Introduction
The incidence and mortality rates of prostate cancer
are among the highest for all cancers worldwide (1). In males, prostate cancer currently has
the second highest cancer mortality rate after lung cancer. It is
highly heterogeneous with a disease-specific mortality of
approximately one in seven cases, and its incidence is expected to
rise as a result of lifestyle changes and the aging of the global
population (2). Long non-coding
RNAs (lncRNAs) are heterogeneous transcripts, several of which
function as master regulators of gene expression and contribute to
biological processes, including carcinogenesis (3). Numerous lncRNAs have demonstrated an
association with the development of diverse types of cancer in
genome-wide association studies (4). The presence of mutations and the
aberrant expression of lncRNAs play important roles in
tumorigenesis and metastasis. Notably, numerous lncRNAs have been
linked with the occurrence and progression of prostate cancer
(5).
The lncRNA sprouty4-intron 1 (SPRY4-IT1) has been
shown to have proto-oncogenic or anticancer activity, which varies
according to tumor type (6). Our
previous study showed that SPRY4-IT1 sponges microRNA (miR)-101-3p
to promote the proliferation and metastasis of bladder cancer cells
via upregulation of the expression of enhancer of zeste homolog 2
(7). Previous studies have
confirmed that the expression of SPRY4-IT1 is upregulated in
primary human prostatic adenocarcinomas and is higher in the PC3
prostatic cancer cell line than in normal prostate epithelial cells
(8). In addition, the knockdown of
SPRY4-IT1 expression has been shown to reduce the proliferation and
invasive ability of PC3 cells and promote their apoptosis (9); however, the underlying mechanism is
unclear. The receptor tyrosine kinase-mediated PI3K/AKT signaling
pathway could be involved in the regulation of prostate cancer cell
proliferation and differentiation, since in cancer, the activation
of this pathway is known to promote cell proliferation, survival,
invasion and metastasis (10).
Tumor cells actively proliferate in vivo and
it is generally considered that hypoxia plays a vital role in this
process (11). A hypoxic
microenvironment facilitates tumor invasiveness and reduces the
sensitivity of tumors to chemotherapy (12,13).
Hypoxia is common in human prostate cancer, in which it is
associated with disease progression and treatment resistance
(14). Various types of solid
tumors, including prostate cancer, contain substantial hypoxic
regions due to their tortuous and undeveloped vasculature (15).
In the present study, the role of SPRY4-IT1 in the
development of prostate cancer was investigated. In particular, the
expression levels of SPRY4-IT1 in prostate cancer tissues and cell
lines were compared with the corresponding expression in
pair-matched benign adjacent prostate tissues and in an
immortalized non-cancerous prostatic epithelial cell line,
respectively. Furthermore, the expression levels of SPRY4-IT1 were
evaluated in the prostate cancer cells following culture under
hypoxic conditions. To simulate the hypoxic microenvironment in
vivo, a hypoxia incubator was used. The changes in the cell
cycle and in the expression levels of cell cycle-associated
proteins and PI3K/AKT signaling pathway components were
investigated. The viability of SPRY4-IT1-overexpressing prostate
cell lines was also monitored under hypoxic conditions.
Materials and methods
Clinical samples
A total of 36 pairs of fresh prostate cancer tissues
and matched benign adjacent prostate tissues were collected from
patients with prostate cancer at the Department of Urology of
Shanghai Pudong Hospital affiliated with Fudan University
(Shanghai, China) between May 2018 and November 2020. The protocols
used in the present study were approved by the Shanghai Pudong
Hospital Ethics Review Committee and written informed consent to
participate was obtained from all patients prior to surgery. The
specimens were classified according to the 2016 World Health
Organization criteria and the TNM staging system (16). The size and Gleason score of each
tumor was recorded (17). The
clinicopathological features of the patients are shown in Table I. The inclusion criteria were as
follows: Aged between 50–79 years; pathologically confirmed
prostate cancer; accepted prostatectomy; and willing to participate
in the study. The exclusion criteria were as follows: Aged <50
or >79 years; another active malignancy, with the exception of
non-melanoma skin cancer, in addition to prostate cancer; did not
accept prostatectomy; and unwilling to participate in the
study.
 | Table I.Associations between SPRY4-IT1
expression and the clinicopathological features of patients with
prostate cancer. |
Table I.
Associations between SPRY4-IT1
expression and the clinicopathological features of patients with
prostate cancer.
|
|
| SPRY4-IT1
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total | High | Low | P value |
|---|
| Age (years) |
|
|
| 0.99 |
|
<69 | 12 | 9 | 3 |
|
| ≥69 | 24 | 19 | 5 |
|
| Gleason score |
|
|
| 0.04 |
| 6 or
7 | 11 | 6 | 5 |
|
| 7-10 | 25 | 22 | 3 |
|
| Tumor stage |
|
|
| 0.69 |
| T2 | 12 | 10 | 2 |
|
| T3 or
T4 | 24 | 18 | 6 |
|
| Tumor size (cm) |
|
|
| 0.05 |
|
<0.6 | 8 | 4 | 4 |
|
| ≥0.6 | 28 | 24 | 4 |
|
Cell culture
The PC3, DU145 and LNCaP human prostatic cancer cell
lines and the RWPE-1 human immortalized non-cancerous prostatic
epithelial cell line were obtained from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. The PC3
cells were cultured in F12K medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). The DU145 cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) in the
presence of 10% FBS. The LNCaP cells were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) in the presence of 10% FBS.
The RWPE-1 cells were cultured in keratinocyte serum-free medium
(Gibco; Thermo Fisher Scientific, Inc.). All cell media were
supplemented with 1% streptomycin/penicillin. The incubator
temperature was set to 37°C. An anaerobic environment was created
in a hypoxic incubator in the presence of 1% O2. The
durations of hypoxia were 1, 6, 12 and 24 h.
Overexpression and short hairpin RNA
(shRNA) plasmids
An overexpression vector targeting SPRY4-IT1 was
purchased from GeneChem, Inc., and an empty PLVX vector was used as
a control. Two shRNAs targeting SPRY4-IT1 and a negative control
shRNA (shNC) with no specific target were synthesized by GeneChem,
Inc. The following shRNA sequences were used: sh-SPRY4-IT1-1,
GGTGGTTGAAAGGAATCCT; sh-SPRY4-IT1-2, GCCTGTGAATGCCAACATC; and shNC,
ATCGACTAGCCACTCAGAC. PC3 or DU145 cells were seeded in a 6-well
plate for 24 h to reach a density of 30–50%, after which they were
transfected with 2.5 µg overexpression vector, shRNA or respective
control using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C according to the manufacturer's
instructions. The transfected cells were harvested at 48 h
following transfection. Stable cell lines were selected by
treatment with 0.5‰ puromycin for 3 days, and the concentration
used for maintenance was 0.1‰ puromycin
Hypoxic culture
A hypoxic environment was established using an
anaerobic incubator with a 1% O2 concentration. The
cells were plated in a 6-well plate 24 h prior to the induction of
hypoxia at 70–80% confluence. The time periods for hypoxic
induction were set at 1, 6, 12 and 24 h.
Cell viability
Cell viability was assessed using a Cell Counting
Kit (CCK)-8 assay (Dojindo Laboratories, Inc.) following the
manufacturer's instructions. The cells were seeded in a 96-well
plate at a density of 5,000 cells per well. Following the induction
of hypoxia for 24 h, the CCK-8 solution was added to every well and
the cells were cultured at 37°C in an incubator in the dark under
normal conditions for 2 h. The absorbance was detected at 450 nm
using a microplate reader.
Flow cytometry analysis
For analysis of the cell cycle using flow cytometry,
a Cell Cycle Staining Kit (CCS012; MultiSciences Biotech Co., Ltd.)
was used. All steps were performed following the manufacturer's
specifications. The analysis was performed using the BD
FACSCalibur™ Flow Cytometer (BD Biosciences). The flow cytometry
data were analyzed using FlowJo v10 software (FlowJo LLC).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) assays
Total RNA was isolated from cells using a SteadyPure
Universal RNA Extraction Kit (cat. no. AG21017; Accurate Biology)
according to the manufacturer's instructions. Complementary DNA was
synthesized with random primers using the Evo M-MLV RT Kit with
gDNA Clean for qPCR (Accurate Biology). The RT temperature protocol
included gDNA removal at 42°C for 2 min, and reverse transcription
at 37°C for 15 min and 85°C for 5 sec. qPCR was carried out using
the SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.). The
qPCR thermocycling conditions included initial denaturation at 95°C
for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec. The primer set for SPRY4-IT1 was as follows: Forward,
5′-AGCCACATAAATTCAGCAGA-3′ and reverse,
5′-CGATGTAGTAGGATTCCTTTCA-3′. Primers for β-actin were obtained
from Accurate Biology (cat. no. AG11722) and had the following
sequences: Forward, 5′-TATTTTGAATGATGAGCCTTCGT-3′ and reverse,
5′-TGCACTTTTATTCAACTGGTCT-3′. All data analyses were performed
using the StepOnePlus Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The expression levels of SPRY4-IT1
were normalized against those of β-actin as the reference gene. The
data were analyzed using the 2−ΔΔCq method (18).
Western blot assays
Following the induction of hypoxia, the cells were
quickly collected and lysed using RIPA protein extraction reagent
(Epizyme, Inc.; Ipsen) supplemented with protease inhibitor and
phosphatase inhibitor cocktails (both Epizyme. Inc.; Ipsen). The
concentrations of the protein samples were detected using a BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). Protein
extracts (15 µg/lane) were separated by 10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked for 25 min at room temperature in protein-free
blocking buffer (Epizyme, Inc.; Ipsen) and incubated with primary
antibodies at 4°C for 12 h. Antibodies targeting CDK2 (cat. no.
A0094), cyclin D1 (cat. no. A19038), AKT1 (cat. no. A20799),
phosphorylated-AKT (cat. no. AP1172) and β-actin (cat. no. AC026)
were used. All primary antibodies were diluted 1:1,000. All the
primary antibodies were acquired from ABclonal Biotech Co., Ltd.
Membranes were incubated with Anti-rabbit IgG, HRP-linked Antibody
(dilution, 1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.)
at room temperature for 60 min. Immobilon Western HRP Substrate
(WBKLS0050) was purchased from MilliporeSigma. The membrane was
exposed to an autoradiography film and autoradiograms were
quantified by densitometry using Quantity One software 4.4.6
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times. Patients
were divided into high and low SPRY4-IT1 groups based on the Cq
value of the RWPE-1 cells and clinicopathological data were
analyzed according to using Fisher's exact test. Comparisons
between the prostate cancer and normal adjacent tissues was
performed using paired Student's t-test, and other comparisons
between two groups were performed using unpaired Student's tests.
One-way ANOVA with Tukey's post hoc test was used to determine the
significance of differences among multiple groups. P<0.05 was
considered to indicate a statistically significant result. All data
were analyzed using Excel 2019 (Microsoft Corporation) and SPSS
Statistics 21 (IBM Corp.).
Results
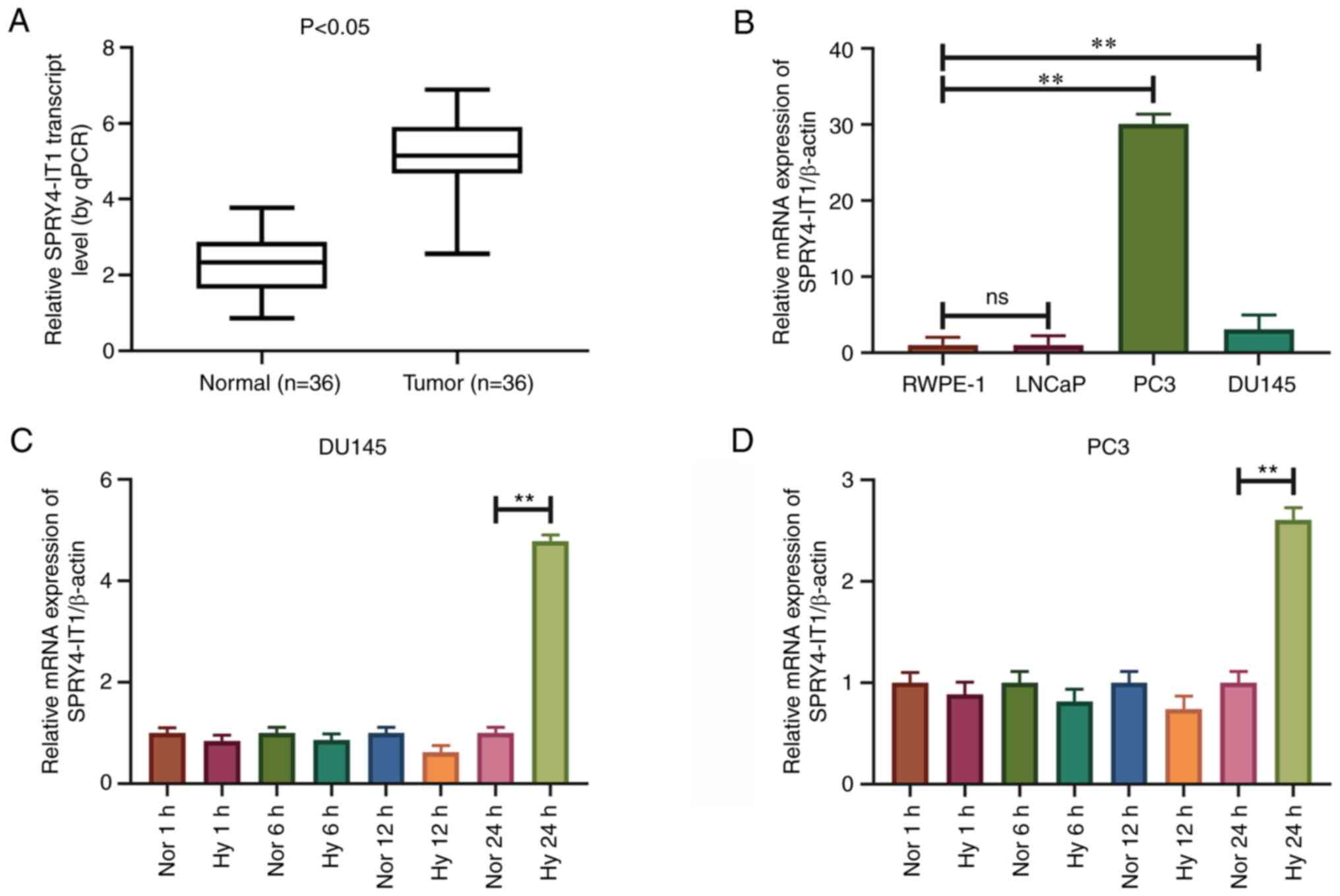
SPRY4-IT1 is highly expressed in
prostate cancer tissues and cell lines
RT-qPCR was used to detect the expression levels of
SPRY4-IT1 in prostate cancer tissues and cell lines. The expression
levels of SPRY4-IT1 were significantly higher in prostate cancer
tissues than in the pair-matched normal adjacent tissues
(P<0.05; Fig. 1A). SPRY4-IT1
expression was associated with the Gleason score of patients with
prostate cancer (P<0.04; Table
I), and was independent of patient age, tumor stage and tumor
size. In addition, SPRY4-IT1 was expressed at higher levels in the
DU145 and PC3 prostate cancer cell lines than in the RWPE-1 human
immortalized non-cancerous prostate epithelial cell line
(P<0.01). However, SPRY4-IT1 expression was not upregulated in
LNCaP cells (Fig. 1B).
SPRY4-IT1 expression is upregulated
under hypoxic conditions
A hypoxic microenvironment is common in prostate
cancer. To assess the effect of hypoxia on the expression of
SPRY4-IT1, DU145 and PC3 cells were cultured in an anaerobic
incubator for various time periods. Following 24 h of cell culture
under hypoxia, the expression levels of SPRY4-IT1 were
significantly increased in the DU145 and PC3 cells compared with
those cultured under normoxic conditions (P<0.01). However, the
expression levels of SPRY4-IT1 did not change significantly in
DU145 and PC3 cell lines following 1, 6 and 12 h of culture under
hypoxic conditions (Fig. 1C and
D).
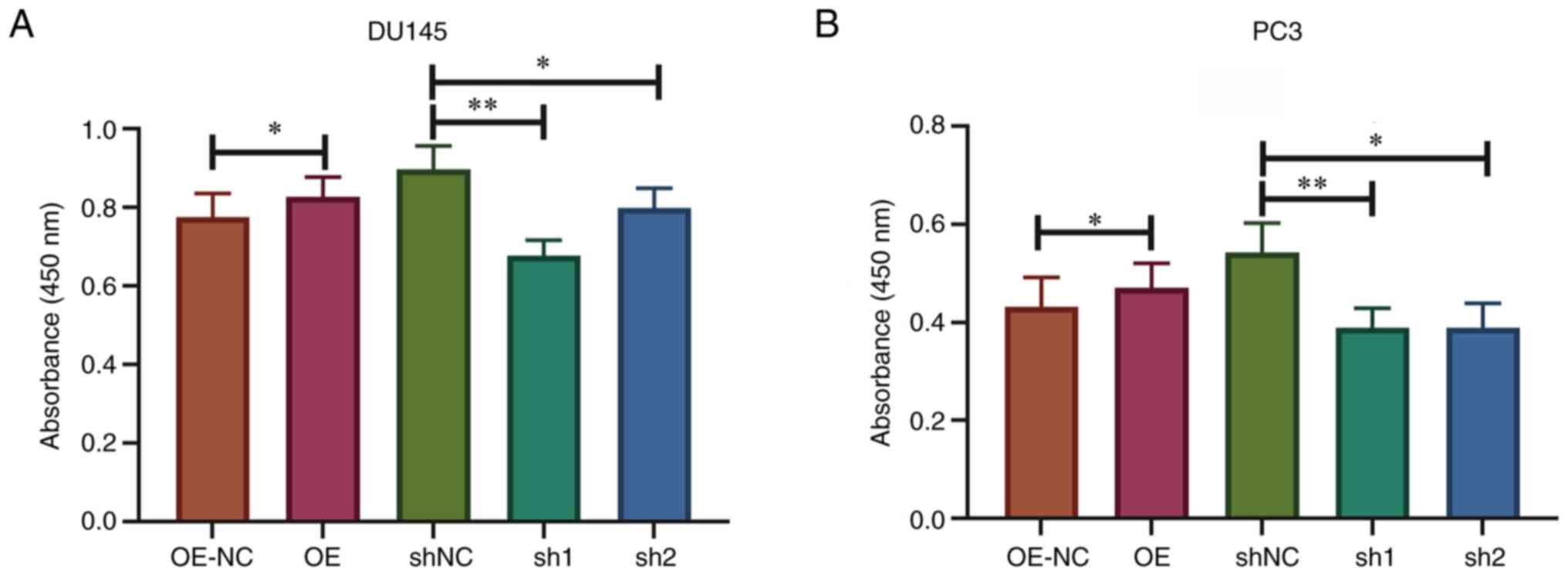
Knockdown of SPRY4-IT1 suppresses
prostate cancer cell viability
DU145 and PC3 cells were transfected with SPRY4
overexpression vector or shRNA, and the transfection efficiency is
presented in Fig. S1. To
effectively simulate the hypoxic microenvironment in vitro,
the transfected cells were cultivated in an anaerobic incubator for
24 h and the cell viability was evaluated using CCK-8 assays. The
results demonstrated that the cell viability was significantly
lower in DU145 and PC3 cells transfected with sh-SPRY4-IT1 compared
with the corresponding control cells transfected with shNC
(P<0.01). Overexpression of SPRY4-IT1 in DU145 and PC3 cells
increased resistance to the hypoxic environment (P<0.05). This
result indicates that SPRY4-IT1 had a protective effect on prostate
cancer cell viability under hypoxic conditions (Fig. 2).
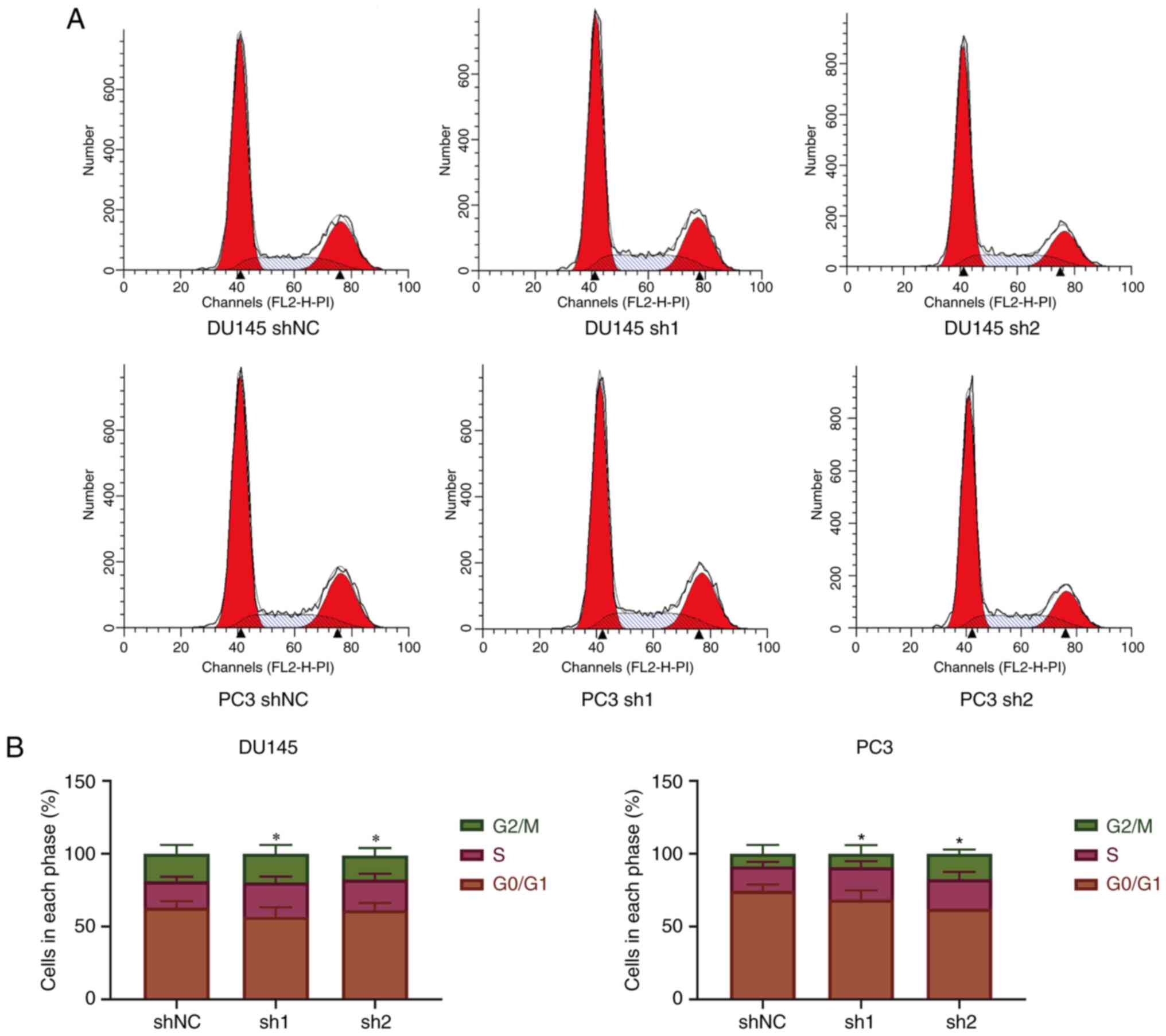
Knockdown of SPRY4-IT1 leads to
S-phase arrest in prostate cancer cells
Flow cytometry was used to evaluate the cell cycle
following the knockdown of SPRY4-IT1 expression in DU145 and PC3
cells. The results indicated that the knockdown of SPRY4-IT1
expression led to S-phase arrest in prostate cancer cells
(P<0.05; Fig. 3).
Knockdown of SPRY4-IT1 inhibits cell
cycle-associated protein expression and AKT phosphorylation
The transfected DU145 and PC3 cell lines were
cultured in an anaerobic incubator for 24 h and the total protein
was then rapidly extracted for western blot analysis. After
SPRY4-IT1 was overexpressed in the DU145 cell line, the expression
levels of CDK2 and cyclin D1 were increased (P<0.05). In
SPRY4-IT1-overexpressing PC3 cells, cyclin D1 expression was
upregulated (P<0.05), while no significant difference in CDK2
expression was observed. The expression levels of CDK2 and cyclin
D1 were lower in DU145 and PC3 cells transfected with sh-SPRY4-IT1
compared with cells transfected with shNC (P<0.01), indicating
that downregulation of SPRY4-IT1 expression affected the cell cycle
progression of prostate cancer cells. This is consistent with the
flow cytometry results. After overexpression of SPRY4-IT1, the
phosphorylation levels of AKT in PC3 cells were increased
(P<0.05), while those in DU145 cells were not significantly
altered. AKT phosphorylation was also reduced following the
knockdown of SPRY4-IT1 expression (Fig.
4).
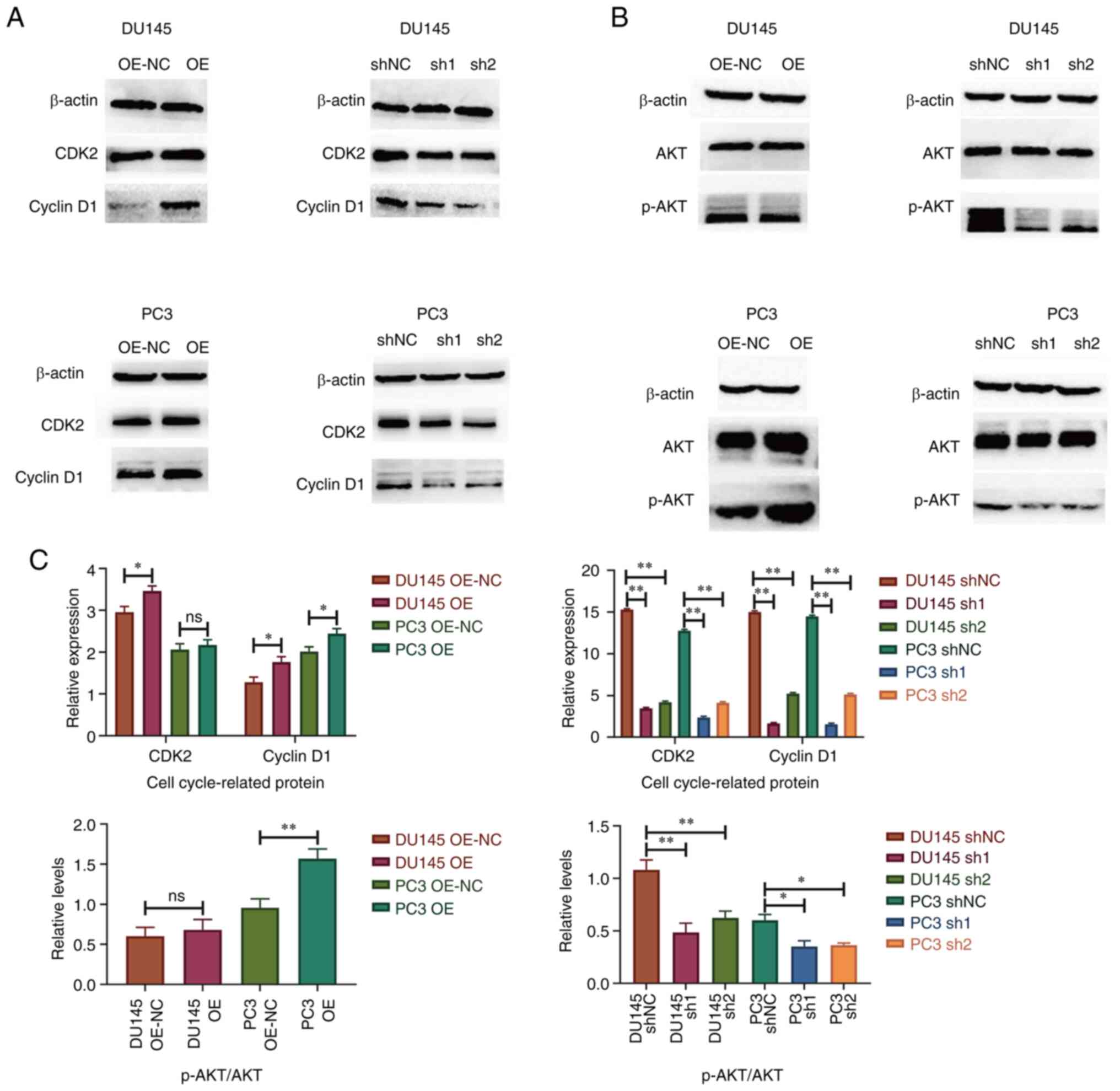 | Figure 4.Western blotting results of cell
cycle-associated proteins and AKT phosphorylation. Representative
blots for (A) CDK2 and cyclin D1 and (B) AKT phosphorylation. (C)
Quantification of the western blot results. Knockdown of SPRY4-IT1
in DU145 and PC3 cell lines led to reductions in CDK2 and cyclin D1
expression and AKT phosphorylation. *P<0.05, **P<0.01, ns, no
significance. SPRY4-IT1, sprouty4-intron 1; OE, overexpression;
OE-NC, OE negative control (empty vector); shNC, short hairpin
negative control; sh1, sh-SPRY4-IT1-1; sh2, sh-SPRY4-IT1-2; p-,
phosphorylated. |
Discussion
The present study revealed that the expression
levels of SPRY4-IT1 expression were higher in prostate cancer
tissues and cell lines than in pair-matched adjacent benign
prostate tissues and the RWPE-1 human immortalized non-cancerous
prostate epithelial cell line, respectively. SPRY4-IT1 has been
reported to be highly expressed in prostate cancer tissues and the
PC3 cell line (19). However, to
the best of our knowledge, the expression levels of SPRY4-IT1 in
the DU145 cell line have not been previously reported. The present
study further confirmed that SPRY4-IT1 was highly expressed in two
prostate cancer cell lines. SPRY4-IT1 expression was significantly
associated with the Gleason score in prostate cancer but not with
age, tumor stage or tumor size. These results suggest that
SPRY4-IT1 is potentially involved in the progression of prostate
cancer. However, the sample size of the present study was
relatively small and the relationships between SPRY4-IT1 and the
clinical parameters require further examination in subsequent
studies.
Adaptation to hypoxic stress is pivotal in tumor
progression and malignancy (20).
The current study is a preliminary analysis demonstrating the
effect of hypoxia on SPRY4-IT1 expression in prostate cancer. At
the beginning of the hypoxic culture period, the expression of
SPRY4-IT1 exhibited a slight reduction. However, following 24 h of
cell culture under hypoxic conditions, the expression levels of
SPRY4-IT1 in the DU145 and PC3 prostatic cancer cells were
increased; thus, during adaptation to hypoxia, the expression
levels of SPRY4-IT1 in the prostate cancer cell lines were
increased. The knockdown of SPRY4-IT1 expression in DU145 and PC3
cells resulted in a reduction in cell viability following cell
culture under hypoxic conditions for 24 h. This finding indicates
that SPRY4-IT1 promotes prostate cancer cell viability when the
cells are exposed to hypoxia. Therefore, it appears that SPRY4-IT1
plays an important role in the adaptation of prostate cancer cells
to hypoxic stress and in the maintenance of cell activity in a
hypoxic environment. Considering hypoxia is common in human
prostate cancer, we hypothesize that SPRY4-IT1 plays the same role
in vivo.
The present study revealed that the knockdown of
SPRY4-IT1 expression led to S-phase arrest in prostate cancer cells
under hypoxic conditions. This result is in accordance with a study
performed on human melanoma, in which the suppression of SPRY4-IT1
impaired cell proliferation and invasion (20). The present study also demonstrated
that following the knockdown of SPRY4-IT1 expression in prostate
cancer cells, the expression levels of the cell cycle-associated
proteins CDK2 and cyclin D1 were decreased. The aberrant expression
of CDK2 has been detected in a variety of tumors and is associated
with the proliferation of tumor cells (21), while the upregulation of cyclin D1
expression has been shown to accelerate cell cycle progression and
lead to tumor cell proliferation (22). Furthermore, CDK2 and cyclin D1 have
both been shown to drive cell cycle progression through the S phase
(23,24). The flow cytometry results and
western blot assay results were consistent regarding the effects of
SPRY4-IT1 on the cell cycle. Knockdown of sprouty4 and SPRY4-IT1
expression in tumor cells has been reported to potently inhibit AKT
phosphorylation (6), with the
latter increasing the expression levels of cyclin D1 (25). Changes in the total and
phosphorylated levels of AKT, a member of the PI3K/AKT signaling
pathway, following the knockdown of SPRY4-IT1 expression were
investigated in prostate cancer cells using western blot analysis.
The knockdown of SPRY4-IT1 expression in DU145 and PC3 cells
inhibited AKT phosphorylation under hypoxic conditions. The AKT
pathway promotes cell survival via AKT phosphorylation and the
subsequent inhibition of apoptosis (26); this mechanism of action may explain
the decreased viability of prostate cancer cells following the
knockdown of SPRY4-IT1 expression.
The present study verified that SPRY4-IT1 expression
was elevated in prostate cancer tissues and the DU145 and PC3 cell
lines compared with pair-matched adjacent benign prostate tissues
and the non-cancerous prostate epithelial cell line RWPE-1. This
finding improves our understanding of SPRY4-IT1 expression in
prostate cancer. Based on these results, it is suggested that
SPRY4-IT1 is likely to be involved in the progression of prostate
cancer. Furthermore, the results indicated that SPRY4-IT1
expression was upregulated under hypoxic conditions and could
regulate the viability of prostate cancer cells, possibly via the
regulation of CDK2, cyclin D1 and AKT phosphorylation. The results
suggest the underlying mechanism by which SPRY4-IT1 functions in a
hypoxic microenvironment. Finally, the results suggest a novel
application for SPRY4-IT1 in the clinical diagnosis and treatment
of prostate cancer. Prostate-specific antigen screening and
ultrasound-guided prostate puncture are the main methods of
prostate cancer diagnosis, which often lead to excessive medical
treatment. Detection of the expression level of SPRY4-IT1 in the
urine of prostate cancer patients is potentially an auxiliary means
for the diagnosis of prostate cancer, which may reduce the risk of
over-diagnosis to some extent. To explore the feasibility of urine
SPRY4-IT1 levels in the diagnosis of prostate cancer, SPRY4-IT1
levels in the urine of patients with prostate cancer should be
detected in the future. The results of the present study also
suggested that SPRY4-IT1 may be associated with a worse prognosis
in prostate cancer patients. Therefore, the measurement of
SPRY4-IT1 levels in patients with prostate cancer could potentially
be used to select a more suitable treatment at an early stage to
reduce mortality. However, further mechanistic studies of SPRY4-IT1
are required to fully understand its role in the pathogenesis of
prostate cancer. Additional studies investigating the molecular
interactions of SPRY4-IT1 with other genes may also improve our
comprehension of the mechanisms underlying prostate cancer
occurrence and development.
In summary, the present study demonstrates that the
lncRNA SPRY4-IT1 is upregulated in prostate cancer tissues and cell
lines. It also suggests that the upregulation of SPRY4-IT1 promotes
the proliferation of prostate cancer cells under hypoxia in
vitro. Therefore, SPRY4-IT1 may be a potential target for the
diagnosis and treatment of prostate cancer.
lncRNAs play a variety of roles in tumor
progression. Previous studies have shown that the lncRNA SPRY4-IT1
can bind to a variety of molecules, including miRs and proteins. A
limitation of the present study is that it did not explore the
potential molecules to which SPRY4-IT1 may bind. In addition, the
number of cases included was relatively small and a higher number
of cases are required to support the conclusions of the study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by the Natural Science Foundation of
China (project no. 81802547) and the Science and Technology
Development Fund of Shanghai Pudong New Area (grant no.
PKJ2018-Y34).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and DL were responsible for the design and
completion of the experiment, while RZ and MG were responsible for
the data analysis and article writing. All authors read and
approved the final version of the manuscript. MG and WS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by Shanghai Pudong
Hospital Ethics Review Committee. The patients provided written
informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brandão A, Paulo P and Teixeira MR:
Hereditary predisposition to prostate cancer: From genetics to
clinical implications. Int J Mol Sci. 21:50362020. View Article : Google Scholar
|
|
2
|
Wilson KM, Giovannucci EL and Mucci LA:
Lifestyle and dietary factors in the prevention of lethal prostate
cancer. Asian J Androl. 14:365–374. 2012. View Article : Google Scholar
|
|
3
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng Y, Tang D, Zhao M, Kajiyama H,
Kikkawa F and Kondo Y: Long non-coding RNA: A recently accentuated
molecule in chemoresistance in cancer. Cancer Metastasis Rev.
39:825–835. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y,
Brown M, Chen Y and Liu XS: Integrative genomic analyses reveal
clinically relevant long noncoding RNAs in human cancer. Nat Struct
Mol Biol. 20:908–913. 2013. View Article : Google Scholar
|
|
6
|
Das MK, Furu K, Evensen HF, Haugen ØP and
Haugen TB: Knockdown of SPRY4 and SPRY4-IT1 inhibits cell growth
and phosphorylation of Akt in human testicular germ cell tumours.
Sci Rep. 8:246220186 View Article : Google Scholar
|
|
7
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F, et al: LncRNA SPRY4-IT1 sponges
miR-101-3p to promote proliferation and metastasis of bladder
cancer cells through up-regulating EZH2. Cancer Lett. 388:281–291.
2017. View Article : Google Scholar
|
|
8
|
Li Z, Shen J, Chan MTV and Wu WKK: The
long non-coding RNA SPRY4-IT1: An emerging player in tumorigenesis
and osteosarcoma. Cell Prolif. 51:e124462018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee B, Mazar J, Aftab MN, Qi F, Shelley J,
Li JL, Govindarajan S, Valerio F, Rivera I, Thurn T, et al: Long
noncoding RNAs as putative biomarkers for prostate cancer
detection. J Mol Diagn. 16:615–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Regad T: Targeting RTK signaling pathways
in cancer. Cancers (Basel). 7:1758–1784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Guo S, Zhu X, Qiu J, Deng G and
Qiu C: Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α
axis. J Cell Mol Med. 24:8430–8440. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie C, Ji N, Tang Z, Li J and Chen Q: The
role of extracellular vesicles from different origin in the
microenvironment of head and neck cancers. Mol Cancer. 18:832019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu X, Zhu Y, Huang W, Li J, Zhang B, Li Z
and Yang X: Hyperbaric oxygen potentiates Doxil antitumor efficacy
by promoting tumor penetration and sensitizing cancer cells. Adv
Sci (Weinh). 5:17008592018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krishnamachary B, Mironchik Y, Jacob D,
Goggins E, Kakkad S, Ofori F, Dore-Savard L, Bharti SK, Wildes F,
Penet MF, et al: Hypoxia theranostics of a human prostate cancer
xenograft and the resulting effects on the tumor microenvironment.
Neoplasia. 22:679–688. 2020. View Article : Google Scholar
|
|
15
|
Zhang W, Fan W, Rachagani S, Zhou Z, Lele
SM, Batra SK and Garrison JC: Comparative study of subcutaneous and
orthotopic mouse models of prostate cancer: Vascular perfusion,
vasculature density, hypoxic burden and BB2r-targeting efficacy.
Sci Rep. 9:111172019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buyyounouski MK, Choyke PL, McKenney JK,
Sartor O, Sandler HM, Amin MB, Kattan MW and Lin DW: Prostate
cancer-major changes in the American joint committee on cancer
eighth edition cancer staging manual. CA Cancer J Clin. 67:245–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; ISUP Grading Committee, : The 2005 international society
of urological pathology (ISUP) consensus conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melana JP, Mignolli F, Stoyanoff T,
Aguirre MV, Balboa MA, Balsinde J and Rodríguez JP: The hypoxic
microenvironment induces stearoyl-CoA desaturase-1 overexpression
and lipidomic profile changes in clear cell renal cell carcinoma.
Cancers (Basel). 13:29622021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng Y, Han C, Yang Y, Wang J, Gu Y, Li W
and Guo L: Correlation between LncRNA-LINC00659 and clinical
prognosis in gastric cancer and study on its biological mechanism.
J Cell Mol Med. 24:14467–14480. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li K, Xu X, He Y, Tian Y, Pan W, Xu L, Ma
Y, Gao Y, Gao J, Qi Y, et al: P21-activated kinase 7 (PAK7)
interacts with and activates Wnt/β-catenin signaling pathway in
breast cancer. J Cancer. 9:1821–1835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Bai Q, Xu L, Kakiyama G, Pandak
WM, Zhang Z and Ren S: Cytosolic sulfotransferase 2B1b promotes
hepatocyte proliferation gene expression in vivo and in vitro. Am J
Physiol Gastrointest Liver Physiol. 303:G344–G355. 2012. View Article : Google Scholar
|
|
24
|
Jin H, Zhang X, Su J, Teng Y, Ren H and
Yang L: RNA interference-mediated knockdown of translationally
controlled tumor protein induces apoptosis, and inhibits growth and
invasion in glioma cells. Mol Med Rep. 12:6617–6625. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito Y, Kaji M, Sakamoto E and Terauchi Y:
The beneficial effects of a muscarinic agonist on pancreatic
β-cells. Sci Rep. 9:161802019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Razak NA, Abu N, Ho WY, Zamberi NR, Tan
SW, Alitheen NB, Long K and Yeap SK: Cytotoxicity of eupatorin in
MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle
arrest, anti-angiogenesis and induction of apoptosis. Sci Rep.
9:15142019. View Article : Google Scholar : PubMed/NCBI
|