Introduction
Lung cancer is the leading cause of cancer-related
death and the second most commonly diagnosed cancer worldwide with
an estimated 1.8 million deaths and 2.2 million new cases in 2020
(1). For years, standard treatments
for patients with advanced metastatic non-small-cell lung cancer
(NSCLC) included cytotoxic chemotherapy including platinum
(cisplatin or carboplatin) based regimens with third generation
cytotoxins (paclitaxel, gemcitabine, docetaxel, and vinorelbine)
resulting in a median survival of approximately one year after
chemotherapy (2). Until 2008, all
platinum doublets used to treat NSCLC were considered equal, since
none of the four chemotherapy regimens (cisplatin/paclitaxel,
cisplatin/gemcitabine, cisplatin/docetaxel, and
carboplatin/paclitaxel) offered a significant advantage over the
others (3). Nevertheless, in 2008,
it was shown for the first time that overall survival (OS) was
significantly superior for cisplatin/pemetrexed vs.
cisplatin/gemcitabine in patients with adenocarcinoma histology,
while patients with squamous cell histology had a significant
improvement in OS with cisplatin/gemcitabine vs.
cisplatin/pemetrexed (4). Since
then, it is widely accepted that the cytotoxic efficacy of
different chemotherapeutics depend on NSCLC histology and treatment
should therefore be tailored according to NSCLC subtype.
Recently, immunotherapy with immune checkpoint
inhibitors (ICI) has demonstrated durable responses and
significantly improved OS in patients with NSCLC. The programmed
death receptor 1 (PD-1) and its ligand (PD-L1) are the first
introduced checkpoints being targeted in NSCLC, and according to
several clinical trials and guidelines, antibodies against PD-1 and
PD-L1 have remarkable efficacy both in the first line and second
line treatment of metastatic NSCLC, especially in patients with a
higher tumour proportion score of PD-L1 (2,5–8).
However, despite demonstrated successes, not all patients respond
to immunotherapy interventions or progress while on ICI
monotherapy. Therefore, most of the recent research is focused on
improvement of the activity of immunotherapies with novel
combinations (including cytotoxic agents) together with biomarker
optimization. To date, there are few phase III clinical studies
that have studied ICI combinations with cytotoxic chemotherapy in
NSCLC. For example, in lung adenocarcinoma patients, atezolizumab
combination with carboplatin/nab-paclitaxel showed OS of 18.6
months (9), whereas pembrolizumab
combination with platinum doublet containing pemetrexed extended OS
to 22.0 months (10), showing that
even in one histologic subtype, the efficacy of chemotherapeutics
and/or ICIs may vary when combined.
It is widely thought that cytotoxic treatment can
potentiate immune response while used in combination with
immunotherapy (11,12). It has been shown that conventional
chemotherapy, apart from the cytotoxic effects, may enhance the
activity of immunotherapy by increasing infiltration of CD8 +T
cells, maturation of antigen-presenting cells (APCs), and
downregulation of regulatory T cells (Tregs) (13). Moreover, recent studies have
described the association of non-immune pathways to the outcome of
checkpoint blockade (14).
In a previous study we demonstrated a significant
positive correlation between the PD-1/PD-L1 axis and tumour cell
enzyme DNA-dependent protein kinase (DNA-PK), which is part of a
key pathway involved in the repair of cytotoxic cancer
therapy-induced damage (15).
Therefore, this study aims to explore whether ICIs can affect the
cytotoxicity of chemotherapeutics in clinically relevant lung
adenocarcinoma cell lines with different PD-L1 expression levels
(high: HCC-44, low: A-549) thus specifically addressing the
tumour-cell centred effects of combined therapies.
Materials and methods
Chemicals and equipment
Human non-small cell lung carcinoma (adenocarcinoma)
cell line HCC-44 and human lung carcinoma (adenocarcinoma) cell
line A-549 were from the Leibniz Institute DSMZ (German Collection
of Microorganisms and Cell Cultures GmbH). The following
therapeutic antibodies were used: durvalumab (Imfinzi 50 mg/ml by
Astra Zeneca), atezolizumab (Tecentriq 60 mg/ml by Roche),
pembrolizumab (Keytruda 25 mg/ml by Merck Sharp & Dohme),
nivolumab (Opdiva 10 mg/ml by Bristol-Myers Squibb Pharma). The
following cytotoxic agent stock solutions were used: cisplatin 1
mg/ml, etoposide 20 mg/ml and gemcitabine 100 mg/ml by Accord
(Utrecht, Netherlands), docetaxel 1 mg/ml by Accord (Barcelona,
Spain) and paclitaxel 6 mg/ml by Fresenius Kabi (Warsaw, Poland).
Vinorelbine (tartrate salt) and pemetrexed were from Selleckchem
(Munich, Germany); the cell culture grade DMSO was from AppliChem
(Darmstadt, Germany).
The solutions and growth medium components for the
cell culture were obtained from the following sources:
phosphate-buffered saline (PBS), foetal bovine serum (FBS),
L-glutamine, Dulbecco's Modified Eagle's medium (DMEM), and Roswell
Park Memorial Institute medium (RPMI-1640)-Sigma-Aldrich
(Steinheim, Germany); a mixture of penicillin, streptomycin, and
amphotericin B-Capricorn (Ebsdorfergrund, Germany). Resazurin and
PBS for the viability assay (supplemented with Ca2+,
Mg2+) were from Sigma-Aldrich (St Louis, MO, USA).
The cells were grown at 37°C in 5% CO2
humidified incubator (Sanyo; Osaka, Japan). For the viability
assay, the initial number of cells was counted using TC-10 cell
counter (Bio-Rad; Hercules, CA, USA), and the cells were seeded
onto transparent 96-well clear flat bottom cell culture plates
BioLite 130188 (Thermo Fischer Scientific; Rochester, NY, USA).
Fluorescence intensity and absorbance measurements were carried out
with Synergy NEO or Cytation 5 multi-mode readers (both from
Biotek; Winooski, VT, USA).
The immunohistochemistry (IHC) with PFA-fixed cell
pellets and the following light microscopy were carried out
according to the previously published protocols (15); 22C3 pharmDx primary antibody was
used for staining. The bright-field imaging of IHC samples was
carried out using light microscopy by the Pathology Department of
Tartu University Hospital according to the protocols used for the
analysis of clinical samples (accreditation certificate No M017 by
the Estonian Accreditation Centre). Fluorescence microscopy with
γH2AX-immunostained cells was carried out with Cytation 5
multi-mode reader using 20× air objective. For DAPI, 365 nm LED and
DAPI filter block were used; for Alexa Fluor® 568, 523
nm LED and RFP filter block were used.
Treatment of cells prior to IHC
HCC-44 or A549 cells (passage number below 30) were
seeded in growth medium (RPMI-1640 or DMEM supplemented with 10%
FBS) onto Petri dishes (1/4 dilution from the confluent Petri) and
grown overnight as in culture. Next, treatment with the following
compounds or mixtures in usual growth medium was started: 0.49
mg/ml durvalumab, 1 µM cisplatin, 5 nM docetaxel, mixture of
cisplatin (1 µM) and durvalumab (0.49 mg/ml), or mixture of
docetaxel (5 nM) and durvalumab (0.49 mg/ml). After 48 h, the
treatment mixtures were removed; the cells were rinsed with PBS,
detached from the plates using 0.25% trypsin, and then resuspended
in the culture medium. After pelleting by centrifugation (5 min at
800 rcf), the cells were treated by the fixation solution and the
samples were then treated adhering to the requirements of standard
EVS-EN ISO 15189:2012. Two independent IHC experiments were carried
out.
Treatment of cells prior to the
viability assay
The dose-response curves for chemotherapeutic agents
in the presence or absence of therapeutic antibodies were performed
in the 96-well format. HCC-44 or A549 cells (passage number below
30) were seeded in growth medium (RPMI-1640 or DMEM supplemented
with 10% FBS) onto plate at a density of 2,000 cells or 3,500 cells
per well, respectively [within the linear range of the method
according to the preliminary experiments (16)]. After incubation for 24 h, the
growth medium was exchanged, and dilution series of compounds in
PBS or PBS supplemented with therapeutic antibodies were added onto
the cells (1/10 of the growth medium volume). The following final
total concentrations were used: cisplatin and gemcitabine-6-fold
dilution starting from 3.3 µM; docetaxel and paclitaxel-6-fold
dilution starting from 5 µM; etoposide-6-fold dilution starting
from 34 µM; vinorelbine-6-fold dilution starting from 1 µM;
pemetrexed-6-fold dilution starting from 40 µM. The final total
concentrations of antibodies were chosen according to the
steady-state concentration in serum reported in the literature:
atezolizumab-0.63 mg/ml (17);
durvalumab-0.49 mg/ml (18);
nivolumab-0.13 mg/ml (19);
pembrolizumab-0.09 mg/ml (20).
Pure PBS was added to the negative control (100% viability). The
final volume per well was 200 µl, and the concentration of DMSO in
the treated wells was equal to or below 0.1% by volume; on each
plate, each concentration of each compound was represented in
duplicate. The cells were incubated with compounds in the presence
or absence of therapeutic antibodies for 48 h, and the viability
assay was then carried out.
Viability assay
The growth media was removed from the cells, the
cells were rinsed with PBS, and 50 µM resazurin solution in PBS
(containing Ca2+ and Mg2+) was applied onto
the cells. The plates were placed into a multi-mode reader, and
measurements were performed at 30°C in kinetic mode (reading taken
every 15 min for 2 h) using the following parameters: (A)
fluorescence: excitation 540 nm, emission 590 nm, monochromator,
top optics, gain 50; (B) absorbance at 570 and 600 nm,
monochromator; read height 8.5 mm.
γH2AX immunostaining and
microscopy
For immunofluorescence (IF) studies, the cells were
grown on 96-well Ibidi black µ-plates (ibidi GmbH, Gräfelfing,
Germany); the treatment of cells with dilution series of
gemcitabine, pemetrexed, cisplatin, docetaxel, paclitaxel, or
vinorelbine in the presence or absence of durvalumab was carried
out as described above. Following 48 h treatment, the cells were
fixed and stained with the rabbit monoclonal IgG against
phosphorylated Ser139 of histone H2AX (anti-γH2AX; Sigma-Aldrich,
St Louis, MO, USA) and subsequently goat cross-adsorbed antibody
against rabbit IgG (H+L) conjugated with Alexa Fluor®
568 (Invitrogen; Eugene, OR, USA) as reported previously (21). For the staining of nuclei,
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Eugene, OR, USA)
was used. The imaging was performed in the automated mode; 25
images per well were taken and the DAPI channel was used for
autofocusing. The automated image analysis using the Ilastik model
and the modified version of MembraneTools module of Aparecium 2.0
software (22) was carried out as
reported previously (21).
Statistical analysis
For general data analysis, GraphPad Prism 6 (San
Diego, CA, USA) and Excel 2016 (Microsoft Office 365; Redmond, WA,
USA) were used. In each independent experiment, the fluorescence
intensity measured for the replicate treatments was pooled and the
data obtained for the negative control was plotted against
incubation time with resazurin. One time-point within the duration
of data acquisition was chosen where the signal of the negative
control stayed in the linear range, and only data measured at this
time-point was used for the further analysis.
For normalization of dose-response studies of
compounds in the absence of therapeutic antibodies, data obtained
for wells treated with PBS (in the absence of compounds; negative
control) were considered as 100% viability; in case of
dose-response studies of compounds in the presence of antibodies,
data obtained for wells treated with PBS supplemented with the
corresponding antibodies were considered as 100% viability. For
normalization of the effect of the fixed concentrations of
antibodies in the absence of chemotherapeutics, data obtained for
wells treated with PBS (negative control) was considered as 100%
viability. In all cases, data acquired for the 50 µM resazurin
solution (in the absence of cells) were considered as 0%
viability.
Next, the ratio of absorbance at 570 and 600 nm was
calculated for each well. The ratios were analysed analogously to
the fluorescence intensity data, and the normalized viability
values calculated from the fluorescence intensity and the
absorbance measurements were pooled. Finally, data from all
independent experiments were pooled (n≥3).
The pooled normalized viability obtained for serial
dilutions was plotted against the concentration of compound in the
dilution series and fitted to the logarithmic dose-response
function or biphasic function. The statistical significance of
difference of calculated negative logarithms of IC50
values (pIC50) for compounds in the presence vs. absence
of therapeutic antibodies was assessed using the one-way ANOVA (95%
confidence level) and Dunnett test for multiple comparisons.
For analysis of the IF data, the total intensity of
γH2AX signal in nucleus was plotted against the concentration of a
chemotherapeutic agent by pooling data for all nuclei identified
from the identically treated cells in all the independent
experiments (n=3). The normality of data distribution in each
condition was tested using the D'Agostino-Pearson test and
non-Gaussian distribution was confirmed for most of the tested
conditions. For further comparison, the lowest concentration of
each chemotherapeutic agent was chosen causing significant
elevation of the γH2AX signal relative to the negative control
(PBS-treated cells). For the chosen concentration of each
chemotherapeutic agent, the total intensity of γH2AX signal in
nucleus was compared for treatments with and without durvalumab.
The statistical significance of difference of calculated negative
logarithms of IC50 values (pIC50) for
compounds in the presence vs. absence of ICIs was assessed using
the unpaired two-tailed Mann-Whitney test (95% confidence
level).
Results
Optimization of the viability
assay
Throughout the study, two lung adenocarcinoma cell
lines were used, PD-L1 highly positive HCC-44 and weakly positive
A-549, to demonstrate the dependence of the observed effects on
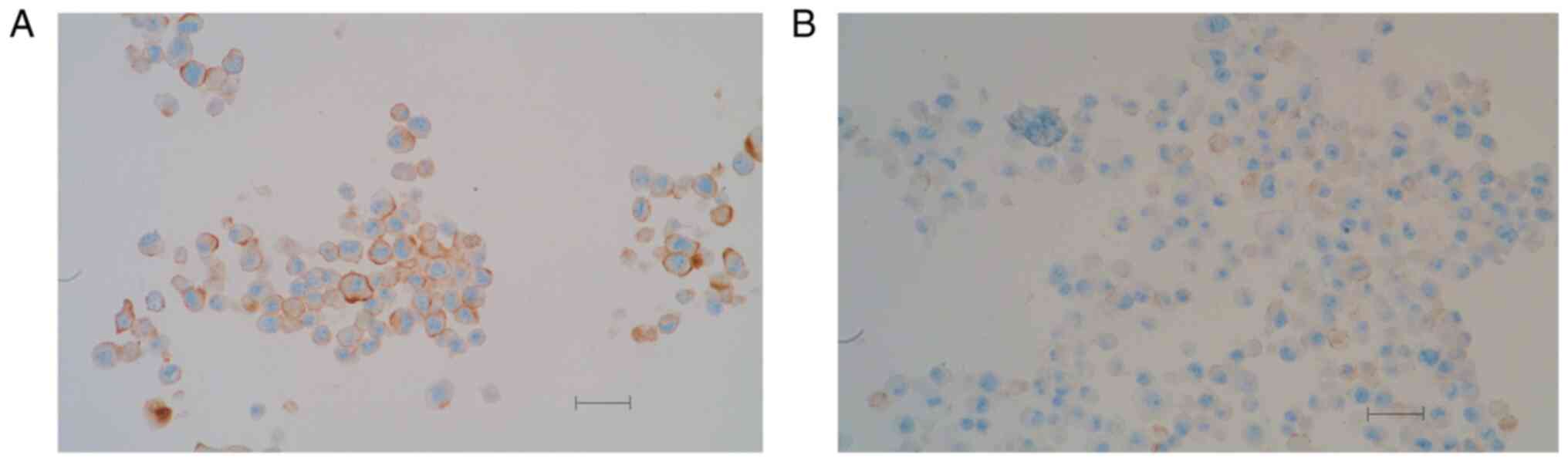
PD-L1 status (Fig. 1).
For assessment of cell viability, resazurin-based
assay was applied. The assay detects changes in fluorescence
intensity and the visible light absorption spectrum that are
associated with the reduction of resazurin in metabolically active
cells. Thus, the higher the fluorescence intensity (excitation 540
nm, emission 590 nm) or 570/600 nm absorption ratio, the more
metabolically active cells the given sample contains, and the
higher the cellular viability (16). According to our previous study, the
resazurin-based assay is superior to the commonly used tetrazolium
dye (MTT)-based assay from at least two aspects (16). First, as resazurin and resorufin
penetrate cell plasma membrane, measurements with resazurin can be
performed in a kinetic mode, without the need for cell lysis after
certain incubation time. Second, based on the comparison of the
Z'-factors of the either assay, resazurin assay is more sensitive
and robust, which is especially important in case if the measured
potentiation/depotentiation effects are relatively small.
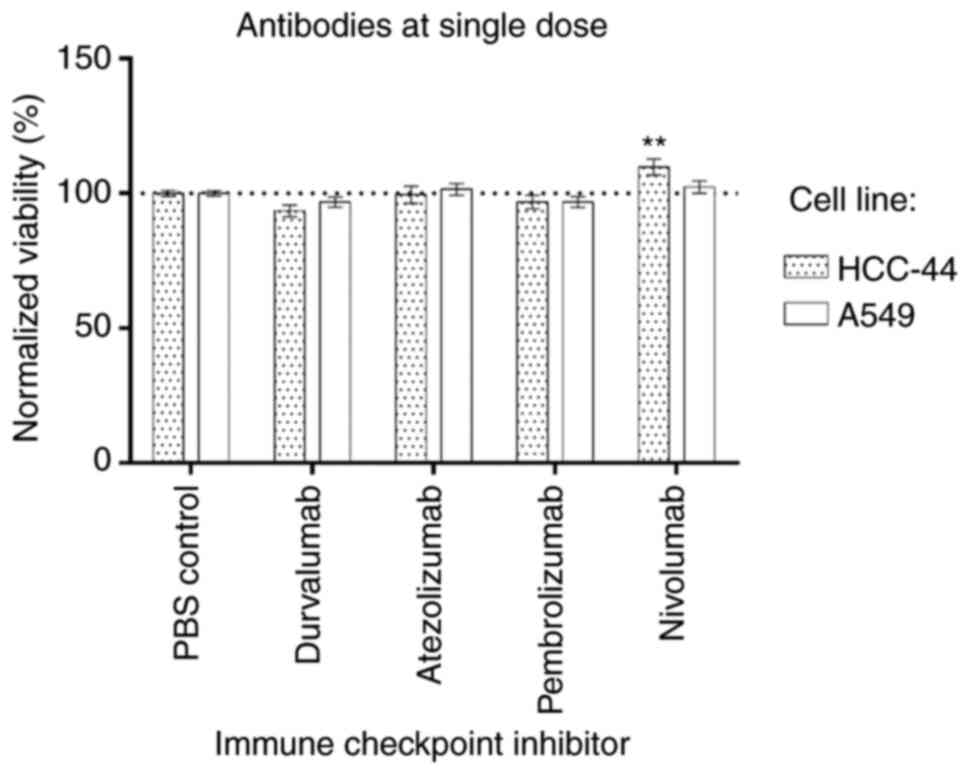
Initially, the effect of immune checkpoint
inhibitors applied to the individually chosen single doses on
viability of the cell lines used in the study was assessed
(Fig. 2). The viability was
normalized to the negative control (treatment with PBS); normalized
mean viability ± SEM is shown (for each condition in each cell
line, n≥7). We found that single antibodies had no effect on cell
viability in the A549 cell line. In the HCC-44 cell line, only
nivolumab had a significant effect (P<0.01), apparently
increasing the viability to 110%.
The results regarding the combined antibody effect
and chemotherapy in two lung cancer cell lines are summarized in
Table I and Fig. 3, as well as in the Figs. S1 and S2.
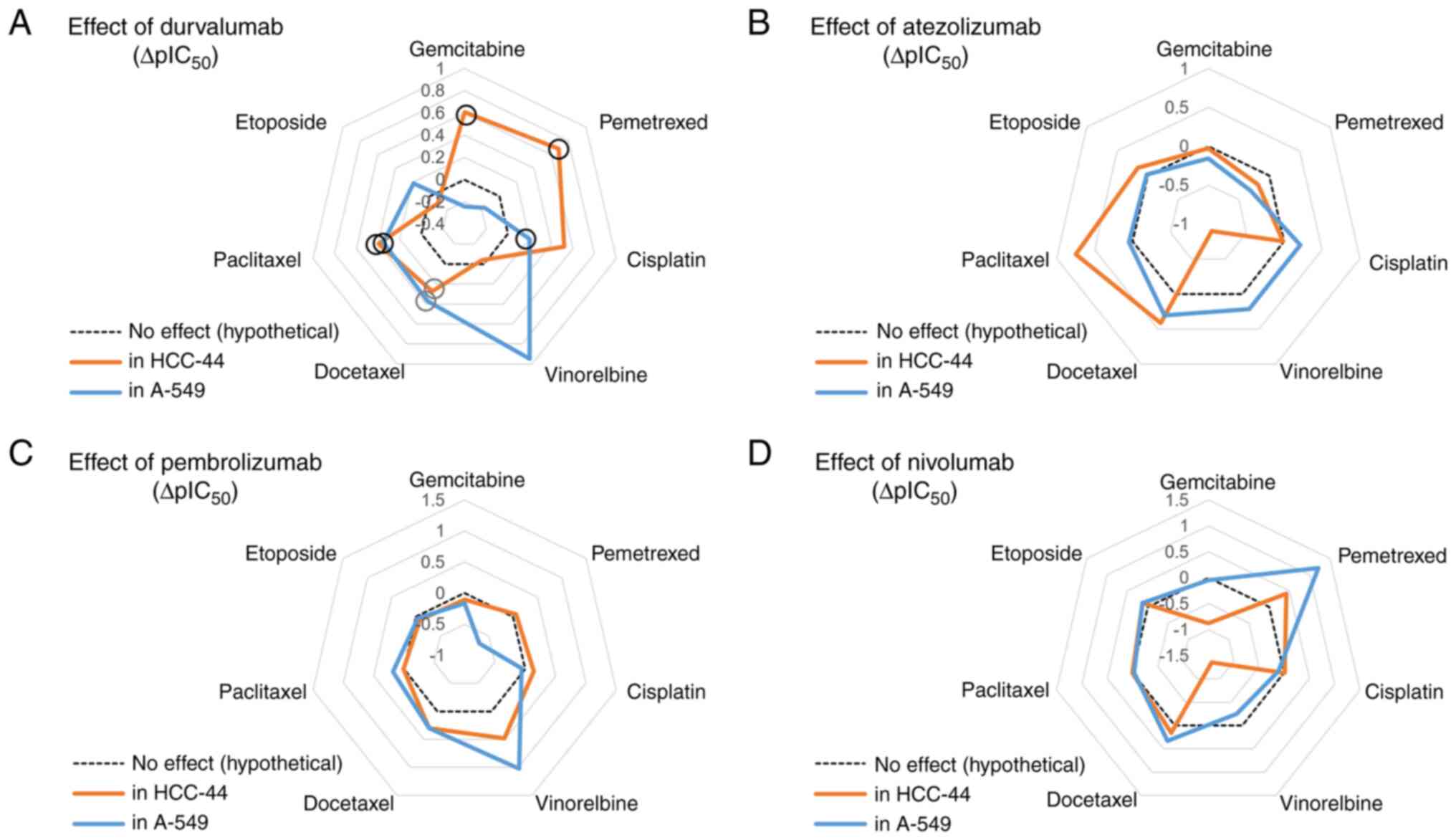
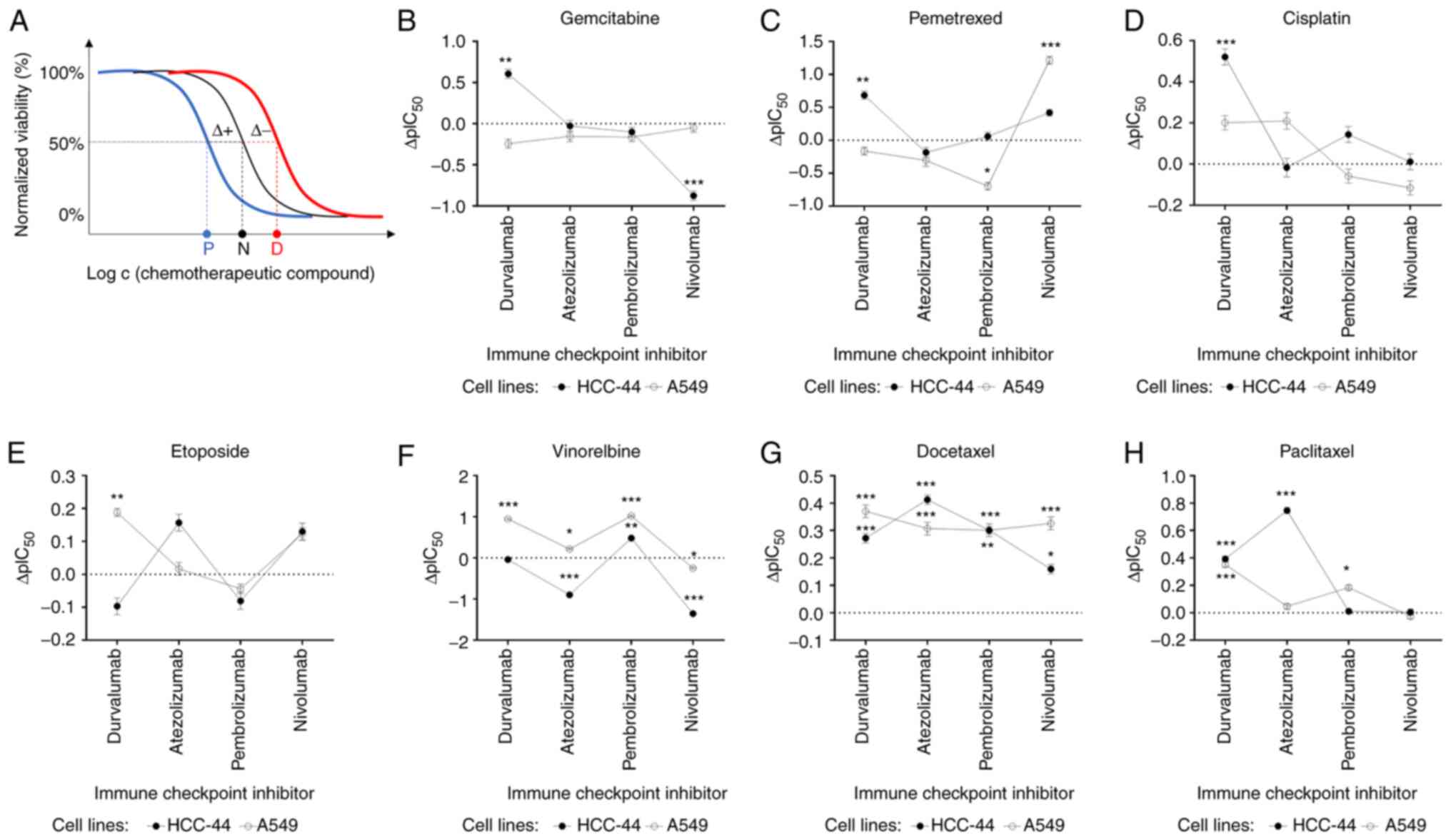 | Figure 3.Assessment of the potentiating or
depotentiating effect of the ICIs on the viability dose-response
curves corresponding to various chemotherapeutic compounds in two
cell lines. (A) Principle of the assay: the shift of the normal
dose-response curve (black) to lower concentrations (blue) or
higher concentrations (red) of a chemotherapeutic compound upon
addition of a fixed concentration of an ICI was assessed. The
letters P, N, D denote the IC50 values established for
the potentiated, normal, or depotentiated effect; Δ+ indicates
shift of IC50 value in the case of potentiation and
Δ-indicates shift of IC50 value in the case of
depotentiation. (B-H) Graphs summarizing the shift of negative
logarithms of IC50 values (ΔpIC50 ± SEM) for
different chemotherapeutic compounds: (B) Gemcitabine, (C)
pemetrexed, (D) cisplatin, (E) etoposide, (F) vinorelbine, (G)
docetaxel, (H) paclitaxel and ICIs. For each condition in each cell
line, n≥3; positive ΔpIC50 indicates potentiation and
negative ΔpIC50 indicates depotentiation. The
statistical significance of difference of calculated
pIC50 values for chemotherapeutic compounds in the
presence vs. absence of a therapeutic antibody was assessed using
the one-way ANOVA (95% confidence level) and Dunnett test for
multiple comparisons (only significant comparisons are shown):
*P<0.05, **P<0.01, ***P<0.001 relative to the
pIC50 value in the absence of ICIs (indicated in each
graph as a dotted line). ICI, immune checkpoint inhibitor. |
 | Table I.Changes in pIC50 values of
chemotherapeutic agents in the presence of therapeutic
antibodies. |
Table I.
Changes in pIC50 values of
chemotherapeutic agents in the presence of therapeutic
antibodies.
| A, Cell line:
HCC-44 (PD-L1 highly positive) |
|---|
|
|---|
|
|
| ΔpIC50
in the presence of the ICIb |
|---|
|
|
|
|
|---|
| Compound | pIC50 ±
SEMa | Durvalumab | Atezolizumab | Pembrolizumab | Nivolumab |
|---|
| Gemcitabine | 8.86±0.09 | 0.61c | ns | ns | −0.87d |
| Pemetrexed | 7.21±0.10 | 0.69c | ns | ns | ns |
| Cisplatin | 5.93±0.06 | 0.52d | ns | ns | ns |
| Vinorelbine | 10.46±0.03 | ns | −0.90d | 0.48d | −1.4d |
| Docetaxel | 9.38±0.03 | 0.27d | 0.41d | 0.30d | 0.16e |
| Paclitaxel | 8.60±0.03 | 0.39d | 0.75d | ns | ns |
| Etoposide | 5.88±0.04 | ns | ns | ns | ns |
|
| B, Cell line:
A549 (PD-L1 weakly positive) |
|
|
|
|
ΔpIC50 in the presence of
the ICIb |
|
|
|
|
|
Compound | pIC50
± SEMa |
Durvalumab |
Atezolizumab |
Pembrolizumab |
Nivolumab |
|
| Gemcitabine | 8.11±0.09 | ns | ns | ns | ns |
| Pemetrexed | 5.10±0.09 | ns | ns | −0.70e | 1.2d |
| Cisplatin | 5.46±0.06 | ns | ns | ns | ns |
| Vinorelbine | 8.47±0.04 | 0.95d | 0.22e | 1.0d | −0.25e |
| Docetaxel | 8.40±0.04 | 0.37d | 0.31d | 0.30c | 0.33d |
| Paclitaxel | 7.95±0.03 | 0.35d | ns | 0.18e | ns |
| Etoposide | 5.02±0.03 | 0.19c | ns | ns | ns |
Effects of chemotherapeutic agents and
mixtures with ICIs on the viability of the HCC-44 cell line
Using chemotherapeutics only, we found that
vinorelbine and docetaxel had the strongest effect on lung
adenocarcinoma cells with high levels of PD-L1 expression
(pIC50 values of 10.46 and 9.38, respectively), followed
by gemcitabine, paclitaxel and pemetrexed (Table I).
In the case of combinations of ICIs with
chemotherapeutics, durvalumab potentiated significantly the
cytotoxic effect of docetaxel and paclitaxel (ΔpIC50
values of 0.27 and 0.39, respectively; P≤0.001), and even more when
added to gemcitabine, pemetrexed (ΔpIC50 values of 0.61
and 0.69, respectively; P≤0.01) or cisplatin (ΔpIC50
value of 0.52; P≤0.001). Only the combination with vinorelbine and
etoposide indicated no changes when the antibody was added to
chemotherapy. For mixtures containing atezolizumab, significant
potentiating effect was seen in the case of docetaxel and
paclitaxel (ΔpIC50 values of 0.41 and 0.75,
respectively; P≤0.001), yet a significant depotentiating effect was
observed for vinorelbine (ΔpIC50 value of −0.90;
P≤0.001). Pembrolizumab potentiated significantly the cytotoxic
effect of vinorelbine and docetaxel (ΔpIC50 values of
0.48 and 0.30, respectively; P≤0.001), yet no significant changes
were detected with other chemotherapy agents. Other combinations
where depotentiating effect was observed after adding the antibody
were represented by nivolumab with vinorelbine or gemcitabine
(ΔpIC50 values of −1.4 and −0.87, respectively; P≤0.001)
(Fig. 3B-H).
Effects of chemotherapeutic agents and
mixtures with ICIs on the viability of the A549 cell line
Overall, we found that the effect of
chemotherapeutics was weaker in the cell line with lower PD-L1
expression, yet most of the observed trends were in line with those
measured in the HCC-44 cell line. For instance, vinorelbine and
docetaxel affected the viability of lung adenocarcinoma cells the
most, with pIC50 values of 8.47 and 8.40 (Table I).
In the case of combinations of ICIs with
chemotherapeutics, similar to the HCC-44 cell line, durvalumab
potentiated significantly the cytotoxic effect of most chemotherapy
agents in A549: e.g. vinorelbine, docetaxel and paclitaxel
(ΔpIC50 values of 0.95, 0.37 and 0.35, respectively;
P≤0.001). The potentiation was also seen in combination with
etoposide but to a smaller extent (ΔpIC50 value of 0.19;
P<0.01) as compared to the effect of the durvalumab plus
pemetrexed mixture in HCC-44. In contrast to HCC-44 studies,
combinations of durvalumab with gemcitabine, pemetrexed and
cisplatin indicated no changes when the antibody was added to
chemotherapy in A549 cells. Atezolizumab significantly potentiated
the cytotoxic effect of docetaxel (ΔpIC50 value of 0.31;
P≤0.001) and vinorelbine (ΔpIC50 value of 0.22; P≤0.05),
while no significant changes were detected with other chemotherapy
agents. Pembrolizumab had a significant potentiating effect when
added to vinorelbine (ΔpIC50 value of 1.0; P≤0.001) and
to a smaller extent in combinations with docetaxel
(ΔpIC50 value of 0.30; P≤0.01) or paclitaxel
(ΔpIC50 value of 0.18; P≤0.05). A depotentiating effect
was seen when pembrolizumab was added to pemetrexed
(ΔpIC50 value of-0.70; P≤0.05). Nivolumab had
significant potentiating effect when added to pemetrexed and
docetaxel (ΔpIC50 values of 1.2 and 0.33, respectively;
P≤0.001), while a depotentiating effect with vinorelbine was
observed (ΔpIC50 value of −0.25; P≤0.05) (Fig. 3B-H).
Investigation of the mechanisms behind
the ICI-mediated potentiation of chemotherapeutic agent
cytotoxicity
In order to clarify the possible molecular
mechanisms in which durvalumab could contribute to an increase in
the cytotoxicity of chemotherapeutic agents, we performed two
additional experiments.
First, to establish whether exposure to
chemotherapeutic agents can trigger changes in PD-L1 expression, we
carried out PD-L1 staining in both cell lines after 48-h treatment
of cells with cisplatin, docetaxel, or the corresponding mixtures
with durvalumab. To ensure the survival of a sufficient number of
cells for analysis, a single concentration of cisplatin (1 µM) or
docetaxel (5 nM) was chosen, corresponding to the IC50
value of the chemotherapeutic agents in the HCC-44 cell line (which
has higher chemosensitivity; Table
I). The IHC results are summarized in the Figs. S3 and S4. Overall, no increase in PD-L1 levels
was observed for any treatment in either cell line, indicating that
the potentiating effects of durvalumab cannot be explained by the
increased concentration of the target in cells.
Second, motivated by the previously reported
positive correlation between the PD-1/PD-L1 axis and tumour cell
DNA-PK expression (15), we
examined whether the potentiating effects of ICIs can occur via
augmentation of the chemotherapy-induced DNA damage. For that, we
carried out immunostaining of a well-recognized DNA damage marker
γH2AX in both the HCC-44 and A549 cell lines after 48-h treatment
with chemotherapy agents in the presence or absence of durvalumab.
The quantification of total signal intensity of γH2AX in individual
nuclei was carried out using an automated image analysis algorithm.
The latter identifies locations of nuclei in images based on the
DNA staining in the DAPI channel and quantifies the γH2AX signal at
the same location in the same field of view according to the
fluorescently labelled secondary antibody channel. Because such an
algorithm is not biased towards the high-intensity nuclei, there is
no bottom limit for a detectable γH2AX signal threshold; hence, the
mean γH2AX signal of the nuclei population can be relatively low.
Therefore, given that the chosen validation assay is not suitable
for assessment of minor trends, for validation we chose six
chemotherapeutic agents for which the ΔpIC50 value
measured in the viability assay was above 0.2 logarithmic units in
at least one cell line (Table
I).
The cell lines were treated with dilution series of
gemcitabine, pemetrexed, cisplatin, docetaxel paclitaxel or
vinorelbine in the presence or absence of durvalumab (0.49 mg/ml).
The spread of data in each treatment condition is shown in the
Figs. S5 and S6. As expected, the profile of the γH2AX
signal changes followed the dose-response curves; in HCC-44, higher
levels of DNA damage were observed than in A549, confirming the
generally higher chemosensitivity of HCC-44. Consistently with the
mechanism of action of the chemotherapy agents, the highest DNA
damage levels were evident for gemcitabine and cisplatin, whereas
the cells treated with the microtubule-binding agents (taxanes and
vinorelbine) featured the lowest γH2AX signal.
For quantification of the durvalumab effect, we
chose the lowest concentration of the chemotherapy agent from the
dose-response curve at which the mean γH2AX signal was
significantly higher than that in the negative control (P<0.05)
and compared for this concentration the average γH2AX signal in the
presence vs. absence of durvalumab. Such an approach is more
reliable than quantification of the γH2AX signal at higher
concentrations of chemotherapy agents (examples of microscopy
images can be seen in the Fig.
S7). Specifically, the increase in DNA damage is associated
with prevalence of cellular death, and the comparison of dead cell
populations in IF methods is problematic due to extensive washing
procedures that result in loss of the objects of interest.
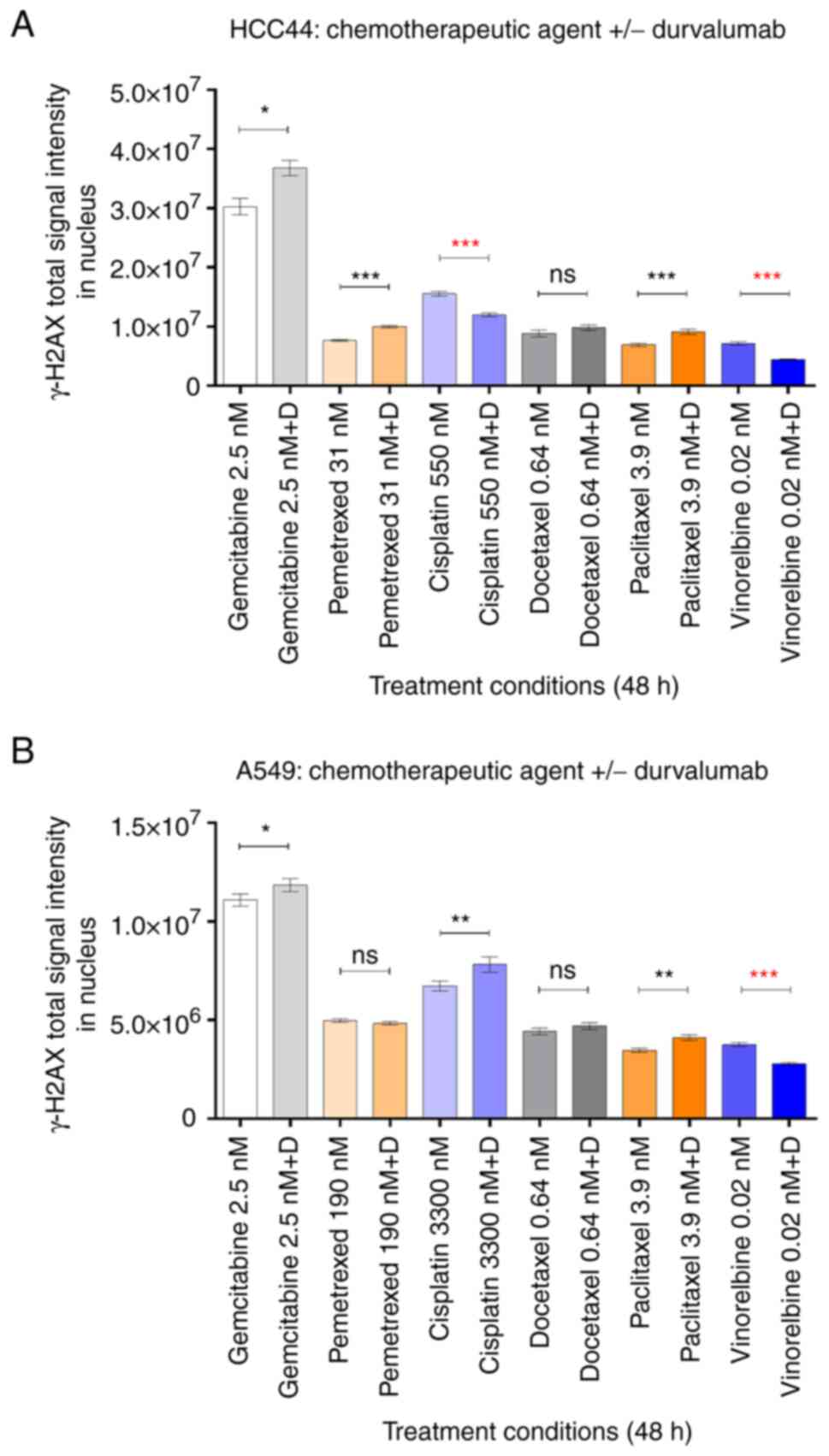
The comparisons are summarized in Fig. 4. Overall, according to the increase
in the γH2AX signal in the presence of durvalumab, we could confirm
that the potentiating effect of the ICI at least partially occurs
via augmentation of the chemotherapy-induced DNA damage in the
following treatments: gemcitabine, pemetrexed and paclitaxel in
HCC-44, and cisplatin and paclitaxel in A549 (in all cases,
P<0.05). In the case of docetaxel treatment of both cell lines,
the γH2AX signal was slightly higher in the presence vs. absence of
durvalumab, yet the statistical significance of the trend could not
be achieved. Interestingly, validation indicated that durvalumab
could also somewhat potentiate the DNA-damaging effect of
gemcitabine in the A549 cell line (P<0.05), although the
corresponding trend was not evident in the viability assay. It is
possible that upon prolonged incubation of cells, the trend would
also have become evident on the level of cell viability.
It should also be noted that while increase in γH2AX
can be unequivocally correlated to increase in DNA damage and thus
interpreted as a mechanism explaining the potentiating effects of
the ICI, the decrease in γH2AX does not indicate depotentiation.
Specifically, the decrease in γH2AX can also occur due to the
inability of cells to detect the DNA damage [e.g. as a result of
inhibition of ATM kinase that catalyses formation of γH2AX loci
(23)]. Therefore, for treatments
such as cisplatin in HCC-44 or vinorelbine in both cell lines where
decrease of γH2AX in the presence of durvalumab was observed, more
detailed examination of the molecular mechanisms behind the ICI
effect is required.
Discussion
Currently, immunotherapy with PD-1 and PD-L1
antibodies that enhance the function of anti-tumour T lymphocytes
and thereby cause immune activation is in clinical use for the
treatment of various tumour types, including lung cancer (2,8,24).
However, despite the demonstrated success, especially in patients
with a higher tumour proportion score of PD-L1, not all patients
respond to immunotherapy interventions or progress while on ICI
monotherapy. Therefore, immunotherapy is increasingly used in
combination with cytotoxic chemotherapy, thus improving overall
survival and prognosis in patients with NSCLC (9,10,12,25).
The combination of ICIs with chemotherapy has been
considered a successful strategy due to enhancement of the
recognition and elimination of tumour cells by the host immune
system and reduction of the immunosuppressive tumour
microenvironment (26). It is also
believed that cytotoxic treatment can potentiate immune response
while used in combination with immunotherapy, and anticancer drugs
can induce immunogenic cell death in sensitive tumour cells
(11,12,27).
Furthermore, chemotherapeutics have different underlying immune
modulating molecular mechanisms such as increased T cell
recognition (cisplatin, carboplatin), decreased Tregs (paclitaxel),
increased T cell infiltrate and antigen presentation (pemetrexed)
or enhanced expression of tumour-antigens (gemcitabine), etc
(26). However, all the mechanisms
underlying synergistic interactions in immunotherapy and
chemotherapy combinations are not completely understood and
described. We have demonstrated earlier a significant positive
correlation between the PD-1/PD-L1 axis and tumour cell enzyme
DNA-PK, which is part of a key pathway involved in the repair of
cytotoxic cancer therapy induced damage (15). Therefore, to confirm our hypothesis
about an alternative role of ICIs in addition to immune activation,
we performed the study to test whether ICIs can affect the
cytotoxicity of chemotherapeutics. For this, we used clinically
relevant lung adenocarcinoma cell lines in the absence of immune
cells and tested modulation of the cytotoxic effects of 4 ICIs.
Our study showed that ICIs can indeed modulate
cytotoxic effects by potentiating or depotentiating chemotherapy
agents. We found that out of all tested ICIs, durvalumab most
markedly potentiated cytotoxic effects of chemotherapy agents in
both cell lines; however, the effect was slightly stronger in the
HCC-44 cell line which has high PD-L1 expression (in 5 out of 7
chemotherapeutics) compared to the cell line A549 with lower levels
of PD-L1 (in 4 out of 7 chemotherapeutics) (Fig. 5).
To the best of our knowledge, there are no similar
studies that have assessed ICI effects on chemotherapy
cytotoxicity. Nevertheless, emerging clinical data indirectly
support our findings. For example, compared to chemotherapy alone,
the addition of atezolizumab to cisplatin and pemetrexed did not
improve OS in lung cancer patients with adenocarcinoma in the phase
3 IMpower132 study (28). In the
context of our data, these findings may also be explained by
improper chemotherapy selection since atezolizumab did not
potentiate the cytotoxic efficacy of either of the
chemotherapeutics used.
Somewhat surprisingly, next to some potentiating
actions of atezolizumab, pembrolizumab and nivolumab, these ICIs
also exerted depotentiating effects in combination with some
chemotherapeutics. Nivolumab had the biggest depotentiating effect,
reducing the cytotoxicity of vinorelbine in both cell lines and the
effect of gemcitabine only in PD-L1 highly positive cells. The
latter was in line with the fact that nivolumab was the only ICI
whose monotreatment increased the proliferation of HCC-44 cells
compared to the non-treated control. Yet again, unfortunately,
there are no studies that can be discussed in comparison to our
data. However, in clinical setting, it is well known that in NSCLC
patients, hyperprogressive disease can occur presenting as a fast,
unexpected, and dramatic increase in tumour burden immediately
after exposure to ICIs (29). This
paradoxical boost in tumour growth following the use of ICIs has
been reported in 4–29% of cancer patients; however, the underlying
molecular mechanisms are not fully understood (30). Therefore, our in vitro model
may serve as an additional tool to study such basic mechanisms.
In clinical practice, it has been shown that
PD-L1-positive NSCLC patients might get a higher benefit from
PD-1/PD-L1 inhibitors compared to patients with no or very low
PD/L1 expression (31). However,
PD-L1 expression level may not be the only or the optimal biomarker
in predicting and evaluating the efficacy of ICIs: in some studies,
patients with negative and weak expression of PD-L1 did also
benefit from immunotherapy or immunotherapy and chemotherapy
(6,32,33).
The cell lines used in this work were chosen to represent either
weak (A549) or strong (HCC-44) PD-L1 positivity (Fig. 1). Interestingly, treatment of cells
with durvalumab, cisplatin and docetaxel, or combinations thereof
somewhat decreased PD-L1 signal in the cell lines (Figs. S3 and S4). This can be explained by two
hypotheses, which need to be validated further. Firstly, at this
concentration, both cisplatin, its mixture with durvalumab, and a
mixture of docetaxel with durvalumab result in a significant drop
in cell viability (Table I). It is
well known that the cytotoxic mode of action of cisplatin is
mediated by its interaction with DNA to form DNA adducts, which
activate several signal transduction pathways, including those
involving ATR, p53, p73, and MAPK, and culminate in the activation
of apoptosis (34). Also, docetaxel
causes apoptosis that follows the cancer cell mitotic arrest
(35). As the apoptosis can result
in alterations of the cell membrane integrity (36), the latter can trigger the apparent
decrease in the PD-L1 membranous signal. Furthermore, in the case
of treatment with durvalumab alone or durvalumab-containing
mixtures, some degree of competition might occur between the
durvalumab and the antibody against PD-L1 used for IHC, which would
manifest as an apparent decrease in the IHC-detected PD-L1 signal.
Still, the difference in PD-L1 status between the cell lines used
was overall conserved after the treatment of cells. Given the fact
that potentiation of certain chemotherapeutic agents by ICIs was
evident in the A549 cell line according to both the viability assay
(Table I) and the IF assay
(Fig. 4), our study points to the
fact that both adenocarcinoma subtypes may potentially benefit from
the ICI and chemotherapy combinations.
In terms of immunomodulatory effects, previous in
vitro studies have shown that PD-L1 antibodies are superior to
the PD-1 antibodies in reverting PD-1/PD-L1 signalling, whereas
pembrolizumab is a slightly more effective PD-1 blocker than
nivolumab (37). Our in
vitro study in clinically relevant lung adenocarcinoma cell
lines showed heterogeneous modulation of the cytotoxic effects of
ICI-chemotherapy combinations that depended on the ICI and
chemotherapeutical drugs used and the PD-L1 status of cells,
pointing toward the need for careful selection of drugs into
combinations. The variations in results for combinations of
different ICIs with chemotherapeutics could be explained by
differences in affecting receptors, epitopes, and binding sites of
ICIs. Nivolumab and pembrolizumab disrupt the PD-1 immune
checkpoint pathway through blockade of PD-1 receptors on T
lymphocytes, while durvalumab and atezolizumab target PD-L1, thus
improving the effector activity of anti-tumour T cells (24). Moreover, PD-1 antibodies are IgG4,
whereas the PD-L1 antibodies harbour unmodified (avelumab) or
modified IgG1 Fc sequences (durvalumab and atezolizumab) (37). Also, although durvalumab and
atezolizumab are both PD-L1 antibodies, those bind to PD-L1
viadifferent binding sites (38).
How much and in which way these differences in ICI binding
mechanisms affect cytotoxic effects in combinations with
chemotherapeutics remains to be discovered.
Recent clinical data by Banchereau et al
revealed that DNA damage repair genes are associated with good ICI
response in PD-L1-positive tumours (14). This is in line with our previous
findings that showed a similar link between PD-1/PD-L1 axis and
tumour cell DNA repair enzyme DNA-PK (15). Within this work, we confirmed that
potentiation of gemcitabine, pemetrexed and taxane cytotoxicity can
be explained by augmentation of the DNA damage in the presence of
the ICI (Fig. 4). However, the
levels of the damage marker γH2AX following treatment with
cisplatin in the HCC-44 cell line or vinorelbine in both cell lines
were significantly reduced in the presence of durvalumab, urging
the need to explore the molecular pathways behind potentiation in
greater detail. For instance, given the fact that the CDK4/6
natural inhibitor CDKN2A has been reported as a significant
transcriptional correlate of ICI response (14), and that cisplatin is known to cause
cell cycle arrest (39), the
targets related to the cell cycle might be of special interest. In
our further studies, we will address the specific mechanisms behind
the observed potentiating or depotentiating effects of ICIs on
chemotherapy cytotoxicity using the large-scale proteomics
approach.
This report has several limitations, including the
in vitro experimental setting and the lack of data on PD-L1
negative adenocarcinoma cells. Nevertheless, we have used
clinically relevant human cell lines that represent 2/3 of lung
adenocarcinoma patients (PD-L1 highly and PD-L1 weakly positive
subgroups). Moreover, we have tested and compared 4 ICIs, based on
patients' steady-state serum concentrations after clinically
approved antibody dosages, making our data more valuable. The use
of preclinical animal models for evaluation of the potentiating or
depotentiating effects of ICI and chemotherapy combinations remains
an open challenge. Given our intention to examine specifically the
effects of ICIs on the level of tumour cells and not the immune
system, relevant animal models are currently not available-since
even the most appropriate human immune system-modelling mice still
show infiltrating T-cells and inflammatory signature that would not
allow to distinguish between the immune-related vs. direct effects
of therapy on tumour cells (40).
Despite the aforementioned limitations, we have
shown for the first time that ICIs can directly modulate cytotoxic
effects by either potentiating or depotentiating chemotherapy
agents used in lung adenocarcinoma cell lines. Durvalumab was the
most promising ICI, potentiating most chemotherapy agents in both
cell lines, especially in the presence of high PD-L1 expression.
Nivolumab, on the other hand, showed depotentiating trends in
several combinations, pointing toward the need for careful
selection of chemotherapeutics into possible combinations with
ICIs. Out of 7 tested chemotherapeutics, only docetaxel showed
increased cytotoxicity in combination with all ICIs irrespective of
the strength of PD-L1 positivity in lung adenocarcinoma cells.
Whether it holds true in clinical setting has to be validated in
prospective clinical trials.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by internal financing from the Institute
of Clinical Medicine, University of Tartu, Estonia (2019–2022; PI:
Dr Jana Jaal).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the FigShare repository, https://doi.org/10.6084/m9.figshare.17099042. All
other data are available from the corresponding author on
reasonable request.
Authors' contributions
MS, DL and JJ conceptualized and designed the study,
analysed and interpreted the data, and drafted and revised the
manuscript. HL carried out work in cell culture, DL performed
viability measurements and HT performed IHC studies. MS, DL and JJ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MS, DL, HL, and HT declare no competing interests.
JJ is an advisory board member for AstraZeneca, Amgen, Johnson
&
Johnson and MSD and has received research funding
from AstraZeneca.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Planchard D, Popat S, Kerr K, Novello S,
Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD,
et al: Metastatic non-small cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29 (Suppl 4):iv192–iv237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brahmer JR, Rodríguez-Abreu D, Robinson
AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A,
Cuffe S, et al: Health-related quality-of-life results for
pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC
(KEYNOTE-024): A multicentre, international, randomised, open-label
phase 3 trial. Lancet Oncol. 18:1600–1609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Incorvaia L, Fanale D, Badalamenti G,
Barraco N, Bono M, Corsini LR, Galvano A, Gristina V, Listì A,
Vieni S, et al: Programmed death ligand 1 (PD-L1) as a predictive
biomarker for pembrolizumab therapy in patients with advanced
non-small-cell lung cancer (NSCLC). Adv Ther. 36:2600–2617. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated analysis from KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hadash-Bengad R, Hajaj E, Klein S, Merims
S, Frank S, Eisenberg G, Yakobson A, Orevi M, Caplan N, Peretz T,
et al: Immunotherapy potentiates the effect of chemotherapy in
metastatic melanoma-a retrospective study. Front Oncol. 10:702020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Judd J and Borghaei H: Combining
immunotherapy and chemotherapy for non-small cell lung cancer.
Thorac Surg Clin. 30:199–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tseng CW, Hung CF, Alvarez RD, Trimble C,
Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, et al:
Pretreatment with cisplatin enhances E7-specific CD8+
T-cell-mediated antitumor immunity induced by DNA vaccination. Clin
Cancer Res. 14:3185–3192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banchereau R, Leng N, Zill O, Sokol E, Liu
G, Pavlick D, Maund S, Liu LF, Kadel E III, Baldwin N, et al:
Molecular determinants of response to PD-L1 blockade across tumor
types. Nat Commun. 12:39692021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saar M, Narits J, Mägi L, Aaspõllu H,
Vapper A, Kase M, Minajeva A, Vooder T, Tamm H, Buldakov M, et al:
Expression of immune checkpoint PD-1 in non-small cell lung cancer
is associated with tumor cell DNA-dependent protein kinase. Mol
Clin Oncol. 15:2112021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lavogina D, Lust H, Tahk MJ, Laasfeld T,
Vellama H, Nasirova N, Vardja M, Eskla KL, Salumets A, Rinken A and
Jaal J: Revisiting the resazurin-based sensing of cellular
viability: Widening the application horizon. Biosensors (Basel).
12:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
European Medicines Agency: Tecentriq.
https://www.ema.europa.eu/en/medicines/human/EPAR/tecentriqNovember
11–2021
|
|
18
|
European Medicines Agency: Imfinzi.
https://www.ema.europa.eu/en/medicines/human/EPAR/imfinziNovember
11–2021
|
|
19
|
European Medicines Agency: Opdivo.
https://www.ema.europa.eu/en/medicines/human/EPAR/opdivoNovember
11–2021
|
|
20
|
European Medicines Agency: Keytruda.
https://www.ema.europa.eu/en/medicines/human/EPAR/keytrudaNovember
11–2021
|
|
21
|
Lavogina D, Laasfeld T, Vardja M, Lust H
and Jaal J: Viability fingerprint of glioblastoma cell lines: Roles
of mitotic, proliferative, and epigenetic targets. Sci Rep.
11:203382021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aparecium. GPCR Workgroup UT; https://gpcr.ut.ee/aparecium.htmlDecember
19–2022
|
|
23
|
Noubissi FK, McBride AA, Leppert HG,
Millet LJ, Wang X and Davern SM: Detection and quantification of
γ-H2AX using a dissociation enhanced lanthanide fluorescence
immunoassay. Sci Rep. 11:89452021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hargadon KM, Johnson CE and Williams CJ:
Immune checkpoint blockade therapy for cancer: An overview of
FDA-approved immune checkpoint inhibitors. Int Immunopharmacol.
62:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leonetti A, Wever B, Mazzaschi G, Assaraf
YG, Rolfo C, Quaini F, Tiseo M and Giovannetti E: Molecular basis
and rationale for combining immune checkpoint inhibitors with
chemotherapy in non-small cell lung cancer. Drug Resist Updat.
46:1006442019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fucikova J, Kralikova P, Fialova A,
Brtnicky T, Rob L, Bartunkova J and Spísek R: Human tumor cells
killed by anthracyclines induce a tumor-specific immune response.
Cancer Res. 71:4821–4833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishio M, Barlesi F, West H, Ball S,
Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S,
Orlandi F, et al: Atezolizumab plus chemotherapy for first-line
treatment of nonsquamous NSCLC: Results from the randomized phase 3
IMpower132 trial. J Thorac Oncol. 16:653–664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abbar B, De Castelbajac V, Gougis P,
Assoun S, Pluvy J, Tesmoingt C, Théou-Anton N, Cazes A, Namour C,
Khalil A, et al: Definitions, outcomes, and management of
hyperprogression in patients with non-small-cell lung cancer
treated with immune checkpoint inhibitors. Lung Cancer.
152:109–118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Denis M, Duruisseaux M, Brevet M and
Dumontet C: How can immune checkpoint inhibitors cause
hyperprogression in solid tumors? Front Immunol. 11:4922020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J and Gu J: PD-L1 expression and EGFR
status in advanced non-small-cell lung cancer patients receiving
PD-1/PD-L1 inhibitors: A meta-analysis. Future Oncol. 15(14):
1667–1678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S, Bai X and Shan F: The progress
and confusion of anti-PD1/PD-L1 immunotherapy for patients with
advanced non-small cell lung cancer. Int Immunopharmacol.
80:1062472020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodríguez-Abreu D, Powell SF, Hochmair MJ,
Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M,
Cheng SY, et al: Pemetrexed plus platinum with or without
pembrolizumab in patients with previously untreated metastatic
nonsquamous NSCLC: Protocol-specified final analysis from
KEYNOTE-189. Ann Oncol. 32:881–895. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Henriques AC, Ribeiro D, Pedrosa J,
Sarmento B, Silva PMA and Bousbaa H: Mitosis inhibitors in
anticancer therapy: When blocking the exit becomes a solution.
Cancer Lett. 440–441. 64–81. 2019.PubMed/NCBI
|
|
36
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Sousa Linhares A, Battin C, Jutz S,
Leitner J, Hafner C, Tobias J, Wiedermann U, Kundi M, Zlabinger GJ,
Grabmeier-Pfistershammer K and Steinberger P: Therapeutic PD-L1
antibodies are more effective than PD-1 antibodies in blocking
PD-1/PD-L1 signaling. Sci Rep. 9:114722019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee HT, Lee JY, Lim H, Lee SH, Moon YJ,
Pyo HJ, Ryu SE, Shin W and Heo YS: Molecular mechanism of
PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and
durvalumab. Sci Rep. 7:55322017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mohiuddin M and Kasahara K: Cisplatin
activates the growth inhibitory signaling pathways by enhancing the
production of reactive oxygen species in non-small cell lung cancer
carrying an EGFR exon 19 deletion. Cancer Genomics Proteomics. 18
(3 Suppl):S471–S486. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marín-Jiménez JA, Capasso A, Lewis MS,
Bagby SM, Hartman SJ, Shulman J, Navarro NM, Yu H, Rivard CJ, Wang
X, et al: Testing cancer immunotherapy in a human immune system
mouse model: Correlating treatment responses to human chimerism,
therapeutic variables and immune cell phenotypes. Front Immunol.
12:6072822021. View Article : Google Scholar : PubMed/NCBI
|