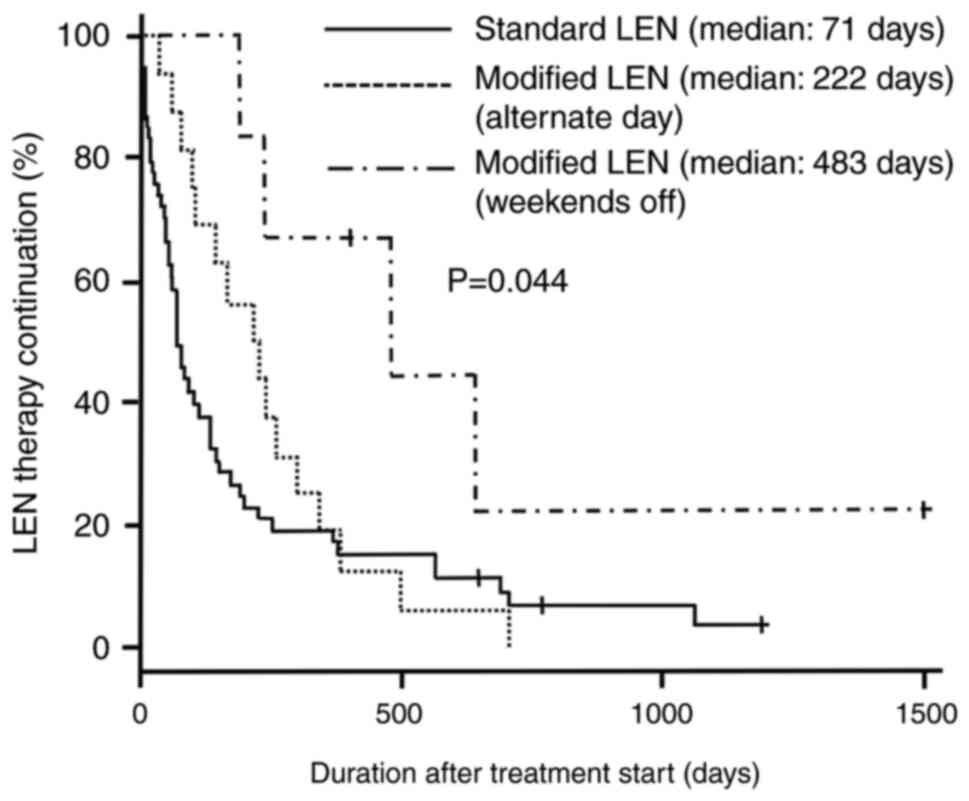
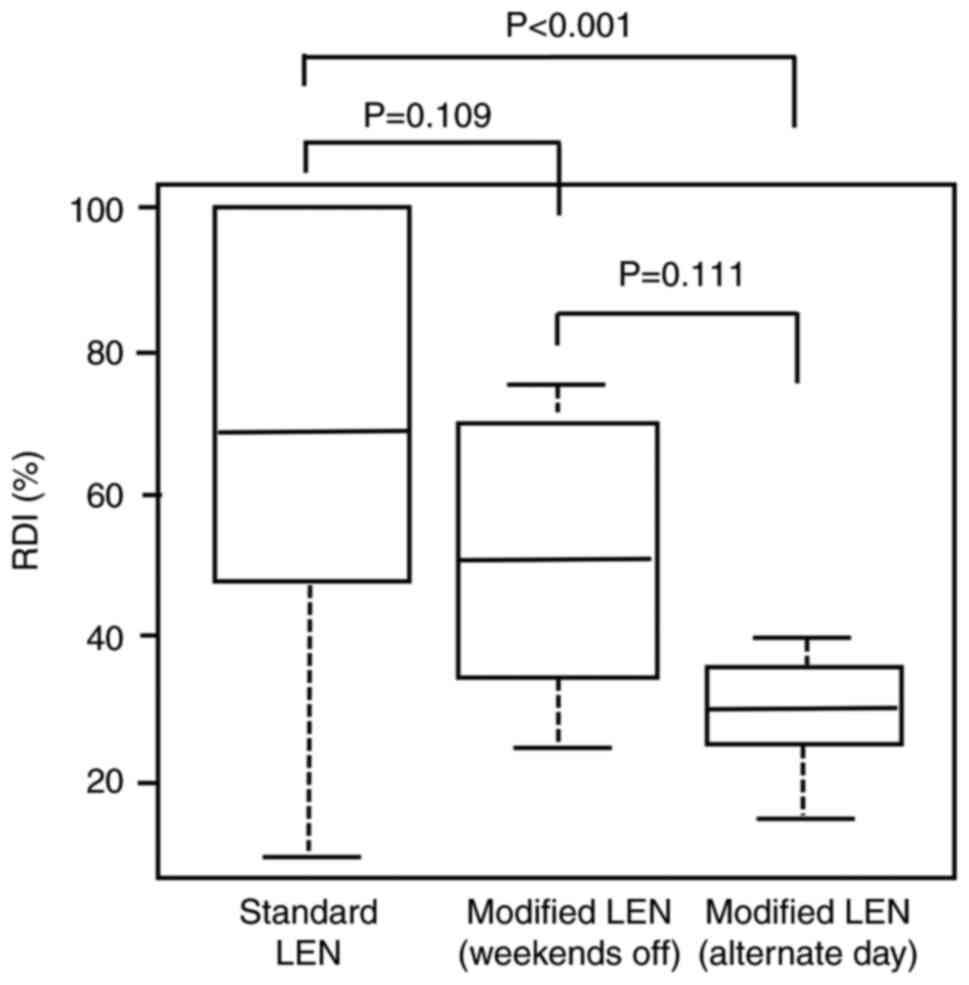
|
1
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogushi K, Chuma M, Uojima H, Hidaka H,
Numata K, Kobayashi S, Hirose S, Hattori N, Fujikawa T, Nakazawa T,
et al: Safety and efficacy of lenvatinib treatment in child-pugh A
and B patients with unresectable hepatocellular carcinoma in
clinical practice: A multicenter analysis. Clin Exp Gastroenterol.
13:385–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwamoto H, Suzuki H, Shimose S, Niizeki T,
Nakano M, Shirono T, Okamura S, Noda Y, Kamachi N, Nakamura T, et
al: Weekends-Off lenvatinib for unresectable hepatocellular
carcinoma improves therapeutic response and tolerability toward
adverse events. Cancers (Basel). 12:10102020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
US Department Of Health and Human
Services, . Common terminology criteria for adverse events (CTCAE)
version 5.0. United States: National Cancer Institute; 2017,
https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
|
|
7
|
Tamai T, Hayato S, Hojo S, Suzuki T,
Okusaka T, Ikeda K and Kumada H: Dose finding of lenvatinib in
subjects with advanced hepatocellular carcinoma based on population
pharmacokinetic and exposure-response analyses. J Clin Pharmacol.
57:1138–1147. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuragawa M, Oshita K, Yamashita Y,
Ishizuka N, Matsuki Y, Okubo R, Shibanami A and Hiura K: Usefulness
of outpatient pharmacist interventions for Lenvatinib-treated
patients. Iryoyakugaku. 47:623–630. 2021.
|
|
10
|
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki R, Fukushima M, Haraguchi M, Miuma
S, Miyaaki H, Hidaka M, Eguchi S, Matsuo S, Tajima K, Matsuzaki T,
et al: Response to lenvatinib is associated with optimal
relativedose intensity in hepatocellular carcinoma: Experience in
clinical settings. Cancers (Basel). 11:17692019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirino S, Tsuchiya K, Kurosaki M, Kaneko
S, Inada K, Yamashita K, Osawa L, Hayakawa Y, Sekiguchi S, Okada M,
et al: Relative dose intensity over the first four weeks of
lenvatinib therapy is a factor of favorable response and overall
survival in patients with unresectable hepatocellular carcinoma.
PLoS One. 15:e02318282020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono A, Aikata H, Yamauchi M, Kodama K,
Ohishi W, Kishi T, Ohya K, Teraoka Y, Osawa M, Fujino H, et al:
Circulating cytokines and angiogenic factors based signature
associated with the relative dose intensity during treatment in
patients with advanced hepatocellular carcinoma receiving
lenvatinib. Ther Adv Med Oncol. 12:17588359209220512020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Havrilesky LJ, Reiner M, Morrow PK, Watson
H and Crawford J: A review of relative dose intensity and survival
in patients with metastatic solid tumors. Crit Rev Oncol Hematol.
93:203–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsuka T, Eguchi Y, Kawazoe S, Yanagita K,
Ario K, Kitahara K, Kawasoe H, Kato H and Mizuta T; Saga Liver
Cancer Study Group, : Skin toxicities and survival in advanced
hepatocellular carcinoma patients treated with sorafenib. Hepatol
Res. 42:879–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho JY, Paik YH, Lim HY, Kim YG, Lim HK,
Min YW, Gwak GY, Choi MS, Lee JH, Koh KC, et al: Clinical
parameters predictive of outcomes in sorafenib-treated patients
with advanced hepatocellular carcinoma. Liver Int. 33:950–957.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Costanzo GG, de Stefano G, Tortora R,
Farella N, Addario L, Lampasi F, Lanza AG, Cordone G, Imparato M
and Caporaso N: Sorafenib off-target effects predict outcomes in
patients treated for hepatocellular carcinoma. Future Oncol.
11:943–951. 2015. View Article : Google Scholar : PubMed/NCBI
|