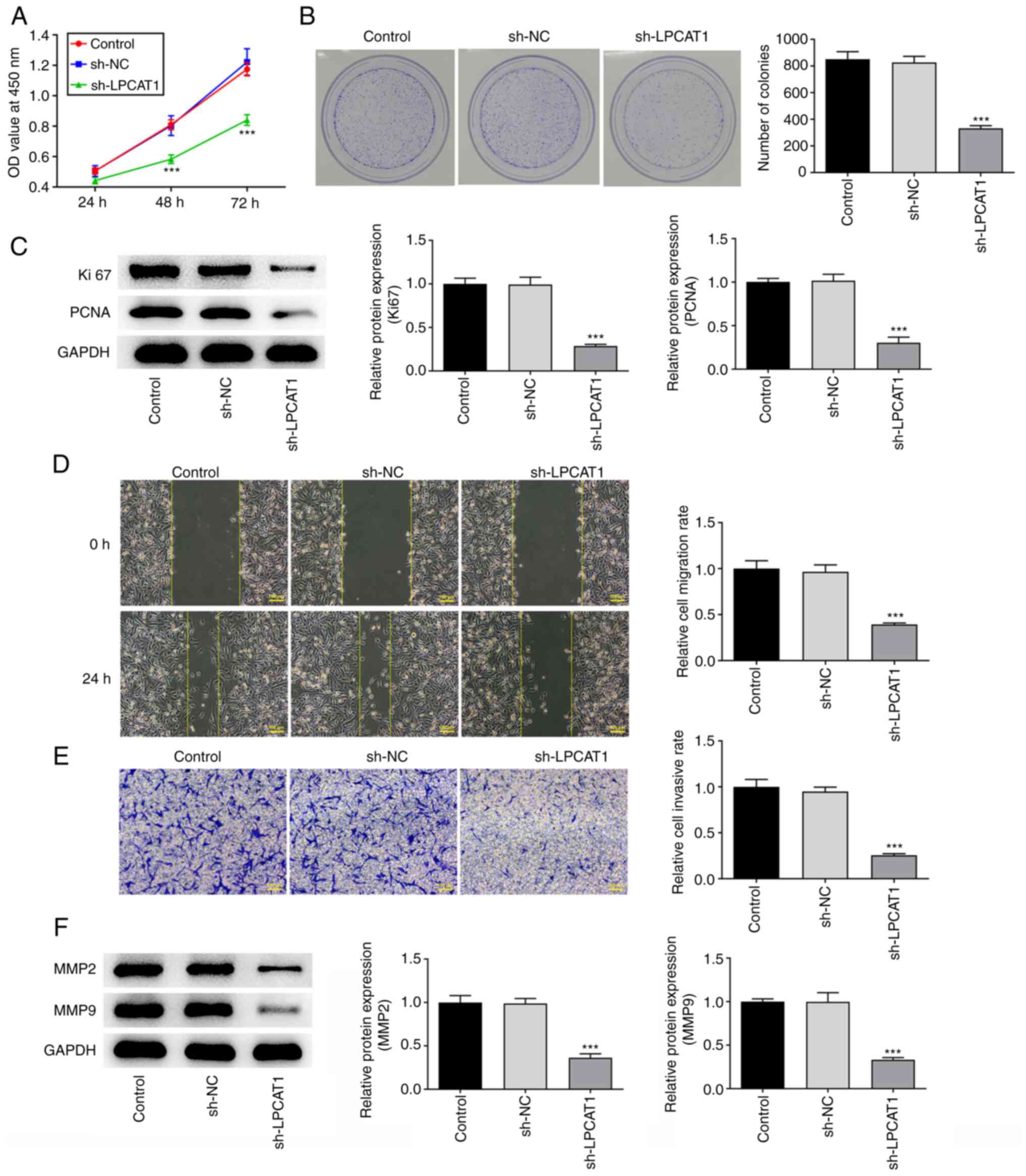
CCK-8 (Fig. 2A) and
colony formation (Fig. 2B) assay
results showed that LPCAT1 knockdown significantly suppressed the
proliferation and colony formation of the cells. The expression
levels of Ki67 and PCNA were also significantly decreased due to
the reduction in LPCAT1 expression (Fig. 2C). In addition, the knockdown of
LPCAT1 inhibited the migration and invasion of the cells (Fig. 2D and E), which was supported by a
reduction in MMP2 and MMP9 expression levels (Fig. 2F).
Role of LPCAT1 in PTX resistance
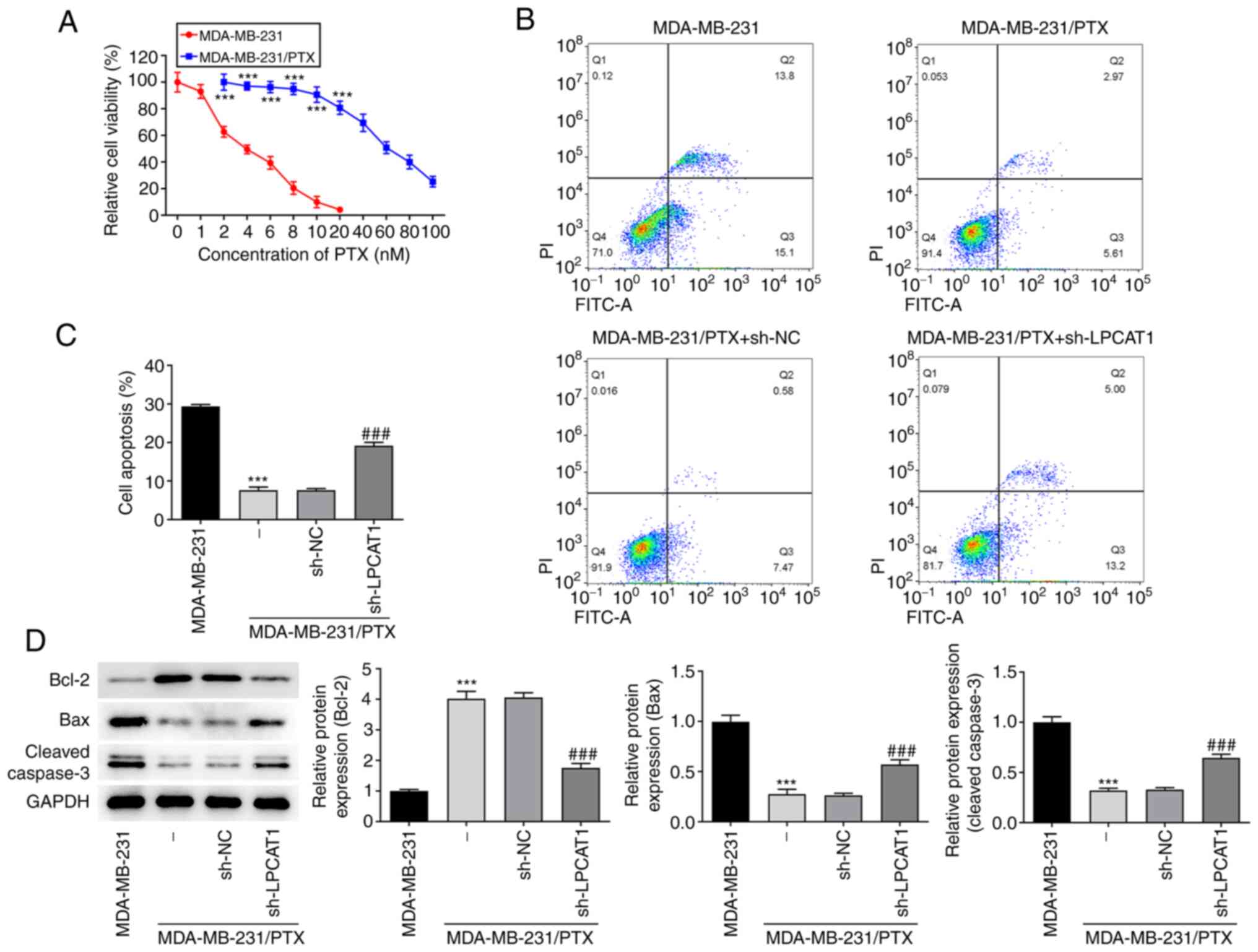
The viability of MDA-MB-231 and MDA-MB-231/PTX cells
following treatment with PTX (0–100 nM) was detected by a CCK-8
assay. The results indicated that MDA-MB-231 cells were
significantly more sensitive to PTX than were the MDA-MB-231/PTX
cells (Fig. 3A). The level of
apoptosis following treatment with 4 nM PTX was assessed by flow
cytometry. The apoptosis rate of the MDA-MB-231/PTX cells was
significantly lower than that of the MDA-MB-231 cells, and LPCAT1
knockdown increased the apoptosis rate of the MDA-MB-231/PTX cells
compared with that of the MDA-MB-231/PTX cells transfected with
sh-NC (Fig. 3B and C). The levels
of apoptosis-associated proteins in the cells treated with PTX were
also determined. Western blot results revealed that Bcl-2 was more
abundant in MDA-MB-231/PTX cells compared with MDA-MB-231 cells,
whereas Bax and cleaved caspase 3 levels were lower. Furthermore,
LPCAT1 knockdown reduced the expression of Bcl-2 and increased Bax
and cleaved caspase 3 levels in MDA-MB-231/PTX cells (Fig. 3D).
Association between FOXA1 and
LPCAT1
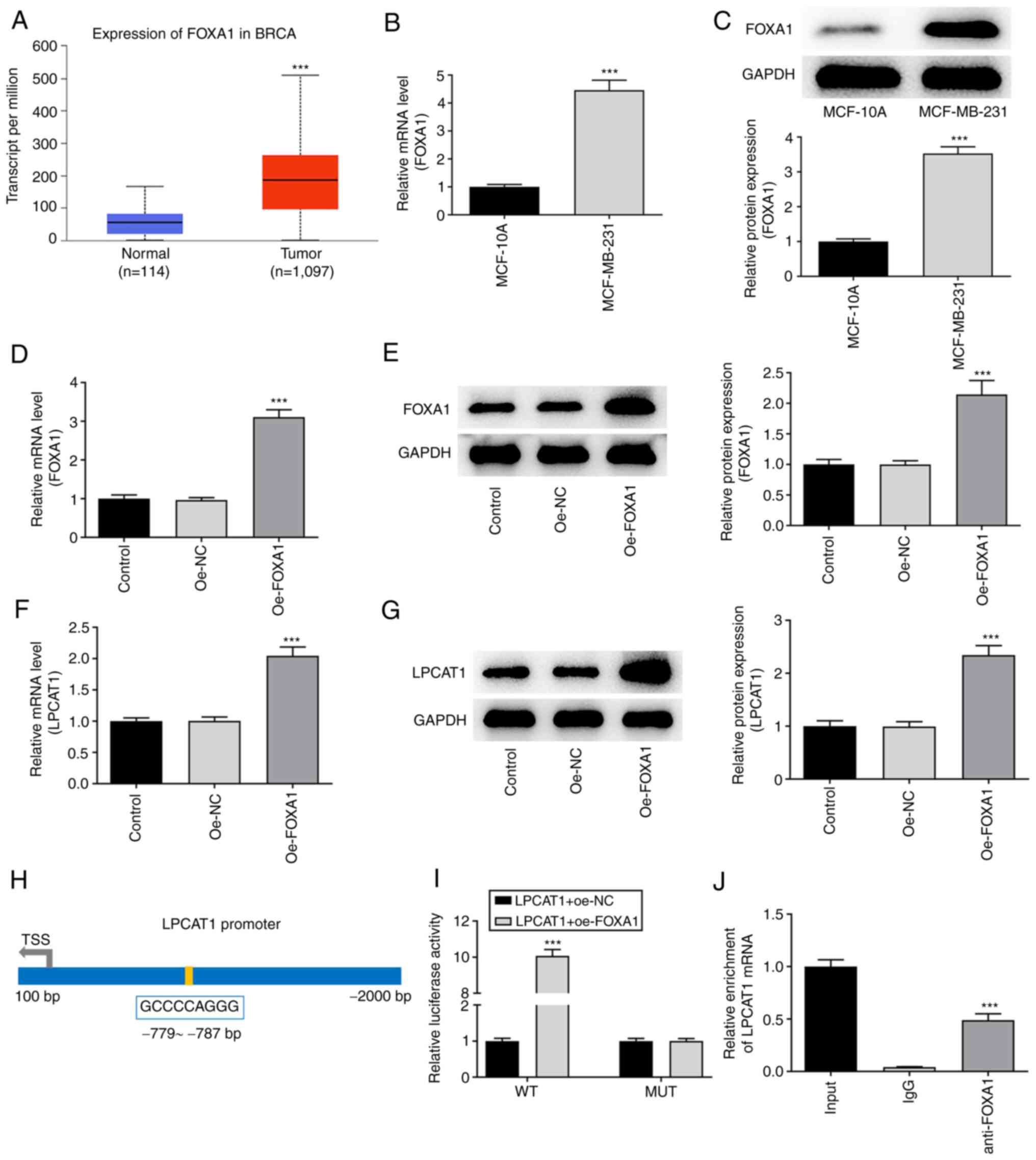
Analysis performed using the UALCAN database
suggested that FOXA1 was also highly expressed in breast cancer
tissues compared with normal breast tissues (Fig. 4A). The expression level of FOXA1 in
MCF-10A and MDA-MB-231 cells was assessed using RT-qPCR (Fig. 4B) and western blotting (Fig. 4C). FOXA1 expression was
significantly higher in MDA-MB-231 cells compared with MCF-10A
cells. Following confirmation that FOXA1 was successfully
overexpressed in the MDA-MB-231 cells transfected with oe-FOXA1
compared with those transfected with oe-NC (Fig. 4D and E), the levels of LPCAT1 were
determined. FOXA1 overexpression boosted the increase of LPCAT1 in
the MDA-MB-231 cells (Fig. 4F and
G). The HumanTFDB website predicted binding sites between
transcription factor FOXA1 and the LPCAT1 promoter (Fig. 4H). Thereafter, LPCAT1 promoter
activity was determined using a luciferase reporter assay. The
activity in the LPCAT1-WT + oe-FOXA1 group was significantly higher
than that in the LPCAT1-WT + oe-NC group (Fig. 4I). The binding of FOXA1 to LPCAT1
was then evaluated using a ChIP assay (Fig. 4J). The relative enrichment of LPCAT1
in the anti-FOXA1 group was significantly higher compared with that
in the lgG group.
FOXA1 regulates LPCAT1
To evaluate the effect of FOXA1 overexpression on
the regulatory role of LPCAT, the proliferation and colony
formation ability of MDA-MB-231 cells co-transfected with sh-LPCAT1
and oe-FOXA1 were assessed. The co-transfection increased cell
proliferation (Fig. 5A) and colony
formation (Fig. 5B) and enriched
the expression of Ki67 and PCNA proteins (Fig. 5C and D) compared with those in cells
transfected with sh-LPCAT1 and oe-NC. These results indicate that
FOXA1 overexpression attenuated the effects of LPCAT knockdown on
cell proliferation. Furthermore, FOXA1 overexpression attenuated
the effects of sh-LPCAT1 on cell migration (Fig. 5E) and invasion (Fig. 5F), which was accompanied by
increased levels of MMP2 and MMP9 (Fig.
5G). The effect of FOXA1 overexpression on the drug resistance
of the cells was also evaluated. The flow cytometry results
indicated that the level of apoptosis was decreased in
MDA-MB-231/PTX cells subjected to co-transfection with sh-LPCAT1
and oe-FOXA1 compared with that in cells transfected with sh-LPCAT1
plus oe-NC (Fig. 5H). In addition,
the expression levels of Bcl-2 in the sh-LPCAT1 and oe-FOXA1
co-transfected cells were increased whereas those of Bax and
cleaved caspase 3 were decreased compared with those in the
sh-LPCAT1 plus oe-NC group (Fig.
5I).
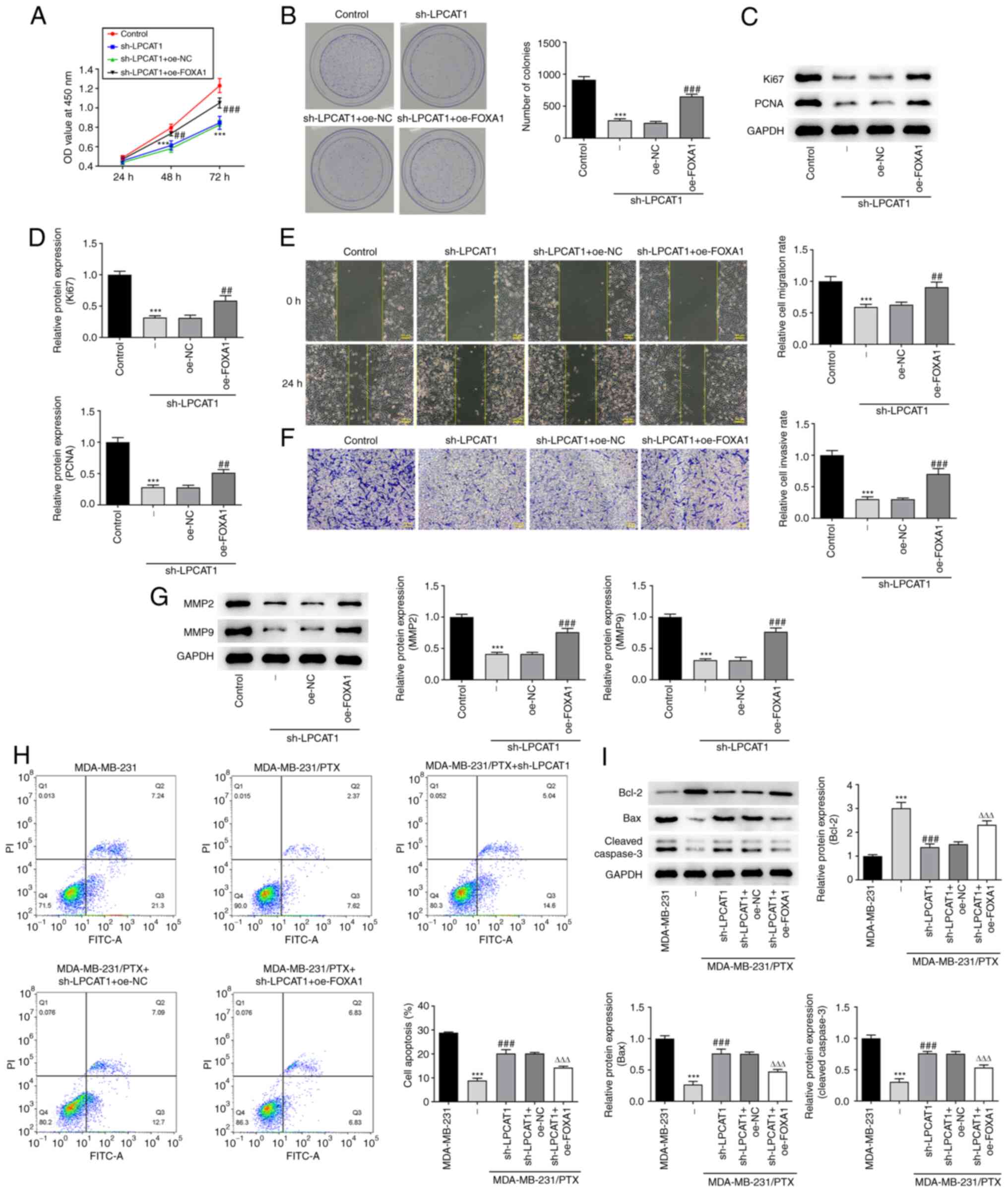 | Figure 5.FOXA1 regulates LPCAT1. (A)
Proliferation and (B) colony formation of MDA-MB-231 cells
co-transfected with sh-LPCAT1 and oe-FOXA1 was assessed. (C and D)
Expression levels of Ki67 and PCNA were determined using western
blotting. (C) Representative images and (D) densitometrically
quantified results are presented. (E) Cell migration and (F)
invasion potential were assessed using wound healing and Transwell
assays, respectively. Scale bar, 100 µm. (G) Expression levels of
MMP2 and MMP9 were determined using western blotting. ***P<0.001
vs. control; ##P<0.01 and ###P<0.001
vs. sh-LPCAT1 + oe-NC. (H) Apoptosis after 4 nM PTX treatment was
assessed by flow cytometry. (I) Enrichment of apoptosis-associated
proteins in the co-transfected cells was determined using western
blotting. ***P<0.001 vs. MDA-MB-231; ###P<0.001
vs. MDA-MB-231/PTX + sh-NC; ΔΔΔP<0.001 vs. sh-LPCAT1
+ oe-NC. FOXA1, forkhead box A1; LPCAT1, lysophosphatidylcholine
acyltransferase 1; sh, short hairpin; oe, overexpression; NC,
negative control; PCNA, proliferating cell nuclear antigen; OD,
optical density; PI, propidium iodide; FITC-A, fluorescein
isothiocyanate-Annexin V. |
Discussion
PTX has been extensively known for its antitumor
activity. It has a broad range of anticancer properties and can be
employed in the chemotherapy of various solid tumors (20), including non-small cell lung cancer,
ovarian cancer and esophageal cancer. At present, it is the
first-line drug in the chemotherapy of breast cancer (21). Resistance to chemotherapy drugs is a
serious issue in cancer treatment, and recurrence and metastasis
are the predominant causes of mortality in patients with breast
cancer (22). Primary resistance is
resistance that is present prior to therapy (23), whereas acquired resistance develops
over time after drug usage. As a consequence, the long-term effects
of PTX use may be unsatisfactory (24). The mechanisms underlying
chemotherapy resistance have not been fully elucidated and require
additional investigation. The key to improving the prognosis of
breast cancer is the effective control of metastases and treatment
resistance (25). Therefore,
investigating the molecular mechanisms of breast cancer invasion,
metastasis and drug resistance, as well as identifying specific
targets for the reversal of chemotherapy resistance (25), may provide new research and
development options for gene-targeted breast cancer therapy.
The findings of the present study indicate that
LPCAT1 modulates breast cancer cell proliferation, metastatic
potential and drug resistance. Given the previous findings of
LPCAT1 in various tumors (26), it
may be inferred that LPCAT1 is a novel target that is prevalent in
a wide range of cancers. Furthermore, it is considered to be an
enzyme that is associated with genetic and metabolic anomalies in
cancer cells (11,27), contributing to aggressive tumor
growth. A novel finding of the present study is that LPCAT1 is
implicated in chemoresistance. Furthermore, FOXA1 was discovered to
alter the function of LPCAT1 in breast cancer through rescue
experiments.
The role of FOXA1 in breast cancer has been reported
in previous studies. For example, one study showed that microRNA
(miR)-100 inhibits the proliferation, migration and invasion of
breast cancer cells by targeting FOXA1 (28). Another study demonstrated that by
sponging miR-23a-3p and thereby promoting FOXA1, the long
non-coding RNA NEAT1 enhances drug resistance in breast cancer
cells (29). Furthermore, somatic
point mutations in FOXA1 occur at a rate of 4–8%, and FOXA1
mutations boost cancer progression by reprogramming functions such
as the androgen receptor (AR) (30,31).
The AR is the therapeutic target in some types of breast cancer,
which indicates that FOXA1 mutation may result in the failure of
targeted AR therapy (32).
According to recent research, increased levels of FOXA1 are
associated with reduced interferon activity and T-cell infiltration
in patients with estrogen-positive luminal breast cancer treated
with neoadjuvant chemotherapy, indicating that the role of FOXA1 in
the cancer immune response contributes to immune evasion and
therapeutic resistance (33).
Nevertheless, it is uncertain if LPCAT1 also mediates immune
responses in breast cancer, which necessitates further research in
the future.
In summary, the present study reveals that the
presence of LPCAT1 contributes to breast cancer cell proliferation,
metastatic potential and PTX resistance. Moreover, LPCAT1 is
transcriptionally regulated by FOXA1, and the identification of
this signaling pathway in PTX resistance suggests a new potential
target for the alleviation of chemotherapy resistance. Follow-up
experiments using animals with gene overexpression or knockdown are
planned to verify this discovery, and new therapies may become
available to patients in terms of this potential novel target.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YZ contributed to the study concept,
experiments and analysis. HZ drafted the manuscript. HZ and YZ
confirm the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisusi FA and Akala EO: Drug combinations
in breast cancer therapy. Pharm Nanotechnol. 7:3–23. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonald ES, Clark AS, Tchou J, Zhang P
and Freedman GM: Clinical diagnosis and management of breast
cancer. J Nucl Med. 57 (Suppl 1):9S–16S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma R, Sharma R, Khaket TP, Dutta C,
Chakraborty B and Mukherjee TK: Breast cancer metastasis: Putative
therapeutic role of vascular cell adhesion molecule-1. Cell Oncol
(Dordr). 40:199–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rashid NS, Grible JM, Clevenger CV and
Harrell JC: Breast cancer liver metastasis: Current and future
treatment approaches. Clin Exp Metastasis. 38:263–277. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugie T: Immunotherapy for metastatic
breast cancer. Chin Clin Oncol. 7:282018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abu Samaan TM, Samec M, Liskova A, Kubatka
P and Büsselberg D: Paclitaxel's mechanistic and clinical effects
on breast cancer. Biomolecules. 9:7892019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chi Y, Xue J, Huang S, Xiu B, Su Y, Wang
W, Guo R, Wang L, Li L, Shao Z, et al: CapG promotes resistance to
paclitaxel in breast cancer through transactivation of PIK3R1/P50.
Theranostics. 9:6840–6855. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
AlFakeeh A and Brezden-Masley C:
Overcoming endocrine resistance in hormone receptor-positive breast
cancer. Curr Oncol. 25 (Suppl 1):S18–S27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia ZH, Wang XG and Zhang H: Overcome
cancer drug resistance by targeting epigenetic modifications of
centrosome. Cancer Drug Resist. 2:210–224. 2019.PubMed/NCBI
|
|
11
|
Bi J, Ichu TA, Zanca C, Yang H, Zhang W,
Gu Y, Chowdhry S, Reed A, Ikegami S, Turner KM, et al: Oncogene
amplification in growth factor signaling pathways renders cancers
dependent on membrane lipid remodeling. Cell Metab. 30:525–538.e8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Wang Y, Wang Y, Wang N, Duan Q,
Wang S, Liu M, Bilal MA and Zheng Y: LPCAT1 promotes cutaneous
squamous cell carcinoma via EGFR-mediated protein kinase B/p38MAPK
signaling pathways. J Invest Dermatol. 142:303–313.e9. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He RQ, Li JD, Du XF, Dang YW, Yang LJ,
Huang ZG, Liu LM, Liao LF, Yang H and Chen G: LPCAT1 overexpression
promotes the progression of hepatocellular carcinoma. Cancer Cell
Int. 21:4422021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao T, Zhang Y, Ma X, Wei L, Hou Y, Sun R
and Jiang J: Elevated expression of LPCAT1 predicts a poor
prognosis and is correlated with the tumour microenvironment in
endometrial cancer. Cancer Cell Int. 21:2692021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu H, Miao YR, Jia LH, Yu QY, Zhang Q and
Guo AY: AnimalTFDB 3.0: A comprehensive resource for annotation and
prediction of animal transcription factors. Nucleic Acids Res.
47(D1): D33–D38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Wu N, Zhang J, Wang H and Men X:
MiR-153-5p enhances the sensitivity of triple-negative breast
cancer cells to paclitaxel by inducing G2M phase arrest. Onco
Targets Ther. 13:4089–4097. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verco S, Maulhardt H, Baltezor M, Williams
E, Iacobucci M, Wendt A, Verco J, Marin A, Campbell S, Dorman P and
diZerega G: Local administration of submicron particle paclitaxel
to solid carcinomas induces direct cytotoxicity and immune-mediated
tumoricidal effects without local or systemic toxicity: Preclinical
and clinical studies. Drug Deliv Transl Res. 11:1806–1817. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dan VM, Raveendran RS and Baby S:
Resistance to intervention: Paclitaxel in breast cancer. Mini Rev
Med Chem. 21:1237–1268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mishra A, Srivastava A, Pateriya A, Tomar
MS, Mishra AK and Shrivastava A: Metabolic reprograming confers
tamoxifen resistance in breast cancer. Chem Biol Interact.
347:1096022021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Li Z, Deng M, Liu B, Xin X, Zhao
Z, Zhang Y and Lv Q: Downregulation of GPSM2 is associated with
primary resistance to paclitaxel in breast cancer. Oncol Rep.
43:965–974. 2020.PubMed/NCBI
|
|
24
|
Ge X, Cao Z, Gu Y, Wang F, Li J, Han M,
Xia W, Yu Z and Lyu P: PFKFB3 potentially contributes to paclitaxel
resistance in breast cancer cells through TLR4 activation by
stimulating lactate production. Cell Mol Biol (Noisy-le-grand).
62:119–125. 2016.PubMed/NCBI
|
|
25
|
Nedeljković M and Damjanović A: Mechanisms
of chemotherapy resistance in triple-negative breast cancer-how we
can rise to the challenge. Cells. 8:9572019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang B and Tontonoz P: Phospholipid
remodeling in physiology and disease. Annu Rev Physiol. 81:165–188.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao M, Luo J, Gu T, Yu X, Song Z, Jun Y,
Gu H, Han K, Huang X, Yu W, et al: LPCAT1 reprogramming cholesterol
metabolism promotes the progression of esophageal squamous cell
carcinoma. Cell Death Dis. 12:8452021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie H, Xiao R, He Y, He L, Xie C, Chen J
and Hong Y: MicroRNA-100 inhibits breast cancer cell proliferation,
invasion and migration by targeting FOXA1. Oncol Lett. 22:8162021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu L, Wang F, Fan W, Jin Z, Teng C and
Zhang J: lncRNA NEAT1 promotes the Taxol resistance of breast
cancer via sponging the miR-23a-3p-FOXA1 axis. Acta Biochim Biophys
Sin (Shanghai). 53:1198–1206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams EJ, Karthaus WR, Hoover E, Liu D,
Gruet A, Zhang Z, Cho H, DiLoreto R, Chhangawala S, Liu Y, et al:
FOXA1 mutations alter pioneering activity, differentiation and
prostate cancer phenotypes. Nature. 571:408–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parolia A, Cieslik M, Chu SC, Xiao L,
Ouchi T, Zhang Y, Wang X, Vats P, Cao X, Pitchiaya S, et al:
Distinct structural classes of activating FOXA1 alterations in
advanced prostate cancer. Nature. 571:413–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arruabarrena-Aristorena A, Maag JLV,
Kittane S, Cai Y, Karthaus WR, Ladewig E, Park J, Kannan S,
Ferrando L, Cocco E, et al: FOXA1 mutations reveal distinct
chromatin profiles and influence therapeutic response in breast
cancer. Cancer Cell. 38:534–550.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He Y, Wang L, Wei T, Xiao YT, Sheng H, Su
H, Hollern DP, Zhang X, Ma J, Wen S, et al: FOXA1 overexpression
suppresses interferon signaling and immune response in cancer. J
Clin Invest. 131:e1470252021. View Article : Google Scholar : PubMed/NCBI
|