Introduction
Cystic brain metastases (BMs) are rare in lung
carcinoma cases. Adenocarcinoma is the leading pathological type
that results in cystic BMs, followed by squamous cell carcinoma
(1). However, pulmonary sarcomatoid
carcinoma or its rare subtype of spindle cell carcinoma has not
been previously reported with cystic BMs. Sarcomatoid carcinomas
occupied approximately 0.2-0.3% of all lung carcinomas, while
spindle cell carcinomas (SpCCs) are rarer, accounting for 13.3% of
pulmonary sarcomatoid carcinomas (2,3).
According to the World Health Organization, pulmonary SpCCs are
resected samples consisting solely of spindle cells, whereas biopsy
samples with similar pathological findings are referred to as
non-small cell carcinoma with SpCC (2). However, biopsy-proven SpCCs,
consisting solely of spindle cells, have been widely discussed in
the literature since 70% of patients with lung cancer present at
advanced and unresectable stages (2). Clinical studies of biopsy-proven rare
malignancies are particularly important for providing clinical
information to guide treatment of patients with advanced disease
stages.
Cystic BMs are characterized by larger tumor sizes
with increased peritumoral edema compared with solid BMs, resulting
in poor prognosis (4,5). Therefore, specific treatment
strategies are clinically examined including cyst aspiration and
stereotactic radiotherapy for cystic BMs (6–8). Also,
the crucial role of immunotherapy targeting programmed cell death-1
(PD-1)/programmed cell death-ligand 1 (PD-L1) is recently
highlighted in treating pulmonary sarcomatoid carcinomas based on
high PD-L1 expression in these tumors (9).
Herein, we report a rare case of biopsy-proven
pulmonary SpCC with solitary cystic BM. BM was initially managed
with stereotactic irradiation, and subsequent immunotherapy
targeting PD-1 conferred long-term disease control. The present
report provides the first description of the successful management
with focal and systemic therapies for pulmonary SpCC with cystic
BM.
Case report
An 82-year-old male was diagnosed with pulmonary
SpCC at the clinical stage of T3N2M0 in August of Year X-1 based on
the pathological findings of a tumor specimen obtained by
transcutaneous core needle biopsy. The biopsy sample was entirely
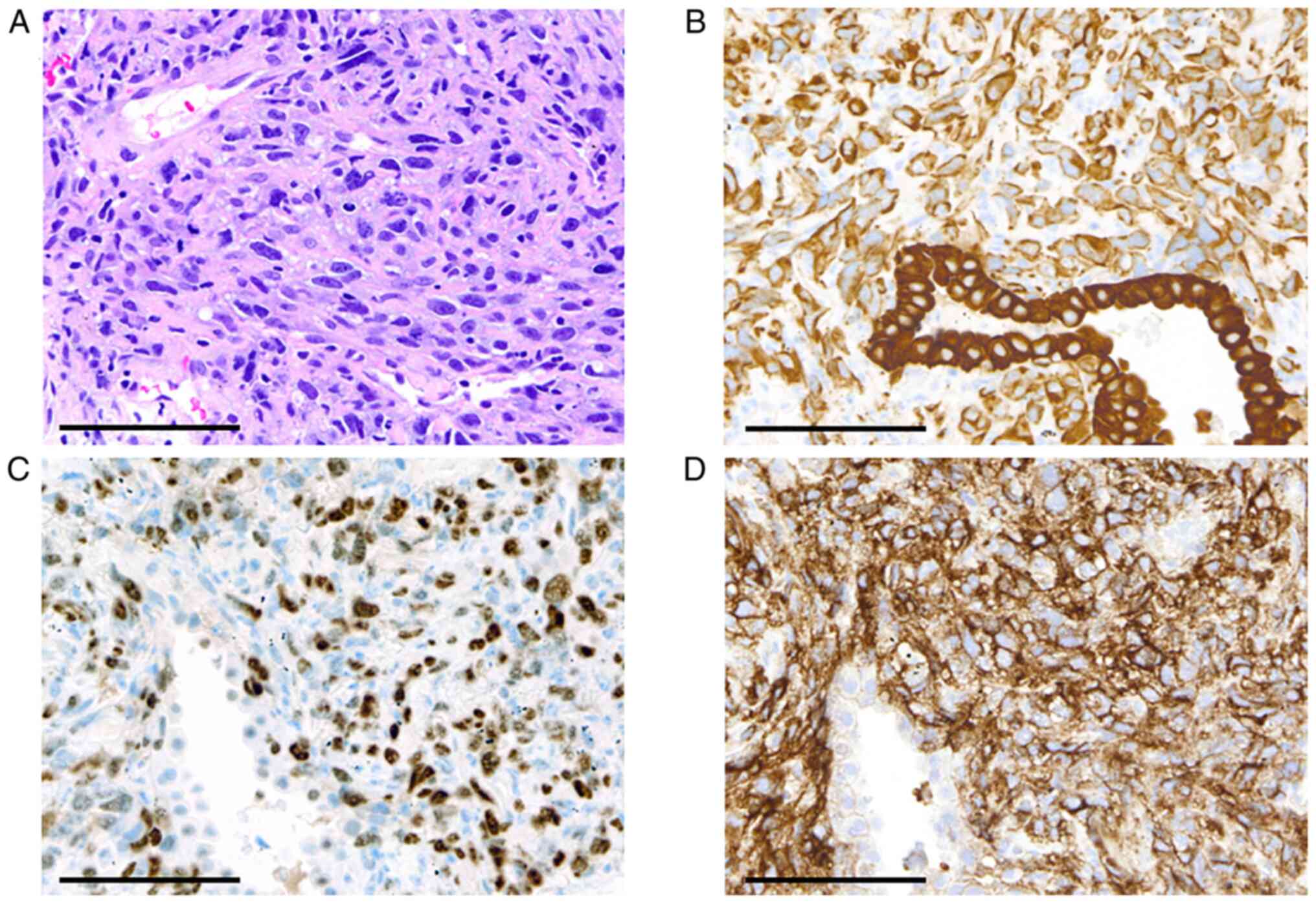
composed of monotonous spindle-shaped tumor cells that were
positive for AE1/AE, CK7, and p53 and negative for CK5/6, p40,
TTF-1, or Napsin A by immunostaining, which was indicative of SpCC
(Fig. 1A-C). The programmed cell
death ligand-1 (PD-L1) expression in tumor cells was evaluated by
immunostaining using 22C3 PharmDx Dako (Agilent, Santa Clara, CA,
United States) (Fig. 1D). The
tissue proportion score (TPS) was calculated by dividing the total
tumor cells into PD-L1-positive tumor cells and expressed as a
percentage, which was high at 90% in the present case. In contrast,
oncogene addiction was negative. Owing to the age of the
asymptomatic patient, the patient was receiving supportive care at
Toranomon Hospital Kajigaya.
In March of Year X, the patient was admitted to Hino
Municipal Hospital complaining of a walking disturbance. The
patient was conscious, and his vital signs were intact.
Neurological examination revealed unilateral spatial neglect and
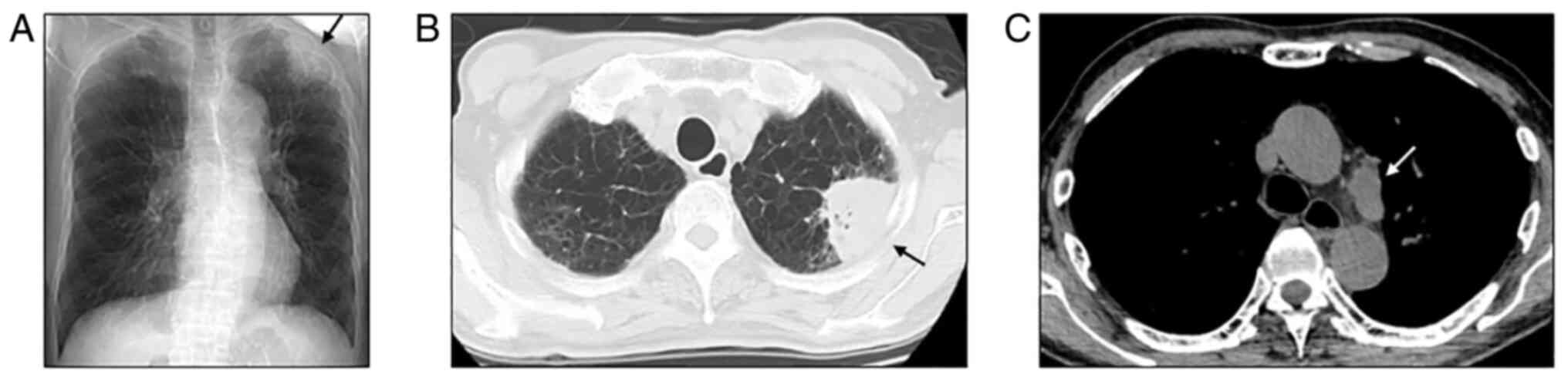
mild hemiparesis of the left extremities. Chest radiography and
computed tomography revealed a primary lung tumor (maximum
diameter, 60 mm) invading the chest wall in the left upper lobe and
subaortic lymphadenopathy (Fig.
2A-C) without any other metastatic sites. Gadolinium-enhanced
magnetic resonance imaging showed a solitary brain tumor in the
right parietal lobe (maximum diameter, 40 mm) consisting of solid
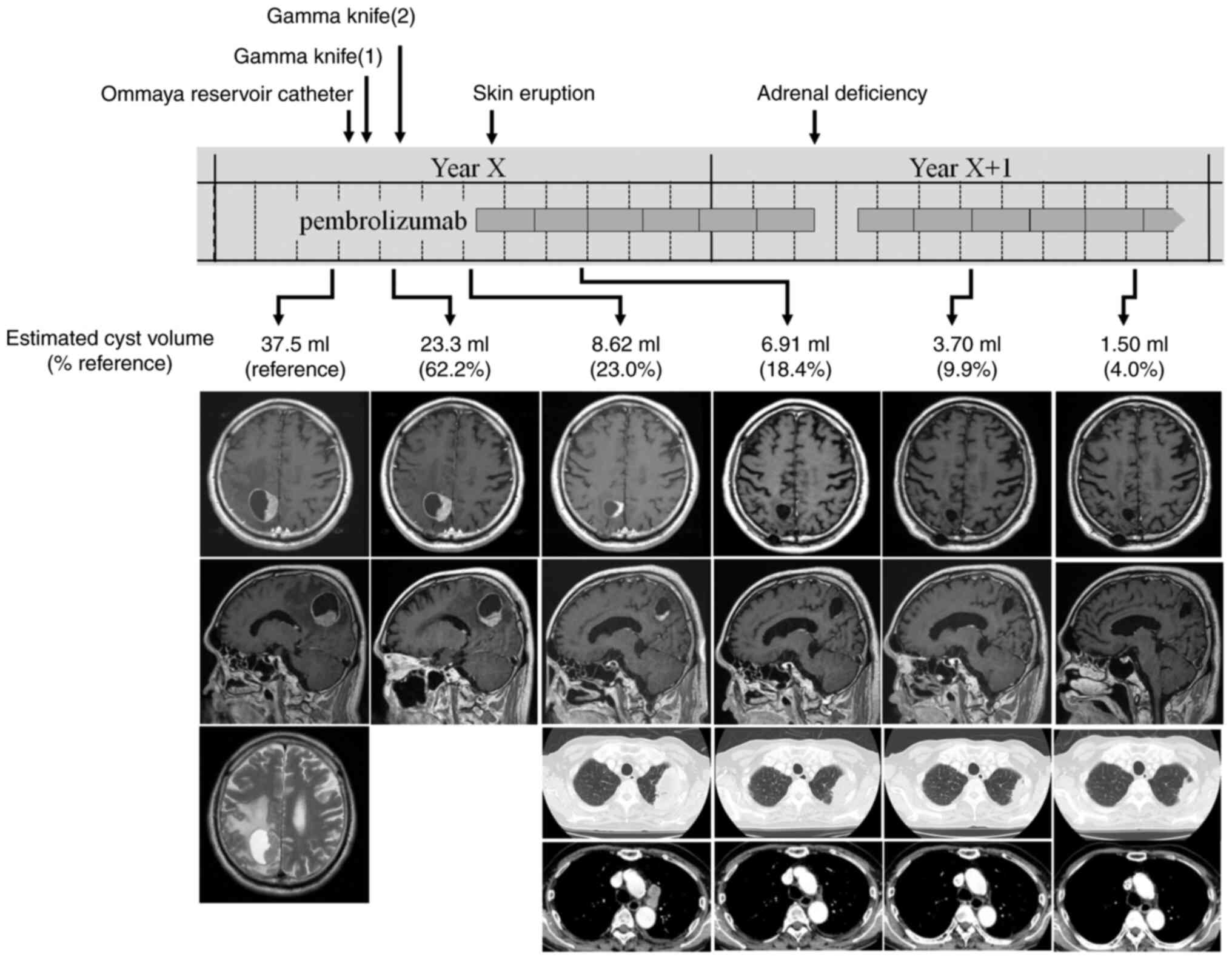
and cystic components (estimated cyst volume, 37.5 ml) (Fig. 3, reference group). A retrospective
review of imaging examinations revealed that the lung tumor was 20
mm in diameter, and brain metastasis was absent 1 year ago. Cyst
aspiration of the BM followed by surgical resection or stereotactic
irradiation was recommended for the rapid control of neurological
symptoms. The patient underwent placement of an Ommaya reservoir
catheter, and 7 ml of bloody-yellowish aspirate was collected, in
which cohesive pleomorphic malignant cells were detected.
Unfortunately, the catheter collapsed early, resulting in
insufficient tumor shrinkage. While catheter replacement or
surgical resection of the BM were treatment options, split gamma
knife (GK) was performed to minimize the patient's therapeutic
burden and allow an earlier initiation of immunotherapy. A
twice-split GK was performed with a prescription dose to tumor
margin of 14 Gy each, resulting in a shrinkage of the solid portion
and reduction in cyst volume by 62.2 and 23.0% following the first
and second rounds of GK, respectively (Fig. 3). During the period of GK treatment,
the patient's neurological symptoms improved to the point where he
was able to walk independently and was discharged from the
hospital. In July of Year X, the patient started anti-PD-1
immunotherapy (pembrolizumab, 400 mg intravenous administration,
every 6 weeks). After two cycles of treatment, substantial
regression was observed in the primary tumor and a mediastinal
lymph node, thereby indicating a partial response (Fig. 3). In addition, the solitary
compartment of the brain metastasis disappeared, and the cyst
volume further decreased by 18.4% during this period. Skin eruption
and adrenal deficiency appeared after the first and sixth cycles of
pembrolizumab treatment, respectively, and were treated
appropriately. The patient is currently continuing pembrolizumab
for 12 cycles and has maintained progression-free survival. The
patient did not complain of any neurological or physical
symptoms.
Discussion
The present report provides clinical observations of
biopsy-proven SpCC with cystic BM. Cystic BMs are rare and account
for 1.7-18.8% of all metastatic brain tumors (4–6,10–12).
The lungs and breasts were the two leading primary cancer sites
that resulted in cystic BMs, and non-small cell lung carcinomas
were less frequently accompanied by cystic BMs (7/1099 patients,
0.6%) than breast cancers (11/317 patients, 3.5%) (6,10–12).
Xu et al (1) evaluated the
histopathological types of 33 cases of lung cancer with cystic BMs,
including 26 adenocarcinomas (78.8%) and seven squamous cell
carcinomas (21.2%). Few studies have reported small cell lung
carcinomas with cystic BMs (13,14).
Cystic BM has not been reported with any subtype of pulmonary
sarcomatoid carcinoma including SpCC. The mechanisms underlying
cyst formation in non-mucinous carcinomas are not fully understood;
however, literature suggests that exudate collection results from
the breakdown of blood-brain barriers by tumor invasion (15).
BMs are frequently associated with neurological
symptoms and poor prognosis without appropriate treatment (16). Poor prognosis has been reported in
patients with lung carcinomas (5)
and breast cancers (4) with cystic
BMs compared to those with solid BMs. Other studies have reported
comparable overall survivals of patients with either type of BM
following stereotactic radiosurgery (SRS); however, these studies
also reported a lower local control rate, slower tumor shrinkage,
and higher recurrence rate in cystic BMs (11,12,15).
Predictive factors associated with SRS failure include large tumor
size, non-lung primary tumor, prior history of whole brain
irradiation, and the presence of cystic components (11,15,17).
Therefore, prior cyst aspiration was clinically examined to
minimize the target volumes and eventually showed time-efficient
tumor shrinkage, minimized peritumoral edema, and rapid symptom
relief following SRS treatment (6–8). In
the present case, the patient had a symptomatic BM with a large
cyst volume (37.5 ml), indicative that cyst aspiration was
required; however, the Ommaya reservoir catheter collapsed early,
resulting in insufficient tumor shrinkage. The catheter was not
replaced because the direction of the tube route is one of the
relevant factors for successful drainage (18), suggesting that simple replacement
would not be sufficient. Alternatively, the patient was
dispositioned to receive twice-split GK treatment based on previous
reports showing favorable outcomes and safe management by
hypo-fractionated SRS for large BMs (19,20).
The present case showed effective and safe management of a large
cystic BM of pulmonary SpCC using split GK.
Although pulmonary SpCCs are treated using the
guidelines for non-small cell lung carcinomas, SpCCs are usually
chemoresistant, leading to poor prognosis. In contrast, the use of
immunotherapy targeting PD-1/PD-L1 has emerged with recent studies
showing high PD-L1 expression and tumor mutation burden in
pulmonary sarcomatoid carcinomas (9). Two studies estimated an objective
response rate of 26.1-64.8%, disease control rate of 64.8-69.6%,
and overall survival of 12.7-18.2 months in patients with
sarcomatoid carcinoma following immunotherapy (21,22).
Notably, immunotherapy was the only treatment that prolonged the
overall survival of patients with pulmonary sarcomatoid carcinomas,
whereas platinum-based chemotherapy or molecular targeted therapy
for oncogene addiction did not (22). Furthermore, an additive platinum
doublet in immunotherapy did not improve the overall survival
(22). Six reported cases of
pulmonary SpCC have been treated with immune checkpoint inhibitors
(Table I) (23–27).
Of these cases and the present case, four were diagnosed using
surgical specimens and three were diagnosed using biopsy-proven
SpCCs. Tumor PD-L1 expression was high (TPS >90%) in all the
cases. All cases except one used monotherapy with pembrolizumab in
the first (n=5) or second (n=1) treatment line. Notably, all cases
showed partial or complete responses for the maximum response, and
four cases, including the present case, maintained progression-free
survival for more than one year at the reported date. While the
therapeutic perspective for pulmonary SpCC is still in debate,
immunotherapy is expected to provide a good prognosis for SpCC as
other types of sarcomatoid carcinomas.
 | Table I.Pulmonary spindle cell carcinoma cases
treated with immune checkpoint inhibitors. |
Table I.
Pulmonary spindle cell carcinoma cases
treated with immune checkpoint inhibitors.
| First author/s,
year | Age, years | Sex | Samples | PD-L1, % | Stages | BMs | Regimen | Treatment lines | Maximum response | Outcomes | (Refs.) |
|---|
| Mizushina et
al, 2021 | 52 | M | Surgical
specimen | >95 | Post-operation
recurrence | + | Pemb (q3w) | 2nd | PR | 11 cycles, dead | (23) |
| Oshiro et al,
2021 | 70 | M | Surgical
specimen | 100 | Post-operation
recurrence | - | Pemb (q3w) | 1st | PR | 23 cycles, alive | (24) |
| Akaba et al,
2021 | 72 | F | Surgical
specimen | 100 | Post-operation
recurrence | - | CBDCA/PTX/Bev/Atezo
(induction therapy); Bev/Atezo (maintenance therapy) | 1st | PR | 4 cycles; 15 months,
alive | (25) |
| Oshiro et al,
2021 | 75 | F | Surgical
specimen | 90 | Stage IVB | - | Pemb (q3w) | 1st | CR | 29 cycles, alive | (24) |
| Awobajo et al,
2020 | 69 | F | Biopsy | 90 | Post-radiotherapy
recurrence | + | Pemb (q3w) | 1st | PR | 2 cycles, alive | (26) |
| Tsurumi et al,
2020 | 76 | M | Biopsy | >90 | Stage IVB | - | Pemb (q3w) | 1st | PR | 7 cycles,
alive | (27) |
| Present study | 82 | M | Biopsy | 90 | Stage IVB | + | Pemb (q6w) | 1st | PR | 12 cycles,
alive | - |
In summary, we concluded that the large cystic BM of
SpCC was radiosensitive and successfully managed using split GK.
The present case and current literature indicated the crucial role
of immunotherapy targeting PD-1/PD-L1 in treating pulmonary
SpCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH, YK, HT and NM substantially conceived and
designed the study. YK, HT, SM, SO and TK acquired, analyzed and
interpreted the data. SM reviewed the pathological specimens. KH,
SM and NM drafted the manuscript. SM, SO and TK created the
figures. YK, HT, SO and TK critically revised the manuscript for
important intellectual content. KH and NM confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for his information to be published in this case
report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BM
|
brain metastasis
|
|
GK
|
gamma knife
|
|
PD-1
|
programmed cell death-1
|
|
PD-L1
|
programmed cell death ligand-1
|
|
SpCC
|
spindle cell carcinoma
|
|
SRS
|
stereotactic radiosurgery
|
|
TPS
|
tissue proportion score
|
References
|
1
|
Xu YB, Zhang Y, Song Z, Wang W and Shao L:
Treatment and prognosis of solid and cystic brain metastases in
patients with non-small-cell lung cancer. Cancer Manag Res.
13:6309–6317. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization (WHO), . WHO
Classification of Tumours. Thoracic Tumours. 5th Edition.
International Agency for Research on Cancer; Lyon: 2021
|
|
3
|
Rossi G, Cavazza A, Sturm N, Migaldi M,
Facciolongo N, Longo L, Maiorana A and Brambilla E: Pulmonary
carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements:
A clinicopathologic and immunohistochemical study of 75 cases. Am J
Surg Pathol. 27:311–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brigell RH, Cagney DN, Martin AM, Besse
LA, Catalano PJ, Lee EQ, Wen PY, Brown PD, Phillips JG, Pashtan IM,
et al: Local control after brain-directed radiation in patients
with cystic versus solid brain metastases. J Neurooncol.
142:355–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun B, Huang Z, Wu S, Ding L, Shen G, Cha
L, Wang J and Song S: Cystic brain metastasis is associated with
poor prognosis in patients with advanced breast cancer. Oncotarget.
7:74006–74014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franzin A, Vimercati A, Picozzi P, Serra
C, Snider S, Gioia L, Ferrari da Passano C, Bolognesi A and
Giovanelli M: Stereotactic drainage and Gamma Knife radiosurgery of
cystic brain metastasis. J Neurosurg. 109:259–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebinu JO, Lwu S, Monsalves E, Arayee M,
Chung C, Laperriere NJ, Kulkarni AV, Goetz P and Zadeh G: Gamma
knife radiosurgery for the treatment of cystic cerebral metastases.
Int J Radiat Oncol Biol Phys. 85:667–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horiguchi T, Yanagi S, Yatsushiro K,
Tsubouchi H, Matsumoto N and Nakazato M: A case of impaired
consciousness due to large cystic metastatic brain tumors from lung
adenocarcinoma successfully controlled with Ommaya reservoir
placement. Respir Med Case Rep. 30:1010692020.PubMed/NCBI
|
|
9
|
Ağaçkıran Y, Aksu F, Akyürek N, Ercan C,
Demiröz M and Aksu K: Programmed death ligand-1 expression levels,
clinicopathologic features, and survival in surgically resected
sarcomatoid lung carcinoma. Asia Pac J Clin Oncol. 17:280–288.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higuchi F, Kawamoto S, Abe Y, Kim P and
Ueki K: Effectiveness of a 1-day aspiration plus Gamma Knife
surgery procedure for metastatic brain tumor with a cystic
component. J Neurosurg. 117 (Suppl):S17–S22. 2012. View Article : Google Scholar
|
|
11
|
Yamanaka Y, Shuto T, Kato Y, Okada T,
Inomori S, Fujino H and Nagano H: Ommaya reservoir placement
followed by Gamma Knife surgery for large cystic metastatic brain
tumors. J Neurosurg. 105 (Suppl):S79–S81. 2006. View Article : Google Scholar
|
|
12
|
Wang H, Liu X, Jiang X, Song Y, Wang X,
Wang J, Dong Y, Li F, Wu Z, Zhang Y and Yuan Z: Cystic brain
metastases had slower speed of tumor shrinkage but similar
prognosis compared with solid tumors that underwent radiosurgery
treatment. Cancer Manag Res. 11:1753–1763. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ismailoglu O, Albayrak BS and Ciris M:
Cerebral metastasis of small-cell lung carcinoma mimicking a
supratentorial cystic astrocytoma. Am J Med Sci. 342:5202011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda T, Saitoh M and Takeda S: Solitary
cystic brain metastasis of small-cell lung carcinoma controlled by
a stereotactically inserted Ommaya reservoir. Am J Med Sci.
337:215–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gardner WJ, Collis JS Jr and Lewis LA:
Cystic brain tumors and the blood-brain barrier. Comparison of
protein fractions in cyst fluids and sera. Arch Neurol. 8:291–298.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owonikoko TK, Arbiser J, Zelnak A, Shu HK,
Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, et
al: Current approaches to the treatment of metastatic brain
tumours. Nat Rev Clin Oncol. 11:203–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan HC, Sheehan J, Stroila M, Steiner M
and Steiner L: Gamma knife surgery for brain metastases from lung
cancer. J Neurosurg. 102 (Suppl):S128–S133. 2005. View Article : Google Scholar
|
|
18
|
Oshima A, Kimura T, Akabane A and Kawai K:
Optimal implantation of Ommaya reservoirs for cystic metastatic
brain tumors preceding gamma knife radiosurgery. J Clin Neurosci.
39:199–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minniti G, D'Angelillo RM, Scaringi C,
Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM
and Trodella L: Fractionated stereotactic radiosurgery for patients
with brain metastases. J Neurooncol. 117:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feuvret L, Vinchon S, Martin V, Lamproglou
I, Halley A, Calugaru V, Chea M, Valéry CA, Simon JM and Mazeron
JJ: Stereotactic radiotherapy for large solitary brain metastases.
Cancer Radiother. 18:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domblides C, Leroy K, Monnet I, Mazières
J, Barlesi F, Gounant V, Baldacci S, Mennecier B, Toffart AC,
Audigier-Valette C, et al: Efficacy of immune checkpoint inhibitors
in lung sarcomatoid carcinoma. J Thorac Oncol. 15:860–866. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CL, Hsieh MS, Shih JY, Lee YH, Liao
WY, Hsu CL, Yang CY, Chen KY, Lee JH, Ho CC, et al: Real-world
treatment patterns and outcomes among patients with advanced
non-small-cell lung cancer with spindle cell and/or giant cell
carcinoma. Ther Adv Med Oncol. 14:175883592211338892022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizushina Y, Ohyanagi F, Shiihara J,
Nomura M, Ohta H, Oshiro H, Tsubochi H, Kusaka G and Yamaguchi Y:
Clinical case of lung spindle cell carcinoma markedly responsive to
pembrolizumab. Thorac Cancer. 12:2279–2282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oshiro Y, Suzuki S, Sakurada A, Hamada K,
Yamamoto R and Kazama A: A long-term survival in two cases of
pulmonary spindle cell carcinoma treated with pembrolizumab. Jpn J
Lung Cancer. 16:327–335. 2021.(In Japanese). View Article : Google Scholar
|
|
25
|
Akaba T, Shiota Y, Onizawa F, Isaka T,
Nagashima Y and Tagaya E: Recurrent spindle cell carcinoma of the
lung successfully treated by chemoimmunotherapy. Respirol Case Rep.
9:e007572021. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Awobajo MD, Vaporciyan AA, Lu C and Gandhi
SJ: Stereotactic body radiation therapy (SBRT) in the management of
pulmonary spindle cell carcinoma. BMJ Case Rep. 13:e2347792020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsurumi K, Kawashima Y, Akahira J, Saito
R, Toi Y, Nakamura A, Yamanda S, Kimura Y, Honda Y and Sugawara S:
A remarkable clinical response to pembrolizumab in a rare spindle
cell carcinoma of the lung. JMA J. 3:83–86. 2020.PubMed/NCBI
|