Introduction
KIN17 was initially discovered due to the cross
reaction with antibodies against the RecA protein of Escherichia
coli. In addition, KIN17 is a highly conserved gene across
evolution. Previous studies have indicated that KIN17 is involved
in global genome repair, DNA replication, transcription and
regulation of the cell cycle as part of a multi-protein complex.
The above biological processes have important roles in
tumorigenesis, cancer development and chemoresistance in tumor
cells. Indeed, KIN17 is abundantly expressed in a variety of
tumors, including breast, thyroid, colorectal, liver, cervical,
lung and ovarian cancer. It is worth mentioning that KIN17 has
vital impacts on tumor genesis and development. The present review
was the first, to the best of our knowledge, to systematically
summarize and clarify the relevance between KIN17 and tumor
development from different aspects (Table I), including the structure,
function, expression level and regulatory mechanisms of KIN17, so
as to explore the potential of KIN17 as a tumor diagnostic and
prognostic biomarker and as a potential therapeutic target. The
present study aimed to summarize the influence of KIN17 on tumor
development and the possibility of KIN17 as a tumor diagnostic and
prognostic biomarker and as a potential therapeutic target.
 | Table I.Effects of KIN17 in cancers. |
Table I.
Effects of KIN17 in cancers.
| Cancer type | KIN17
expression | Function | Pathway | (Refs.) |
|---|
| Luminal-A breast
cancer | High | High expression
promotes migration and invasion | - | (60) |
| Triple-negative
breast cancer | High | Knockdown of KIN17
promotes apoptosis of MDA-MB-231 cells | Mitochondrial
pathway of apoptosis | (66) |
| Thyroid cancer | High | High expression
promotes proliferation, migration and invasion | p38 MAPK signaling
pathway | (68) |
| Colorectal
cancer | High | High expression
associated with poor prognosis | - | (57) |
| Hepatocellular | High | i) High expression
promotes proliferation; | TGF-β/Smad2
signaling | (65,69) |
| carcinoma |
| ii) High expression
associated with poor prognosis; | pathway |
|
|
|
| iii) Knockdown of
KIN17 inhibits migration and invasion, on the contrary,
overexpression of KIN17 promotes migration and invasion |
|
|
| Cervical
cancer | High | i) High expression
associated with poor prognosis; | NF-κB-Snail
pathway | (61,62,67,73) |
|
|
| ii) Knockdown of
KIN17 inhibits proliferation, migration and invasion, but promotes
apoptosis |
|
|
| Ovarian cancer | High | i) High expression
associated with poor prognosis; | - | (64) |
|
|
| ii) KIN17 knockdown
sensitized SKOV3 cells to cisplatin and inhibited the proliferation
ability and migration ability of epithelial ovarian cancer
cells |
|
|
| Non-small cell
lung | High | i) High expression
associated with poor prognosis; | MEK/ERK
signaling | (63) |
| cancer |
| ii) Knockdown of
KIN17 inhibits migration and invasion | pathway |
|
KIN17, a DNA and RNA binding protein
Structure of KIN17
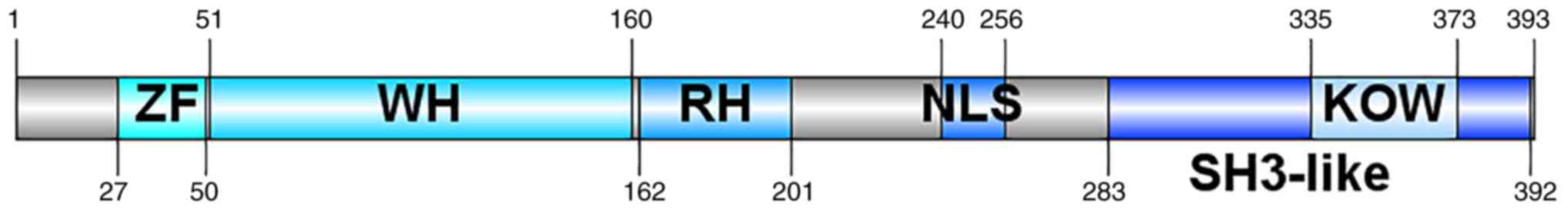
KIN17 gene is highly conserved across evolution
(1). It is mainly composed of three
parts, including a zinc finger (ZF) domain (27–50 nt) (2,3), a
nuclear location signal (240–257 nt) and a motif named
Kyprides-Onzonis-Woese (KOW; 335–373 nt), which mediates protein
and RNA interaction (Fig. 1)
(4), and these three motifs
determine that KIN17 are 45 kDa nuclear proteins and highly
conserved across evolution. There is a domain with homology to the
RecA protein from Escherichia coli (162–201 nt; Fig. 1) (5–7), which
is how KIN17 was initially discovered, i.e. because of its
cross-reaction with Escherichia coli RecA protein antibody
(8). Based on the phylogenetic
analysis, the mean identity of the ZF domain and the winged helix
(WH) domain (51–160 nt) (Fig. 1) of
KIN17 was respectively 90.37 and 65.36%, which means that the two
domains are highly conserved, whereas this conservative phenomenon
in the KOW motif was only found in the higher eukaryote,
speculating that the KOW motif appeared late in evolution. >50%
of the secondary structure of KIN17 consists of random coil and
β-turns, while the components of α-helices and β-strands make up
for <50% of the structure of KIN17 (9).
Function of KIN17
KIN17 preferentially binds to the curved DNA found
at the illegitimate recombination positions in the chromosomes of
eukaryotic cells (10,11), inferencing that KIN17 has key roles
in global genome repair. In addition to binding with the curved DNA
and in regulating the association with chromatin, KIN17 also has
roles in RNA binding via the tandem SH3 domain (12). Based on the above findings, KIN17 is
known as a DNA and RNA binding protein.
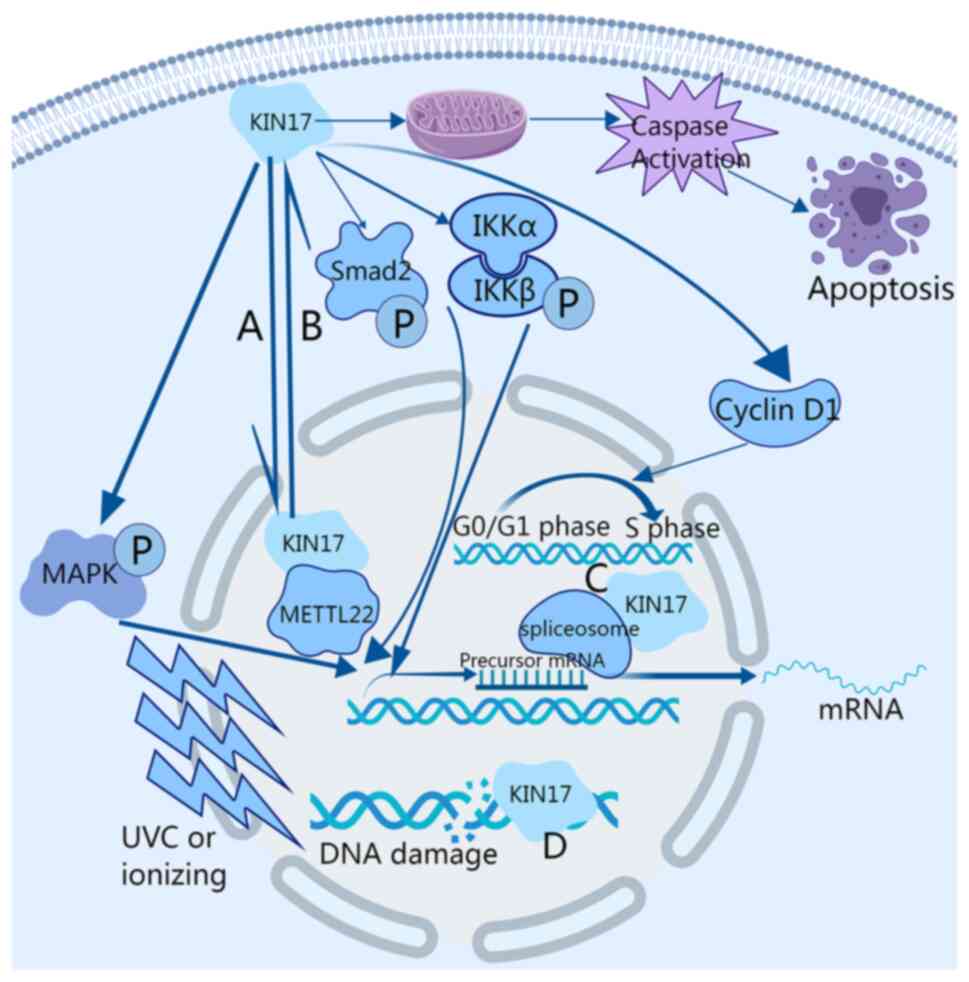
The DNA damage increased after ultra-violet C
radiation or ionizing radiation, resulting in an increase in the
expression level of KIN17. In fact, KIN17 gathered in the nucleus
forming lesions in cells (Fig. 2)
(13); therefore, KIN17 also
participates in the general response to genotoxic stress (14–17),
but this depends on the genome integrity in the DNA repair
mechanism; particularly the existence of XPA and XPC is of great
importance for the increase of KIN17 after exposure to ultra-violet
C radiation or ionizing radiation (14). In several studies, KIN17 was
co-mentioned with DNA damage repair-associated proteins (18–22),
and even forms complexes with DNA damage repair-associated
proteins. In addition, KIN17 is able to efficiently repair DNA
double strand breaks (DSBs) caused by ionizing radiation or
activation-induced cytidine deaminase (AID). Of note, low
expression levels of KIN17 lead to an increase in the frequency of
deletions in the mutation of AID. Furthermore, low expression of
KIN17 influences multiple repair pathways of DSBs, particularly
homologous recombination and non-homologous end joining (23).
In the detection and analysis of proteomics, KIN17
was found to exist in the spliceosome, inferring that it is of
great importance in the regulation of transcription (Fig. 2) (4). Besides this, KIN17 was observed to
accumulate with replication protein A, forming intranuclear foci
following γ-irradiation, using electron microscopy (8). In addition, low expression of KIN17
was also associated with a long S phase in the cell cycle due to
the increased sensitivity following γ-irradiation and decreased DNA
synthesis rate (24). Another study
reported that a physical interaction between human KIN17 and simian
virus 40 (SV40) large T antigen led to DNA synthesis inhibition
both in vitro and in vivo (25). KIN17 has also been found to be part
of a multi-protein complex participating in DNA replication and
regulation of the cell cycle (26–28).
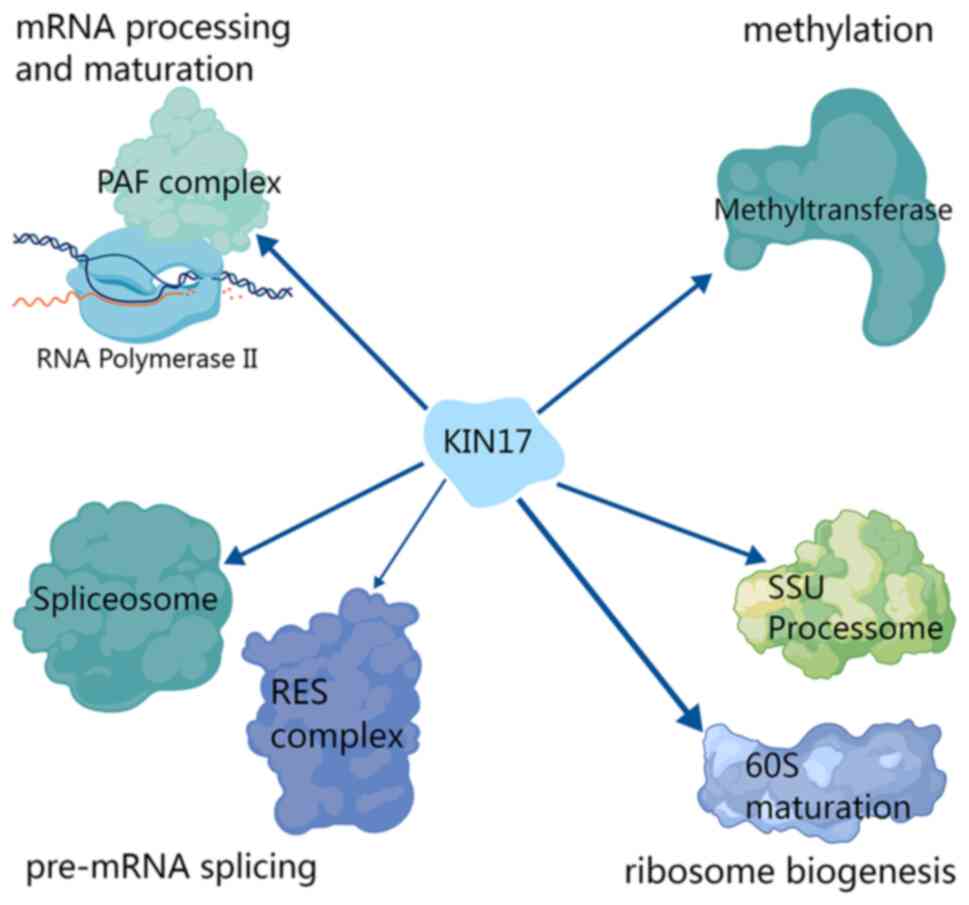
New interactions between KIN17 and other proteins have also been
found, particularly proteins related to RNA processing, such as
certain proteins associated with pre-mRNA splicing and ribosome
biogenesis (Fig. 3) (29).
In addition, it was reported that KIN17 was
methylated on lysine 135 and transferred from the nucleus to the
cytoplasm after overexpression of methyltransferase 22, which means
that lysine 135 of KIN17 has key roles in regulating methylation
and chromatin association (Figs. 2
and 3) (30). It appears that KIN17 is closely
related to the involvement of methyltransferase in methylation
(31–36), and histone methylation is an
epigenetic modification (37–41),
which refers to the process of transferring methyl to the
N-terminal arginine or lysine residues of H3 and H4 histones under
the catalysis of histone methyltransferase (42–46).
Methylation at different sites and different degrees of methylation
lead to different effects. Histone methylation is related to the
activation, extension or inhibition of gene expression, which has
an important role in the occurrence and development of cancer
(47–55). Of note, it was reported that the WH
domain of human KIN17 may mediate protein-protein interactions
between KIN17 and a series of methyltransferases (56).
To sum up, KIN17 has been discovered to participate
in several vital cell behaviors, including DNA replication, damage
repair, regulation of the cell cycle, epigenetic modification,
ribosome biogenesis and RNA processing, including pre-mRNA
splicing.
Distribution of KIN17
Under normal circumstances, KIN17 is mainly
expressed in cardiac, testicular and skeletal muscle, while
exhibiting lower expression in other organs (5). Of note, the DNA damage increased after
ultra-violet C radiation or ionizing radiation, resulting in an
increase in the expression level of KIN17; in fact, KIN17 gathered
in the nucleus forming lesions in cells (13). Furthermore, one study on colorectal
cancer revealed that KIN17 is mainly expressed in the nucleus in
para-carcinoma tissues, whereas it is expressed both in the nucleus
and cytoplasm in the tumor tissues (57). In another study, the components of
nuclear and chromatin-related proteins were analyzed, the
expression pattern of KIN17 was detected in all components and an
increase in the concentration of chromatin-related components of
clones with low metastatic potential was observed (58).
Roles of KIN17 in cancer cells
Expression of KIN17 in tumors and its
relationship with survival and prognosis
Immunohistochemical analysis of clinical specimens
from patients with colorectal cancer revealed that tumor tissues
exhibited higher expression levels of KIN17 than para-carcinoma
tissues. In addition, patients in the T1 and T2 groups exhibited
high expression levels of KIN17 compared with patients in the T3
and T4 groups. Furthermore, compared with patients without lymph
node metastasis, patients with lymph node metastasis exhibited high
expression levels of KIN17. Finally, patients with distant
metastasis exhibited higher expression levels of KIN17 than
patients without distant metastasis. The TNM staging system is
based on the primary tumor, lymph node metastasis and distant
metastasis; therefore, the above immunohistochemical results
indicate that KIN17 expression is associated with tumor genesis and
development (57).
Univariate analysis and Kaplan-Meier survival
analysis suggested that KIN17 may serve as a prognostic biomarker
for colorectal cancer. In addition to univariate analysis and
Kaplan-Meier survival analysis, multivariate Cox regression
analysis also indicated that KIN17 was an independent prognostic
factor in colorectal cancer (57).
Similarly, the immunohistochemical analysis of
clinical breast tumor specimens also demonstrated that the
expression of KIN17 was significantly increased in breast cancer
tissues and related to the expression levels of Ki-67 and
progesterone receptor, the mutation status of p53 and the tumor
stage (59).
Survival analysis of clinical data from The Cancer
Genome Atlas (TCGA) database revealed that high expression of KIN17
was associated with a low overall survival rate in patients with
breast cancer, particularly the subtype of luminal-A among the 4
subtypes. Furthermore, besides overall survival, high expression of
KIN17 was also found to be associated with poor relapse-free
survival, distant metastasis-free survival and post-progression
survival, indicating that the high expression of KIN17 has
important roles in disease progression and prognosis (60).
In addition to colorectal cancer and breast cancer,
the immunohistochemical analysis of clinical cervical tumor
specimens also indicated that the expression level of KIN17 in
invasive cervical cancer and cervical intraepithelial neoplasia
(CIN) or carcinoma in situ was higher than that in normal
cervical tissues, indicating that KIN17 may serve as a biomarker
for progression to cervical carcinoma. Furthermore, it was found
that the expression level of KIN17 was associated with the
expression of Ki-67, the degree of differentiation and the status
regarding lymph node metastasis (61).
Of note, a recent study found that the expression
level of KIN17 was positively associated with the severity of
cervical lesions, indicating that KIN17 is a novel protein
biomarker for high-grade CIN (62).
High-grade CIN is considered to be the precursor of cervical
carcinoma, which further suggests that KIN17 may be used as a
predictive biomarker for cervical cancer.
Elevated KIN17 mRNA and protein expression was
detected in non-small cell lung cancer (NSCLC). Furthermore, it was
found that the expression of KIN17 was relevant to the grading of
tumors and the status of lymph node metastasis, which are both
indicators related to poor survival prognosis (63).
Analysis of clinical data from patients with ovarian
tumor in the TCGA database indicated that KIN17 expression was
significantly increased in ovarian serous adenocarcinoma tissues
compared with normal ovary tissues (P<0.05). It was found that
the DNA expression level of KIN17 in ovarian serous adenocarcinoma
was also higher than that in normal ovarian tissues. Furthermore,
the transcription level of KIN17 in ovarian serous adenocarcinoma
was also higher than that in borderline epithelial stromal tumor of
the ovary. However, the mRNA expression of KIN17 exhibited no
difference between normal ovary tissues and other epithelial
ovarian cancer (EOC) tissues. Using the Kaplan-Meier method and a
log-rank test, the results indicated that, similar to colorectal
cancer, KIN17 may also serve as a prognostic biomarker in EOC. In
addition to univariate analysis and Kaplan-Meier survival analysis,
multivariate Cox regression analysis suggested that KIN17 and the
tumor stage were independent prognostic factors in EOC. However,
there was no association between the localization (cytoplasm or
nucleus) of KIN17 and the overall survival (64).
A recent study related to KIN17 demonstrated that
the expression level of KIN17 in hepatocellular carcinoma (HCC)
tissues was higher than that in para-carcinoma tissues, as proved
by multiple analytic methods, including Bioinformatics, western
blot analyses and immunohistochemistry (65). In addition, the expression levels of
KIN17 in portal vein tumor thrombus tissues and intrahepatic
metastasis tissues were higher than those in the primary tumor
tissues, suggesting that KIN17 is related to metastasis in HCC.
Furthermore, Kaplan-Meier survival analysis, univariate analysis
and multivariate Cox regression analysis indicated that KIN17 is
significantly relevant to the survival rates and prognosis.
The above immunohistochemical analysis and survival
analysis results reveal that KIN17 is abundantly expressed in
neoplasms and closely associated with clinical features and
prognosis of neoplasms.
Roles of KIN17 in tumor cell apoptosis
induction
A study on triple-negative breast cancer revealed
that knockdown of KIN17 promoted the apoptosis rates of MDA-MB-231
cells. Furthermore, not only the caspase activity (Fig. 2), but also the expression levels of
cleaved poly ADP ribose polymerase (PARP) in the KIN17-knockdown
group were both higher than those in the mock and negative control
groups (66).
In addition to triple-negative breast cancer,
Annexin V-APC staining performed in cervical cancer demonstrated
that the apoptosis rates of HeLa cells in the KIN17 knockdown group
were also higher than those in the negative control group (61,67).
These results suggest that KIN17 has key roles in
induction of cell apoptosis and a PARP-related mechanism may be
attributed to it.
Roles of KIN17 in regulation of tumor
cell proliferation
A study on thyroid cancer revealed that in the
colony-formation assay, the number of colonies in the
KIN17-knockdown group was lower than that in the
KIN17-overexpression group (68).
Furthermore, cell-cycle analysis by flow cytometry suggested that
the cell number in G1 phase in the KIN17-knockdown group was higher
than that in the negative control group, while the cell number in S
phase and G2 phase in the KIN17-knockdown group was lower than that
in the negative control group. On the contrary, the cell number in
G1 phase in the KIN17-overexpression group was lower than that in
the negative control group, while the cell number in S phase and G2
phase in the KIN17-overexpression group was greater than that in
the negative control group. Western blot analysis demonstrated that
the expression levels of phosphorylated (p)-p38, cyclin D1 and p27
were downregulated following the knockdown of KIN17 in 8505C cells
and SW579 cells, while the expression levels of p-p38, cyclin D1
and p27 were upregulated following the overexpression of KIN17 in
8505C cells and SW579 cells. These results demonstrated that KIN17
is able to promote the proliferation of thyroid cancer cells
(Fig. 2) (68).
In addition to the effects of KIN17 in vitro, in
vivo xenograft assays also proved that the tumor weight in the
KIN17 knockdown group was markedly lower than that in the control
group. Correspondingly, the abundance of Ki-67-positive cells in
the KIN17 knockdown group was lower than that in the control group.
In addition, the level of p-p38 in KIN17 knockdown group was lower
than that in the control group. In conclusion, these findings
indicate that knockdown of KIN17 may repress the progression of
thyroid cancer in vivo (68).
Overexpression of KIN17 also promoted the growth of
hepatoma cells in vitro and in vivo. The number of
colonies in the KIN17-overexpression group was significantly
increased compared with that in the negative control group in both
HepG2 cells and SMMC-7721 cells, indicating that overexpression of
KIN17 promoted the cell proliferation ability in both HepG2 cells
and SMMC-7721 cells. Further study indicated that the expression of
cyclin D1 and p27 in the KIN17-overexpression group was markedly
higher than that in the negative control group in both HepG2 cells
and SMMC-7721 cells. Taken together, KIN17 may be relevant to the
cell proliferation activity in hepatocellular carcinoma (69).
Ki-67 is regarded as a cell proliferation marker
(70). In one study, the expression
of KIN17 and Ki-67 in breast cancer tissues was detected by a
double-labeled immunofluorescence assay (59). The analysis revealed that KIN17 and
Ki-67 were both mainly located in the nucleus and they were
co-positive in MDA-MB-231 cells, indicating that KIN17 and Ki-67
were co-expressed in the nucleus. Co-expression of KIN17 and Ki-67
in both tumor tissues and cells suggested that KIN17 may be
associated with cellular proliferation. Western blot analysis
revealed that overexpression of KIN17 increased the expression of
ERK1/2 autophosphorylation and cyclin D1 in MCF-10A and BT474
cells, whereas knockdown of KIN17 decreased the expression level of
ERK1/2 autophosphorylation and cyclin D1 in MCF-10A and BT474
cells. This revealed that KIN17 may be relevant to the cell
proliferation activity in breast cancer.
Epidermal growth factor (EGF) has been reported as a
major growth factor associated with cell proliferation and
tumorigenesis in breast cancer (71,72).
The expression level of KIN17 was significantly increased following
the stimulation of EGF in MDA-MB-231, BT474 and MCF-10A cells. It
was also found that the effect of EGF on stimulating cell
proliferation was weakened following knockdown of KIN17, which
means that KIN17 is a prerequisite for EGF to stimulate cell
proliferation (59).
Overall, these data indicate that KIN17 participates
in cancer cell proliferation by regulating the expression levels of
cell cycle-related proteins.
Roles of KIN17 in the regulation of
tumor cell migration and invasion
In thyroid cancer, the cell migratory ability was
markedly suppressed following knockdown of KIN17, while the cell
migratory ability was markedly enhanced following overexpression of
KIN17 in 8505C and SW579 cells, as indicated by a wound-healing
assay. The Transwell assay results indicated that the cell invasion
ability was inhibited following knockdown of KIN17, while the cell
invasion ability was promoted following overexpression of KIN17.
Furthermore, silencing of KIN17 upregulated the expression of
epithelial to mesenchymal transition (EMT)-associated E-cadherin,
while overexpression of KIN17 downregulated the expression of
EMT-associated E-cadherin in 8505C and SW579 cells. On the
contrary, silencing of KIN17 downregulated the expression of
EMT-associated N-cadherin, while overexpression of KIN17
upregulated the expression of EMT-associated N-cadherin in 8505C
and SW579 cells. These findings suggest that KIN17 promoted cell
migration and invasion in thyroid cancer (68).
In cervical cancer, the migration rates in the
KIN17-knockdown group were lower than those in the negative control
group in both HeLa cells and SiHa cells in the wound-healing assay
and Transwell assay without Matrigel. Furthermore, the number of
invasive cells was also downregulated in both HeLa cells and SiHa
cells following knockdown of KIN17 (73).
The migration and invasion ability were suppressed
in the NSCLC cell line A549 following the knockdown of KIN17. In
addition, the expression levels of MMP-7, EGF receptor and MYC were
downregulated following the knockdown of KIN17, as proved by
reverse transcription-quantitative PCR and western blot analysis
(63).
A latest study related to KIN17 demonstrated that
the migration and invasion ability were inhibited following the
knockdown of KIN17 in Huh7 cells and HepG2 cells both in
vitro and in vivo, while the migration and invasion
ability were enhanced following overexpression of KIN17 in MHCC-97L
cells and HepG2 cells both in vitro and in vivo
(65).
Taken together, the expression of KIN17 has a vital
impact on the migration and invasion ability of tumor cells.
Roles of KIN17 in chemoresistance
A study on ovarian cancer revealed that the mRNA
expression of KIN17 in cisplatin-resistant ES-2 cells was higher
than that in cisplatin-sensitive cells (OVCAR-3, FU-OV-1 and OA W42
cells) and moderately sensitive cells (SKOV3 cells). KIN17
knockdown sensitized SKOV3 cells to cisplatin and inhibited the
proliferation and migration ability of EOC cells in ovarian cancer
(64).
The DNA damage detected in breast cancer cells
treated with adriamycin (ADM) was greater than that in breast
cancer cells without ADM induction. Of note, the DNA damage
detected in BT474 cells was greater compared with that in MCF-10A
cells. In addition to DNA damage, the expression level of KIN17 was
upregulated following induction with ADM. The DNA damage was
enhanced in the MCF-10A cells and BT474 cells following knockdown
of KIN17. However, the DNA damage in BT474 cells was greater
compared with that in MCF-10A cells following knockdown of KIN17.
In addition, the sensitivity to ADM in BT474 cells was increased
following knockdown of KIN17, while the sensitivity to ADM
treatment in MCF-10A cells exhibited no significant change
following knockdown of KIN17 (59).
Similarly, in addition to breast cancer cells, KIN17
knockdown significantly enhanced the inhibitory effects of
Doxorubicin on the cell viability and proliferation in the thyroid
cancer cell lines 8505C and SW579. Silencing of KIN17 also promoted
the suppressive effects of Doxorubicin on the protein levels of
p-p38, cyclin D1 and p27 in 8505C cells and SW579 cells. The
inhibitory effects of Doxorubicin on the cell migration and
invasion abilities of 8505C cells and SW579 cells were enhanced by
knockdown of KIN17 proved by a Transwell assay. Silencing of KIN17
also promoted the downregulation effect of Doxorubicin on the
expression of N-cadherin and the upregulation effect of Doxorubicin
on the expression level of E-cadherin. These findings indicate that
the sensitivity to the treatment of Doxorubicin in thyroid cancer
cells was enhanced by knockdown of KIN17 (68). The above study highlights the
changes in chemosensitivity of tumor cells following knockdown of
KIN17, indicating that KIN17 may have a key role in chemoresistance
and is expected to alleviate chemoresistance in tumor cells to a
certain extent.
Regulatory mechanism of KIN17 in tumor
cells
The metastasis of luminal-A breast cancer was
promoted by EMT signaling activated by KIN17. The expression levels
of β-catenin, Vimentin and claudin-1 in the KIN17-knockdown group
were lower than those in the mock and negative control groups,
while the expression levels of β-catenin, Vimentin and claudin-1 in
the KIN17-overexpression group were higher than those in the mock
and negative control groups (60).
KIN17 facilitates thyroid cancer cell migration and
invasion by activating the p38 MAPK signaling pathway (Fig. 2). P79350, a p38 agonist, reversed
the suppressive effects of knockdown of KIN17 on the
colony-forming, migratory and invasive ability of 8505C and SW579
cells. In addition, P79350 also reversed the effect of knockdown of
KIN17 to increase the G1-phase population and to decrease the S-
and G2/M-phase populations in 8505C and SW579 cells. Furthermore,
western blot analysis revealed that P79350 rescued the
downregulation effect of knockdown of KIN17 on the protein levels
of p-p38, cyclin D1, p27 and N-cadherin in 8505C and SW579 cells.
These findings suggest that the inhibitory effects of knockdown of
KIN17 on the cell proliferation, migration and invasion of thyroid
cancer cells were abolished by P79539 and activation of the MAPK
signaling pathway (68).
KIN17 knockdown suppressed the migration and
invasion of cervical cancer cells through the NF-κB-Snail pathway
(Fig. 2). Knockdown of KIN17
repressed the phosphorylation level of certain molecules related to
the NF-κB pathway and downregulated the expression level of Snail
in HeLa and SiHa cells. In addition, migration and invasion were
inhibited in cervical cancer cells following knockdown of KIN17,
indicating that KIN17 may promote cell migration and invasion in
cervical cancer cells by activating the NF-κB-Snail pathway
(73).
A study about KIN17 demonstrated that recombinant
human TGF-β1 reversed the suppressive effects of knockdown of KIN17
on HCC cell migration and invasion (65). In addition, TGF-β1 also reversed the
promotion effects of knockdown of KIN17 on the expression levels of
epithelial-related proteins and the suppressive effects of
knockdown of KIN17 on the expression levels of mesenchymal-related
proteins. On the contrary, Y2109761, a selective TGF-β receptor
type I/II dual inhibitor, reversed the promotion effects of
overexpression of KIN17 on the cell migration and invasion ability.
In addition, Y2109761 also reversed the suppressive effects of
overexpression of KIN17 on the expression levels of
epithelial-related proteins and the promotion effects of
overexpression of KIN17 on the expression levels of
mesenchymal-related proteins. In conclusion, these findings
revealed that KIN17 may impact cell migration and invasion in HCC
cells through stimulating the TGF-β/Smad2 signaling pathway
(Fig. 2).
In conclusion, KIN17 facilitates cell proliferation,
migration and invasion in cancers by activating various processes,
including EMT, the p38-MAPK signaling pathway, NF-κB-Snail pathway
and the TGF-β/Smad2 signaling pathway.
Application prospect and limitation
As mentioned above, immunohistochemical analysis of
clinical specimens confirmed that the expression level of KIN17 in
tumor tissues is higher than that in para-carcinoma tissues, and
the expression level of KIN17 is related to TNM stage. In addition,
survival analysis also indicated that high expression of KIN17 in
various tumor types is related to poor prognosis. Therefore, it is
worth mentioning that KIN17 is expected to be a novel target and
diagnostic marker and serve as a novel prognostic biomarker in
neoplasms.
It was verified in vivo and in vitro
that KIN17 has impacts on the genesis and development of various
tumors in numerous aspects, such as apoptosis, proliferation,
migration and invasion. Knockdown of KIN17 may inhibit the
proliferation, migration and invasion of tumor cells. Because of
this, it may be speculated that using small-molecule inhibitors to
reduce the expression of KIN17 may effectively inhibit the
proliferation and metastasis of tumor cells in the clinic, thereby
inhibiting the development of tumors and improving the survival
rate of patients. However, to the best of our knowledge, to date,
no further in-depth clinical experiments have been performed and
their findings applied in clinical practice. Therefore, it may be
proposed that KIN17 may serve as a potential therapeutic target,
but further in-depth research is required to verify this.
In addition, KIN17 is a highly conserved gene across
evolution and is expressed in almost all types of cells, which
indicates its limitation as a therapeutic target in tumor therapy
because of its non-specific properties; its interference may have
detrimental effects, such as destroying the balance of the
organism, reducing the overall responsiveness of the organism and
bringing about unknown side effects.
However, the prospect of KIN17 as a tumor diagnostic
and prognostic biomarker and as a potential therapeutic target in
neoplasms is clinically significant and promising.
Conclusion and prospect
KIN17 is highly expressed in a variety of tumor
types and is closely related to the clinical and pathological
features of patients, which indicates that KIN17 is expected to be
a novel target and serve as a novel diagnostic and prognostic
biomarker in neoplasms. In addition, knockdown of KIN17 may inhibit
cell proliferation and metastasis in various tumor types. As
mentioned above, although more in-depth clinical experiments are
required to verify its clinical therapeutic effects, cell and
molecular experiments in vitro and xenograft assays in
vivo proved that KIN17 may be a potential therapeutic
target.
Acknowledgements
Not applicable.
Funding
This research was funded by the Start-up Fund for High-level
Talents of the Affiliated Hospital of Guangdong Medical University
(grant no. 51301Z20200007), Medical Science and Technology Research
Project of Guangdong Province (grant no. B2021180), Guangdong Basic
and Applied Basic Research Foundation (grant no. 2023A1515010235),
and Basic and Applied Basic Research Project of GuangZhou (grant
no. SL2022A04J00207). The funders had no role in the study design,
data collection and analysis, manuscript preparation or decision to
publish.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
TZ and QH conceived the study. XH, ZD and QL
prepared the original draft. XL reviewed and edited the original
draft. XH conducted the literature search. QH was responsible for
the visualization. TZ and QH, acquired the funding and were the
supervisor and project administrator. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Angulo JF, Moreau PL, Maunoury R, Laporte
J, Hill AM, Bertolotti R and Devoret R: KIN, a mammalian nuclear
protein immunologically related to E. coli RecA protein. Mutat Res.
217:123–134. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seetharam A and Stuart GW: A study on the
distribution of 37 well conserved families of C2H2 zinc finger
genes in eukaryotes. BMC Genomics. 14:4202013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angulo JF, Rouer E, Mazin A, Mattei MG,
Tissier A, Horellou P, Benarous R and Devoret R: Identification and
expression of the cDNA of KIN17, a zinc-finger gene located on
mouse chromosome 2, encoding a new DNA-binding protein. Nucleic
Acids Res. 19:5117–5123. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tissier A, Kannouche P, Mauffrey P,
Allemand I, Frelat G, Devoret R and Angulo JF: Molecular cloning
and characterization of the mouse Kin17 gene coding for a Zn-finger
protein that preferentially recognizes bent DNA. Genomics.
38:238–242. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kannouche P, Mauffrey P, Pinon-Lataillade
G, Mattei MG, Sarasin A, Daya-Grosjean L and Angulo JF: Molecular
cloning and characterization of the human KIN17 cDNA encoding a
component of the UVC response that is conserved among metazoans.
Carcinogenesis. 21:1701–1710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tissier A, Kannouche P, Biard DS,
Timchenko T, Mazin A, Araneda S, Allemand I, Mauffrey P, Frelat G
and Angulo JF: The mouse Kin-17 gene codes for a new protein
involved in DNA transactions and is akin to the bacterial RecA
protein. Biochimie. 77:854–860. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angulo JF, Rouer E, Benarous R and Devoret
R: Identification of a mouse cDNA fragment whose expressed
polypeptide reacts with anti-recA antibodies. Biochimie.
73:251–256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biard DS, Miccoli L, Despras E, Frobert Y,
Creminon C and Angulo JF: Ionizing radiation triggers
chromatin-bound kin17 complex formation in human cells. J Biol
Chem. 277:19156–19165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pattaro Júnior JR, Caruso ÍP, de Lima Neto
QA, Duarte Junior FF, Dos Santos Rando F, Gerhardt ECM, Fernandez
MA and Seixas FAV: Biophysical characterization and molecular
phylogeny of human KIN protein. Eur Biophys J. 48:645–657. 2019.
View Article : Google Scholar
|
|
10
|
Mazin A, Milot E, Devoret R and Chartrand
P: KIN17, a mouse nuclear protein, binds to bent DNA fragments that
are found at illegitimate recombination junctions in mammalian
cells. Mol Gen Genet. 244:435–438. 1994. View Article : Google Scholar
|
|
11
|
Mazin A, Timchenko T, Ménissier-de Murcia
J, Schreiber V, Angulo JF, de Murcia G and Devoret R: Kin17, a
mouse nuclear zinc finger protein that binds preferentially to
curved DNA. Nucleic Acids Res. 22:4335–4341. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
le Maire A, Schiltz M, Stura EA,
Pinon-Lataillade G, Couprie J, Moutiez M, Gondry M, Angulo JF and
Zinn-Justin S: A tandem of SH3-like domains participates in RNA
binding in KIN17, a human protein activated in response to
genotoxics. J Mol Biol. 364:764–776. 2006. View Article : Google Scholar
|
|
13
|
Kannouche P, Pinon-Lataillade G, Tissier
A, Chevalier-Lagente O, Sarasin A, Mezzina M and Angulo JF: The
nuclear concentration of kin17, a mouse protein that binds to
curved DNA, increases during cell proliferation and after UV
irradiation. Carcinogenesis. 19:781–789. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masson C, Menaa F, Pinon-Lataillade G,
Frobert Y, Chevillard S, Radicella JP, Sarasin A and Angulo JF:
Global genome repair is required to activate KIN17, a
UVC-responsive gene involved in DNA replication. Proc Natl Acad Sci
USA. 100:616–621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biard DS, Saintigny Y, Maratrat M, Paris
F, Martin M and Angulo JF: Enhanced expression of the Kin17 protein
immediately after low doses of ionizing radiation. Radiat Res.
147:442–450. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masson C, Menaa F, Pinon-Lataillade G,
Frobert Y, Radicella JP and Angulo JF: Identification of KIN
(KIN17), a human gene encoding a nuclear DNA-binding protein, as a
novel component of the TP53-independent response to ionizing
radiation. Radiat Res. 156((5 Pt 1)): 535–544. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blattner C, Kannouche P, Litfin M, Bender
K, Rahmsdorf HJ, Angulo JF and Herrlich P: UV–Induced stabilization
of c-fos and other short-lived mRNAs. Mol Cell Biol. 20:3616–3625.
2000. View Article : Google Scholar
|
|
18
|
Johmura Y, Yamashita E, Shimada M,
Nakanishi K and Nakanishi M: Defective DNA repair increases
susceptibility to senescence through extension of Chk1-mediated G2
checkpoint activation. Sci Rep. 6:311942016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trevino V: Integrative genomic analysis
identifies associations of molecular alterations to APOBEC and
BRCA1/2 mutational signatures in breast cancer. Mol Genet Genomic
Med. 7:e8102019. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elshimali YI, Wu Y, Khaddour H, Wu Y,
Gradinaru D, Sukhija H, Chung SS and Vadgama JV: Optimization of
cancer treatment through overcoming drug resistance. J Cancer Res
Oncobiol. 1:1072018.
|
|
21
|
Phadnis N, Hyppa RW and Smith GR: New and
old ways to control meiotic recombination. Trends Genet.
27:411–421. 2011. View Article : Google Scholar
|
|
22
|
Biard DS: Untangling the relationships
between DNA repair pathways by silencing more than 20 DNA repair
genes in human stable clones. Nucleic Acids Res. 35:3535–3550.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le MX, Haddad D, Ling AK, Li C, So CC,
Chopra A, Hu R, Angulo JF, Moffat J and Martin A: Kin17 facilitates
multiple double-strand break repair pathways that govern B cell
class switching. Sci Rep. 6:372152016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Despras E, Miccoli L, Créminon C,
Rouillard D, Angulo JF and Biard DS: Depletion of KIN17, a human
DNA replication protein, increases the radiosensitivity of RKO
cells. Radiat Res. 159:748–758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miccoli L, Biard DS, Créminon C and Angulo
JF: Human kin17 protein directly interacts with the simian virus 40
large T antigen and inhibits DNA replication. Cancer Res.
62:5425–5435. 2002.PubMed/NCBI
|
|
26
|
Miccoli L, Frouin I, Novac O, Di Paola D,
Harper F, Zannis-Hadjopoulos M, Maga G, Biard DS and Angulo JF: The
human stress-activated protein kin17 belongs to the multiprotein
DNA replication complex and associates in vivo with mammalian
replication origins. Mol Cell Biol. 25:3814–3830. 2005. View Article : Google Scholar
|
|
27
|
Miccoli L, Biard DS, Frouin I, Harper F,
Maga G and Angulo JF: Selective interactions of human kin17 and RPA
proteins with chromatin and the nuclear matrix in a DNA damage- and
cell cycle-regulated manner. Nucleic Acids Res. 31:4162–4175. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biard DS, Miccoli L, Despras E, Harper F,
Pichard E, Créminon C and Angulo JF: Participation of kin17 protein
in replication factories and in other DNA transactions mediated by
high molecular weight nuclear complexes. Mol Cancer Res. 1:519–531.
2003.PubMed/NCBI
|
|
29
|
Gaspar VP, Ramos AC, Cloutier P, Pattaro
Junior JR, Duarte Junior FF, Bouchard A, Seixas FAV, Coulombe B and
Fernandez MA: Interactome analysis of KIN (Kin17) shows new
functions of this protein. Curr Issues Mol Biol. 43:767–781. 2021.
View Article : Google Scholar
|
|
30
|
Cloutier P, Lavallée-Adam M, Faubert D,
Blanchette M and Coulombe B: Methylation of the DNA/RNA-binding
protein Kin17 by METTL22 affects its association with chromatin. J
Proteomics. 100:115–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cloutier P, Lavallée-Adam M, Faubert D,
Blanchette M and Coulombe B: A newly uncovered group of distantly
related lysine methyltransferases preferentially interact with
molecular chaperones to regulate their activity. PLoS Genet.
9:e10032102013. View Article : Google Scholar
|
|
32
|
Huttlin EL, Bruckner RJ, Paulo JA, Cannon
JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, et
al: Architecture of the human interactome defines protein
communities and disease networks. Nature. 545:505–509. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo M: Chemical and biochemical
perspectives of protein lysine methylation. Chem Rev.
118:6656–6705. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Malecki J, Aileni VK, Ho AYY, Schwarz J,
Moen A, Sørensen V, Nilges BS, Jakobsson ME, Leidel SA and Falnes
PØ: The novel lysine specific methyltransferase METTL21B affects
mRNA translation through inducible and dynamic methylation of
Lys-165 in human eukaryotic elongation factor 1 alpha (eEF1A).
Nucleic Acids Res. 45:4370–4389. 2017.PubMed/NCBI
|
|
35
|
Fusser M, Kernstock S, Aileni VK,
Egge-Jacobsen W, Falnes PØ and Klungland A: Lysine methylation of
the valosin-containing protein (VCP) is dispensable for development
and survival of mice. PLoS One. 10:e01414722015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jakobsson ME, Davydova E, Małecki J, Moen
A and Falnes PØ: Saccharomyces cerevisiae eukaryotic elongation
factor 1A (eEF1A) is methylated at Lys-390 by a METTL21-Like
Methyltransferase. PLoS One. 10:e01314262015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Lu Q and Chang C: Epigenetics in
health and disease. Adv Exp Med Biol. 1253:3–55. 2020. View Article : Google Scholar
|
|
38
|
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang
Y, Fang Y and Fang D: Overview of histone modification. Adv Exp Med
Biol. 1283:1–16. 2021. View Article : Google Scholar
|
|
39
|
Hyun K, Jeon J, Park K and Kim J: Writing,
erasing and reading histone lysine methylations. Exp Mol Med.
49:e3242017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Liu K, Qin S, Xu C and Min J:
Epigenetic targets and drug discovery: Part 1: Histone methylation.
Pharmacol Ther. 143:275–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kimura H: Histone modifications for human
epigenome analysis. J Hum Genet. 58:439–445. 2013. View Article : Google Scholar
|
|
42
|
Gong F and Miller KM: Histone methylation
and the DNA damage response. Mutat Res Rev Mutat Res. 780:37–47.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trievel RC: Structure and function of
histone methyltransferases. Crit Rev Eukaryot Gene Expr.
14:147–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang W and Ernst P: Distinct functions of
histone H3, lysine 4 methyltransferases in normal and malignant
hematopoiesis. Curr Opin Hematol. 24:322–328. 2017. View Article : Google Scholar
|
|
45
|
Wang MY, Liow P, Guzman MIT and Qi J:
Exploring methods of targeting histone methyltransferases and their
applications in cancer therapeutics. ACS Chem Biol. 17:744–755.
2022. View Article : Google Scholar
|
|
46
|
Martinez NJ and Simeonov A: Cell-based
assays to support the profiling of small molecules with histone
methyltransferase and demethylase modulatory activity. Drug Discov
Today Technol. 18:9–17. 2015. View Article : Google Scholar
|
|
47
|
Dawson MA and Kouzarides T: Cancer
epigenetics: From mechanism to therapy. Cell. 150:12–27. 2012.
View Article : Google Scholar
|
|
48
|
Ilango S, Paital B, Jayachandran P, Padma
PR and Nirmaladevi R: Epigenetic alterations in cancer. Front
Biosci (Landmark Ed). 25:1058–1109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Darılmaz Yüce G and Ortaç Ersoy E: Lung
cancer and epigenetic modifications. Tuberk Toraks. 64:163–170.
2016.(In Turkish). View Article : Google Scholar
|
|
51
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View Article : Google Scholar
|
|
52
|
Sun L, Zhang H and Gao P: Metabolic
reprogramming and epigenetic modifications on the path to cancer.
Protein Cell. 13:877–919. 2022. View Article : Google Scholar
|
|
53
|
Jones PA, Issa JP and Baylin S: Targeting
the cancer epigenome for therapy. Nat Rev Genet. 17:630–641. 2016.
View Article : Google Scholar
|
|
54
|
Park JW and Han JW: Targeting epigenetics
for cancer therapy. Arch Pharm Res. 42:159–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang P and Zhang M: Epigenetic
alterations and advancement of treatment in peripheral T-cell
lymphoma. Clin Epigenetics. 12:1692020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Carlier L, Couprie J, le Maire A,
Guilhaudis L, Milazzo-Segalas I, Courçon M, Moutiez M, Gondry M,
Davoust D, Gilquin B and Zinn-Justin S: Solution structure of the
region 51–160 of human KIN17 reveals an atypical winged helix
domain. Protein Sci. 16:2750–2755. 2007. View Article : Google Scholar
|
|
57
|
Ruan L, Jiang Q and Zhang H: Relationships
of kin17 protein expression with clinical features and prognosis of
colorectal cancer. Transl Cancer Res. 7:2018. View Article : Google Scholar
|
|
58
|
Ramos AC, Gaspar VP, Kelmer SM, Sellani
TA, Batista AG, De Lima Neto QA, Rodrigues EG and Fernandez MA: The
kin17 protein in murine melanoma cells. Int J Mol Sci.
16:27912–27920. 2015. View Article : Google Scholar
|
|
59
|
Zeng T, Gao H, Yu P, He H, Ouyang X, Deng
L and Zhang Y: Up-regulation of kin17 is essential for
proliferation of breast cancer. PLoS One. 6:e253432011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang Q, Zahid KR, Chen J, Pang X, Zhong
M, Huang H, Pan W, Yin J, Raza U, Zeng J, et al: KIN17 promotes
tumor metastasis by activating EMT signaling in luminal-A breast
cancer. Thorac Cancer. 12:2013–2023. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang Y, Gao H, Gao X, Huang S, Wu K, Yu
X, Yuan K and Zeng T: Elevated expression of Kin17 in cervical
cancer and its association with cancer cell proliferation and
invasion. Int J Gynecol Cancer. 27:628–633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Marques LS, Violin VGA, Meireles LEF, de
Souza MVF, Mari NL, Damke GMZF, Damke E, Pereira MW, Mesquita CSS,
da Silva VRS and Consolaro MEL: Kin17 high expression as a
potential biomarker for high-grade cervical intraepithelial
neoplasia. Int J Gynaecol Obstet. 160:339–341. 2023. View Article : Google Scholar
|
|
63
|
Zhang Y, Huang S, Gao H, Wu K, Ouyang X,
Zhu Z, Yu X and Zeng T: Upregulation of KIN17 is associated with
non-small cell lung cancer invasiveness. Oncol Lett. 13:2274–2280.
2017. View Article : Google Scholar
|
|
64
|
Chen J, Xia Y, Peng Y, Wu S, Liu W, Zhang
H, Wang T, Yang Z, Zhao S and Zhao L: Analysis of the association
between KIN17 expression and the clinical features/prognosis of
epithelial ovarian cancer, and the effects of KIN17 in SKOV3 cells.
Oncol Lett. 21:4752021. View Article : Google Scholar
|
|
65
|
Dai Z, Huang Q, Huang X, Zhu C, Zahid KR,
Liu T, Li Q, Wu C, Peng M, Xiao X, et al: KIN17 promotes cell
migration and invasion through stimulating the TGF-β/Smad2 pathway
in hepatocellular carcinoma. Mol Carcinog. 62:369–384. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao X, Liu Z, Zhong M, Wu K, Zhang Y, Wang
H and Zeng T: Knockdown of DNA/RNA-binding protein KIN17 promotes
apoptosis of triple-negative breast cancer cells. Oncol Lett.
17:288–293. 2019.
|
|
67
|
Su B, Zhong M, Zhang Y, Wu K, Huang Q, Zhu
C and Zeng T: Deficiency of kin17 facilitates apoptosis of cervical
cancer cells by modulating caspase 3, PARP, and Bcl-2 Family
Proteins. J Oncol. 2022:31569682022. View Article : Google Scholar
|
|
68
|
Jiang QG, Xiong CF and Lv YX: Kin17
facilitates thyroid cancer cell proliferation, migration, and
invasion by activating p38 MAPK signaling pathway. Mol Cell
Biochem. 476:727–739. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kou WZ, Xu SL, Wang Y, Wang LW, Wang L,
Chai XY and Hua QL: Expression of Kin17 promotes the proliferation
of hepatocellular carcinoma cells in vitro and in vivo. Oncol Lett.
8:1190–1194. 2014. View Article : Google Scholar
|
|
70
|
de Azambuja E, Cardoso F, de Castro G Jr,
Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D,
Piccart-Gebhart MJ and Paesmans M: Ki-67 as prognostic marker in
early breast cancer: A meta-analysis of published studies involving
12,155 patients. Br J Cancer. 96:1504–1513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Price JT, Tiganis T, Agarwal A, Djakiew D
and Thompson EW: Epidermal growth factor promotes MDA-MB-231 breast
cancer cell migration through a phosphatidylinositol 3′-kinase and
phospholipase C-dependent mechanism. Cancer Res. 59:5475–1578.
1999.PubMed/NCBI
|
|
72
|
Kolev V, Mandinova A, Guinea-Viniegra J,
Hu B, Lefort K, Lambertini C, Neel V, Dummer R, Wagner EF and Dotto
GP: EGFR signalling as a negative regulator of Notch1 gene
transcription and function in proliferating keratinocytes and
cancer. Nat Cell Biol. 10:902–911. 2008. View Article : Google Scholar
|
|
73
|
Zhong M, Liu Z, Wu K, Hong Z, Zhang Y, Qu
J, Zhu C, Ou Z and Zeng T: Kin17 knockdown suppresses the migration
and invasion of cervical cancer cells through NF-κB-Snail pathway.
Int J Clin Exp Pathol. 13:607–615. 2020.
|