Introduction
Gastric cancer is one of the most common
malignancies worldwide and the fourth leading cause of
cancer-related death worldwide (1).
In China, gastric cancer is the fourth and third most common cancer
in terms of incidence and mortality, respectively (2). Although the morbidity and mortality
rates resulting from gastric cancer are exhibiting a significant
downward trend, the 5-year overall survival rate of patients with
advanced distant metastasis is still <5% (3). Therefore, it is urgent to elucidate
the molecular mechanism underlying the occurrence of gastric cancer
and reduce its incidence (4).
Gastric cancer development results from a combination of factors,
of which Helicobacter pylori (Hp) infection is the
most important risk factor (5). Our
research group previously found that Hp infection causes
upregulation of the fat mass and obesity-associated (FTO) gene. The
FTO gene, also known as ALKBH9, is located on chromosome 16q12.2
and was the first N6-methyladenosine (m6A) demethylase to be
identified (6). Recent research
suggests that variants of the FTO gene are not only associated with
obesity and metabolic disorders in humans, but also with cancer
(7). Abnormal expression of FTO
promotes tumorigenesis, progression and chemotherapy resistance in
several cancer types, including breast (8), ovarian (9) and colorectal cancer (10). Previous research also suggests that
FTO may be a potential marker of gastric cancer (11–13).
The increased expression of FTO in gastric cancer promotes cell
proliferation, migration and invasion (14) and is associated with tumor stage and
poor prognosis (15).
To the best of our knowledge, there are currently no
published data determining whether Hp infection affects FTO
expression in gastric cancer. Therefore, the present study aimed to
investigate this question by using Hp-infected cells and a
gerbil animal model to analyze FTO expression in gastric cancer and
adjacent tissues, and to examine the effect of FTO gene knockdown
on cell migration and invasion in the context of Hp
infection.
Materials and methods
Human cell lines and cell culture
Human gastric mucosal epithelial cell line GES-1 and
gastric adenocarcinoma cell line MKN45 were obtained from the Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
The human gastric epithelial adenocarcinoma cell line AGS and the
human kidney 293T cell line were purchased from The American Type
Culture Collection. All cell lines were cultured in RPM1640 medium
with 10% fetal bovine serum (both Gibco; Thermo Fisher Scientific)
and 100 U/ml penicillin and streptomycin (Gibco; Thermo Fisher
Scientific) at 37°C, and in a humidified incubator at 10%
CO2.
Hp culture and infection
The Hp GZ7 strain (GenBank accession ID,
KR154737.1) was determined to be a typical East Asian strain
(CagA+) by sequencing in our previous study (16), where it was isolated from clinical
gastric cancer tissues with informed consent from the patients and
the approval of The Ethics Committee of Guizhou Medical University
[Guiyang, China; approval no. 2017(43)]. Hp GZ7 was cultured
for 48–72 h at 37°C in a microaerophilic atmosphere on a Columbia
agar plate (Thermo Fisher Scientific) containing 10% sheep's blood.
Gastric mucosal GES-1, AGS and MKN45 cells were cultured for 24 h
in 6-well plates containing antibiotic-free medium and subsequently
infected with Hp at a multiplicity of infection (MOI) of 40.
The cells were infected for 24 h before they were harvested for
western blotting and reverse transcription-quantitative PCR
(RT-qPCR). The uninfected cells served as a negative control. All
cells were incubated at 37°C and in a humidified incubator at 10%
CO2.
Plasmid construction and lentiviral
coating
The sequence of short hairpin FTO (shFTO) was
5′-CACCAAGGAGACTGCTATTTA-3′ and that of nonsense shRNA (sh-NC) was
5′-GCUUCGCGCCGUAGUCUUA-3′. In the design of shFTO and sh-NC,
restriction sites (BamHI and EcoRI) and a loop at
both the 5′ and 3′ ends and in the middle of the sequence were
added. For plasmid construction, the shRNA was added to a 0.2 ml
centrifuge tube, placed in a PCR machine at 95°C for 5 min, and
then allowed to stand at room temperature for 20 min to form a
double-stranded oligo fragment. The psi-LVRU6GP vector (Guangzhou
Fulengen Co., Ltd.) was digested with BamHI and EcoRI
at 37°C for 1 h, fragments were separated by 0.8% agarose gel
electrophoresis, and were then purified using a gel recovery kit
(Tiangen Biotech Co.). The double-stranded oligo fragment was mixed
with the digested vector, and the ligase reaction was carried out
at 16°C overnight with T4 DNA ligase. The ligated product was
transformed into competent Escherichia coli DH5α cells and
positive clones were selected for using ampicillin. Plasmid DNA was
extracted by DNA plasmid extraction kit (Tiangen Biotech Co.) for
sequencing and identification. 293T cells were cultured until 80%
confluency and then transfected with 2 µg shFTO plasmid or empty
vector plasmid and lentivirus package plasmids psPASX2 (1.5 µg) and
PMD2G (0.5 µg) at the ratio of 4:3:1 in the presence of 5 µl H4000
(Engreen Biosystem Ltd.). After 48 h of transfection, the virus
supernatant was extracted and used to infect AGS and MKN45 cells at
an MOI of 40. When the AGS and MKN45 cells reached 90% confluency,
puromycin (final concentration, 1 µg/ml) was added and stable cell
lines with knocked down FTO expression were isolated after 1
week.
Stomach tissue sections of Mongolian
gerbils infected with Hp
In our previous study (17), Hp NCTC 11637 (ATCC 43504,
CagA-positive) was used to intragastrically infect Mongolian
gerbils for 24 months to establish Hp-related gastric
disease models successfully. In these gerbils, chronic superficial
gastritis, erosive gastritis, atrophic gastritis, intestinal
metaplasia and well-differentiated gastric cancer were
pathologically observed at 3, 6, 12 and 24 months after Hp
infection. At different time points, the gerbils (3 gerbils per
time point) were sacrificed after anesthesia, and their stomach
tissues were removed, fixed and embedded in paraffin (17). In the present study, the
paraffin-embedded gerbil stomach tissues of 3 months after
Hp infection were cut into 5-µm sections for IHC staining.
The animal study was approved by the Animal Care Welfare Committee
of Guizhou Medical University (Guiyang, China; approval no.
1900801).
Human gastric tissue
A total of 20 cases of surgically resected gastric
cancer and corresponding para-cancerous tissue specimens were
collected from patients with gastric adenocarcinoma (13 male and 7
female patients; age, 51.2±9.6 years; range, 40–73 years), who were
admitted to the Affiliated Hospital of Guizhou Medical University
from March, 2021, to December, 2021. Informed and signed consent
was obtained before surgery, and the research protocol was approved
by The Ethics Committee of Guizhou Medical University [Guiyang,
China; approval no: 2019(19)].
Immunohistochemistry
Paraffin-embedded tissue sections were
deparaffinized in xylene for three 10 min intervals, incubated in
100% ethanol for three 10 min intervals, and then in a series of
graded ethanol solutions (90, 70, 50 and 30% ethanol) for 3 min
each. The tissue sections were placed in a boiling pressure cooker
for 2.5 min for antigenic repair. After cooling for 10 min in 3%
H2O2, sections were washed in PBS for 10 min
and then incubated with the FTO antibody (1:3,000; cat. no.
27226-1-AP; Proteintech Group, Inc.; Wuhan Sanying) for 2 h at room
temperature. Sections were then washed three times with PBS for 10
min intervals and incubated with the goat anti-rabbit IgG antibody
labeled with peroxidase HRP (1:10,000; cat. no. SA00001-2;
Proteintech Group, Inc.; Wuhan Sanying) at room temperature for 2
h. Sections were then washed three times with PBS for 10 min
intervals. A total of 1 ml reagent A and 50 µl reagent B from the
DAB Detection Kit (cat. no. ZLI-9018; OriGene Technologies, Inc.)
was mixed, two drops were added to the tissue sections for 1–3 min,
and then sections were incubated in PBS to stop the reaction.
Slides were stained in hematoxylin for 3 min at room temperature,
washed in distilled water, stained in ammonia reverse blue solution
for 1–2 min, washed in distilled water, dehydrated in a graded
ethanol series (50, 70, 90% and absolute ethanol), incubated three
times in xylene solution for 10 min intervals, and then mounted.
The FTO expression level of each sample was judged according to the
number of stained cells and the depth of staining using an optical
microscope. Image-Pro Plus 6.0 software (Media Cybernetics, Inc.)
was used to calculate the mean integrated optical density of active
areas in each group, and the average optical density was taken for
analysis.
RT-qPCR
The total RNA of GES-1 and AGS cells was isolated
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific). A
Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics,
Ltd.) was used for the reverse transcription of RNA according to
the manufacturer's instructions. qPCR was next performed using a 2X
SYBR Green Master Mix (Roche Diagnostics, Ltd.) in an Illumina Eco™
Real-Time PCR system (Gene Company, Ltd.). The amplification
program was as follows: 95°C for 10 min then 38 cycles of 95°C for
12 sec, 60°C for 18 sec and 72°C for 30 sec. GAPDH served as a
normalization control, and the 2−ΔΔCq approach (18) was employed to calculate relative
gene expression. Primer 5.0 (PREMIER Biosoft) was used to design
primers for qPCR analysis. The FTO primer sequence was
5′-AACACCAGGCTCTTTACGGTC-3′, which was synthesized by Sangon
Biotech Co., Ltd.
Western blotting
Total protein of GES-1 and AGS cells was extracted
according to the instructions of the high potency RIPA lysate
(tissue/cell) research reagent kit (Beijing Solarbio Science &
Technology Co., Ltd.). Protein was quantified using a BCA protein
quantification kit (Beijing Solarbio Science & Technology Co.,
Ltd.). For denaturation, 5X loading buffer was added to the protein
sample at a 4:1 ratio and boiled for 10 min. A total of 20 µg
protein was run on a 10% gel using SDS-PAGE at 80 V for 2 h,
proteins were transferred to a polyvinylidene fluoride membrane for
another 2 h and the membrane was then blocked with a solution of 20
ml PBS containing 0.05% Tween20 (1X PBST) with 1 g non-fat milk
powder at room temperature for 2 h. Incubation with the FTO primary
antibody (1:3,000; cat. no. 27226-1-AP; Proteintech Group, Inc.;
Wuhan Sanying) was conducted at 4°C overnight. The membrane was
washed three times with 1X TBST solution and incubated with the
goat anti-rabbit IgG antibody labeled with peroxidase HRP
(1:10,000; cat. no. SA00001-2: Proteintech Group, Inc.; Wuhan
Sanying) at room temperature for 2 h. Signals were visualized using
chemiluminescence ECL (Thermo Fisher Scientific) and imaged using a
chemiluminescence imager (Amersham; Cytivia). ImageJ software
(version 1.41; National Institutes of Health) was used to quantify
the gray value of the image, and the relative protein expression
using the ratio of the FTO to HRP-conjugated mouse anti-ACTB
monoclonal antibody (1:10,000; cat. no. HRP-60008: Proteintech
Group, Inc.; Wuhan Sanying) or HRP-conjugated mouse anti-GAPDH
monoclonal antibody (1:10,000; cat. no. HRP-60004: Proteintech
Group, Inc.; Wuhan Sanying) was calculated.
Immunofluorescence
The cell culture medium in the confocal dish was
discarded and the GES-1 and AGS cells were washed with PBS three
times for 5 min intervals. To fix the cells, 1 ml 4%
paraformaldehyde was added and incubated for 30 min at room
temperature, before washing with PBS three times for 5 min
intervals. Cells were permeated with 0.3% Triton X-100 for 50 min
at room temperature, before washing with PBS three times for 5 min
intervals. The cells were blocked with 5% Bovine Serum Albumin
BSA-V (cat. no. A8020; Beijing Solarbio Science & Technology
Co., Ltd.) for 1 h at room temperature. The cells were incubated
with FTO primary antibody (1:50; cat. no. 27226-1-AP; Proteintech
Group, Inc.; Wuhan Sanying) overnight at 4°C and then washed with
PBS three times for 5 min intervals. CoraLite594-conjugated goat
anti-rabbit IgG(H+L) (1:200; cat. no. SA00013-4; Proteintech Group,
Inc.; Wuhan Sanying) was added to the cells and incubated for 1 h
at room temperature. Then, 100 µl of Antifade Mounting Medium with
DAPI (cat. no. P0131-25 ml; Beyotime Institute of Biotechnology)
was added to each well in turn. The cells were carefully covered
with a slide and imaged by a laser confocal microscope. Image
fluorescence intensity was quantified using ImageJ software
(version 1.41; National Institutes of Health).
Bioinformatics analysis-acquisition
and preprocessing of gastric cancer data in the Cancer Genome Atlas
(TCGA) database
FTO RNA-seq V2 mRNA expression data from specimens
in the gastric cancer dataset (including 408 gastric cancer
specimens and adjacent tissues) were downloaded from the TCGA
database (https://tcga-data.nci.nih.gov/tcga/) and preprocessed.
From these, 211 specimens were screened for sex, age, tumor size
(T1, T2, T3 and T4), lymph node metastasis (N0, N1 and Nx), distant
metastasis (M0, M1 and Mx) and American Joint Committee on Cancer
(AJCC) pathological feature stage (AJCC stage I + II, III + IV)
(19). A total of 378 cases from
TCGA database were selected as the gastric cancer group. In
addition, according to the level of FTO mRNA expression, cases in
the gastric cancer group were divided into the high expression
group and the low expression group according to the median value,
with 180 cases in each group.
Bioinformatics analysis-FTO-related
gene screening and gene function enrichment analysis
To analyze the genes that were positively and
negatively correlated with FTO expression the LinkedOmics database
(http://linkedomics.org/login.php) was
used. The CANCER COHORT was selected as ‘TCGA_STAD’, the SEARCH
DATASET as ‘HiSeq RNA’, the SEARCH DATASET ATTRIBUTE as ‘FTO’, the
TARGET DATASET as ‘HiSeq RNA’ and the STATISTICAL METHOD as
‘Pearson Correlation test’. Gene function enrichment analysis was
performed using the STRING website (https://cn.string-db.org/). The website operation
steps were followed to carry out Gene Ontology (GO) Enrichment
analysis for ‘Molecular Function’, ‘Biological Process’ and ‘Cell
Component’.
Wound healing assay
A total of 1×106 AGS and MKN45 cells were
cultured in a 6-well plate overnight. When the degree of confluence
reached ~90%, a horizontal cell scraping strip of equal width (~500
µm) was drawn through the center of the well, and the wounded cells
were washed with PBS and incubated for a further 48 h in the medium
with 4% FBS. Imaging was conducted using an optical microscope at 0
and 48 h to measure the cell damage. The migration ability of cells
was evaluated by comparing the relative wound width at 48 h with
the wound width at 0 h. The relative scratch healing rate was
calculated as follows: Percentage relative scratch healing
rate=[gap distance (T0-T48)/gap distance at T0] ×100%, with T0 and
T48 corresponding to the width of the wound at 0 and 48 h,
respectively.
Invasion assay
Invasion assays were performed in Transwell plates
(Costar; Corning, Inc.) precoated with Matrigel (BD Bio sciences).
The chamber coating was performed at 37°C for 1 h. A total of
2×104 of each cell line (AGS and MKN45) diluted in
serum-free DMEM medium was added to the upper chamber and 500 µl
DMEM containing 10% FBS was added to the lower chamber as a
nutritional attractant. After incubating for 48 h at 37°C, the
cells inside the upper chamber were removed and the cells invading
through the membrane were fixed with 4% paraformaldehyde for 30 min
at room temperature. The fixed cells were then stained with 0.1%
crystal violet for 30 min at room temperature and counted under an
optical microscope.
Statistical analysis
The SPSS 18.0 software (SPSS, Inc.) was used for
data analysis. Unpaired t-test was used to compare the two
independent Hp infected and uninfected groups. To analyze
the differences in FTO expression between gastric cancer tissues
and paired adjacent tissue, paired t-test was used. One-way ANOVA
followed by Dunnett's post hoc tests was used when multiple groups
were compared respectively with control groups. One-way ANOVA
followed by Tukey's post hoc test was used to compare all pairs of
columns containing multiple groups. The following settings for
GEPIA were used as: ‘Expression on Box Plots’, ‘Gene as FTO’,
‘|log2FC| Cutoff as 1’, ‘P-value Cutoff as 0.01’, ‘Datasets as
STAD’, ‘Log Scale as log2(TPM + 1)’, ‘Jitter Size as 0.4’ and
‘Matched normal date’ as ‘TCGA normal and GTEx data’. Survival
curve analysis was performed using the Kaplan-Meier method and the
log-rank test using GEPIA data. To analyze the relationship between
FTO expression and clinicopathological parameters, χ2
test was used. Spearman's correlation test was used for analysis of
FTO-related gene screening and Fisher's exact test was used for
gene function enrichment analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Hp infection upregulates FTO
expression
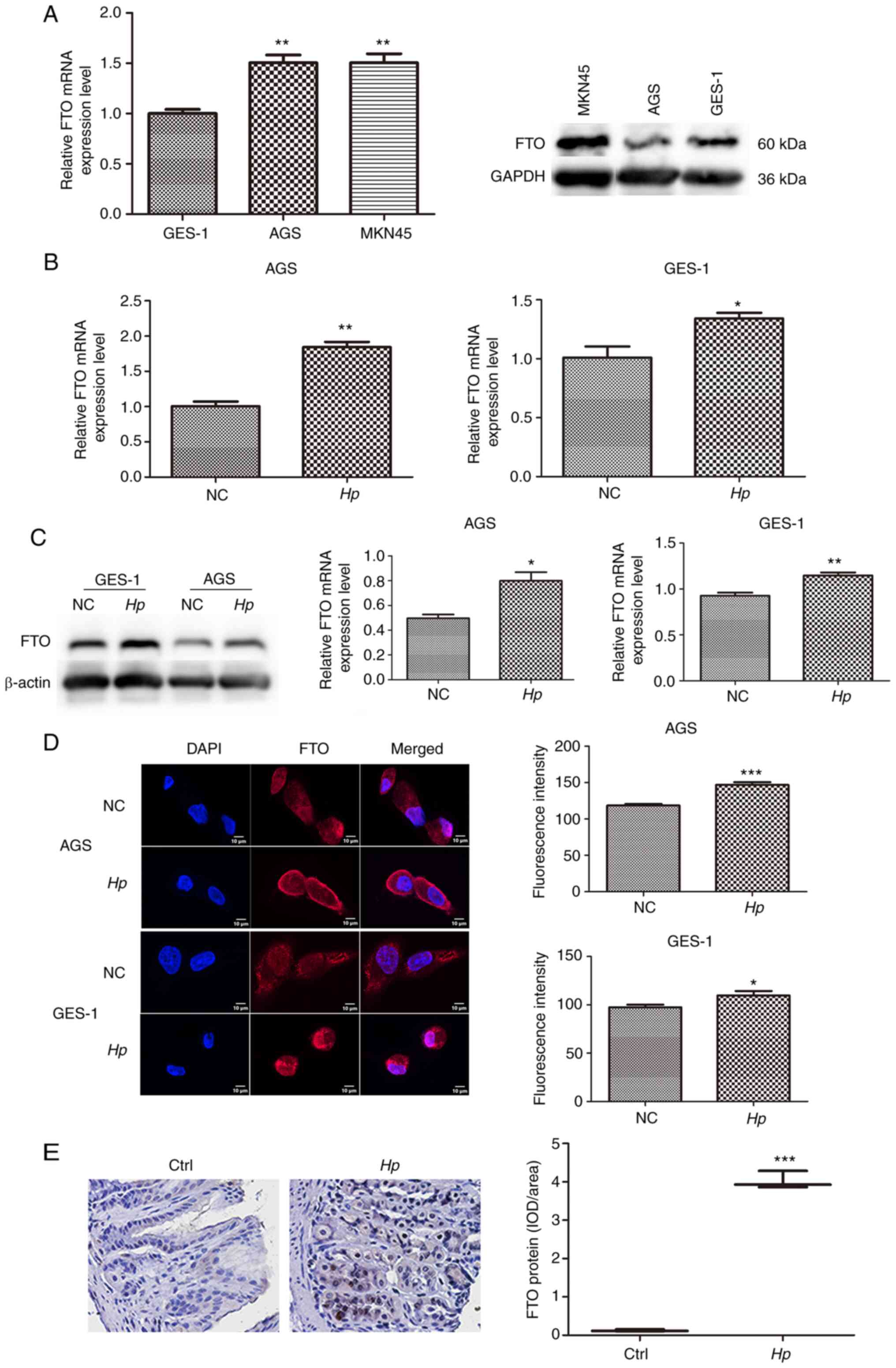
Of the cell lines used, the cell lines with lowest
FTO expression were the AGS and GES-1 cell lines. Therefore, these
two cell lines were selected for follow-up experiments (Fig. 1A). Following GES-1 and AGS infection
with the Hp GZ7 strain at MOI 40:1 for 24 h, FTO mRNA and
protein levels were detected by RT-qPCR and western blotting,
respectively, and both the mRNA and protein levels in infected
cells were significantly higher than those in uninfected cells
(Fig. 1B and C). Immunofluorescence
demonstrated that FTO was mainly expressed in the cytoplasm of
gastric epithelial cells, and the total fluorescence intensity of
FTO in Hp infected cells increased (Fig. 1D). The expression level of FTO
protein in the gastric tissues of Mongolian gerbils infected with
Hp was also significantly higher than that of the control
group (Fig. 1E).
FTO expression in gastric cancer is
associated with patient prognosis
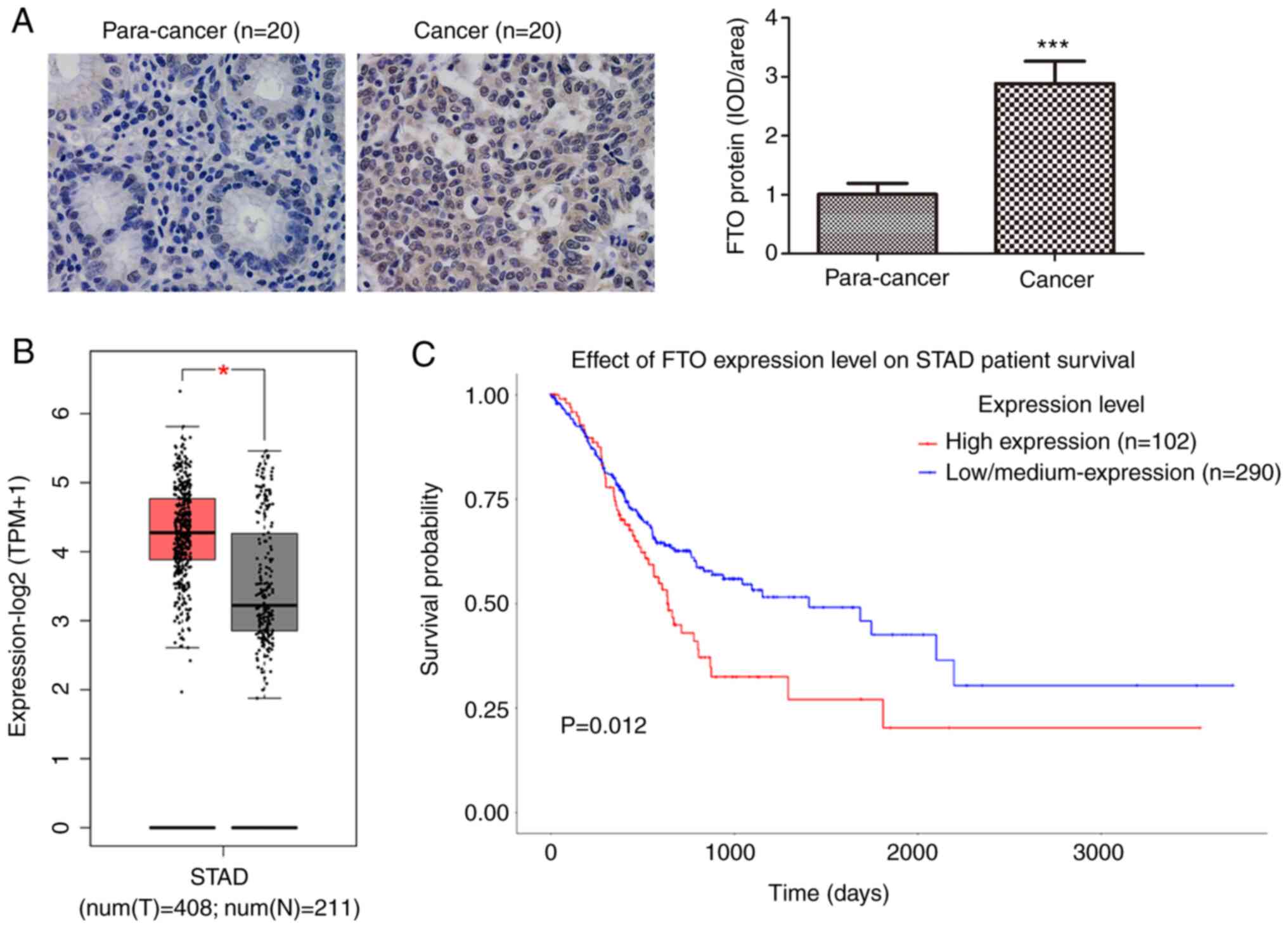
A total of 20 gastric cancer specimens and 20
corresponding adjacent tissue sections were examined by
immunohistochemistry to determine the differences in FTO expression
levels. Positive staining of FTO protein was visualized as
brown-yellow granules, mostly located in the cytoplasm and nucleus
(Fig. 2A). The protein level of FTO
in gastric cancer tissues was also significantly higher than that
in the adjacent tissues (Fig. 2A).
FTO mRNA expression data based on a total of 408 gastric cancer
cases and 211 normal tissues were available from the TCGA database.
Bioinformatics analysis indicated that the mRNA expression of FTO
in gastric cancer was significantly higher than that in normal
tissues (Fig. 2B). Kaplan-Meier
survival curves were used to explore the prognostic value of FTO
expression on the overall survival rate of patients with gastric
cancer. The overall survival rate of patients with high FTO
expression was significantly lower than that of patients with low
FTO expression (Fig. 2C).
FTO expression is associated with some
clinicopathological parameters in patients with gastric cancer
The gastric cancer group included 242 males and 136
females; 118 cases aged ≤60 years and 260 aged >60 years; 102
cases of T1+T2 stage and 276 of T3+T4 stage; 121 cases of N0 stage,
99 of N1 stage, 81 of N2 stage, 72 of N3 stage and 5 of Nx stage;
334 cases in M0 stage, 26 in M1 stage and 18 in Mx stage; 182 cases
in Stage I+II and 196 in Stage III+IV. The χ2 test was
used to examine the relationship between FTO mRNA expression and
the clinicopathological characteristics of patients with gastric
cancer in the TCGA database. The results demonstrated a positive
association between the level of FTO mRNA expression and
clinicopathological parameters in patients with gastric cancer,
such as T stage, M stage and AJCC stage, and a high expression
level of FTO mRNA was associated with higher T stage, M stage and
AJCC stage. There was no association between FTO mRNA expression
level and sex, age or N stage of patients (Table I).
 | Table I.Relationship between the expression
level of FTO mRNA and the clinicopathological parameters of
patients with gastric cancer. |
Table I.
Relationship between the expression
level of FTO mRNA and the clinicopathological parameters of
patients with gastric cancer.
|
| FTO mRNA |
|
|
|---|
| Clinicopathological
feature |
|
|
|
|---|
| Low | High | χ2 | P-value |
|---|
| Sex, n |
|
| 0.46 | 0.83 |
|
Male | 122 | 120 |
|
|
|
Female | 67 | 69 |
|
|
| Age, n |
|
| 3.154 | 0.076 |
| >60
years | 138 | 122 |
|
|
| ≤60
years | 51 | 67 |
|
|
| T stage, n |
|
| 6.499 | 0.011 |
|
T1+T2 | 62 | 40 |
|
|
|
T3+T4 | 127 | 149 |
|
|
| N stage, n |
|
| 5.417 | 0.2471 |
| N0 | 67 | 54 | |
|
| N1 | 52 | 47 |
|
|
| N2 | 40 | 41 |
|
|
| N3 | 28 | 44 |
|
|
| NX | 2 | 3 |
|
|
| M stage, n |
|
| 6.758 | 0.0341 |
| MO | 174 | 160 |
|
|
| M1 | 11 | 15 |
|
|
| MX | 4 | 14 |
|
|
| AJCC stage, n |
|
| 5.129 | 0.024 |
|
I+II | 102 | 80 |
|
|
|
III+IV | 87 | 109 |
|
|
FTO gene in gastric cancer is related
to genes associated with RNA stability and RNA metabolism
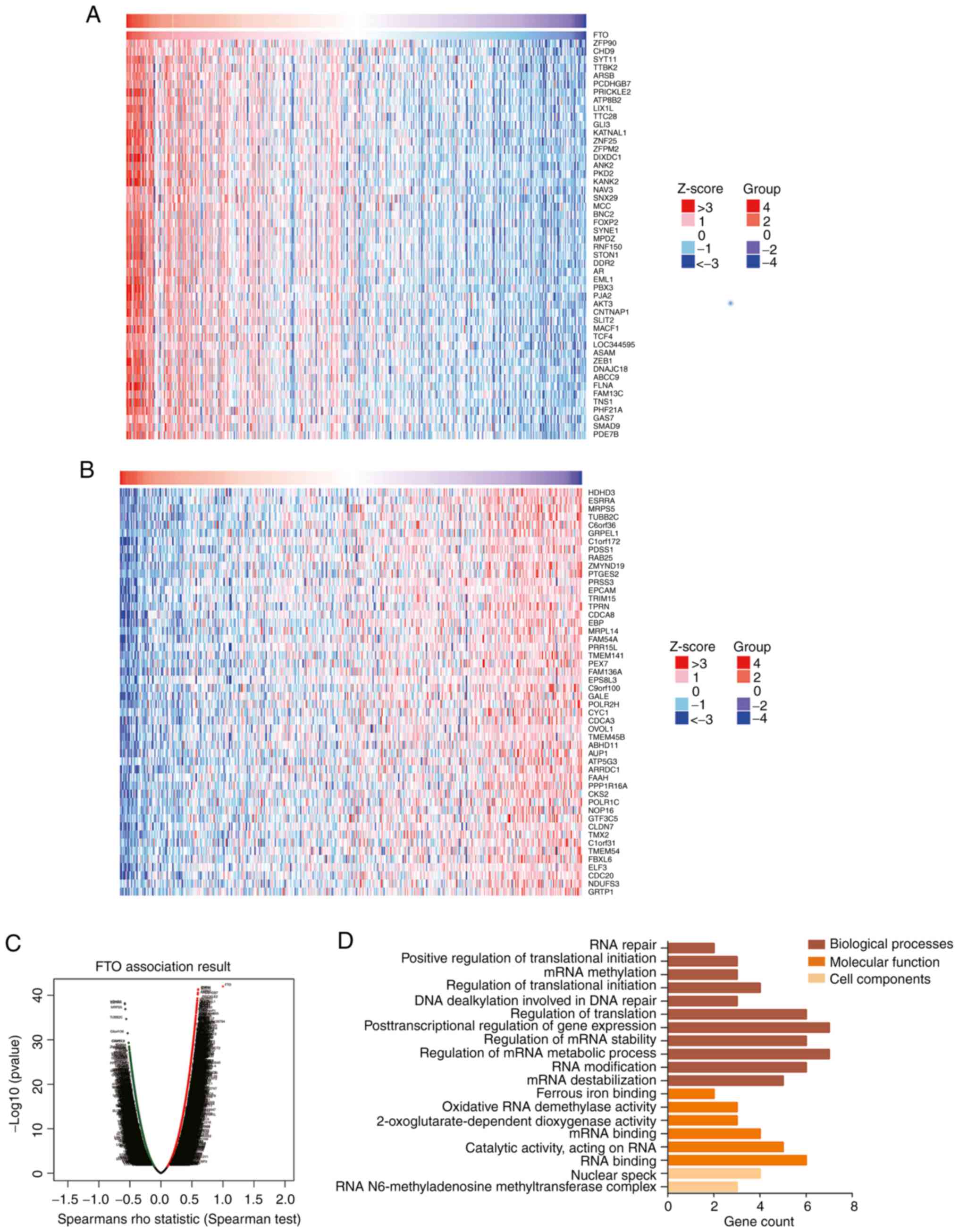
The LinkedOmics database was used to analyze genes
related to FTO in patients with gastric cancer, based on Spearman's
correlation test. The FTO gene in gastric cancer was positively
correlated with 16,601 genes, including ZFP90, chromodomain
helicase DNA binding protein 9 (CHD9) and protocadherin γ subfamily
B7 (PCDHGB7) (Fig. 3A), and was
negatively correlated with 3,623 genes, including halo acid
dehalogenase like hydrolase domain containing 3 (HDHD3) and
estrogen related receptor α (ESRRA) (Fig. 3B). GO enrichment demonstrated that
RNA stability and RNA metabolism were the biological processes in
which FTO related genes were most significantly enriched during
gene expression regulation (Fig.
3D). The molecular functions of FTO-related genes that were
most significantly enriched were oxidative RNA demethylase activity
and mRNA binding. The cellular components most significantly
enriched were the RNA m6a methyltransferase complex and nuclear
speckles. The enrichment results of FTO-related genes suggest a
possible function for FTO in cell proliferation and metabolism and
a role in the tumorigenesis and progression of gastric cancer.
FTO gene knockdown decreases
Hp-induced migration and invasion of gastric cancer cells
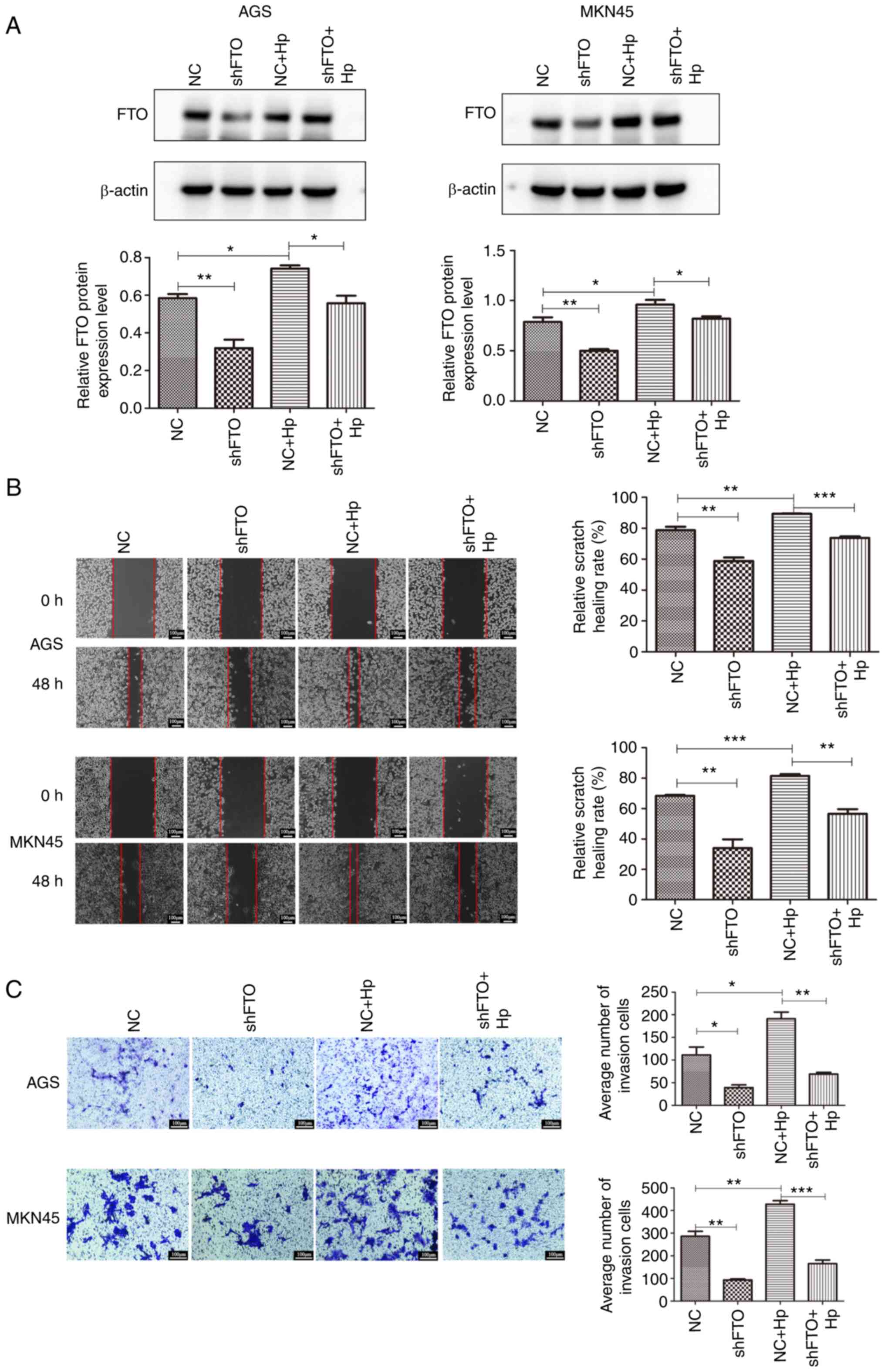
Lentiviral expression vectors were used to establish
stable AGS and MKN45 cell lines containing a knocked down FTO gene.
Western blot analysis demonstrated that FTO was successfully
knocked down in AGS and MKN45 cells, which was rescued by Hp
infection (Fig. 4A). Hp
infection of cell lines with or without a knocked down FTO gene
were used to investigate the role of FTO on Hp-induced cell
migration and invasion abilities by wound healing and Transwell
assays. The results demonstrated that Hp enhanced cell
migration and invasion abilities in AGS and MKN45 cells (Fig. 4B and C). However, the migration and
invasion abilities of cells were reduced in cell lines where the
FTO gene had been knocked down. Altogether, these findings
suggested that knocking down the FTO gene may inhibit the promoting
effect of Hp on the migration and invasion ability of
gastric cancer cells.
Discussion
The occurrence and development of gastric cancer is
a complex process, involving a variety of factors and molecular
pathways (20,21), most of which remain to be elucidated
(22). Hp is a gram-negative
bacterium that can selectively colonize the gastric mucosa. A study
showed that Hp infection is one of the most important risk
factors for gastric cancer (23).
In total, ~90% of non-cardia cancers are associated with Hp
infection (24). Hp
infection can lead to chronic immune pathological damage to the
gastric mucosa, and to the secretion of a variety of cytokines and
chemokines, such as interleukin (IL)-8 (25) and IL-6 (26), which cause chronic inflammation
(27). There is a stepwise process
from early phenotype to the development of gastric cancer: Normal
gastric mucosa to chronic atrophic gastritis to intestinal
metaplasia to gastric mucosal epithelium atypical hyperplasia to
gastric cancer (28,29). Although Hp has been listed as
a class I carcinogen since 1994 (30), the specific mechanisms underlying
carcinogenesis remain unclear.
Due to the lack of effective biomarkers, the
prognosis of patients with early gastric cancer is still poor
(31). In the absence of effective
therapies, the search for new biomarkers is critical for the
optimization of targeted therapies. The FTO gene encodes an
α-ketoglutarate-dependent hydroxylase-related non-heme iron
nucleoprotein, originally identified as a regulator of body weight
and obesity (32). Studies have
shown that FTO can act as a demethylase of m6A to participate in
the development and progression of various cancer types such as
breast cancer (8,33,34).
FTO can demethylate m6A to increase the mRNA stability in tumor
pathways and promote tumor progression (35). Zhang et al (36) demonstrated that FTO removes the m6A
modification from homeobox B13 (HOXB13) mRNA, abolishes YTH
domain-containing family protein 2-mediated HOXB13 degradation,
promotes HOXB13 protein expression, activates the WNT signaling
pathway and promotes endometrial cancer invasion and metastasis.
FTO has also been demonstrated to promote breast cancer cell
invasion and migration through the FTO/micro-RNA −181b-3p/ADP
ribosylation factor-like GTPase 5B signaling pathway (8). The pro-apoptotic gene, BCL2
interacting protein 3 (BNIP3), is a downstream target of the
FTO-mediated m6A modification (15). FTO can demethylate BNIP3 m6A mRNA
and induce its degradation, thereby promoting proliferation of
breast cancer cells and inhibiting apoptosis. Tang et al
(37) demonstrated that knockdown
of FTO reduces the proliferation of pancreatic cancer cells, and
identified c-Myc as the main target of FTO, suggesting that FTO may
be necessary for pancreatic cancer progression. Although it is
widely appreciated that FTO is involved in multiple pathways
involved in the development and progression of various cancer
types, the exact physiological function of this gene remains
unclear.
In previous research by our group, Hp-CagA
transfected gastric cancer cells were used for transcriptome
sequencing and it was found that the expression of FTO mRNA was
increased (38). It was speculated
that Hp may play a role in the development and progression
of gastric cancer by increasing FTO expression. In the present
study, gastric epithelial cells were infected with Hp and an
increase in FTO mRNA and protein levels were observed, compared
with uninfected cells. Using immunofluorescence, it was also
observed that Hp infection increased the level of FTO
protein. These findings were further confirmed using a gerbil
model. These results add to the hypothesis that FTO may play an
important role in Hp-related gastric cancer.
In the present study, using immunohistochemistry on
20 gastric cancer and adjacent tissue sections, it was observed
that the expression level of FTO in human gastric cancer tissue was
higher than that in adjacent normal tissues. The analysis using the
TCGA database also demonstrated that patients with gastric cancer
had a higher expression level of FTO than the controls. Survival
curve analysis showed an association between a high expression
level of FTO and poor survival rate of patients with gastric
cancer. Altogether, these data suggest a role of FTO in the
occurrence of gastric cancer. A comparison of clinicopathological
parameters in patients with gastric cancer with high and low FTO
expression levels indicated a statistically significant association
between FTO expression and T stage, M stage and AJCC stage, but no
association with age, sex or N stage. These results were consistent
with the research of Xu et al (14).
Using the LinkedOmics database, genes closely
related to FTO were examined and it was determined that the FTO
gene in gastric cancer was positively correlated with 16,601 genes,
including ZFP90, CHD9 and PCDHGB7. A study demonstrated that
PCDHGB7 induces apoptosis of breast cancer cells and is involved in
cancer development and progression (39). However, it was also found that FTO
was negatively correlated with 3,623 genes, including HDHD3 and
ESRRA. Chen et al (40)
demonstrated that ESRRA enhanced the metabolism and proliferation
abilities of cancer cells. Wang et al (41) also demonstrated that ESRRA may be
mainly involved in cellular metabolism processes, and demonstrated
a role for ESRRA in the tumorigenesis and progression of uteri
corpus endometrial carcinoma. In the present study, the GO
enrichment results demonstrated that the biological processes of
FTO-related genes were associated with gene expression regulation,
RNA stability and RNA metabolism, and the molecular functions of
FTO-related genes were associated with oxidative RNA demethylase
activity and mRNA binding. Regarding the cellular components,
enrichment to the RNA m6a methyltransferase complex and nuclear
speckles was observed. These results suggest that FTO may have a
role in cell proliferation and metabolism and possibly in the
tumorigenesis and progression of gastric cancer. The in
vitro experiments confirmed that FTO knockdown reduced the
invasion and migration abilities of AGS and MKMN45 cells induced by
Hp infection. Hp may affect the stability of
downstream gene transcription levels by upregulating the expression
of the FTO gene (from the transcription and translation level),
causing changes in the m6A level of downstream target genes and
therefore a biological behavioral change of gastric cancer cells.
Therefore, high FTO expression induced by Hp infection may
be related to the progression of gastric cancer tumors, suggesting
that FTO may be an important biomarker in the occurrence,
development and prognosis of gastric cancer.
In conclusion, the present study demonstrated that
Hp infection can lead to an increase in the expression level
of FTO in gastric cancer cells, with a high expression of FTO being
associated with poor prognosis of patients with gastric cancer.
High FTO expression levels induced by Hp might play an
important role in regulating the proliferation and migration of
gastric cancer cells. These results suggest a novel molecular
mechanism underlying gastric cancer induced by Hp infection
that may be of therapeutic interest.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural Science
Foundation of China (grant nos. 31960028 and 32160166), The Key
Project of Science and Technology of Guizhou Province [grant no.
(2020)1Z010] and The Science and Technology of Guizhou Province
[grant no. (2020)1Y333].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and JZ conceived and designed the experiments.
SG, QW and LB conducted most of the experiments and analyses. XH,
ZW, LL, LW and YZ confirm the authenticity of all the raw data. YX
and SG drafted the manuscript. XH, ZW, LL, LW and YZ were
responsible for overseeing the manuscript. YX, SG, QW, LB, XH, ZW,
LL, LW, YZ and JZ were responsible for writing and revising the
manuscript, and contributed significantly to the study design, data
collection, data analysis and interpretation of data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experimental processes with human specimens were
checked and authorized by The Ethics Committee of Guizhou Medical
University [Guiyang, China; approval no, 2019(19)]. All subjects
gave their informed consent for inclusion before participating in
the study. The study was conducted following the Declaration of
Helsinki. The animal study was approved by the Animal Care Welfare
Committee of Guizhou Medical University (Guiyang, China; approval
no. 1900801).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y,
Zhao R, Duan Y, Zeng Z, Li X, et al: Analysis of status and
countermeasures of cancer incidence and mortality in China. Sci
China Life Sci. 62:640–647. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Feng A, Zheng S, Chen C and Lyu J:
Recent estimates and predictions of 5-year survival in patients
with gastric cancer: A model-based period analysis. Cancer Control.
29:107327482210992272022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GBD 2017 Stomach Cancer Collaborators, .
The global, regional, and national burden of stomach cancer in 195
countries, 1990–2017: A systematic analysis for the global burden
of disease study 2017. Lancet Gastroenterol Hepatol. 5:42–54. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukamoto T, Nakagawa M, Kiriyama Y,
Toyoda T and Cao X: Prevention of gastric cancer: Eradication of
Helicobacter pylori and beyond. Int J Mol Sci. 18:16992017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu
H, Ni T, Zhang ZS, Zhang T, Li C, et al: Small-molecule targeting
of oncogenic FTO demethylase in acute myeloid leukemia. Cancer
Cell. 35:677–691.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou
K, Wang L, Cao Y, Sun P and Wang T: The FTO/miR-181b-3p/ARL5B
signaling pathway regulates cell migration and invasion in breast
cancer. Cancer Commun (Lond). 40:484–500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang H, Wang Y, Kandpal M, Zhao G,
Cardenas H, Ji Y, Chaparala A, Tanner EJ, Chen J, Davuluri RV and
Matei D: FTO-Dependent N (6)-Methyladenosine modifications inhibit
ovarian cancer stem cell self-renewal by blocking cAMP signaling.
Cancer Res. 80:3200–3214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue C, Chen J, Li Z, Li L, Chen J and Guo
Y: MicroRNA-96 promotes occurrence and progression of colorectal
cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. J Exp Clin
Cancer Res. 39:2402020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Y, Huang J and Hu J: M6A RNA
methylation regulators contribute to malignant progression and have
clinical prognostic impact in gastric cancer. Front Oncol.
9:10382019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimura T, Kandimalla R, Okugawa Y, Ohi M,
Toiyama Y, He C and Goel A: Novel evidence for m(6)A methylation
regulators as prognostic biomarkers and FTO as a potential
therapeutic target in gastric cancer. Br J Cancer. 126:228–237.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Yan X, Wang Y, Zhou J and Yu Y:
Denocarcinoma. Front Oncol. 11:7260182022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu D, Shao W, Jiang Y, Wang X, Liu Y and
Liu X: FTO expression is associated with the occurrence of gastric
cancer and prognosis. Oncol Rep. 38:2285–2292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun
L, Wang Y, Li X, Xiong XF, Wei B, et al: RNA N6-methyladenosine
demethylase FTO promotes breast tumor progression through
inhibiting BNIP3. Mol Cancer. 18:462019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long NY, Xiong L, Zhao Y, Yuan H, Li YJ,
Chen XS, Zhang XY, Xie Y and Zhou JJ: Sequences difference of CagA
between East Asian strain and Western strain of Helicobacter
pylori and its impact on the proliferation and apoptosis.
Microbiology. 45:848–855. 2018.(In Chinese).
|
|
17
|
Zhao Y, Xie Y, Chen X, Xu W, Wang Y and
Zhou J: Establishment of Mongolian gerbil model of gastric cancer
induced by Helicobacter pylori infection and its proteomics
analysis. Zhonghua Bing Li Xue Za Zhi. 43:820–826. 2014.(In
Chinese). PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer International Publishing; New York, NY: 2017, View Article : Google Scholar
|
|
20
|
Amieva M and Peek RM JR: Pathobiology of
Helicobacter pylori-induced gastric cancer.
Gastroenterology. 150:64–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process-first american cancer society
award lecture on cancer epidemiology and prevention. Cancer Res.
52:6735–6740. 1992.PubMed/NCBI
|
|
22
|
Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang
X, Li H, Li Q, Wang N and Ji J: Gastric cancer: Epidemiology, risk
factors and prevention strategies. Chin J Cancer Res. 32:695–704.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loor A and Dumitraşcu DL: Helicobacter
pylori infection, gastric cancer and gastropanel. Rom J Intern
Med. 54:151–156. 2016.PubMed/NCBI
|
|
24
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
Helicobacter pylori. Int J Cancer. 136:487–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Outlioua A, Badre W, Desterke C, Echarki
Z, El Hammani N, Rabhi M, Riyad M, Karkouri M, Arnoult D, Khalil A
and Akarid K: Gastric IL-1β, IL-8, and IL-17A expression in
Moroccan patients infected with Helicobacter pylori may be a
predictive signature of severe pathological stages. Cytokine.
126:1548932020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rasool KH, Alubadi AE and Al-Bayati IFI:
The role of serum interleukin-4 and interleukin-6 in
Helicobacter pylori-infected patients. Microb Pathog.
162:1053622022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonciarz W, Krupa A, Hinc K, Obuchowski M,
Moran AP, Gajewski A and Chmiela M: The effect of Helicobacter
pylori infection and different H. pylori components on the
proliferation and apoptosis of gastric epithelial cells and
fibroblasts. PLoS One. 14:e02206362019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mera RM, Bravo LE, Camargo MC, Bravo JC,
Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG,
Morgan DR, et al: Dynamics of Helicobacter pylori infection
as a determinant of progression of gastric precancerous lesions:
16-year follow-up of an eradication trial. Gut. 67:1239–1246. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ernst PB, Peura DA and Crowe SE: The
translation of Helicobacter pylori basic research to patient
care. Gastroenterology. 130:188–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Infection with Helicobacter pylori.
IARC Monogr Eval Carcinog Risks Hum. 61:177–240. 1994.PubMed/NCBI
|
|
31
|
Battaglin F, Naseem M, Puccini A and Lenz
HJ: Molecular biomarkers in gastro-esophageal cancer: Recent
developments, current trends and future directions. Cancer Cell
Int. 18:992018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dina C, Meyre D, Gallina S, Durand E,
Körner A, Jacobson P, Carlsson LMS, Kiess W, Vatin V, Lecoeur C, et
al: Variation in FTO contributes to childhood obesity and severe
adult obesity. Nat Genet. 39:724–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J and Du B: Novel positioning from
obesity to cancer: FTO, an m(6)A RNA demethylase, regulates tumour
progression. J Cancer Res Clin Oncol. 145:19–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou D, Dong L, Li C, Yin Z, Rao S and Zhou
Q: The m(6)A eraser FTO facilitates proliferation and migration of
human cervical cancer cells. Cancer Cell Int. 19:3212019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng X, Su R, Stanford S and Chen J:
Critical enzymatic functions of FTO in obesity and cancer. Front
Endocrinol (Lausanne). 9:3962018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Wan Y, Zhang Z, Jiang Y, Lang J,
Cheng W and Zhu L: FTO demethylates m6A modifications in HOXB13
mRNA and promotes endometrial cancer metastasis by activating the
WNT signalling pathway. RNA Biol. 18:1265–1278. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang X, Liu S, Chen D, Zhao Z and Zhou J:
The role of the fat mass and obesity-associated protein in the
proliferation of pancreatic cancer cells. Oncol Lett. 17:2473–2478.
2019.PubMed/NCBI
|
|
38
|
Chen D, Li C, Zhao Y, Zhou J, Wang Q and
Xie Y: 2021. Bioinformatics analysis for the identification of
differentially expressed genes and related signaling pathways in H.
pylori-CagA transfected gastric cancer cells. PeerJ. 9:e112032021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou S, Shan M, Gao C, Feng X, Yang Y,
Zhang R, He Y, Zhang G and Zhang L: PCDHGB7 increases
chemosensitivity to carboplatin by inhibiting HSPA9 via inducing
apoptosis in breast cancer. Dis Markers. 2019:61315482019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Zhou Y, Han F, Zhao Y, Tu M, Wang
Y, Huang C, Fan S, Chen P, Yao X, et al: A novel
miR-1291-ERRα-CPT1C axis modulates tumor cell proliferation,
metabolism and tumorigenesis. Theranostics. 10:7193–7210. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S and Huo X: Comprehensive analysis
of ESRRA in endometrial cancer. Technol Cancer Res Treat.
20:15330338219920832021.PubMed/NCBI
|