Introduction
Several biomarkers predict cancer progression,
patient prognosis, and therapeutic efficacy in cancer. Some of the
most useful biomarkers are involved in the growth or metastasis of
cancers (1–4). In breast cancer, the biomarker human
epidermal growth factor receptor-2 (HER2) can be used to predict
both patient prognosis and anti-HER2 therapeutic efficacy (5–11). The
use of trastuzumab, a HER2-targeting agent, for treating patients
with HER2-overexpressed breast cancer that exhibits aggressive
clinical behaviors and poor prognosis has significantly improved
the prognoses of these patients; for these patients, treatment with
trastuzumab has resulted in a prognosis as favorable as that of
patients with luminal HER2-negative breast cancer in both
postoperative and metastatic settings (11–15).
However, for triple-negative (TN) breast cancer that exhibits
clinical aggressiveness and lacks the expression of target
molecules and biomarkers available for treatment, no efficient
therapeutics have been established. Recent studies have found that
the immune response plays a crucial role in the disease progression
and prognosis of patients with cancer. Therefore, studies have
investigated the use of immune checkpoint inhibitors targeting the
programmed cell death 1-programmed cell death-ligand-1 (PD-1-PD-L1)
axis in cancer treatment; this has resulted in dramatic positive
effects against immunogenic cancers (16–27).
Although breast cancer is among the less immunogenic cancers
(28–30), certain aggressive subtypes with
typically poor prognoses, such as TN and HER2-overexpressed breast
cancer, have been found to be potently immunogenic (31–33).
Clinical trials of the therapeutic potential of
immune checkpoint inhibitors, such as atezolizumab and
pembrolizumab, in the treatment of advanced TN breast cancer have
achieved an objective treatment response rate of 53.2-56.0%, with a
significantly longer progression-free survival compared with that
of placebo group patients (34–38).
PD-1 and PD-L1 are molecules responsible for immune checkpoint
processes. PD-L1 is expressed in various cells, including
macrophages, monocytes, T cells, B cells, and tumor cells and binds
to PD-1 receptors on T and B cells. PD-L1 is overexpressed in tumor
cells and binds to PD-1 on cytotoxic T lymphocytes (CTLs). This
initiates the lysis and apoptosis of cancer cells by CTLs. However,
prolonged exposure to cancer cells can lead to CTL exhaustion,
which reduces their ability to kill tumor cells (39). Inhibiting the interaction between
PD-1 and PD-L1 prevents CTLs from becoming less responsive and
helps them restore their cytotoxic activity against cancer cells.
For the body to recognize cancer cells and ensure that CTLs attack
them, immune cells should infiltrate the tumor mesenchyme. Immune
cells can include antigen-presenting cells such as macrophages,
dendritic cells, and B lymphocytes. Moreover, CD4+
helper lymphocytes and CD8+ CTLs can infiltrate the
tumor mesenchyme and recognize antigenic epitopes present on major
histocompatibility complex (MHC) molecules. Therefore,
tumor-infiltrating lymphocytes (TILs) and MHC molecules are
essential for inducing an antitumor immune response. Several
studies have reported the utility of the level of TILs and
CD8+ T lymphocyte infiltrates, and expression of MHC and
PD-1-PD-L1 axis for prognostic prediction in cancer (40–49).
Cancer cells can gradually acquire the ability to evade the immune
surveillance system of antitumor immune cells, thereby leading to
cancer progression (50). Among
these evasive tactics against antitumor immunity is the deletion of
MHC class I molecules on the surface of cancer cells. This prevents
the interaction of CTLs with T cell receptors, which is necessary
for the recognition of the cancer cells by CTLs. Thus, MHC class 1
molecules also have prognostic significance (48–50).
As described above, antitumor immune responses can
affect cancer progression and patient prognosis in immunogenic
subtypes such as TN and HER2-overexpressed breast cancer; however,
antitumor immunity is unlikely to affect luminal HER2-negative
breast cancer, a dominant subtype, because of its lower
immunogenicity. Indeed, compared with other cancer types, breast
cancer including luminal HER2-negative breast cancer, which occurs
in a majority of the population, exhibits fewer mismatch repair
deficiencies and microsatellite instabilities; this is partly
because breast cancer is a well-differentiated and slow-growing
cancer (28,51). This results in a low production of
non-self antigenic proteins during cancer progression. This low
immunogenicity has been verified in a clinical trial evaluating an
immune checkpoint inhibitor; in the trial, the treatment was
inefficacious in patients with luminal HER2-negative breast cancer
compared with the beneficial effects observed in those with TN
breast cancer (30).
In this study, we retrospectively evaluated the
status and prognostic value of the immunological breast cancer
biomarkers, TILs, CD8+ T lymphocyte infiltrates, MHC
molecules, and PD-L1. We compared these biomarkers between patients
with less immunogenic luminal HER2-negative breast cancer and those
with immunogenic non-luminal breast cancer including TN and
non-luminal HER2-overexpressed breast cancers.
Materials and methods
Patients
Seventy-one female patients with primary breast
cancer who had undergone surgery such as mastectomy or partial
resection for primary lesions with either axillary dissection or
sentinel node biopsy from January 2010 to December 2021 at Kagawa
University Hospital were included in this study. The exclusion
criteria were as follows: previous invasive breast cancer or
non-breast cancer within 5 years before surgery for primary breast
cancer; any previous chemotherapy or endocrine therapy for cancer;
any previous anti-HER2 therapy or other previous anticancer
biologic therapy or immunotherapy; and concurrent serious diseases
interfering with adjuvant therapy for breast cancer. The median
patient age was 59 (35–85) years.
At the time of surgery, 27 of the patients were in clinical stage
1, 42 were in stage 2, and 2 were in stage 3 (Table I). The cohort comprised 48 patients
with luminal HER2-negative, 21 with TN, and 2 with non-luminal
HER2-overexpressed breast cancer. Tissue samples of the main breast
tumor obtained by either surgical resection or preoperative biopsy
were examined.
 | Table I.Clinical features and prognoses of
the primary breast cancer patient cohort in this study. |
Table I.
Clinical features and prognoses of
the primary breast cancer patient cohort in this study.
| Variable | All (n=71) | Non-luminal
(n=23) | Luminal HER2 (−)
(n=48) | P-value |
|---|
| Median age,
years | 59 (31–85) | 58 (31–78) | 60 (32–85) | 0.681 |
| Median tumor size,
cm | 2 (0.5-8.5) | 2.1 (0.5-6.5) | 1.7 (0.7-8.0) | 0.269 |
| N-positive, % | 43.6% | 60.9% | 31.3% | 0.018a |
| Stage |
|
|
|
|
| 1 | 27 | 4 | 23 | 0.014a |
| 2 | 42 | 19 | 23 | 0.006a |
| 3 | 2 | 0 | 2 | 0.324 |
| MHC-positive,
% | 70.4% | 78.3% | 66.7% | 0.319 |
| High TILs, % | 60.6% | 82.7% | 50.0% | 0.009a |
| Median no.
CD8+ T, % | 66.0
(1.0-176.3) | 88.0
(17.3-176.3) | 55.7
(1.0-130.0) | 0.001a |
| PDL1-E1L3N (+),
% | 19.7% | 39.1% | 10.4% | 0.005a |
| PDL1-SP263 (+),
% | 54.9% | 60.9% | 47.9% | 0.174 |
| RFS at 10
years | 60.3% | 52.1% | 66.7% | 0.059 |
| OS at 10 years | 78.1% | 60.9% | 87.5% | 0.038a |
Evaluation of TIL levels
TIL levels in patient tissue samples were evaluated.
After the samples were fixed in formalin and embedded in paraffin,
they were sectioned into 4-µm-thick slices and stained in
hematoxylin-eosin solution, as previously described (52). All mononuclear cells, including
lymphocytes and plasma cells, were selected for the evaluation of
TIL levels. Granulocytes and other polymorphonuclear leukocytes
were excluded. As recommended in previous studies, stromal TIL
levels were determined according to the area of stromal tissues
occupied by mononuclear inflammatory cells over the total
intratumoral stromal area (=% stromal TILs). The denominator used
to determine the % stromal TIL level is the area of stromal tissue
and not the number of stromal cells (52–54).
Three representative fields of view were selected and the average
of each TIL level was determined. We used a cutoff score of 10% as
previously established (55).
Therefore, a stromal TIL level ≥10% was designated as a high TIL
level, and that <10% was designated as a low TIL level.
Immunohistochemistry
Serial sections (4-µm-thick) of formalin-fixed
paraffin-embedded tissue specimens were stained via standard
indirect immunoperoxidase procedures for PD-L1, CD8, and MHC class
I molecules, according to the staining kit manufacturer's
instructions. Briefly, each tissue section was deparaffinized in
xylene and rehydrated in ethanol and distilled water. Antigen
retrieval was performed via 10 min of microwave treatment in 10 mM
sodium citrate buffer (pH 6.0) for PD-L1 or 10 mM Tris/1 mM
ethylenediaminetetraacetic acid (pH 9.0) for MHC class I molecules.
Endogenous peroxidase activity was blocked by treatment with 3%
H2O2 for 10 min. After blocking in
Tris-buffered saline with Tween-20 and 5% normal goat serum for 1 h
at room temperature, the sections were incubated at 4°C overnight
with antihuman PD-L1 monoclonal antibodies (clone: E1L3N, diluted
1:200, Cell Signaling Technology, Danvers, MA, USA; SP263, diluted
1:100, Ventana Medical Systems, Tucson, AZ, USA), which were
produced by immunizing rabbits with peptides derived from the
C-terminus of PD-L1 protein, anti-CD8 monoclonal antibody (clone:
SP57, diluted 1:100, Ventana Medical Systems), or an anti-HLA class
I monoclonal antibody (clone: EMR8-5, diluted 1:500, Hokudo Co.,
Ltd., Sapporo, Japan). The sections were then incubated with
SignalStain boost IHC detection reagent (Cell Signaling Technology)
for PD-L1 or Envision Dako ChemMate (Dako Ltd., Kyoto, Japan) for
CD8 and MHC class I molecules. They were visualized using a
SignalStain DAB (3,3′-diaminobenzidine) substrate kit (Cell
Signaling Technology) for PD-L1 or Envision Dako
ChemMate/horseradish peroxidase (HRP) DAB for CD8 and MHC class I
molecules for 1 min. This was followed by counterstaining with
hematoxylin. Isotype-matched control antibodies were used for
immunohistochemistry. These were rabbit immunoglobulin G (IgG)
monoclonal antibody (Cell Signaling Technology) for PD-L1, and
mouse IgG monoclonal antibody (Dako) for CD8 and MHC class I
molecules.
Evaluation of PD-L1, MHC class 1, and
CD8 expression
Serial sections of stained tumor tissues were
independently examined by two researchers, including a pathologist.
To compare the cellular staining intensities of PD-L1, CD8, and MHC
class I molecules, cells from the serial sections were evaluated
microscopically (magnification: ×200). Three representative fields
of view were selected and any expression of PD-L1 and MHC class I
molecules was identified in 100 tumor cells per field. Cases in
which the proportion of tumor cells positive for PD-L1 was ≥1% were
considered PD-L1+ tumor cell-dominant. Cases in which the
proportion of tumor cells positive for MHC class I molecules was
≥80% were considered MHC class I+ tumor cell-dominant, as
previously reported (56). To
evaluate CD8+ T lymphocyte levels, we counted the number
of CD8+ T lymphocytes in three stroma fields of view and
calculated the median number of CD8+ T lymphocytes per
field.
Statistical analysis
All statistical analyses were performed using SPSS
Statistics for Windows (IBM Corp., Armonk, NY, USA) software. For
comparisons between two groups, we used the Mann-Whitney U test or
the χ2 test. The effects of clinical and demographic
variables, clinical responses, and prognostic parameters on the
duration of survival and risk of progression were assessed using
Kaplan-Meier survival analyses and log-rank tests. A 95% confidence
interval for the median of each variable was calculated using the
Brookmeyer and Crowley method (57). All analyses were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association of clinicopathological
patient variables and immunological biomarker status with cancer
progression and prognosis
The patient cohort included 48 (67.6%) patients with
luminal HER2-negative, 21 (29.6%) with TN, and two (2.8%) with
non-luminal HER2-overexpressed breast cancer. At the time of the
study, 30 patients experienced relapsed lesions. The relapse-free
survival (RFS) and overall survival (OS) rates 10 years after the
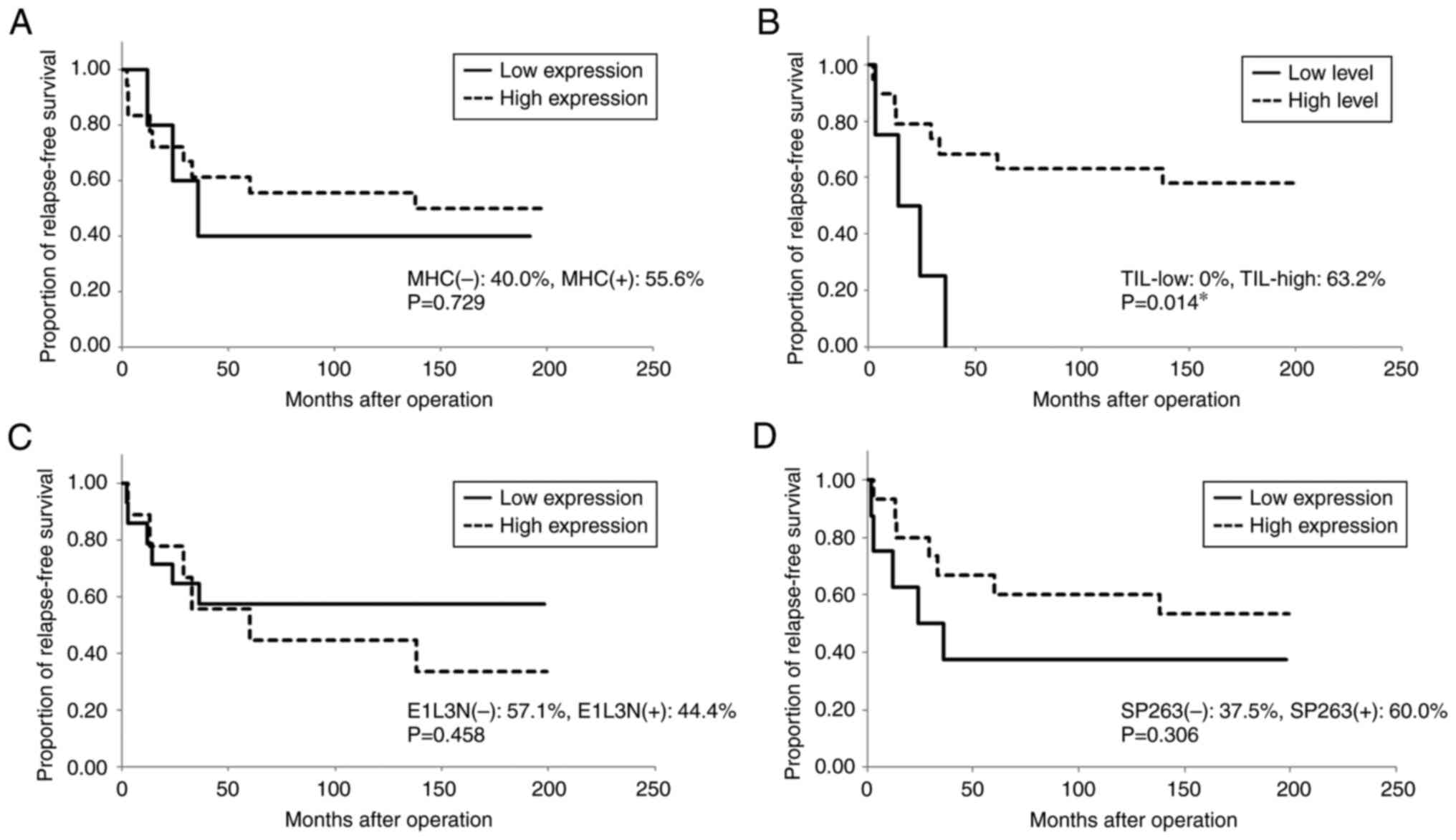
primary operation were 60.3 and 78.1%, respectively (Table I). There were 50 (70.4%) patients
positive for MHC expression and 43 (60.6%) with high TIL levels
(Tables I and II). Microscopic images of low and high
TIL levels are shown in Fig. 1A and
B, respectively. Microscopic images of breast cancer tumors
positive and negative for MHC expression are shown in Fig. 1C and D, respectively. Reactivity to
E1L3N was observed in 14 (19.7%) patients and reactivity to SP263
in 39 (54.9%) patients (Tables I
and III). Microscopic images of
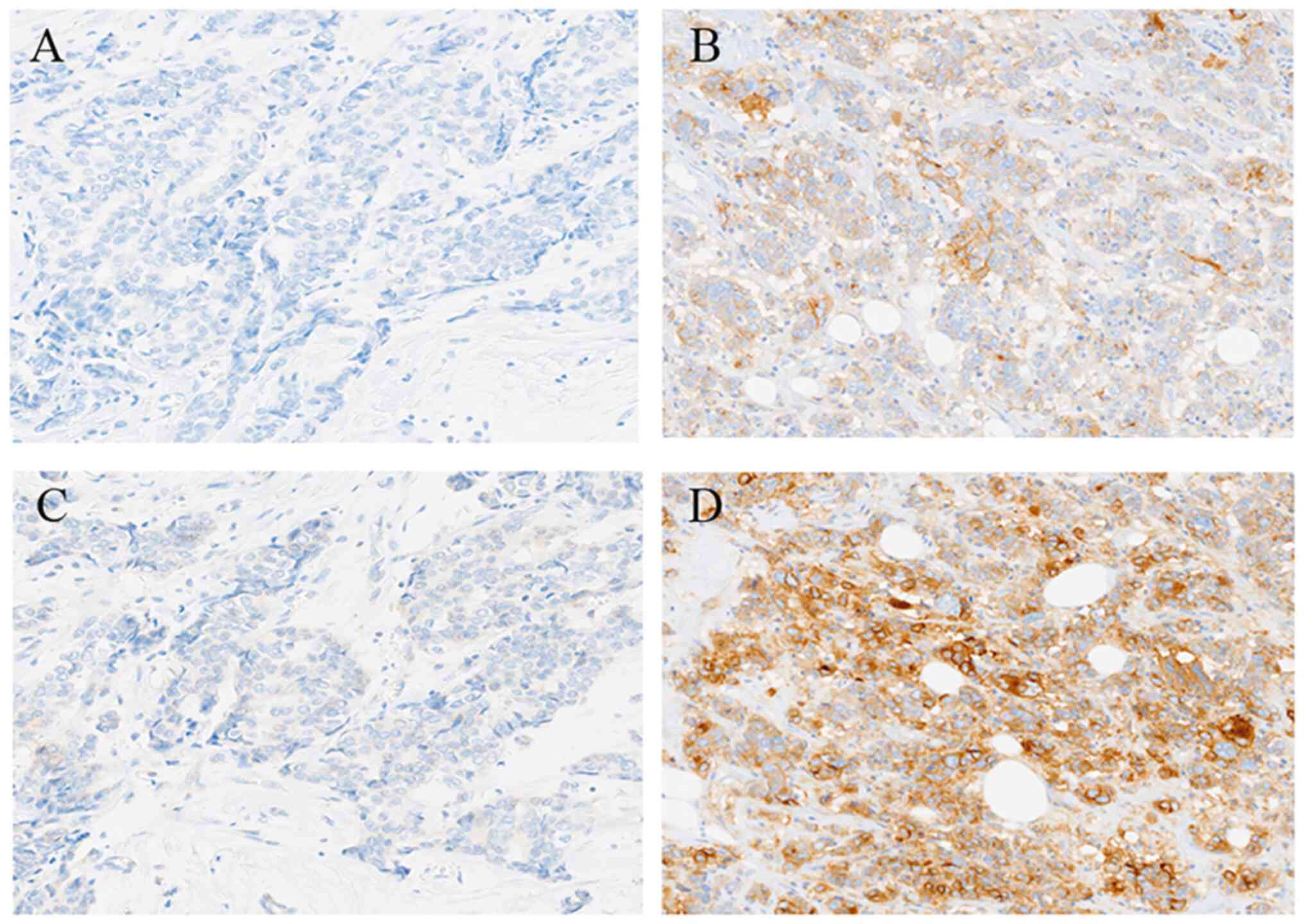
tumors positive and negative for E1L3N and SP263 were shown in
Fig. 2. Although the sensitivity of
reaction of the two monoclonal antibodies observed differed, all
patients responsive to E1L3N exhibited reactivity to SP263 because
both the monoclonal antibodies recognized antigenic determinants
near the C-terminus of PD-L1 protein. Furthermore, MHC expression
was significantly associated with tumor size (P=0.017) and clinical
stage (P=0.046) and TIL level was significantly associated with
tumor size (P=0.021), nodal involvement (P=0.004), and clinical
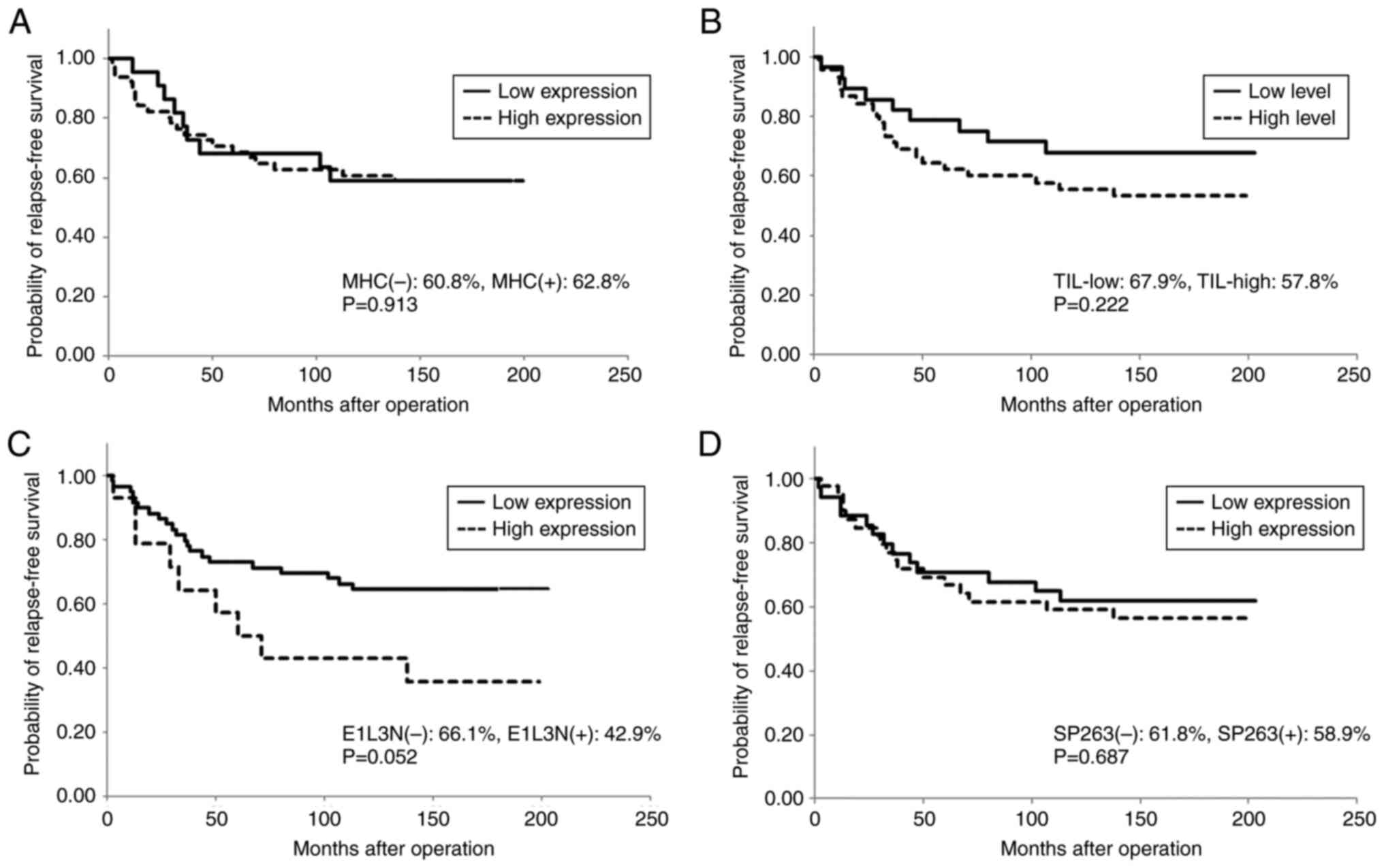
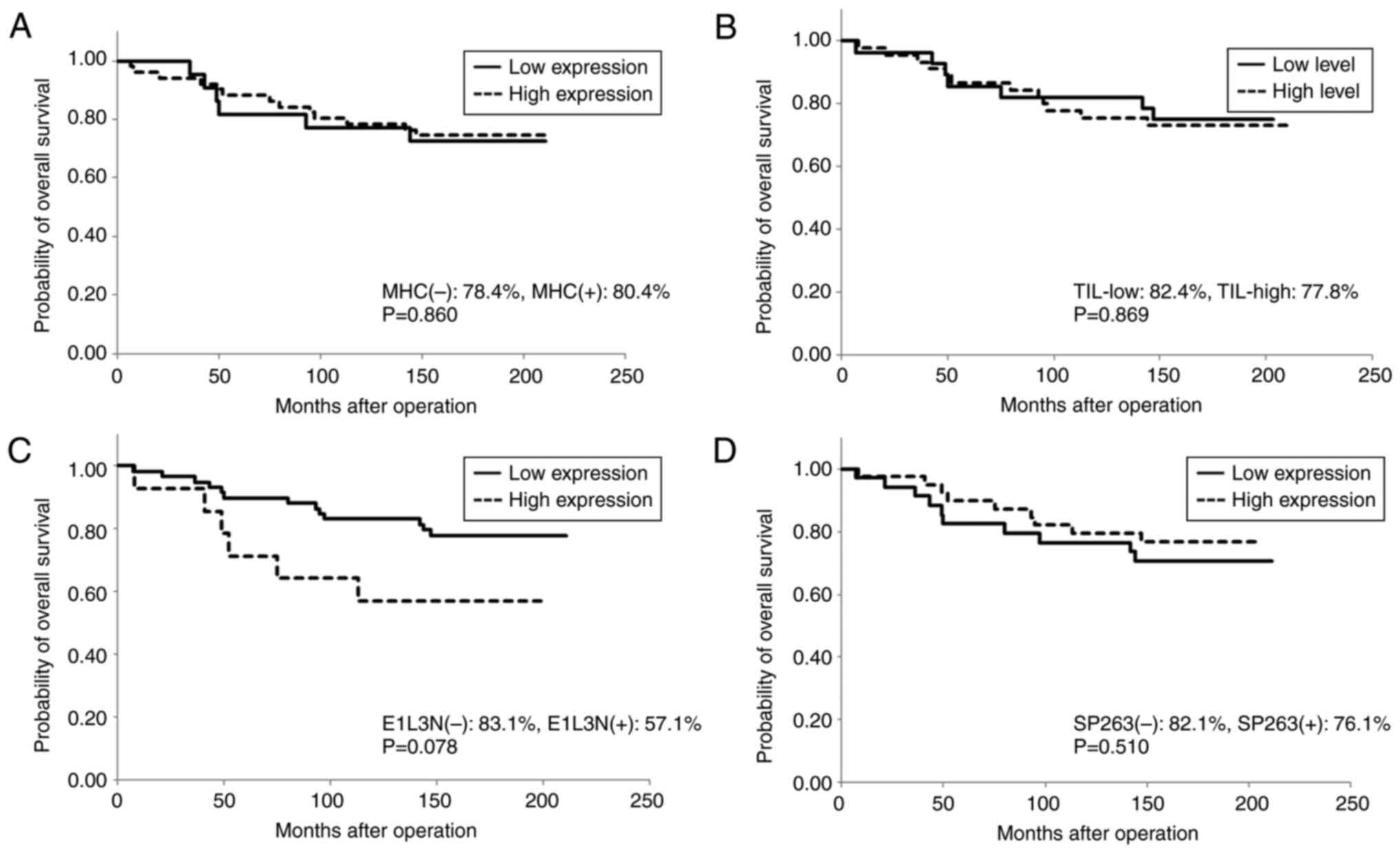
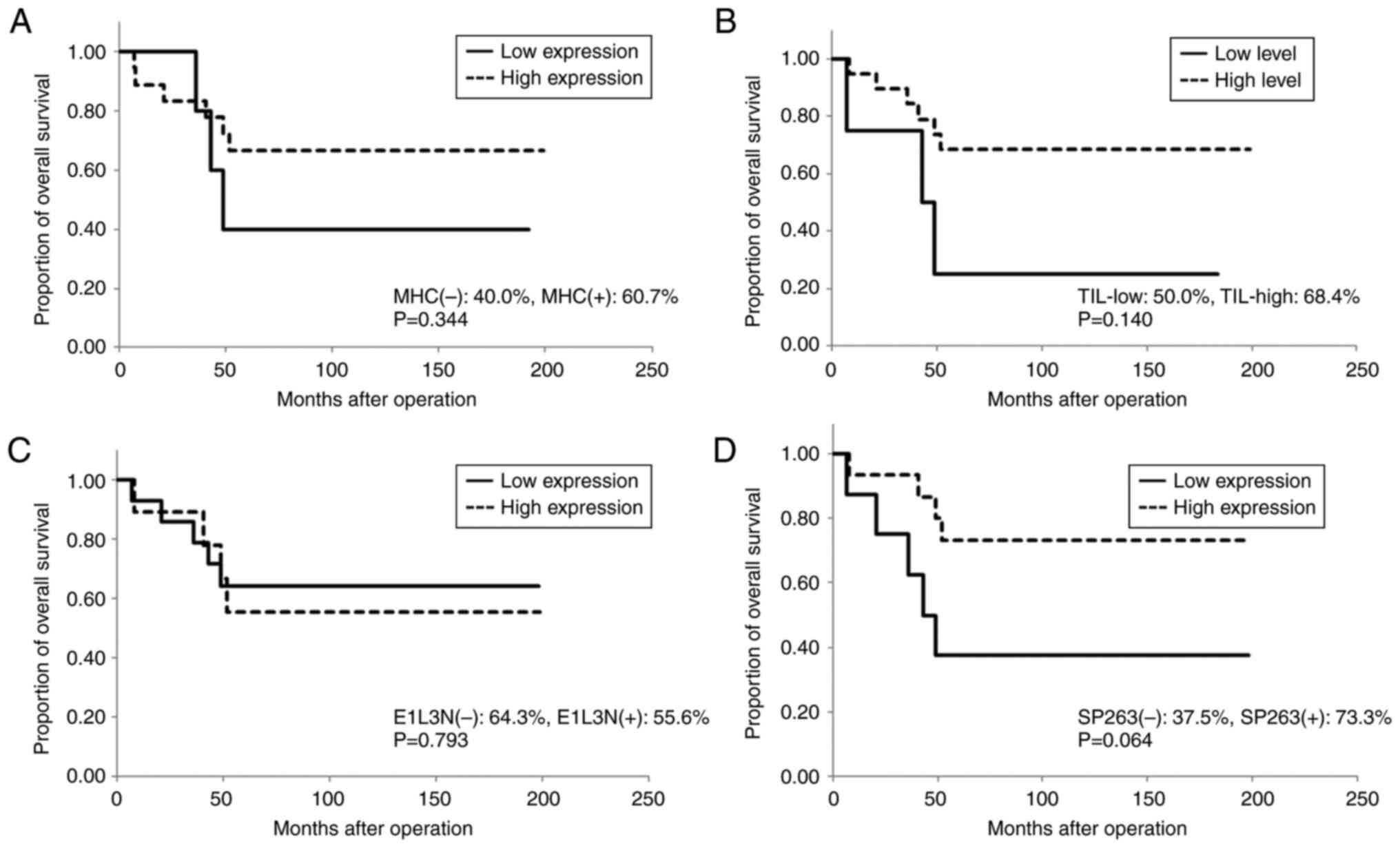
stage (P=0.006); however, neither MHC expression nor TIL level was
associated with RFS or OS (Table
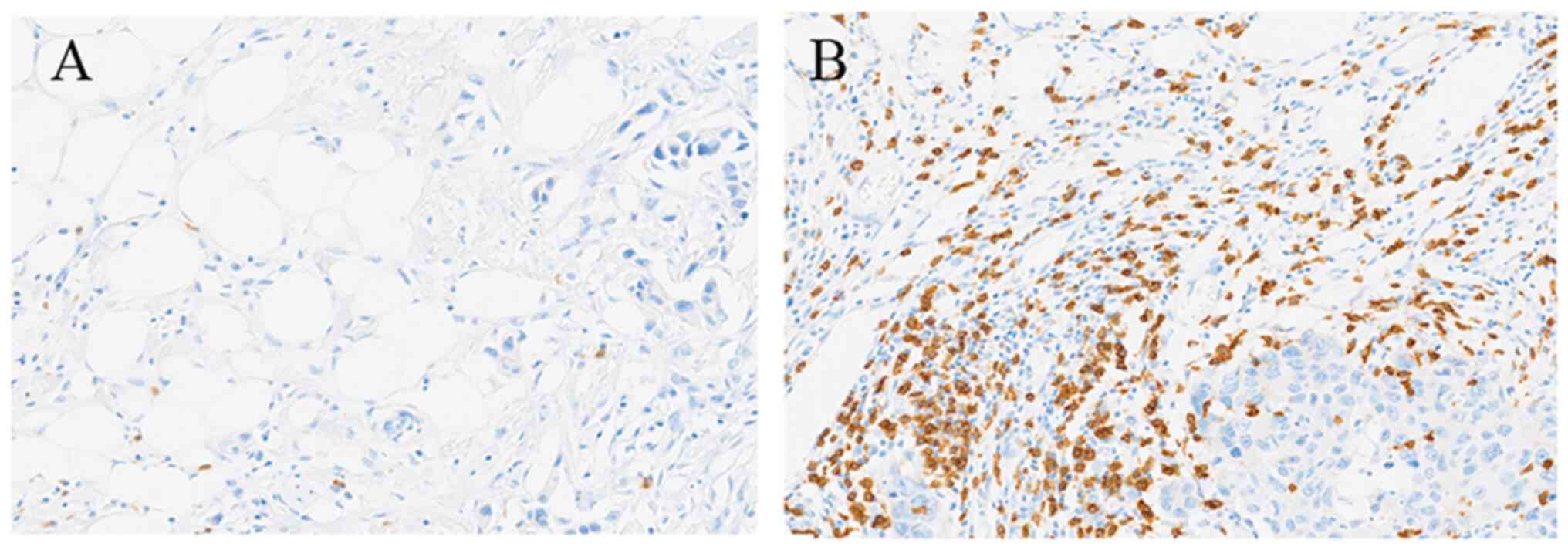
II; Figs. 3 and 4). The number of CD8+ T
lymphocyte infiltrates in the tumor stroma was significantly
associated with MHC and PD-L1 expression and TIL levels (Table II). Microscopic images of low and
high counts of CD8+ T lymphocyte infiltrates are shown
in Fig. 5A and B, respectively. The
proportion of patients positive for SP263 was significantly higher
in patients positive for E1L3N than that in patients negative for
E1L3N (E1L3N-negative and E1L3N-positive patients: 42.1 and 100%,
respectively, P<0.001, Table
III) and the proportion of patients positive for E1L3N was
significantly higher in patients positive for SP263 than that in
patients negative for SP263 (SP263-negative and SP263-positive
patients: 0 and 36.8%, P<0.001). Regarding the status of these
immunological biomarkers, the proportion of patients with high TIL
levels, the proportion of patients reactive to E1L3N, and the
number of CD8+ T lymphocyte infiltrates were
significantly higher in the non-luminal group than in the luminal
group (high TIL levels, 82.7% vs. 50.0%, P=0.009; E1L3N positivity,
39.1% vs. 10.4%, P=0.005; number of CD8+ T lymphocyte
infiltrates, 88.0 vs. 55.7, P=0.001, Table I). However, the status of these
biomarkers showed no prognostic value, except for an almost but not
quite significant association between E1L3N reactivity and shorter
RFS (RFS rate at 10 years: 66.1 and 42.9% for patients negative and
positive for E1L3N, respectively; P=0.052; Figs. 3 and 4).
 | Table II.Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of the patient
cohort. |
Table II.
Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of the patient
cohort.
| Variable | MHC(−) (n=21) | MHC(+) (n=50) | P-value | Low TIL (n=28) | High TIL
(n=43) | P-value |
|---|
| Median age,
years | 61 (46–73) | 57 (31–85) | 0.286 | 60 (37–77) | 58 (32–85) | 0.861 |
| Median tumor size,
cm | 1.7 (0.5–3.5) | 2.1 (0.7–6.6) | 0.017a | 1.5 (0.7–3.5) | 2.1 (0.5–8.0) | 0.021a |
| N-positive, % | 27.2% | 45.1% | 0.229 | 21.4% | 55.6% | 0.005a |
| Median stage | 1 (1–2) | 2 (1–3) | 0.046 a | 1 (1–2) | 2 (1–3) | 0.006a |
| MHC-positive,
% | - | - | - | 50.0% | 83.7% | 0.004a |
| High TILs, % | 31.8% | 70.6% | 0.004 a | - | - | - |
| Median no.
CD8+ T | 39.7
(1.0–109.7) | 74.3
(27.0–176.3) | 0.001a | 33.7
(1.0–74.3) | 83.3
(31.0–176.3) |
<0.001a |
| PDL1-E1L3N (+),
% | 0% | 27.5% | 0.007a | 3.6% | 30.2% | 0.008a |
| PDL1-SP263 (+),
% | 31.8% | 60.8% | 0.016a | 32.1% | 65.1% | 0.018a |
 | Table III.Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of the patient cohort. |
Table III.
Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of the patient cohort.
| Variable | E1L3N(−)
(n=57) | E1L3N(+)
(n=14) | P-value | SP263(−)
(n=32) | SP263(+)
(n=39) | P-value |
|---|
| Median age,
years | 60 (31–75) | 57 (41–85) | 0.714 | 62 (32–77) | 58 (31–85) | 0.328 |
| Median tumor size,
cm | 2.0 (0.5–8.0) | 2.0 (1.3–6.5) | 0.624 | 1.9 (0.5–8.0) | 2.0 (0.7–6.5) | 0.766 |
| N-positive, % | 39.0% | 57.1% | 0.219 | 41.2% | 41.0% | 0.791 |
| Median stage | 2 (1–3) | 2 (1–2) | 0.553 | 2 (1–3) | 2 (1–2) | 0.705 |
| MHC-positive,
% | 63.2% | 100% | 0.007a | 57.6% | 81.6% | 0.016a |
| High TILs, % | 52.6% | 92.9 | 0.008a | 45.5% | 73.7% | 0.018a |
| Median no.
CD8+ T | 58.3
(1.0–125.7) | 100.3
(54.0–176.3) |
<0.001a | 43.3
(12.0–109.7) | 79.0
(1.0–176.3) |
<0.001a |
| PDL1-E1L3N (+),
% | - | - | - | 0% | 36.8% |
<0.001a |
| PDL1-SP263 (+),
% | 42.1% | 100% |
<0.001a | - | - | - |
Associations of immunological
biomarker status with cancer progression and prognosis in patients
with immunogenic non-luminal cancer
The non-luminal group included 21 patients with TN
and 2 with non-luminal HER2-overexpressed breast cancer. The
proportion of patients positive for PD-L1 expression was
significantly higher in patients positive for MHC expression than
that in patients negative for MHC expression (P=0.048 for E1L3N and
P=0.019 for SP263, Table IV).
There was no difference in the proportion of patients with high TIL
levels between MHC status (MHC-negative and MHC-positive patients:
60.0 and 88.9%, respectively, P=0.140).) Reciprocally, the
proportion of patients with MHC expression was significantly higher
in patients positive for E1L3N or SP263 than that in patients
negative for PD-L1 expression (E1L3N-negative and E1L3N-positive
patients: 64.3 and 100%, respectively, P=0.048; SP263-negative and
SP263-positive patients: 50.0 and 93.3%, respectively, P=0.019,
Table V). No association of high
and low TIL levels with MHC and PD-L1 expression was observed
(P=0.140 with MHC, P=0.084 for E1L3N, and P=0.069 for SP263). In
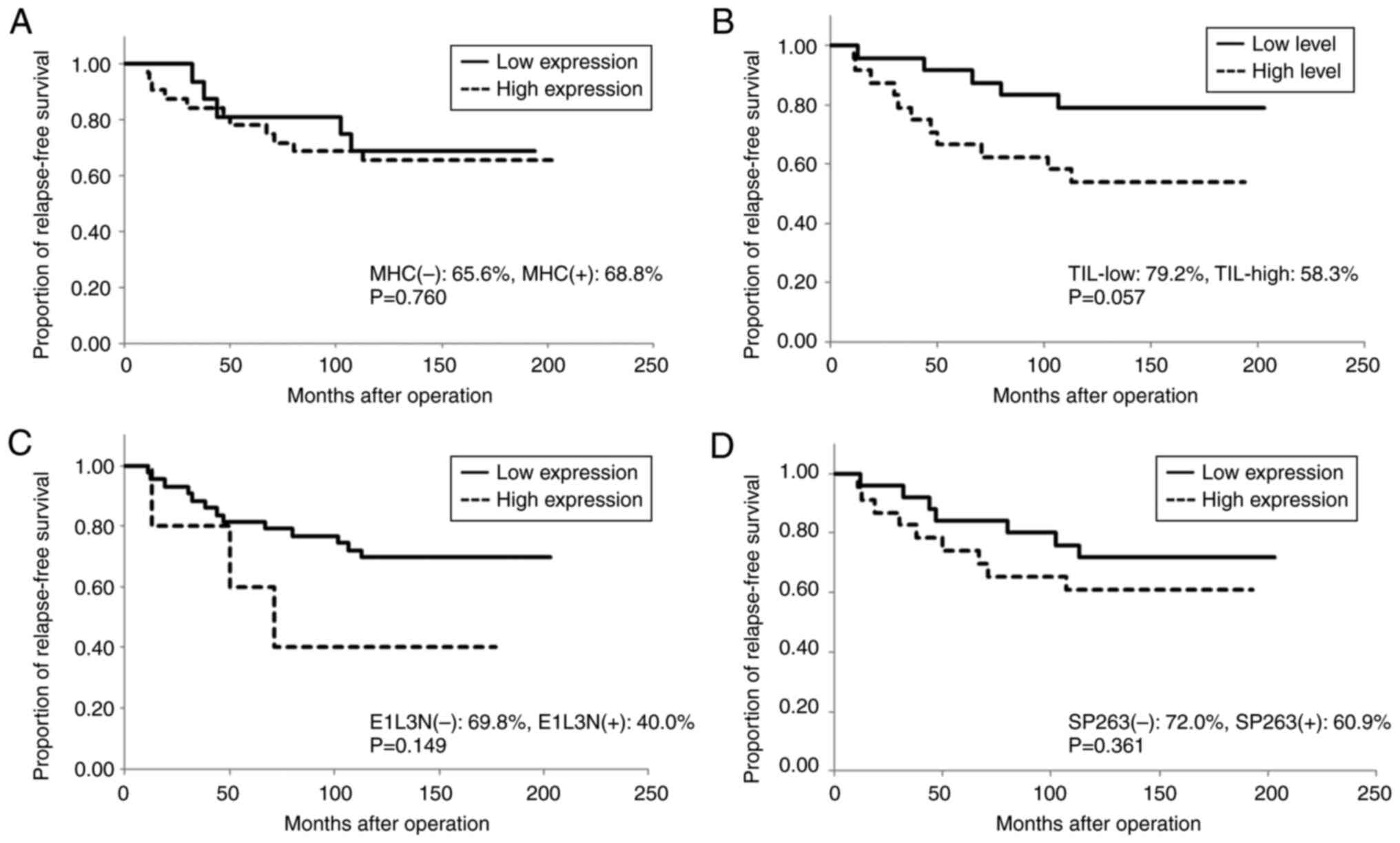
the non-luminal group, compared with patients with low TIL levels,
patients with high TIL levels showed significantly longer RFS (low
levels: median RFS of 14 months; high levels: RFS rate of 63.2% at
10 years, P=0.014; Fig. 6);
however, TIL levels were not associated with cancer progression
(Table IV). Of the remaining
markers in this group, SP263 reactivity was associated with
prognosis, with reactive patients showing slightly better OS rates
10 years after their primary operation compared with nonreactive
patients (37.5 and 73.3%, respectively, P=0.064; Fig. 7).
 | Table IV.Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of patients with
non-luminal breast cancer. |
Table IV.
Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of patients with
non-luminal breast cancer.
| Variable | MHC(−) (n=5) | MHC(+) (n=18) | P-value | Low TIL (n=4) | High TIL
(n=19) | P-value |
|---|
| Median age,
years | 66 (53–77) | 57 (31–78) | 0.141 | 47 (31–60) | 61 (40–78) | 0.109 |
| Median tumor size,
cm | 1.8 (0.5–3.5) | 2.2 (1.2–6.5) | 0.359 | 3.3 (1.0–4.0) | 2.0 (0.5–6.5) | 0.545 |
| N-positive, % | 60.0% | 61.1% | 0.965 | 75.0% | 57.9% | 0.533 |
| Median stage | 2 (1–2) | 2 (1–2) | 0.310 | 1 (1–2) | 2 (1–2) | 0.750 |
| MHC-positive,
% | - | - | - | 50.0% | 84.2% | 0.140 |
| High TILs, % | 60.0% | 88.9% | 0.140 | - | - | - |
| Median no.
CD8+ T | 31.0
(17.3–109.7) | 97.8
(32.7–176.3) | 0.052 | 28.7
(17.3–74.3) | 99.7
(51.7–176.3) | 0.007a |
| PDL1-E1L3N (+),
% | 0% | 50.0% | 0.048a | 0% | 47.3% | 0.084 |
| PDL1-SP263 (+),
% | 20.0% | 77.8% | 0.019a | 25.0% | 73.7% | 0.069 |
 | Table V.Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of patients with non-luminal breast
cancer. |
Table V.
Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of patients with non-luminal breast
cancer.
| Variable | E1L3N(−)
(n=14) | E1L3N(+) (n=9) | P-value | SP263(−) (n=8) | SP263(+)
(n=15) | P-value |
|---|
| Median age,
years | 61 (31–77) | 53 (40–76) | 0.250 | 64 (41–77) | 56 (31–76) | 0.125 |
| Median tumor size,
cm | 2.4 (0.5–3.5) | 2.0 (1.2–6.5) | 0.785 | 2.6 (0.5–3.5) | 2.1 (1.2–6.5) | 0.716 |
| N-positive, % | 57.1% | 66.7% | 0.655 | 62.5% | 60.0% | 0.909 |
| Median stage | 2 (1–2) | 2 (1–2) | 0.520 | 2 (1–2) | 2 (1–2) | 0.150 |
| MHC-positive,
% | 64.3% | 100% | 0.048a | 50.0% | 93.3% | 0.019a |
| High TILs, % | 71.4% | 100% | 0.084 | 62.5% | 93.3% | 0.069 |
| Median no.
CD8+ T | 75.3
(17.3–125.7) | 101.0
(71.7–176.3) | 0.012a | 42.2
(17.3–109.7) | 101.0
(71.7–176.3) | 0.004a |
| PDL1-E1L3N (+),
% | - | - | - | 0% | 60.0% | 0.006a |
| PDL1-SP263 (+),
% | 42.9% | 100% | 0.006a | - | - | - |
Associations of immunological
biomarker status with progression and prognosis in patients with
luminal HER2-negative cancer
In patients with luminal HER2-negative cancer, the
proportion of patients positive for MHC expression and median
number of CD8+ T lymphocyte infiltrates per patient were
significantly higher in patients with high TIL levels than those in
patients with low TIL levels (MHC-positive patients: low TIL and
high TIL levels, 50.0 and 83.3%, respectively, P=0.016; median
CD8+ T lymphocyte counts: low TIL and high TIL levels,
39.8 and 62.3, respectively, P<0.001, Table VI); moreover, a significantly
higher number of patients with high TIL levels were in a more
advanced stage of cancer compared with patients with low TIL levels
(P=0.024). Neither MHC expression nor TIL levels showed any
association with PD-L1 expression (MHC, P=0.099 for E1L3N and
P=0.312 for SP263; TIL, P=0.161 for E1L3N and P=0.153 for SP263).
Furthermore, patients with high TIL levels showed a marginal trend
to significance of having a shorter RFS than those with low TIL
levels (RFS rate at 10 years: 79.2 and 58.3% for low and high TIL
levels, respectively; P=0.057, Fig.
8). Neither MHC nor PD-L1 expression was associated with
progression or prognosis in this group (Figs 8 and 9; Tables
VI and VII). Remarkably, the
association between high TIL levels and shorter RFS in this subtype
group was contrary to that observed in the immunogenic group, in
which high TIL levels were associated with longer RFS.
 | Table VI.Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of patients with
luminal HER2-negative breast cancer. |
Table VI.
Relationships between MHC and TILs
status of breast cancer and cancer progression and prognosis as
determined by the clinicopathological feature of patients with
luminal HER2-negative breast cancer.
| Variable | MHC(−) (n=16) | MHC(+) (n=32) | P-value | Low TIL (n=24) | High TIL
(n=24) | P-value |
|---|
| Median age,
years | 61 (46–73) | 58 (32–85) | 0.641 | 61 (45–77) | 58 (32–85) | 0.411 |
| Median tumor size,
cm | 1.7 (0.7–3.1) | 1.9 (0.7–8.0) | 0.063 | 1.5 (0.7–3.1) | 2.4 (0.8–8.0) | 0.009a |
| N-positive, % | 18.8% | 31.3% | 0.191 | 12.5% | 50.0% | 0.006a |
| Median stage | 1 (1–2) | 2 (1–3) | 0.270 | 1 (1–3) | 2 (1–3) | 0.024a |
| MHC-positive,
% | - | - | - | 50.0% | 83.3% | 0.016a |
| High TILs, % | 25.0% | 62.5% | 0.016a | - | - | - |
| Median no.
CD8+ T | 39.7
(1.0–75.3) | 62.3
(24.7–137.0) | 0.002a | 39.8
(1.0–66.7) | 62.3
(24.7–137.0) |
<0.001a |
| PDL1-E1L3N (+),
% | 0% | 12.5% | 0.099 | 4.2% | 16.7% | 0.161 |
| PDL1-SP263 (+),
% | 37.5% | 53.1% | 0.313 | 37.5% | 58.3% | 0.153 |
 | Table VII.Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of patients with luminal
HER2-negative breast cancer. |
Table VII.
Relationships between PD-L1 status of
breast cancer and cancer progression and prognosis as determined by
the clinicopathological feature of patients with luminal
HER2-negative breast cancer.
| Variable | E1L3N(−)
(n=43) | E1L3N(+) (n=5) | P-value | SP263(−)
(n=25) | SP263(+)
(n=23) | P-value |
|---|
| Median age,
years | 59 (32–77) | 63 (53–85) | 0.354 | 62 (32–77) | 59 (38–85) | 0.904 |
| Median tumor size,
cm | 1.7 (0.5–3.5) | 1.5 (1.3–4.0) | 0.907 | 1.7 (0.7–8.0) | 1.5 (0.7–4.0) | 0.329 |
| N-positive, % | 27.9% | 40.0% | 0.659 | 36.0% | 26.1% | 0.464 |
| Median stage | 2 (1–3) | 1 (1–2) | 0.517 | 2 (1–3) | 1 (1–2) | 0.336 |
| MHC-positive,
% | 62.8% | 100% | 0.098 | 60.0% | 73.9% | 0.312 |
| %High TILs, % | 46.5% | 80.0% | 0.161 | 40.0% | 60.8% | 0.153 |
| Median no.
CD8+ T | 53.3
(1.0–108.0) | 80.0
(54.0–130.0) | 0.097 | 42.0
(12.0–120.3) | 66.0
(1.0–130.0) | 0.021a |
| PDL1-E1L3N (+),
% | - | - | - | 0% | 21.7% | 0.015a |
| PDL1-SP263 (+),
% | 41.9% | 100% | 0.015a | - | - | - |
Discussion
In patients with cancer, the antitumor immune
response is crucial to the regulation of cancer progression and
improvement of prognosis. However, cancer cells possess a wide
range of mechanisms for evading host immune responses including the
modification of cancer phenotypes, reduction or deletion of the
expression of antigenic proteins and MHC molecules, and production
of cytokines and factors that inhibit anticancer immune response
activation (28,58–65).
Recently, immune checkpoint inhibitors that bind to PD-1- or
PD-L1-inactivating CTLs have been found to be efficacious against
reduced antitumor immunity, with the ability to restart the immune
response to cancer when it slows or stops (16–27).
Several clinical trials have demonstrated drastically improved
prognosis in patients with cancer when immune checkpoint inhibitors
are added to chemotherapeutic agents (16–22,24–27).
Breast cancer is among the less immunogenic cancers and tends to be
minimally affected by antitumor immunity (28,29).
In fact, TIL levels in this population are not sufficiently high to
exhibit prognostic value (52,66).
Nevertheless, some subtypes of breast cancer such as TN and
HER2-overexpressed cancer both of which can grow rapidly and show
aggressive behavior have been reported to be sensitive to antitumor
immune responses and have demonstrated favorable responses to
immune checkpoint inhibitors (34–38).
Immunological biomarkers, including TILs, CD8+ T
lymphocyte infiltrates, MHC, and the PD-1-PD-L1 axis, have been
found to be useful for predicting cancer progression and prognosis
of patients with the immunogenic subtypes of breast cancer
(40–49). However, these markers have been
found to predict different prognostic outcomes (35,40–42,67)
owing to differences in patient backgrounds and disease stage as
well as the proportion of each subtype and combination of
biomarkers studied. Furthermore, no report has analyzed
associations of cancer progression and patient prognosis with the
four principal immunological biomarkers simultaneously in each
subtype of breast cancer. Therefore, although immunological
biomarkers are expected to be useful in breast cancer, it remains
unclear whether they have any prognostic value, particularly in
luminal HER2-negative breast cancer.
We evaluated the status of immunological biomarkers,
including TIL levels, CD8+ T lymphocyte infiltrate
count, MHC expression, and PD-L1 expression, in the tumors of 71
patients with primary breast cancer to determine their utility as
predictors of cancer progression and prognosis. To date, only B
cells and macrophages in TILs have been found to predict survival
rates in luminal HER2-negative breast cancer (52); to the best of our knowledge, the
prognostic value of other biomarkers has not been previously
evaluated in this population. This is the first report on the
status and prognostic value of principal immunological biomarkers
such as MHC, TILs, CD8+ T lymphocyte infiltrates, and
PD-L1 in luminal HER2-negative and other breast cancer subtypes.
Monoclonal antibodies against PD-1 or PD-L1 (SP142 and 22C3)
currently available as a companion diagnostic agent for potential
breast cancer treatment with immune checkpoint inhibitors have
demonstrated different prognostic capacities (37–41,67).
These monoclonal antibodies exhibited positivity in <5% of
patients with luminal HER2-negative breast cancer in our
preliminary study (data not shown). Therefore, in the present
study, we used alternative clones (E1L3N and SP263) available for
use with non-small cell lung cancer (35,56,68–70).
We observed that PD-L1 expression (reactive with
both E1L3N and SP263) was generally associated with MHC expression
in tumor cells and with stromal TIL levels (Table III). We further found that
CD8+ T lymphocyte infiltrate counts were significantly
associated with the status of all biomarkers in all breast cancer
subtypes. These results suggest that according to breast cancer
subtype, CD8+ CTLs in stromal TILs can recognize tumor
antigens to varying degrees in an MHC-restricted manner and lyse
cancer cells. Furthermore, the CTL response to breast cancer can be
inactivated by PD-1-PD-L1 interaction.
The present study results suggested that MHC and TIL
status were strongly associated with cancer progression and that
PD-L1 status (in terms of its reactivity with E1L3N) exhibited
possible prognostic value for all breast cancer subtypes. To
determine whether the status of these biomarkers differed between
cancer subtypes and whether they interacted with cancer progression
or prognosis in each subtype, the patients were classified into two
cancer subtype groups according to immunogenicity: patients with
less immunogenic luminal HER2-negative cancer and those with
immunogenic non-luminal breast cancers. In the immunogenic group,
no association was observed between TIL levels and MHC expression;
however, TIL levels and MHC expression were closely associated with
PD-L1 expression (Tables IV and
V). Of the patients who tested
negative for MHC expression, those reactive to E1L3N and SP263
accounted for only 0 and 20% (1 case), respectively (Table IV). Similarly, the number of
patients reactive to E1L3N and SP263 among those with low TIL
levels was quite low (no case and 1 case, respectively). Indeed,
there is a small population who is deficient in biomarker
expression in immunogenic breast cancer subtypes. These patients
are unlikely to be affected by antitumor immunity. Furthermore, TIL
status was found to be a good predictor of prognosis in this group,
with high TIL levels indicating significantly longer RFS. These
results are consistent with those of previous studies in that high
TIL levels were associated with good prognoses in TN and
HER2-overexpressed breast cancer (52,66).
Moreover, high SP263 reactivity was associated with longer OS
(Fig. 7D). The close relationship
of PD-L1 expression with good patient prognosis has previously been
reported in several studies (40–42,67),
and our results are consistent with those findings. Therefore, TIL
levels and PD-L1 expression can be useful prognostic biomarkers in
immunogenic breast cancer. However, contrary to our expectations,
none of the biomarkers was associated with cancer progression in
this subtype. It is possible that antitumor immunity is merely one
of the factors influencing cancer progression. Tumor
characteristics such as growth ability, differentiation grade, and
metastatic ability, are also likely to considerably contribute to
cancer progression.
In the less immunogenic luminal HER2-negative breast
cancer group, high TIL levels were strongly associated with cancer
progression and associated with poor prognoses (Table VI and Fig. 8B). In the luminal group, among
patients negative for MHC expression or those with low TIL levels,
only few patients exhibited reactivity to both anti-PD-L1
monoclonal antibodies (Table VI).
Therefore, it is difficult to arrive at a conclusion from the data
of patients who tested negative for biomarker expression. The other
biomarkers showed no association with either cancer progression or
prognosis. Remarkably, the relationship between high TIL levels and
poor prognosis in the luminal HER2-negative group was contrary to
that observed in the immunogenic non-luminal group, in which high
TIL levels were associated with good prognoses. This suggests that
in the immunogenic non-luminal population, TILs in the tumor stroma
contribute to the immunosuppression of cancer, thereby prolonging
RFS. Conversely, the lower level of immunogenicity in luminal
HER2-negative tumor cells reduces their receptivity to host immune
responses, thus allowing more aggressive growth and progression.
Based on the relationship observed between high TIL levels and poor
patient prognosis as well as the significantly lower proportion of
patients with PD-L1 expression or those with high TIL levels in the
luminal group compared with those in the non-luminal group, we
confirmed that luminal HER2-negative breast cancer is less
immunogenic. Therefore, TIL status in different breast cancer
subtypes appears to reflect the distinct microbiology of tumor
cells of the given subtype, in terms of the marked difference in
their susceptibility to host immune responses. In the literature,
only two studies have reported the relationship between TIL levels
and patient prognosis in luminal HER2-negative breast cancer.
Denkert et al (52) reported
significant correlations between high TIL levels and shorter OS.
The result was consistent with the findings of the present study.
However, the other study reported no significant relationship
between them (66). In both
studies, good correlations were observed between high TIL levels
and favorable patient prognoses in TN and HER2-overexpressed breast
cancer subtypes. To clarify the association between TIL levels and
prognosis, further studies including larger cohorts of patients
with luminal HER2-negative cancer are required; these studies
should aim to perform a detailed analysis for determining the
lymphocyte and antigen-presenting cell populations that infiltrate
the tumor stroma and the specific cytokines (e.g., interferon-gamma
or tumor growth factor-beta) responsible for immune activation.
One of the limitations of our study is the small
sample size (n=71); we thus could not classify a sufficient number
of patients into groups to perform more convincing comparative
analyses. Furthermore, we did not analyze systemic immunological
responses, such as leukocyte profiles in peripheral blood,
immunoglobulin and complement levels, or cytokine production in the
studied patients. By including analysis of systemic immunological
responses in patients with breast cancer in a future study, we will
be able to understand the role of antitumor immunity more
comprehensively in breast cancer. In this study, we used two
anti-PD-L1 monoclonal antibodies, which were produced by immunizing
rabbits with synthetic peptides derived from residues near the
C-terminus of PD-L1 protein. The sensitivity of SP263 in detecting
PD-L1 expression was generally higher than that of E1L3N. Although
the precise epitopes of both monoclonal antibodies has not been
reported, these may be different but located nearby. As these
antibodies recognize their antigenic determinants on the
three-dimensional components of the target protein in immunological
assays, the sensitivity of each monoclonal antibody is expected to
differ. Regarding the prognostic value of the PD-L1 status in
luminal HER2-negative breast cancer, Zhang et al (67) found no association between PD-L1
expression and OS; this finding was consistent with our results.
However, significant associations were observed between PD-L1
expression and survival rates in TN and HER2-overexpressed breast
cancer subtypes.
In conclusion, the immunological biomarkers MHC,
TIL, and PD-L1 exhibited different patterns of expression depending
on the breast cancer subtype of the patient. However,
CD8+ T lymphocyte infiltrate counts were closely
associated with TIL levels and MHC and PD-L1 expression regardless
of the breast cancer subtype. Of these biomarkers, only TIL levels
are expected to be associated with cancer progression and patient
prognosis, regardless of the breast cancer subtype. Although the
PD-L1 protein reactive to SP263 is a potential prognostic biomarker
in immunogenic cancers, it is unrelated to either cancer
progression or patient prognosis in luminal HER2-negative breast
cancer.
Acknowledgements
The authors would like to thank Ms. Hiromi Kita and
Ms. Miho Takigawa (Department of Thoracic, Breast and Endocrine
Surgery, Kagawa University Faculty of Medicine, Kagawa, Japan) for
their editorial assistance with an earlier version of this
manuscript.
Funding
This study was supported in part by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Science, Sports
and Culture, Japan (grant nos. 10671249, 13671380, 14571262 and
15591340).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CM, KeK and KT conceived and designed the present
study. KaK, SN, SH, MM and TM contributed to data acquisition and
analysis. KeK, CM, TY and RH were major contributors in writing the
manuscript. TY RH and NH were involved in data interpretation and
discussion. NA and MI performed the statistical analysis. NH, NA
and MI confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol for this study complied with
the guidelines of the Ethics Committee at Kagawa University
Hospital and was approved by the ethical review board of Kagawa
University (approval no. HEISEI23-085); it conformed to the
provisions in the Declaration of Helsinki in 1995. Written informed
consent to participate was obtained from all study
participants.
Patient consent for publication
When patients were given written information about
the present study, written patient consent for publication was also
obtained.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CD
|
cluster of differentiation
|
|
CTL
|
cytotoxic T lymphocyte
|
|
DAB
|
3,3′-diaminobenzidine
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
MHC
|
major histocompatibility complex
|
|
OS
|
overall survival
|
|
PBS
|
phosphate-buffered saline
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed cell death-ligand-1
|
|
RFS
|
relapse-free survival
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
TN
|
triple-negative
|
References
|
1
|
Molina R, Jo J, Filella X, Zanon G, Pahisa
J, Mu noz M, Farrus B, Latre ML, Escriche C, Estape J and Ballesta
AM: c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast
cancer: Prognostic value. Breast Cancer Res Treat. 51:109–119.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berry DA, Cirrincione C, Henderson IC,
Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB,
Norton L, et al: Estrogen-receptor status and outcomes of modern
chemotherapy for patients with node-positive breast cancer. JAMA.
295:1658–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Ravdin P, Hayes DF, Bates S,
Fritsche H Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG,
Somerfield MR, et al: 2000 Update of recommendations for the use of
tumor markers in breast and colorectal cancer: Clinical practice
guidelines of the American society of clinical oncology. J Clin
Oncol. 19:1865–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Penault-Llorca F, André F, Sagan C,
Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC,
Bibeau F, Antoine M, et al: Ki67 expression and docetaxel efficacy
in patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2809–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yarden Y: The EGFR family and its ligands
in human cancer. Signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37 (Suppl 4):S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sjögren S, Inganäs M, Lindgren A, Holmberg
L and Bergh J: Prognostic and predictive value of c-erbB-2
overexpression in primary breast cancer, alone and in combination
with other prognostic markers. J Clin Oncol. 16:462–469. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roche PC and Ingle JN: Increased HER2 with
U.S. food and drug administration-approved antibody. J Clin Oncol.
17:4341999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slamon D, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: An
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundquist M, Brudin L and Tejler G:
Improved survival in metastatic breast cancer 1985–2016. Breast.
31:46–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith I, Procter M, Gelber RD, Guillaume
S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G,
Baselga J, et al: 2-Year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: A randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schadendorf D, Hodi FS, Robert C, Weber
JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM and Wolchok JD:
Pooled analysis of long-term survival data from phase II and phase
III trials of ipilimumab in unresectable or metastatic melanoma. J
Clin Oncol. 33:1889–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loi S, Adams S, Schmid P, Cortes J, Cescon
DW, Winer EP, Toppmeyer DL, Rugo HS, De Laurentiis M, Nanda R, et
al: Relationship between tumor infiltrating lymphocyte (TIL) levels
and response to pembrolizumab (pembro) in metastatic
triple-negative breast cancer (mTNBC): Results from KEYNOTE-086.
Ann Oncol. 28 (Suppl 5):v6082017. View Article : Google Scholar
|
|
25
|
AlHarbi M, Ali Mobark N, AlMubarak L,
Aljelaify R, AlSaeed M, Almutairi A, Alqubaishi F, Hussain ME,
Balbaid AAO, Said Marie A, et al: Durable response to nivolumab in
a pediatric patient with refractory glioblastoma and constitutional
biallelic mismatch repair deficiency. Oncologist. 23:1401–1406.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fremda C, Hlevnjakb M, Zapatkab M, Zoernig
I, Halamaa N, Fejzibegovica N, Thewesb V, Lichterb P, Schirmacherc
P, Kloorc M, et al: Mismatch repair deficiency drives durable
complete remission by targeting programmed death receptor 1 in a
metastatic luminal breast cancer patient. Breast Care (Basel).
14:53–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bates JP, Derakhshandeh R, Jones L and
Webb TJ: Mechanisms of immune evasion in breast cancer. BMC Cancer.
18:5562018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rugo HS, Delord JP, Im SA, Ott PA,
Piha-Paul SA, Bedard PL, Sachdev J, Tourneau CL, van Brummelen EMJ,
Varga A, et al: Safety and antitumor activity of pembrolizumab in
patients with estrogen receptor-positive/human epidermal growth
factor receptor 2-negative advanced breast cancer. Clin Cancer Res.
24:2804–2811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zacharakis N, Huq LM, Seitter SJ, Kim SP,
Gartner JJ, Sindiri S, Hill VK, Li YF, Paria BC, Ray S, et al:
Breast cancers are immunogenic: Immunologic analyses and a phase II
pilot clinical trial using mutation-reactive autologous
lymphocytes. J Clin Oncol. 40:1741–1754. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoda RS, Brogi E, Dos Anjos CH,
Grabenstetter A, Ventura K, Patil S, Selenica P, Weigelt B,
Reis-Filho JS, Traina T, et al: Clinical and pathologic features
associated with PD-L1 (SP142) expression in stromal
tumor-infiltrating immune cells of triple-negative breast
carcinoma. Mod Pathol. 33:2221–2232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Emens LA, Cruz C, Eder JP, Braiteh F,
Chung C, Tolaney SM, Kuter I, Nanda R, Cassier PA, Delord JP, et
al: Long-term clinical outcomes and biomarker analyses of
atezolizumab therapy for patients with metastatic triple-negative
breast cancer: A phase 1 study. JAMA Oncol. 5:74–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nanda R, Chow LQ, Dees EC, Berger R, Gupta
S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al:
Pembrolizumab in patients with advanced triple-negative breast
cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 34:2460–2467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Emens LA, Kok M and Ojalvo LS: Targeting
the programmed cell death-1 pathway in breast and ovarian cancer.
Curr Opin Obstet Gynecol. 28:142–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schalper KA, Velcheti V, Carvajal D,
Wimberly H, Brown J, Pusztai L and Rimm DL: In situ tumor PD-L1
mRNA expression is associated with increased TILs and better
outcome in breast carcinomas. Clin Cancer Res. 20:2773–2782. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baptista MZ, Sarian LO, Derchain SF, Pinto
GA and Vassallo J: Prognostic significance of PD-L1 and PD-L2 in
breast cancer. Hum Pathol. 47:78–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bae SB, Cho HD, Oh MH, Lee JH, Jang SH,
Hong SA, Cho J, Kim SY, Han SW, Lee JE, et al: Expression of
programmed death receptor ligand 1 with high tumor-infiltrating
lymphocytes is associated with better prognosis in breast cancer. J
Breast Cancer. 19:242–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahmoud SMA, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AHS, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin
Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Z, Dong P, Ren M, Song Y, Qian X, Yang
Y, Li S, Zhang X and Liu F: PD-L1 expression is associated with
tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and
poor prognosis of patient. J Cancer. 7:784–793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qin T, Zeng Y, Qin G, Xu F, Lu JB, Fang
WF, Xue C, Zhan JH, Zhang XK, Zheng QF, et al: High PD-L1
expression was associated with poor prognosis in 870 Chinese
patients with breast cancer. Oncotarget. 6:33972–33981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Madjd Z, Spendlove I, Pinder SE, Ellis IO
and Durrant LG: Total loss of MHC class I is an independent
indicator of good prognosis in breast cancer. Int J Cancer.
117:248–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gudmundsdóttir I, Gunnlaugur Jónasson J,
Sigurdsson H, Olafsdóttir K, Tryggvadóttir L and Ogmundsdóttir HM:
Altered expression of HLA class I antigens in breast cancer:
Association with prognosis. Int J Cancer. 89:500–505. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zitvogel L, Tesniere A and Kroemer G:
Cancer despite immunosurveillance: Immunoselection and
immunosubversion. Nat Rev Immunol. 6:715–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Savas P, Salgado R, Denkert C, Sotinou C,
Darcy PK, Smyth MJ and Loi S: Clinical relevance of host immunity
in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol.
13:228–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Davies H, Morganella S, Purdie CA, Jang
SJ, Borgen E, Russnes H, Glodzik D, Zou X, Viari A, Richardson AL,
et al: Whole-genome sequencing reveals breast cancers with mismatch
repair deficiency. Cancer Res. 77:4755–4762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Denkert C, von Mincwitz G, Darb-Esfahni S,
Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F,
Furlanetto J, et al: Tumour-infiltrating lymphocytes and prognosis
in different subtypes of breast cancer: A pooled analysis of 3771
patients treated with neoadjuvant therapy. Lancet Oncol. 19:40–50.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Denkert C, Wienert S, Poterie A, Loibl S,
Budczies J, Badve S, Bago-Horvath Z, Bane A, Bedri S, Brock J, et
al: Standardized evaluation of tumor-infiltrating lymphocytes in
breast cancer: Results of the ring studies of the international
immuno-oncology biomarker working group. Mod Pathol. 29:1155–1164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
international TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Loi S, Michiels S, Adams S, Loibl S,
Budczies J, Denkert C and Salgado R: The journey of
tumor-infiltrating lymphocytes as a biomarker in breast cancer:
Clinical utility in an era of checkpoint inhibition. Ann Oncol.
32:1236–1244. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Igarashi T, Teramoto K, Ishida M, Hanaoka
J and Daigo Y: Scoring of PD-L1 expression intensity on pulmonary
adenocarcinomas and the correlations with clinicopathological
factors. ESMO Open. 1:e0000832016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brookmeyer R and Crowley J: A k-sample
median test for censored data. J Am Stat Assoc. 77:433–440. 1982.
View Article : Google Scholar
|
|
58
|
Wen ZF, Liu H, Gao R, Zhou M, Ma J, Zhang
Y, Zhao J, Chen Y, Zhang T, Huang F, et al: Tumor cell-released
autophagosomes (TRAPs) promote immunosuppression through induction
of M2-like macrophages with increased expression of PD-L1. J
Immunother Cancer. 6:1512018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vitale I, Manic G, Coussens LM, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Drake CG, Jaffee E and Pardoll DM:
Mechanisms of immune evasion by tumors. Adv Immunol. 90:51–81.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mamessier E, Sylvain A, Thibult ML,
Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P,
Romagné F, Thibault G, et al: Human breast cancer cells enhance
self tolerance by promoting evasion from NK cell antitumor
immunity. J Clin Invest. 121:3609–3622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fang Y, Wang L, Wan C, Sun Y, Van der
Jeught K, Zhou Z, Dong T, So KM, Yu T, Li Y, et al: MAL2 drives
immune evasion in breast cancer by suppressing tumor antigen
presentation. Clin Invest. 131:e1408372021. View Article : Google Scholar
|
|
65
|
Spranger S and Gajewski TF: Impact of
oncogenic pathways on evasion of antitumour immune responses. Nat
Rev Cancer. 18:139–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, et
al: Prognostic and predictive value of tumor-infiltrating
lymphocytes in a phase III randomized adjuvant breast cancer trial
in node-positive breast cancer comparing the addition of docetaxel
to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J
Clin Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang N, Sun H, Zhao S, Wang Y, Pu H, Wang
Y and Zhang Q: Expression of PD-L1 and prognosis in breast cancer:
A meta-analysis. Oncotarget. 8:31347–31354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Igarashi T, Teramoto K, Ishida M, Hanaoka
J and Daigo Y: The mechanism of de novo expression of programmed
cell death-ligand 1 in squamous cell carcinoma of the lung. Oncol
Rep. 38:2189–2196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Teramoto K, Igarashi T, Kataoka Y, Ishida
M, Hanaoka J, Sumimoto H and Daigo Y: Biphasic prognostic
significance of PD-L1 expression status in patients with early- and
locally advanced-stage non-small cell lung cancer. Cancer Immunol
Immunother. 70:1063–1074. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Smith J, Robida MD, Acosta K, Vennapusa B,
Mistry A, Martin G, Yates A and Hnatyszyn HJ: Quantitative and
qualitative characterization of two PD-L1 clones: SP263 and E1L3N.
Diag Pathol. 11:442016. View Article : Google Scholar
|