Introduction
Esophageal carcinoma (ESCA) is the seventh most
commonly encountered cancer worldwide. According to the latest
global cancer statistics, 604,000 estimated new cases of esophageal
cancer and 544,000 related deaths have been reported, with the
mortality rate ranking sixth among all types of cancer (1). Adenocarcinoma (AC) and squamous cell
carcinoma (SCC) are the most frequent histological subtypes of
ESCA, of which SCC cases comprise 90% of all ESCA cases; China,
Central Asia, East Africa and South Africa have the highest rates
of ESCA (2). A large body of
evidence suggests that smoking, alcoholism, obesity, poor nutrition
and viral infection may increase the risk of developing ESCA
(3). At present, the treatment for
ESCA is relatively simple and mainly involves esophagectomy;
however, this may lead to severe post-operative complications and
can markedly increase the risk of mortality (4). Hence, detailed insight into the
mechanisms of the development and progression of ESCA are crucial
for the identification of novel therapeutic targets.
Human papillomavirus (HPV) is a double-stranded DNA
virus that easily infects the mucosal epithelial tissues of the
anogenital and upper aerodigestive tracts (5). High-risk HPV infections are the
leading cause of cervical carcinomas, and almost all cervical
cancers are the result of HPV infection (6). There is increasing evidence to
indicate that HPV is also associated with the development of
vaginal, penile, anal, and head and neck cancers (5). Globally, 570,000 female and 60,000
male cancer-related cases are attributable to HPV each year,
demonstrating an increasing trend (7). Although the association between HPV
and ESCA is generally controversial, studies have found that ESCA
samples are accompanied by HPV infection, and HPV infection is a
negative prognostic factor for patients with ESCA (8,9). It
has been previously demonstrated by the authors' laboratory that
HPV18 E6E7 induces the immortalization and malignant transformation
of human embryonic esophageal epithelial cells (10,11).
Therefore, this evidence may suggest that HPV infection is indeed
one of the key oncogenic factors for ESCA.
Cell death can be categorized as accidental cell
death and regulated cell death (RCD). RCD includes apoptosis,
necrosis, autophagy, pyroptosis, ferroptosis, entotic cell death,
netotic cell death, parthanatos, lysosome-dependent cell death,
alkaliptosis and oxeiptosis (12,13).
Several studies have revealed that HPV inhibits cell death to
promote the malignancy of cancer cells. For example, HPV E7
inhibits the necrosis of SiHa cells, while E7 knockdown induces
cell necrosis (14). The tumor
suppressor p53 has been demonstrated to induce cell cycle arrest
and apoptosis, and p53 is a recognized target protein of HPV E6.
HPV E6 degrades p53 to promote cell proliferation and inhibit
apoptosis (15,16). In autophagy, HPV16 E5 downregulates
the expression of key autophagy genes, including Beclin 1,
autophagy-related (ATG)5, microtubule-associated protein 1 light
chain 3 alpha (LC3), Unc-51 like autophagy activating kinase 1
(ULK1), Unc-51 like autophagy activating kinase 2 (ULK2), autophagy
related 4A cysteine peptidase (ATG4A) and ATG7, while E6E7 inhibits
autophagosome-lysosome fusion (17). Although there are limited studies
available on whether HPV affects pyroptosis, it has also been
confirmed that HPV can inhibit the occurrence of pyroptosis
(18). HPV is one of the key
carcinogenic factors of ESCA, and the majority ESCA samples are
also accompanied by HPV infection (8,9).
However, the mechanisms through which HPV is involved in ESCA
occurrence and particularly the mechanisms through it affects cell
death pathways in ESCA, warrant further investigation.
Transcriptomics is the study of the expression and
regulation of genes overall. Using transcriptomics to study tumor
pathogenesis, explore pathway functions and identify biomarkers for
tumor diagnosis and prevention has always been a hotspot in tumor
research. In the present study, differentially expressed genes were
obtained through the original transcriptomic data of HPV18-positive
and HPV-negative ESCC samples. A bioinformatics analysis of
differentially expressed genes, combined with experimental
verification, was conducted in order to examine the effects of
HPV18 on the death pathway of ESCC at the transcriptional
level.
Materials and methods
RNA-Seq raw data acquisition and
analysis
HPV18-positive and HPV-negative ESCC transcriptomic
raw data were downloaded from the NCBI BioProject database
(https://www.ncbi.nlm.nih.gov/bioproject/) through the
Accession no. PRJNA530677 (SRX5628804, SRX5628801 and SRX5628804).
In these data, HPV18-positive ESCC cells (EC9706 and EC109) and
HPV-negative ESCC cells (HKESC01) were derived from a Chinese
patient with ESCA (19).
Subsequently, the data were processed using Hisat2 (http://www.ccb.jhu.edu/software/hisat/)
+ Stringtie (http://ccb.jhu.edu/software/stringtie). Read counts
were adjusted by a scaling normalization factor using the edgeR
package. Differential expression analysis was performed using edgeR
(http://bioconductor.org).
Bioinformatics analysis
The Gene Ontology analysis (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analysis of differentially
expressed genes were performed using OmicsBean V1.0 (http://www.omicsbean.cn/). The GEPIA2 (http://gepia2.cancer-pku.cn/) website was used to
perform the analysis of The Cancer Genome Atlas (TCGA) gene
expression and Kaplan-Meier survival analysis associated with the
differentially expressed genes in clinical samples of ESCA. The
correlation of differentially expressed genes with tumor-associated
fibroblast infiltration was performed using the TIMER2.0 database
(http://timer.cistrome.org/).
Protein-protein interactions were analyzed using STRING (https://string-db.org/).
Cells, cell culture and
transfection
Two HPV18-positive ESCC cell lines (EC109 and
EC9706) and two HPV-negative ESCC cell lines (KYSE150 and KYSE180)
and 293T cells were cultured in DMEM medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(HyClone; Cytiva), and placed in a 5% CO2 constant
temperature cell culture incubator (Thermo Fisher Scientific, Inc.)
at 37°C. The KYSE150 (TCHu236) and 293T (GNHu17) cells were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The EC109 and EC9706 cells were
obtained from Chinese Center for Disease Control and Prevention.
The KYSE180 cells was obtained from Shantou University Medical
College. The KYSE150, KYSE180, EC109, EC9706 cells originate from
the resected specimens of patients with ESCA (19,20).
The HPV18 E6E7 gene was obtained from the NCBI gene database
(https://www.ncbi.nlm.nih.gov/gene)
and synthesized by Tsingke Biological Technology. The HPV18 E6E7
gene was assembled into a pLVX-puro vector, named pLVX-puro-E6E7.
The pLVX-puro-E6E7 (8 µg), pMD2.G (1.25 µg) and psPAX2 (5.75 µg)
plasmids were transfected into 293T cells using 20 µl jetPRIME
transfection reagent (Polyplus-transfection SA) in 10-cm dish for
lentiviral packaging. The culture medium was changed after 6 h of
transfection and the supernatant was collected at 48 and 72 h,
respectively. The supernatants of two times were mixed and
concentrated with lentivirus concentration reagent (cat. no.
BF06205; Biodragon-Immunotech. Inc.). Following KYSE150 and KYSE180
cell transfection with lentivirus and 8 µg/ml polybrene (cat. no.
HY112735; MedChemExpress) for 37°C, 48 h, 8 µg/ml puromycin (cat.
no. P8230; Beijing Solarbio Science & Technology Co., Ltd.) was
added. The cells stably expressing HPV18 E6E7 were obtained after
10 days of continuous culture, named KYSE150-E6E7 and KYSE180-E6E7.
The LC3-RFP-GFP (2 µg) plasmid was transfected into KYSE150 cells
and KYSE150-E6E7 cells with 4 µl jetPRIME transfection reagent in a
six-well plate for 48 h. The pLVX-puro plasmid was obtained from
the Chinese Center for Disease Control and Prevention. pMD2.G and
psPAX2 plasmids were obtained from the Institute of Biophysics of
the Chinese Academy of Sciences. The LC3-RFP-GFP plasmid was
purchased from Tsingke Biological Technology.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
In total, 11 genes were selected, and were enriched
in pathways related to apoptosis, autophagy and pyroptosis to
verify the NCBI BioProject database PRJNA530677 RNA-seq results
acquired through bioinformatics analysis, as aforementioned. PCR
primers can be found in PrimerBank (https://pga.mgh.harvard.edu/primerbank/) or were
designed using the Oligo7 software according to the instructions
(Table SI). Total RNA was
extracted from the cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). A PrimeScript RT kit
with gDNA Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.)
was used to synthesize 1 µg of total RNA with a final volume of 20
µl, in order to synthesize the first-strand cDNA. In the process of
reverse transcription, the conditions for removing genomic DNA are
2 min at 42°C and 0 sec at 4°C. The conditions for reverse
transcription were 15 min at 37°C, 5 sec at 85°C and 0 sec at 4°C.
SYBR Premix DimerEraserTM (cat. no. RR091A; Takara Biotechnology
Co., Ltd.) was used to detect gene expression with a ViiA7 RT-qPCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
GAPDH and β-actin were used as the internal controls. The KYSE150
and KYSE180 cell lines were used as experimental controls. In
total, four sets of each gene were repeated for a 10 µl PCR
reaction. The pre-denaturation occurred at 95°C for 30 sec, and
then for 40 cycles; each cycle included 5 sec at 95°C, 30 sec at
55°C and 30 sec at 72°C. The melting curve cycle included 0 sec at
95°C, 15 sec at 65°C and 0 sec at 95°C. The 2−ΔΔCq
method was used to calculate relative gene expression levels
(21).
Apoptosis induction and detection
The apoptosis of KYSE150 cells was induced by
cisplatin (DDP; cat. no. HY17394; MedChemExpress). DDP is an
antineoplastic chemotherapy agent by cross-linking with DNA and
causing DNA damage in cancer cells, it also can activate
extracellular-signal-regulated protein kinase (ERK) to cause tumor
cell death (22). After treating
the KYSE150 cells with 20, 40 and 80 µM DDP, and the KYSE150-E6E7
cells with 80 µM DDP for 24 h, the cells and supernatant were
collected and centrifuged at 1,000 × g at room temperature for 5
min, and then were washed twice with PBS (Gibco; Thermo Fisher
Scientific, Inc.) and centrifuged again at 1,000 × g for room
temperature, 5 min each time. An Annexin V FITC Apoptosis detection
kit (cat. no. AD10; Dojindo Laboratories, Inc.) was used to detect
apoptosis, and 5 µl Annexin V FITC and 5 µl PI solution were added
to each group. Apoptosis was analyzed using BD FACSCalibur flow
cytometry (BD Biosciences) and FlowJo v10.6.1 software (https://www.flowjo.com/).
Pyroptosis induction
The metabolites α-ketoglutarate can activate
pyroptosis through the GSDMC pathway (23); herein, 15 mM of α-ketoglutarate
(cat. no. S30041, Shanghai Yuanye Bio-Technology Co., Ltd.) were
added to the KYSE150 and KYSE150-E6E7 cells for 24 h, and the cells
were cultured at 37°C under 5% CO2 conditions.
Subsequently, the morphology of cells undergoing pyroptosis was
observed after 4 h using an inverted fluorescence Axio Observer A1
microscope (Zeiss AG).
Autophagy induction and detection
(using western blot anlaysis)
The LC3-RFP-GFP plasmid was transferred into cells.
Afterwards, following an 24-h time period, the KYSE150 and
KYSE150-E6E7 cells were induced with Hank's Balanced Salt Solution
(HBSS; Gibco; Thermo Fisher Scientific, Inc.) for 3 h, and the
cells were fixed with 4% paraformaldehyde (cat. no. 441244;
MilliporeSigma) and observed and photographed using a laser
confocal microscope LSM 5 (Zeiss AG). Following 3 h of HBSS
induction, each group of cells was washed twice with ice-cold PBS
and a RIPA lysis buffer (Cell Signaling Technology, Inc.)
containing protease inhibitor mixture (Roche Diagnostics GmbH) was
used for processing. The sample was centrifuged at a rate of 12,000
× g for 10 min at 4°C, and the supernatant was extracted. The BCA
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.) was used to measure the total protein concentration of the
sample, and the sample was diluted with PBS to 4 mg/ml.
Subsequently, a 5X sodium sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) Sample Loading Buffer (Applygen
Technologies, Inc.) was added and boiled in a water bath at 100°C
for 5 min. The 40 µg/pore protein sample was added to 10% SDS-PAGE
for processing; a semidry method was used to transfer it to a PVDF
membrane (MilliporeSigma); 5% skimmed milk powder diluted with TBST
was used at room temperature for 1 h for blocking. The membrane
with was incubated with the diluted primary antibodies at 4°C
overnight. The primary antibodies used included a rabbit anti-LC3B
polyclonal antibody (1:1,000; cat. no. EPR18709; Abcam) and rabbit
polyclonal antibody GAPDH (1:5,000; cat. no. 10494-1-AP;
Proteintech Group, Inc.). Subsequently, the membrane was washed
three times with TBST (cat. no. B1009; Applygen Technologies, Inc.)
for 10 min each time, and then incubated with goat anti-rabbit IgG
secondary antibody (cat. no. 072-06-15-06; KPL, Inc.) with marked
Dyelight 680 at room temperature for 1 h. Finally, the Odyssey
infrared imaging system (LI-COR Biosciences) was used for detection
and quantification was performed using ImageJ 1.52a software
(National Institutes of Health).
Statistical analysis and graphing
All the data graphs were constructed using GraphPad
Prism 7.04 (https://www.graphpad.com/), Cytoscape
V3.7.1 (https://cytoscape.org/) and TBtools
v1.108 (24) software. The graphs
of TCGA gene expression, Kaplan-Meier survival analysis and
tumor-associated fibroblast infiltration were obtained
bioinformatics analysis website. All statistical analyses were
performed using GraphPad Prism 7.04 and SPSS 22.0 (IBM Corp.). The
data results are expressed as the mean ± standard deviation.
Comparisons between two groups were made using an unpaired
Student's t-test. Comparisons between multiple groups were made
using one-way ANOVA followed by Tukey's post hoc test. Kaplan-Meier
analysis was performed with the log-rank test. Tumor-associated
fibroblast infiltration data was made using Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
RCD-related pathways are significantly
dissimilar and closely related to classical cancer pathways
By comparing data for HPV18-positive ESCC cells
(EC9706) with HPV-negative cells (HKESCC01), 8,962 distinct genes
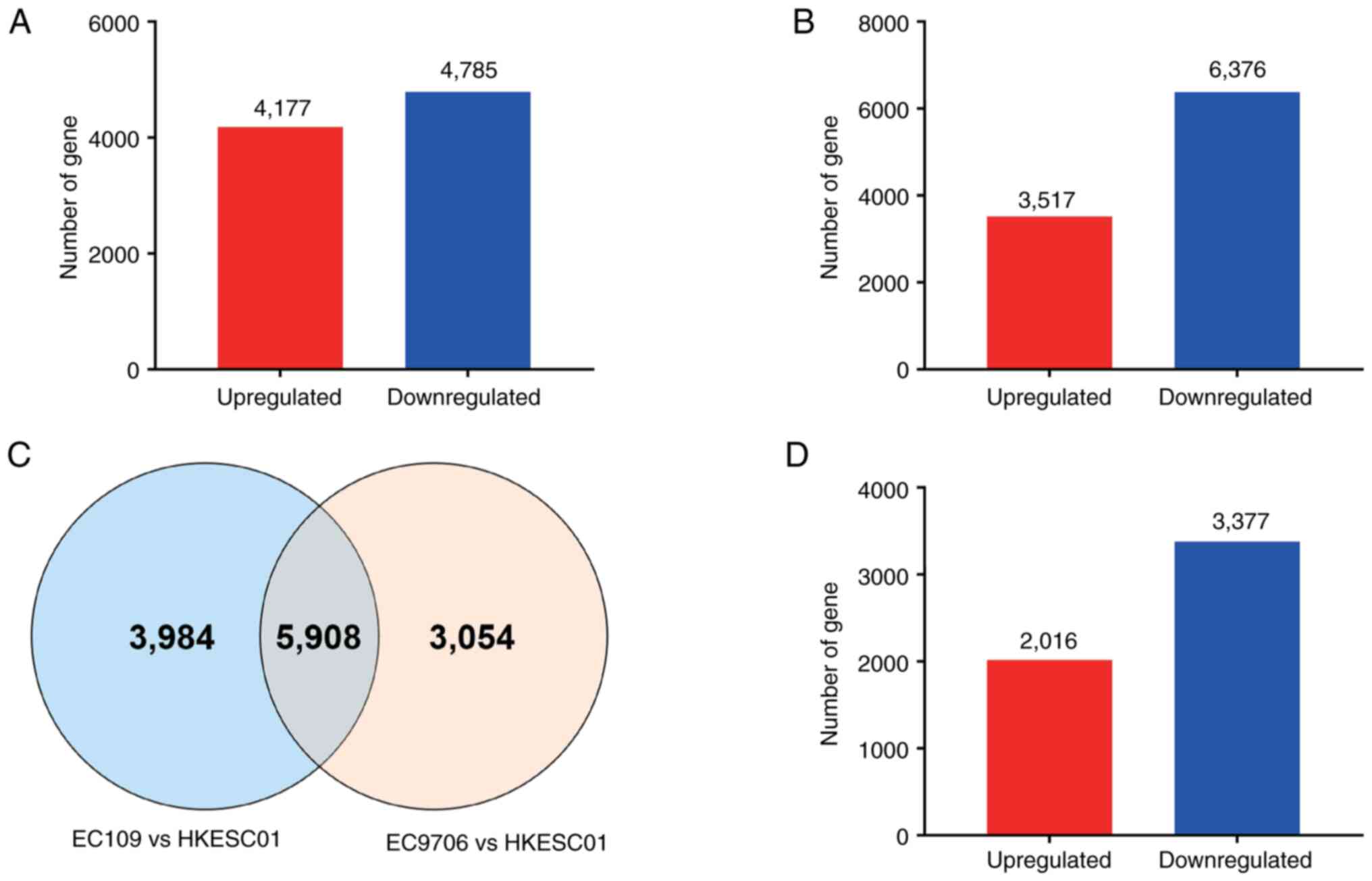
were obtained. Among those genes, 4,177 were upregulated genes and
4,785 downregulated in HPV18-positive ESCC cells (Fig. 1A and Table SII). In total, 9,892 distinct genes
were obtained by comparing HPV18-positive ESCC cells, EC109, with
the HPV-negative HKESCC01 cells, of which 3,516 were upregulated
and 6,376 downregulated (Fig. 1B
and Table SIII). Subsequently,
5,908 genes differentially expressed in the two sets of data
concurrently, were selected (Fig.
1C). Subsequenlty, 5,393 genes with an absolute value of
log2(Foldchange) >2 and padj <0.05 in the two
groups of data were selected, of which 2,016 were found to be
upregulated and 3,377 downregulated (Fig. 1D).
Omicsbean was then used to analyze the combined and
screened differentially expressed genes. KEGG and GO analyses were
performed for the differential genes with log2 (fold change)>2
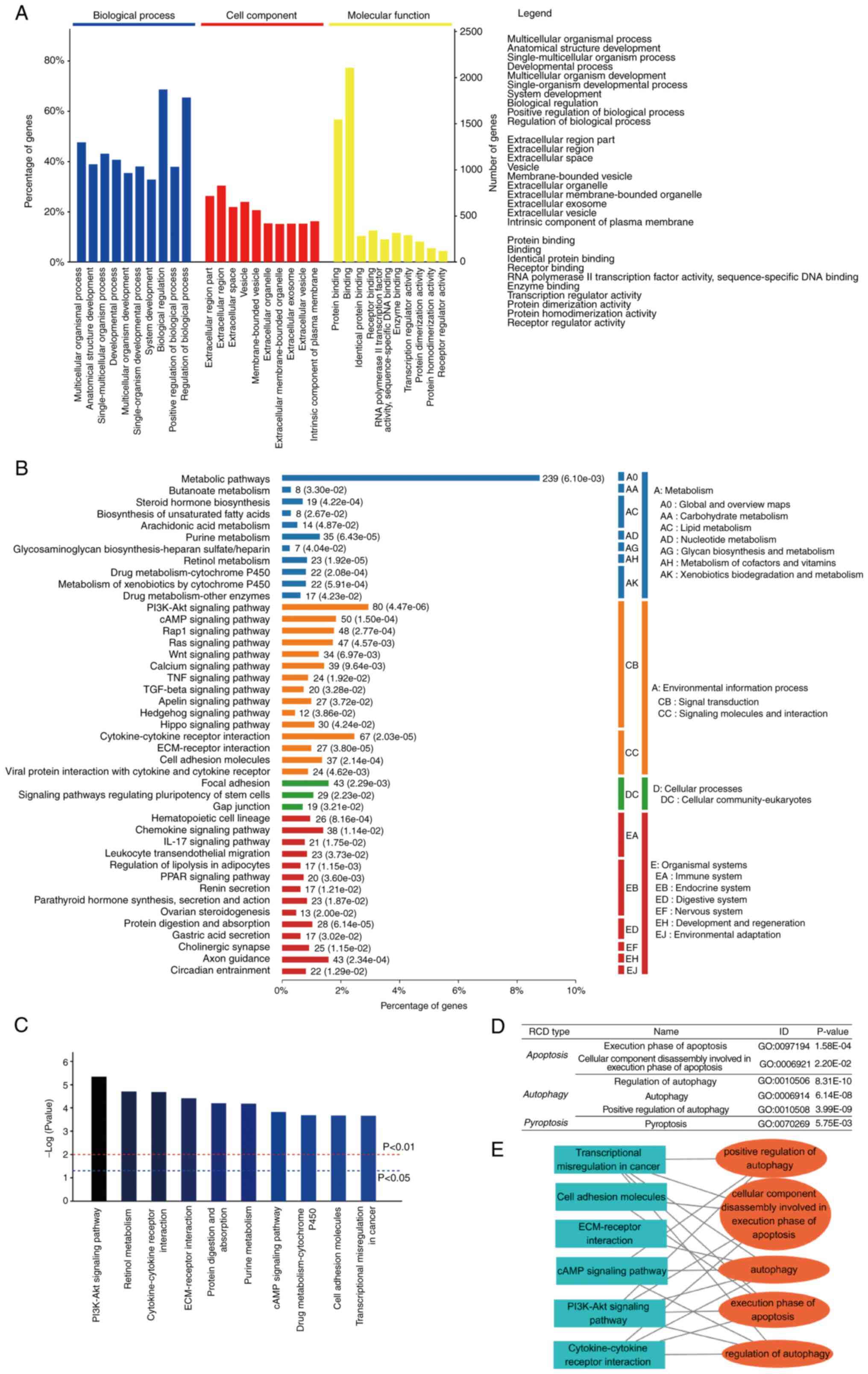
and padj <0.05. In the GO analysis, ‘multicellular organismal
process’ (P=0.00e+00), ‘anatomical structure development’
(P=0.00e+00), ‘single-multicellular organism process’ (P=0.00e+00)
were defined as the top three most significant pathway in
‘biological progress’ category. ‘Extracellular region part’
(P=1.75e-274), ‘extracellular region’ (P=1.28e-273), ‘extracellular
space’ (P=8.20e-238) are the top three most significant pathways in
the ‘cell component’ category. ‘Protein binding’ (P=0.00e+00),
‘binding’ (P=8.60e-165), ‘identical protein binding’ (P=4.22e-99)
were the top three most significant pathways in the ‘molecular
function’ category (Fig. 2A). In
the KEGG analysis, the PI3K/Akt signaling pathway (P=4.47e-06),
retinol metabolism (P=1.92e-05), cytokine-cytokine receptor
interaction (P=2.03e-05), ECM-receptor interaction (P=3.80e-05),
protein digestion and absorption (P=6.14e-05), purine metabolism
(P=6.43e-05), cAMP signaling pathway (P=1.50e-04), drug
metabolism-cytochrome P450 (P=2.08e-04), cell adhesion molecules
(P=2.14e-04) and transcriptional misregulation in cancer
(P=2.20e-04) were the top 10 most significant pathways (Fig. 2B and C). Of note, the development
and progression of cancer involves a large number of significant
pathways. A large number of RCD-related pathways were determined
and these pathways were also closely related to the top 10 pathways
in KEGG (Fig. 2D and E), which may
provide evidence concerning the HPV inhibition of cell death,
thereby promoting cancer progression. At the transcriptional level,
it was suggested that HPV regulates multiple RCD pathways in
ESCC.
HPV18 E6E7 significantly inhibits the
apoptosis pathway
There were two significantly different
apoptosis-related pathways, in which 18 genes were enriched
(Table SIV). Among the enriched
genes, three genes were selected [cyclin dependent kinase inhibitor
2A (CDKN2A), plakophilin 1 (PKP1) and desmoglein 3 (DSG3)] with a
more significant difference and a clearly reported association with
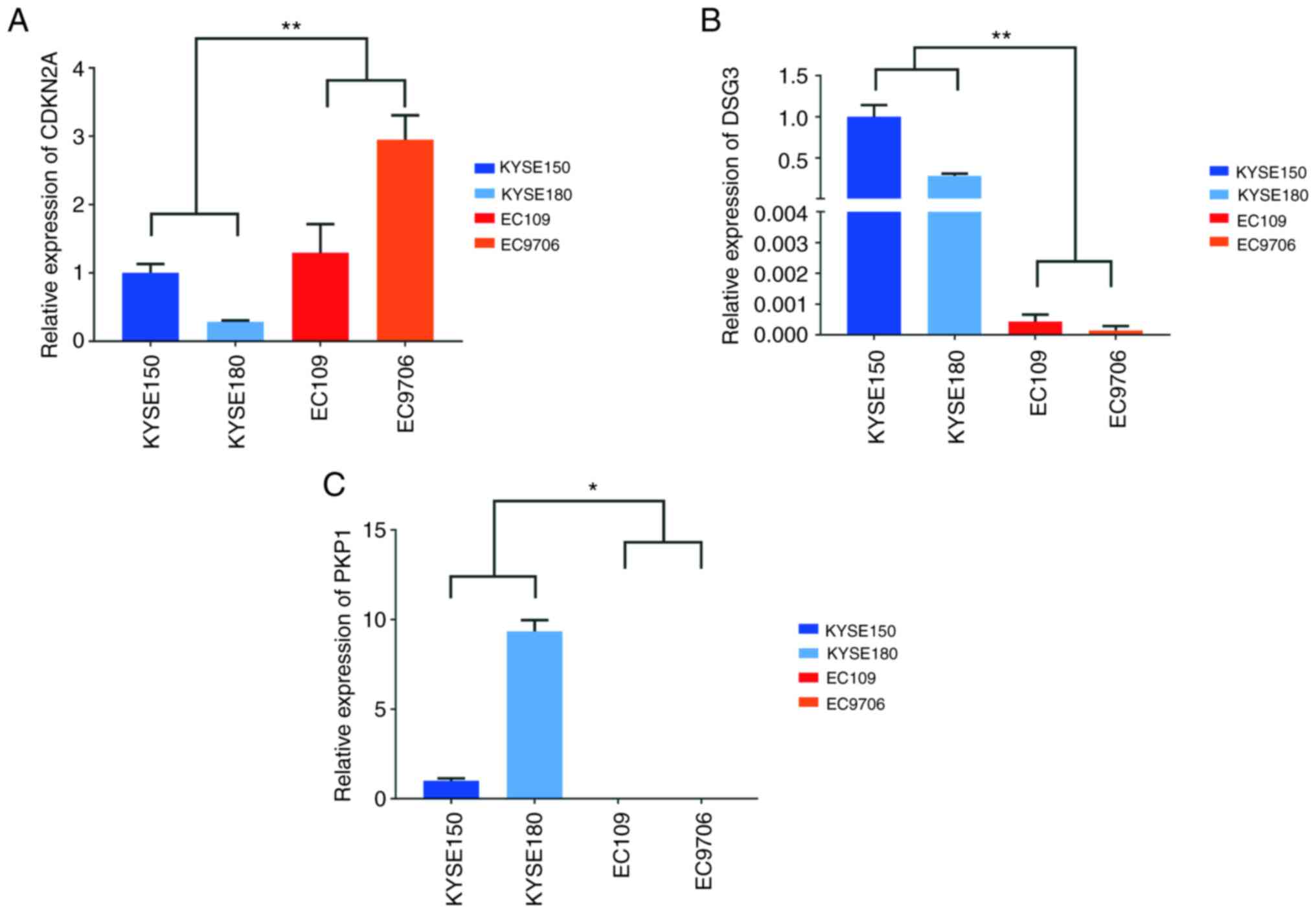
apoptosis (25–27). By validating their expression in
HPV18-positive and HPV-negative ESCC cells, the results of RT-qPCR
revealed that CDKN2A was highly expressed in HPV18-positive cells,
DSG3 expression was comparably reduced in HPV18-positive cells and
PKP1 was barely detected in HPV18-positive cells, although it was
clearly or highly expressed in HPV-negative cells (Fig. 3).
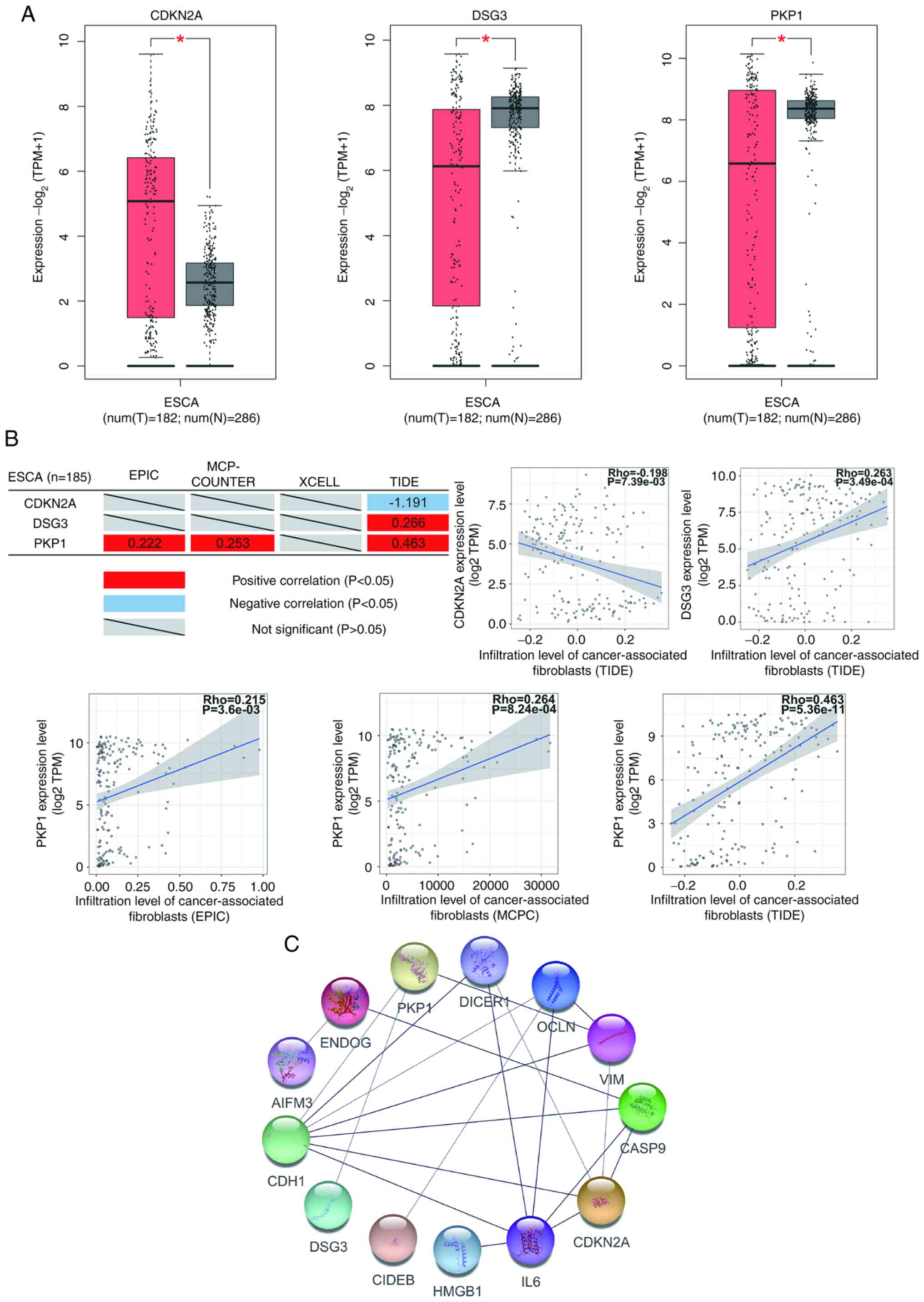
An analysis of primary tumor samples from GEPIA2
website was also performed, which revealed CDKN2A to be
overexpressed, and DSG3 and PKP1 underexpressed in ESCA (Fig. 4A). The correlation analysis of
tumor-associated fibroblast infiltration demonstrated that the
expression of CDKN2A negatively correlated, and the expression of
DSG3 and PKP1 positively correlated with tumor-associated
fibroblast infiltration (Fig. 4B).
The differential expression of genes in tumors and the association
between gene expression and tumor-associated fibroblast
infiltration are important indicators for the evaluation the
association between genes and the occurrence and development of
tumor. These results suggested that the three genes selected were
closely related to and may be important for the occurrence and
development of ESCA. In the protein-protein interaction (PPI)
network analysis, CDKN2A was one of the nodes with the most target
proteins (Fig. 4C). This result
indicated that CDKN2A may be a critical target of HPV18 oncoprotein
and that HPV18 oncoprotein regulates the function of other proteins
via CDKN2A.
The oncogenic ability of HPV is mediated by the HPV
E6 and E7 oncogenes, which are regularly co-expressed (28). In order to investigate which genes
of interest are regulated by HPV E6E7, the HPV18 E6E7 gene was
inserted into a pLVX-puro lentiviral vector to stably obtain
KYSE150-E6E7 and KYSE180-E6E7 cells expressing HPV18 E6E7 (Fig. S1). The mRNA expression of the
CDKN2A, PKP1 and DSG3 genes was detected using RT-qPCR, and the
presence of HPV18 E6E7 significantly promoted CDKN2A expression and
inhibited the expression of DSG3, having no effect on the
transcription levels of PKP1 (Fig. 5A
and B). Since the KYSE150 cells are sensitive to DDP, the early
and late apoptosis degree of KYSE150 cells was detected at three
concentrations of DDP, namely 20, 40 and 80 µM. The results
revealed that the early and late apoptosis level of KYSE150 cells
was positively associated with DDP (Fig. 5C). DDP at a concentration of 80 µM
induced the death of the KYSE150 cells, whereas HPV18 E6E7
significantly inhibited the DDP-induced apoptosis (early and late
stage) of the KYSE150 cells (Fig.
5D). These results indicate that HPV18 may regulate apoptosis
by affecting the expression of apoptosis-related genes.
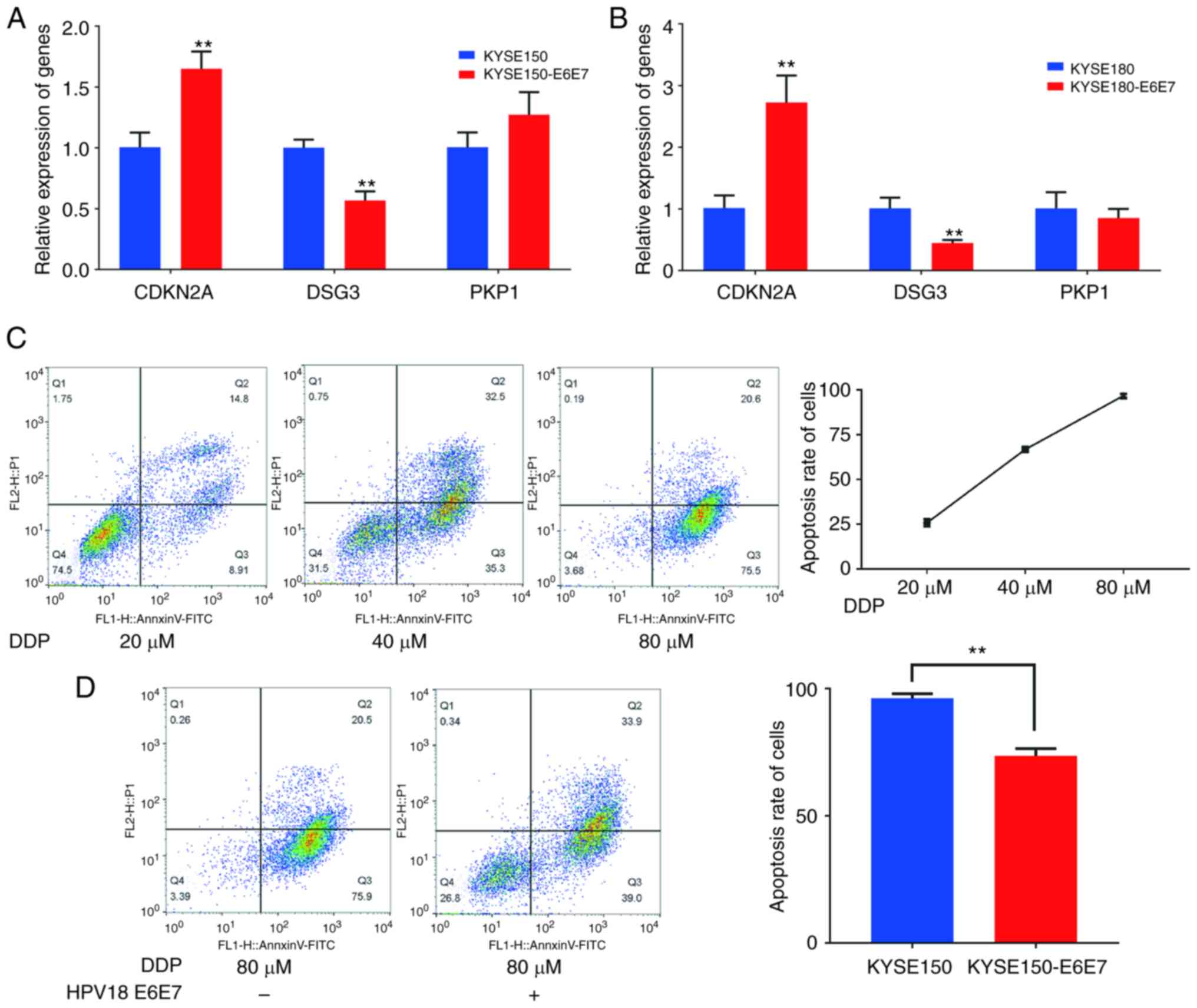 | Figure 5.HPV18 E6E7 inhibits the apoptosis of
ESCC cells. (A) Reverse transcription-quantitative PCR results of
CDKN2A, PKP1 and DSG3 in KYSE150 and KYSE150-E6E7; data represent
the mean ± SD of three independent experiments. **P<0.01, as
compared with the control group. (B) RT-qPCR results of CDKN2A,
PKP1 and DSG3 in KYSE180 and KYSE180-E6E7; data represent the mean
± SD of three independent experiments. **P<0.01, as compared
with the control group. (C) Detection of apoptosis in KYSE150 cell
treated with 20, 40 and 80 µM cisplatin concentrations. (D)
Comparison of the effect of cisplatin-induced apoptosis in KYSE150
cells and KYSE150-E6E7 cells. **P<0.01, as compared with the
KYSE150 group. HPV, human papillomavirus; CDKN2A, cyclin dependent
kinase inhibitor 2A; DSG3, desmoglein 3; PKP1, plakophilin 1. |
HPV18 E6E7 significantly inhibits
autophagy by inhibiting autophagosome-lysosomal fusion
There are three significantly different
autophagy-related pathways, of which 104 genes were found to be
enriched (Table SIV). Among the
enriched genes, two genes with a more significant difference and a
clear reported association with autophagy were selected [ULK1,
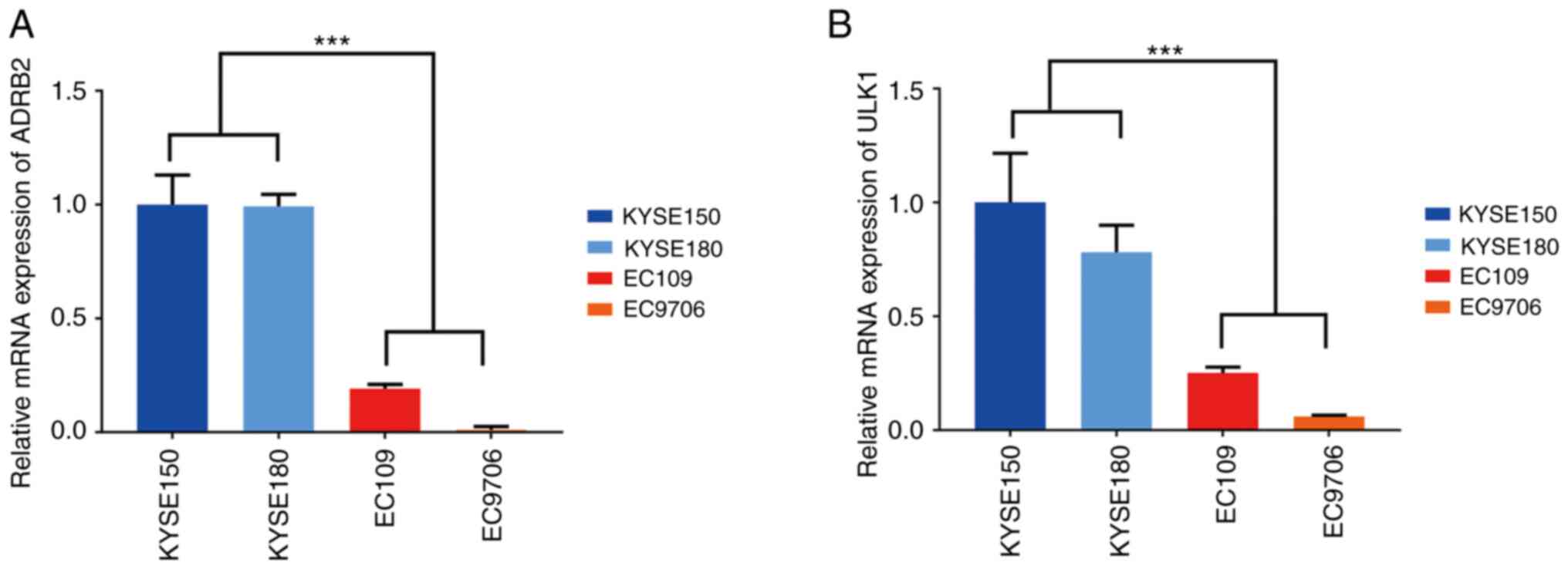
adrenoceptor beta 2 (ADRB2)] (29,30).
The RT-qPCR results demonstrated that the mRNA expression of the
ULK1 and ADRB2 genes was reduced in the HPV18-positive cells
(Fig. 6).
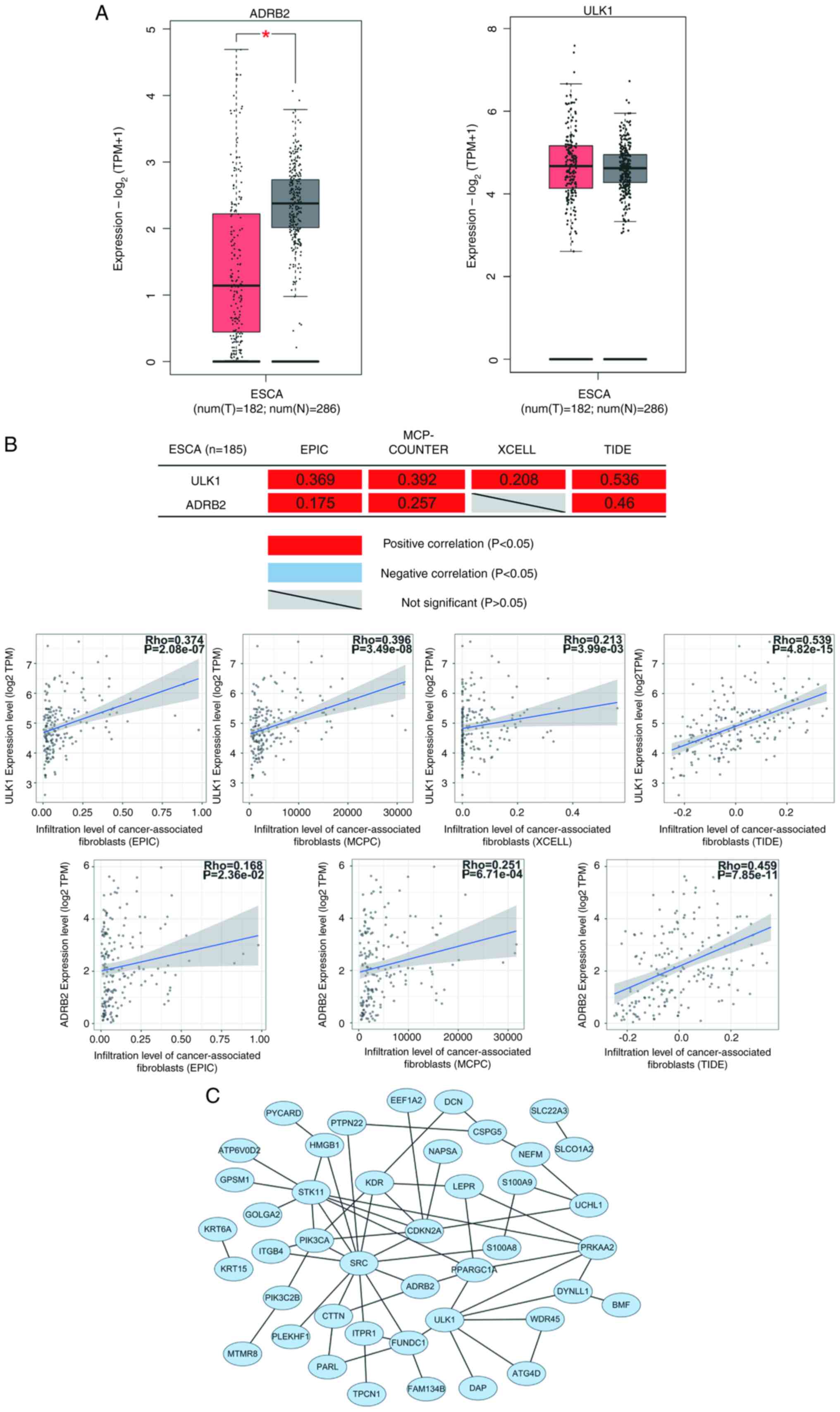
ADRB2 expression was also reduced in primary tumor
samples of ESCA and the expression of ULK1 was not significantly
associated with ESCA (Fig. 7A). The
correlation analysis of tumor-associated fibroblast infiltration
revealed that the expression of the ULK1 and ADRB2 genes positively
correlated with tumor-associated fibroblast infiltration (Fig. 7B). These results suggested that ULK1
and ADRB2 may negatively regulate the occurrence and development of
ESCA. In the PPI network analysis, ULK1 was demonstrated to
interact with several known autophagy-related genes, including
ATG4D, PRKAA2 and WDR45 (Fig.
7C).
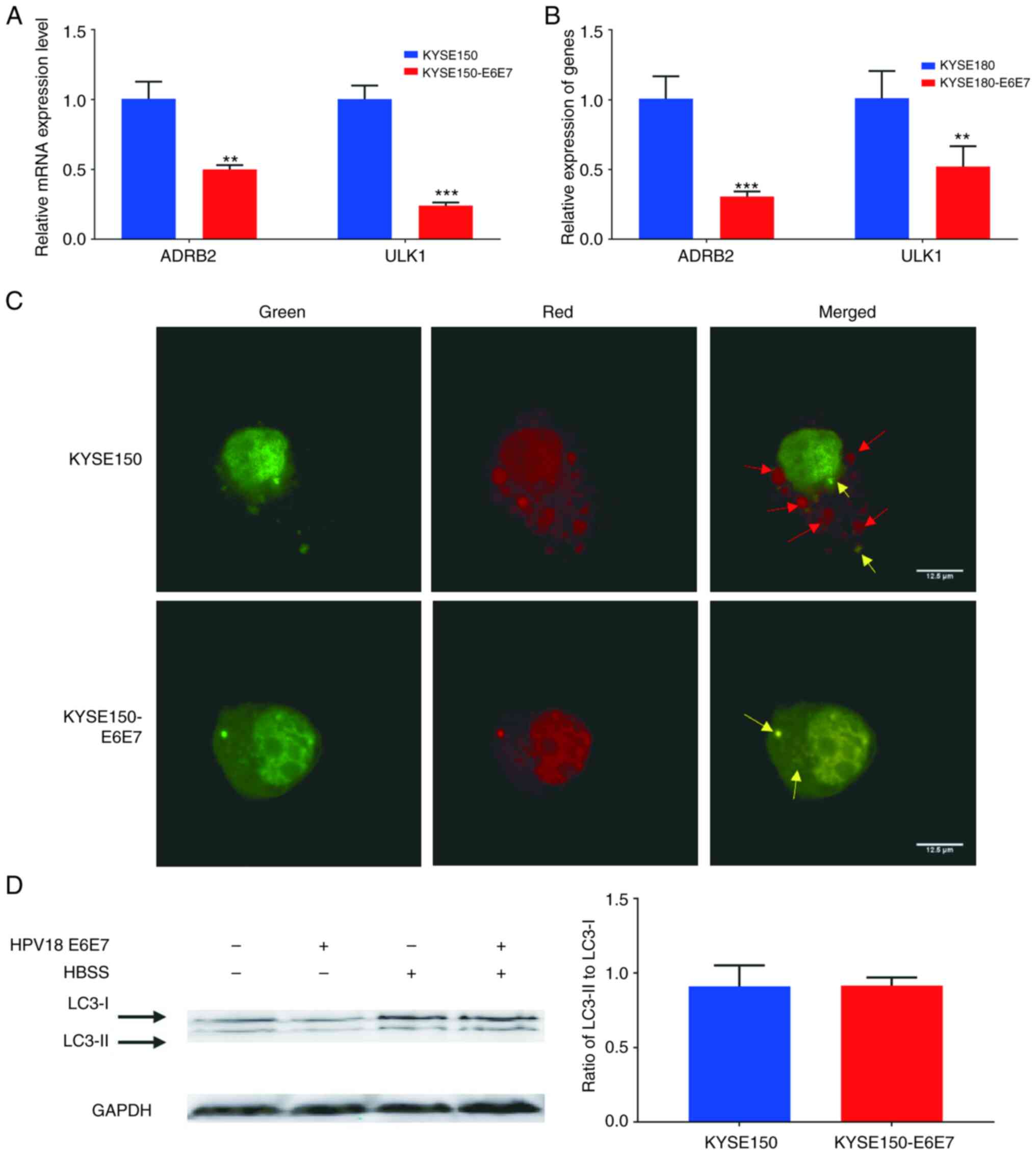
The reduced expression of ULK1 and ADRB2 in
KYSE150-E6E7 and KYSE180-E6E7 was also detected, revealing that
HPV18 E6E7 decreases their mRNA expression levels (Fig. 8A and B). Subsequently, the
LC3-RFP-GFP plasmid was transfected into KYSE150 and KYSE150 E6E7
cells and autophagy was induced using HBSS. LC3-RFP-GFP is an
effective tool for visualizing the autophagy process (31,32).
The simultaneous detection of the green and red fluorescent
proteins was used for the detection of LC3 expression. Prior to the
fusion of the autophagosome and lysosome, LC3 exhibits red and
green signals, and merges into yellow in the autophagosome.
Following the fusion of autophagosome with the lysosome, the acidic
pH environment weakens GFP, and thus LC3 is presented as a single
red dot. In the results of the present study, HPV18 E6E7 inhibited
autophagosome-lysosome fusion in late autophagy without hindering
LC3-I to LC3-II cleavage (Fig. 8C and
D).
HPV18 E6E7 inhibits the pyroptosis
pathway and α-ketoglutarate-induced pyroptosis
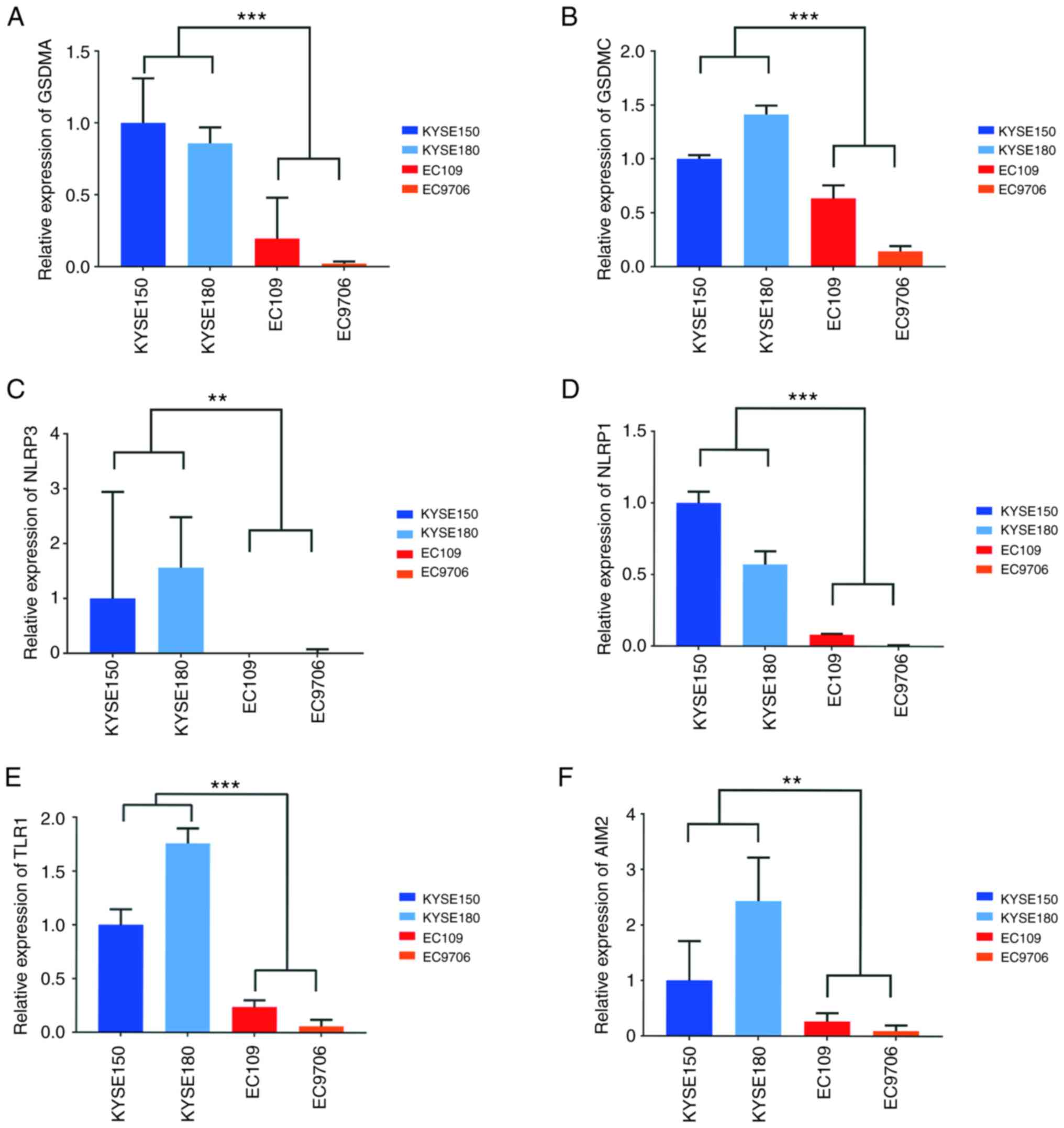
One significantly different pyroptosis-related
pathway was determined, enriching four genes through the GO
analysis (Table SIV). Pyroptosis
is a relatively new concept, leading to the enrichment of
incomplete genes. Other pyroptosis-related genes were identified
based on a detailed review of the published literature (23,33–37).
In total, six genes were obtained [gasdermin A (GSDMA), gasdermin C
(GSDMC), NLR family pyrin domain containing 3 (NLRP3), absent in
melanoma 2 (AIM2), NLR family pyrin domain containing 1 (NLRP1) and
Toll like receptor 1 (TLR1)] for validation through pathway
enrichment and a literature survey. The RT-qPCR results revealed
that the mRNA expression of the aforementioned six genes was
reduced in HPV18-positive cells, with the mRNA expression of NLRP3
being barely detectable in the EC109 and EC9706 cells (Fig. 9).
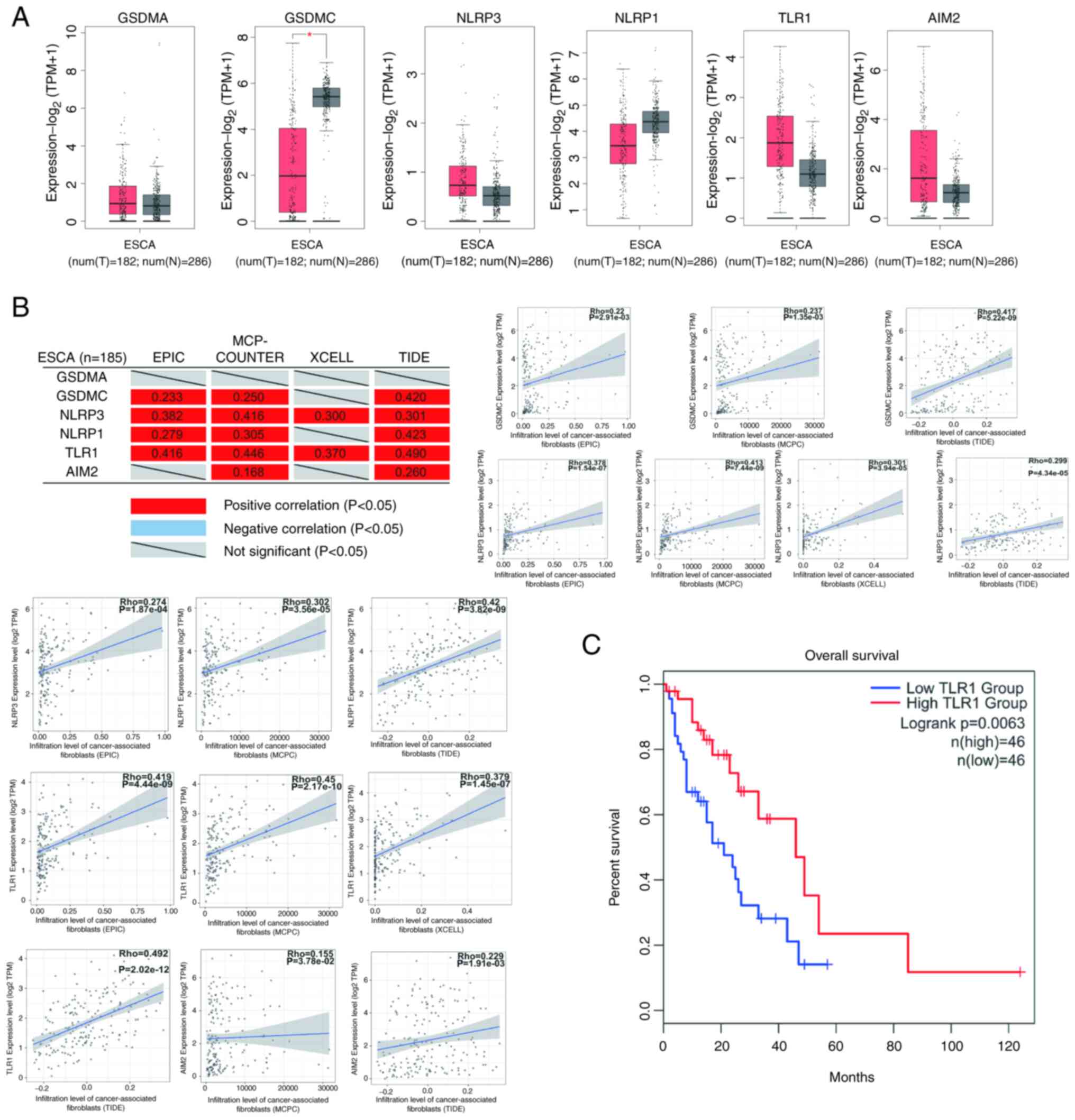
By applying GEPIA2 website analysis, it was defined
that only GSDMC mRNA expression was reduced in ESCA tumors.
However, there was no marked difference in the expression of GSDMA,
NLRP3, AIM2, NLRP1 and TLR1 in ESCA tumors (Fig. 10A). The correlation analysis of
tumor-associated fibroblast infiltration revealed that the
expression of GSDMC, NLRP3, NLRP1, TLR1 and AIM2 positively
correlated, whereas GSDMA expression was not correlated with
tumor-associated fibroblast infiltration (Fig. 10B). The expression of TLR1 was also
closely related to the survival rate of patients with ESCA,
according to an additional a survival analysis (Fig. 10C). The results demonstrated that
all genes, apart from GADMA, may be negatively associated with the
occurrence and development of ESCA.
GSDMA and GSDMC are members of the gasdermin
superfamily and important executive molecules of pyroptosis
(23,33). It was hypothesized that HPV may
inhibit the expression of key executive molecules for the
inhibition of pyroptosis. The mRNA expression of GSDMA, GSDMC,
NLRP3, NLRP1, TLR1 and AIM2 genes was detected in KYSE150-E6E7
cells. The presence of HPV18 E6E7 significantly inhibited the
expression of GSDMC and TLR1, having no marked effect on the
transcription levels of GSDMA, NLRP1, NLRP3 and AIM2 (Fig. 11A and B). α-ketoglutarate has been
demonstrated to induce pyroptosis through the GSDMC pathway
(23). Subsequently, 4 h after the
induction of pyroptosis by α-ketoglutarate, the HPV-negative group
clearly exhibited pyroptosis, whereas in the HPV18 E6E7 group,
pyroptosis was limited (Fig.
11C).
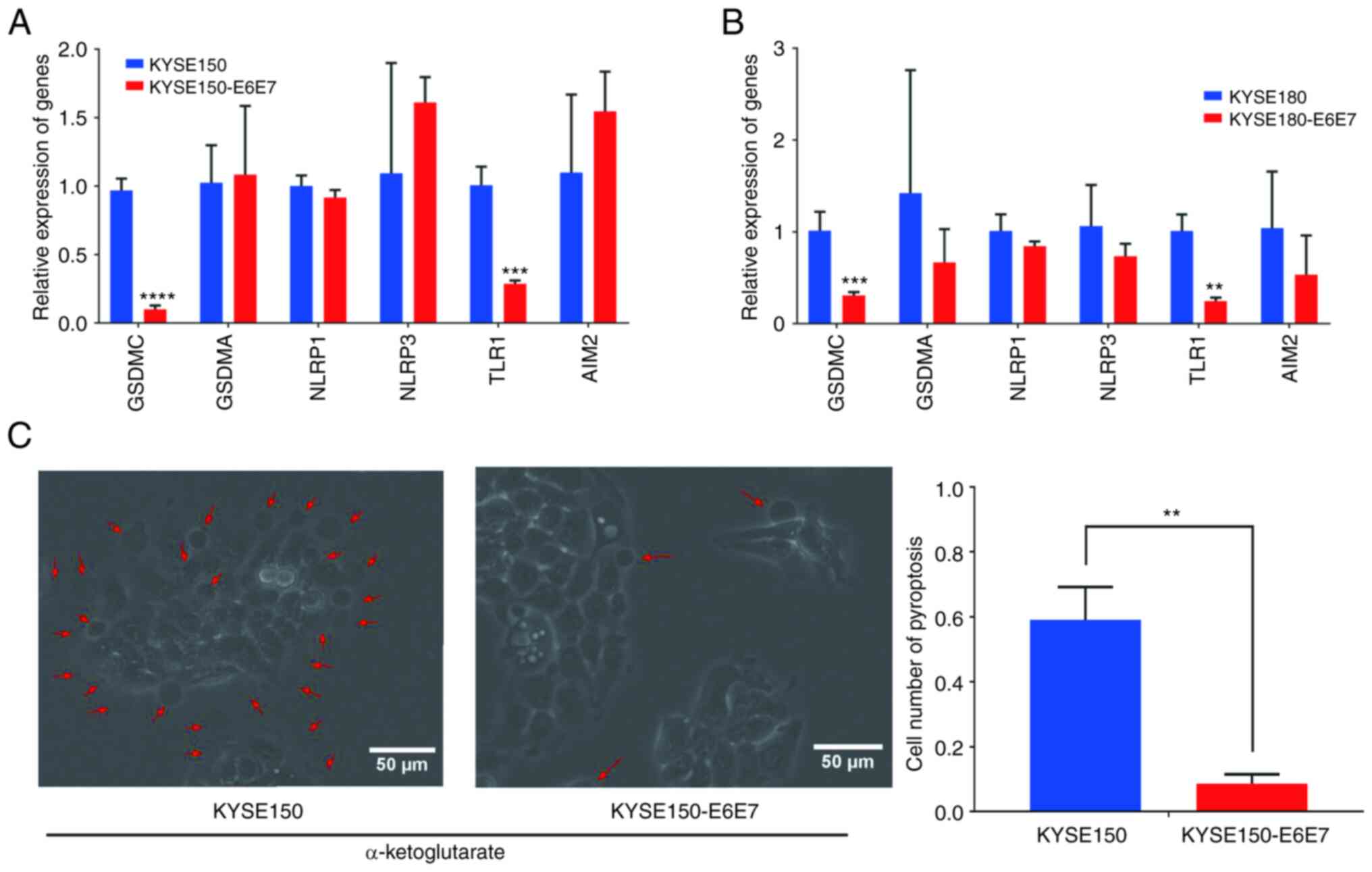 | Figure 11.HPV18 E6E7 inhibits the pyroptosis of
ESCC cells. (A) RT-qPCR results of GSDMA, GSDMC, NLRP3, NLRP1, TLR1
and AIM2 in KYSE150 and KYSE150-E6E7; data represent the mean ± SD
of three independent experiments. ***P<0.001 and
****P<0.0001, compared with the control group. (B) RT-qPCR
results of GSDMA, GSDMC, NLRP3, NLRP1, TLR1 and AIM2 in KYSE180 and
KYSE180-E6E7; data represent the mean ± SD of three independent
experiments. **P<0.01 and ***P<0.001, as compared with the
control group. (C) Comparison of α-ketoglutarate-induced pyroptosis
in KYSE150 cells and KYSE150-E6E7 cells, data represent the mean ±
SD of three random visual fields. **P<0.01, as compared with the
KYSE150 group. HPV, human papillomavirus; ESCC, esophageal squamous
cell carcinoma; RT-qPCR, reverse transcription-quantitative PCR;
GSDMA, gasdermin A; GSDMC, gasdermin C; NLRP3, NLR family pyrin
domain containing 3; NLRP1, NLR family pyrin domain containing 1;
TLR1, Toll like receptor; 1 AIM2, absent in melanoma 2. |
Discussion
ESCA is a very aggressive type of tumor with an
increasing incidence rate, poor prognosis and Single surgical
treatment mode (38). HPV infection
is one of the carcinogenic factors of ESCA, and it has also been
shown to be negatively associated with the prognosis of ESCA.
According to the findings of the present study, HPV may inhibit
ESCC cell death and decrease the cell sensitivity to therapeutics,
thereby weakening the therapeutic effects, and promoting the
development and progression of ESCC.
Using a GO analysis, three types of RCD pathways
were obtained. In the apoptosis-related pathway, three genes
(CDKN2A, PKP1 and DSG3) were verified. CDKN2A has been
alternatively termed in various ways, including ARF, MLM, P14, P16,
P19, CMM2, INK4, MTS1, TP16, CDK4I, CDKN2, INK4A, MTS-1, P14ARF,
P19ARF, P16INK4, P16INK4A, P16-INK4A. The p16 protein encoded by
CDKN2A is usually highly expressed in HPV-related tumors; thus, p16
is an important indicator of HPV infection (39). It has been confirmed that the
overexpression of p16 is caused by the deletion of pRB. The
function of p16 also requires the synergistic effect of pRB, which
is a classical downstream factor of HPV E7. The E7 protein may act
on pRB to inactivate it, thereby exerting the carcinogenic effect
of HPV (40,41). PKP1 expression is less widespread in
lung cancer, with PKP1 having been reported to inhibit cell
proliferation, colony formation, migration and invasion and promote
apoptosis in lung cancer (27).
Concurrently, several studies have demonstrated that PKP1 can
inhibit the expression of SPOCK1 (42). Previous studies have confirmed that
SPOCK1 can promote the occurrence and development of esophageal
squamous cell carcinoma (43). DSG3
is a member of the desmoglein family and cadherin cell adhesion
molecule superfamily, which is predominantly expressed in
stratified epithelia (44). DSG3
has been reported to exhibit an oncogenic function in head and neck
cancer, with DSG3 overexpression promoting tumor growth and
migration (45). Among
autophagy-related pathways, the present study focused on two genes,
ULK1 and ADRB2, which also positively correlated with
tumor-associated fibroblast infiltration. In autophagy regulation,
ULK1 initiates autophagosome formation, whereas ADRB2 negatively
regulates autophagy by disrupting the Akt-dependent
Beclin1/VPS34/Atg14 complex (46,47).
Members of the gasdermin family play a crucial role in pyroptosis.
In the present study, the expression of GSDMA and GSDMC, two genes
that are also direct executors of pyroptosis, was significantly
reduced. At present, there are limited studies available on GSDMA;
however, there have been extensive studies on the induction of
pyroptosis by GSDMC. For example, PD-L1 can mediate GSDMC to
convert apoptosis into pyroptosis, and α-ketoglutarate can induce
pyroptosis through the DDR6-caspase-8-GSDMC pathway (23,48).
NLRP1, NLRP3, AIM2 and TLR1 are upstream factors in different
pyroptotic pathways (49–51). Through TCGA analysis, it was
observed that the genes selected were closely related to the
occurrence and development of ESCA. For example, CDKN2A was highly
expressed in ESCA and negatively correlated with tumor-associated
fibroblast infiltration. DSG3, PKP1, ADRB2, ULK1, NLRP1, GSDMC,
NLRP3, AIM2, and TLR1 positively correlated with tumor-associated
fibroblast infiltration. ESCC cell lines were then used for further
investigation. Through an RNA-Seq analysis and experimental
verification, it was confirmed that CDKN2A was highly expressed and
DSG3, PKP1, ULK1, ADRB2, GSDMA, GSDMC, NLRP3, NLRP1 and TLR1 are
less strongly expressed in HPV18-positive ESCC cells. This result
demonstrates that HPV18 regulates these cell death-related genes at
the transcriptional level, further promoting the malignancy of
ESCC.
The oncogenic ability of HPV is mediated by the HPV
E6 and E7 oncogenes (52). Herein,
to explore the association between HPV18 E6E7 and these
differentially expressed genes, KYSE150-E6E7 cells were constructed
and KYSE180-E6E7 stably expressing HPV18 E6E7 using HPV-negative
KYSE150 cells and KYSE180 cells. EC109 and EC9706 are
HPV18-positive cell lines and were used as negative controls
(53,54). Since the KYSE150 cells and KYSE180
cells lack the expression of HPV all subtypes, these two cells are
excellent cell models for studying the carcinogenic effect of HPV18
E6E7 on ESCC. Detected at the transcriptional level using RT-qPCR,
the presence of HPV18 E6E7 significantly promoted the expression of
CDKN2A and inhibited the expression of DSG3, ADRB2, ULK1, GSDMC and
TLR1, which may be directly regulated by HPV18 E6E7. The
transcription of GSDMA, NLRP3 and NLRP1 was not affected, and these
genes may be regulated by other oncoproteins of HPV18 or other
factors. In future research, the authors aim to construct EC109
cells and EC9706 cells in which HPV18 E6E7 is knocked out, in order
to verify these results and further explore the mechanisms through
which HPV18 E6E7 affects ESCC cell death. DDP is one of the
commonly used drugs in the treatment of ESCC; however, the
resistance of ESCC to DDP has been reported to lead to an
unsatisfactory treatment effect (55). In the present study, by using DDP to
induce KYSE150 and KYSE150-E6E7 cell death, it was observed that
the presence of HPV18 E6E7 significantly inhibited the
apoptosis-inducing effect of cisplatin, indicating that HPV18 could
increase the resistance of esophageal squamous cell carcinoma cells
to cisplatin. Previous studies have revealed that HPV inhibits the
occurrence of autophagy in various ways. HPV E5 interferes with the
transcriptional activation of autophagy-related genes in the early
stage of autophagy, including Beclin 1, ATG5, LC3, ULK1, ULK2,
ATG4A and ATG7, HPV E6E7 inhibits the autophagosome–lysosome fusion
of cells, thereby inhibiting the process of autophagy (17,56).
In the experiments in the present study, it was confirmed that the
presence of HPV18 E6E7 inhibited autophagosome-lysosome fusion at
the late stage of autophagy in ESCC, having no effect on the
cleavage of LC3-I to LC3-II. These results confirmed that HPV18
E6E7 mainly acts in the later stages of autophagy in ESCC cells.
HPV18 E6E7 significantly inhibited the expression of GSDMC, and
α-ketoglutarate was used to induce the pyroptosis of ESCC cells.
The existence of HPV18 E6E7 significantly inhibited the occurrence
of pyroptosis of ESCC cells, which may be related to the inhibition
of GSDMC expression by HPV18 E6E7. Finally, it was considered that
the presence of HPV18 E6E7 significantly inhibits apoptosis,
autophagy and pyroptosis in ESCC cells, which is one of the
important mechanisms for promoting the occurrence and development
of ESCC.
In conclusion, the differentially expressed genes
between HPV18-positive and HPV-negative ESCC samples were explored
through a transcriptomic analysis, and it was observed that several
differentially expressed genes were enriched in various pathways
related to cell death. By constructing HPV18-positive ESCC cells,
it was confirmed that HPV18 E6E7 may increase the resistance of
cisplatin and α-ketoglutarate, inhibits autophagosome-lysosome
fusion, thus inhibiting ESCC apoptosis, autophagy and pyroptosis.
Finally, it was suggested that HPV may inhibit multiple cell death
pathways, which is one of the critical mechanisms for promoting the
occurrence and development of ESCC.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 42077399).
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the NCBI BioProject database
repository through the Accession no. PRJNA530677 (19). The datasets used and/or analyzed
during the current study are also available from the corresponding
author on reasonable request.
Authors' contributions
ZZ and DT designed the study and performed the
experiments. ZL, GW, QW and BW performed the experiments and data
analysis, and contributed significantly to the writing of the
manuscript. XH, YZ, MY and CS were responsible for data collection
and analysis. ZZ and DT confirm the authenticity of all the raw
data. All authors edited the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Short MW, Burgers KG and Fry VT:
Esophageal cancer. Am Fam Physician. 95:22–28. 2017.PubMed/NCBI
|
|
3
|
Uhlenhopp DJ, Then EO, Sunkara T and
Gaduputi V: Epidemiology of esophageal cancer: Update in global
trends, etiology and risk factors. Clin J Gastroenterol.
13:1010–1021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kikuchi H and Takeuchi H: Future
perspectives of surgery for esophageal cancer. Ann Thorac
Cardiovasc Surg. 24:219–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serrano B, Brotons M, Bosch FX and Bruni
L: Epidemiology and burden of HPV-related disease. Best Pract Res
Clin Obstet Gynaecol. 47:14–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castellsagué X: Natural history and
epidemiology of HPV infection and cervical cancer. Gynecol Oncol.
110 (3 Suppl 2):S4–S7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Martel C, Plummer M, Vignat J and
Franceschi S: Worldwide burden of cancer attributable to HPV by
site, country and HPV type. Int J Cancer. 141:664–670. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bognár L, Hegedűs I, Bellyei S, Pozsgai É,
Zoltán L, Gombos K, Horváth ÖP, Vereczkei A and Papp A: Prognostic
role of HPV infection in esophageal squamous cell carcinoma. Infect
Agent Cancer. 13:382018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dinc B, Altay-Kocak A, Aydog G, Kuran S,
Akoglu M, Ozkan S and Bozdayi G: Detection of HPV DNA in esophageal
lesions: A cross-sectional study. Clin Lab. 66:2020. View Article : Google Scholar
|
|
10
|
Shen Z, Cen S, Shen J, Cai W, Xu J, Teng
Z, Hu Z and Zeng Y: Study of immortalization and malignant
transformation of human embryonic esophageal epithelial cells
induced by HPV18 E6E7. J Cancer Res Clin Oncol. 126:589–594. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang D, Wang B, Khodahemmati S, Li J, Zhou
Z, Gao J, Sheng W and Zeng Y: A transcriptomic analysis of
malignant transformation of human embryonic esophageal epithelial
cells by HPV18 E6E7. Transl Cancer Res. 9:1818–1832. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shankar S, Prasad D, Sanawar R, Das AV and
Pillai MR: TALEN based HPV-E7 editing triggers necrotic cell death
in cervical cancer cells. Sci Rep. 7:55002017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li TT, Zhao LN, Liu ZG, Han Y and Fan DM:
Regulation of apoptosis by the papillomavirus E6 oncogene. World J
Gastroenterol. 11:931–937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wawryk-Gawda E, Chylińska-Wrzos P,
Lis-Sochocka M, Chłapek K, Bulak K, Jędrych M and Jodłowska-Jędrych
B: P53 protein in proliferation, repair and apoptosis of cells.
Protoplasma. 251:525–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mattoscio D, Medda A and Chiocca S: Human
papilloma virus and autophagy. Int J Mol Sci. 19:17752018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song Y, Wu X, Xu Y, Zhu J, Li J, Zou Z,
Chen L, Zhang B, Hua C, Rui H, et al: HPV E7 inhibits cell
pyroptosis by promoting TRIM21-mediated degradation and
ubiquitination of the IFI16 inflammasome. Int J Biol Sci.
16:2924–2937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boon SS, Chen Z, Li J, Lee KYC, Cai L,
Zhong R and Chan PKS: Human papillomavirus type 18 oncoproteins
exert their oncogenicity in esophageal and tongue squamous cell
carcinoma cell lines distinctly. BMC Cancer. 19:12112019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada Y, Imamura M, Wagata T, Yamaguchi
N and Tobe T: Characterization of 21 newly established esophageal
cancer cell lines. Cancer. 69:277–284. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang JY, Zhou B, Sun RY, Ai YL, Cheng K,
Li FN, Wang BR, Liu FJ, Jiang ZH, Wang WJ, et al: The metabolite
α-KG induces GSDMC-dependent pyroptosis through death receptor
6-activated caspase-8. Cell Res. 31:980–997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Chen H, Zhang Y, Thomas HR, Frank
MH, He Y and Xia R: TBtools: An integrative toolkit developed for
interactive analyses of big biological data. Mol Plant.
13:1194–1202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Yang J, Liu Y, Fan J and Yang H:
HSV-2-encoded miRNA-H4 regulates cell cycle progression and
Act-D-induced Apoptosis in HeLa cells by targeting CDKL2 and
CDKN2A. Virol Sin. 34:278–286. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Ahmad US, Huang Y, Uttagomol J,
Rehman A, Zhou K, Warnes G, McArthur S, Parkinson EK and Wan H:
Desmoglein-3 acts as a pro-survival protein by suppressing reactive
oxygen species and doming whilst augmenting the tight junctions in
MDCK cells. Mech Ageing Dev. 184:1111742019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haase D, Cui T, Yang L, Ma Y, Liu H, Theis
B, Petersen I and Chen Y: Plakophilin 1 is methylated and has a
tumor suppressive activity in human lung cancer. Exp Mol Pathol.
108:73–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yi SA, Lee DH, Kim GW, Ryu HW, Park JW,
Lee J, Han J, Park JH, Oh H, Lee J, et al: HPV-mediated nuclear
export of HP1γ drives cervical tumorigenesis by downregulation of
p53. Cell Death Differ. 27:2537–2551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen G, Jin X, Luo D, Ai J, Xiao K, Lai J,
He Q, Li H and Wang K: β-Adrenoceptor regulates contraction and
inflammatory cytokine expression of human bladder smooth muscle
cells via autophagy under pathological hydrostatic pressure.
Neurourol Urodyn. 39:2128–2138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li K, Deng Y, Deng G, Chen P, Wang Y, Wu
H, Ji Z, Yao Z, Zhang X, Yu B and Zhang K: High cholesterol induces
apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway
in tendon-derived stem cells. Stem Cell Res Ther. 11:1312020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, He C, Wu Q, Xu G, Kuang M, Wang
T, Xu L, Zhou H and Yuan W: Impaired autophagy induced by
oxLDL/β2GPI/anti-β2GPI complex through PI3K/AKT/mTOR and eNOS
signaling pathways contributes to endothelial cell dysfunction.
Oxid Med Cell Longev. 2021:66622252021.PubMed/NCBI
|
|
33
|
Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng
Z, Min R, Wu Z, Li W, Miao R, et al: Streptococcal pyrogenic
exotoxin B cleaves GSDMA and triggers pyroptosis. Nature.
602:496–502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019:81518362019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z,
Zhang Z and Dai Z: Propofol directly induces caspase-1-dependent
macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell
Death Dis. 10:5422019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu Y, Chen Y, Niu Z, Xing J, Yang Z, Yin
X, Guo L, Zhang Q, Qiu H and Han Y: A novel pyroptotic and
inflammatory gene signature predicts the prognosis of cutaneous
melanoma and the effect of anticancer therapies. Front Med
(Lausanne). 9:8415682022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Li Y, Song C, Hu Y, Dai M, Liu B and
Pan P: Neutrophil extracellular traps augmented alveolar macrophage
pyroptosis via AIM2 inflammasome activation in LPS-induced
ALI/ARDS. J Inflamm Res. 14:4839–4858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harada K, Rogers JE, Iwatsuki M, Yamashita
K, Baba H and Ajani JA: Recent advances in treating oesophageal
cancer. F1000Res 9: F1000 Faculty Rev. 11892020. View Article : Google Scholar
|
|
39
|
Serra S and Chetty R: p16. J Clin Pathol.
71:853–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishida H, Kasajima A, Fujishima F, Akaishi
R, Ueki S, Yamazaki Y, Onodera Y, Gao X, Okamoto H, Taniyama Y, et
al: p16 in highly malignant esophageal carcinomas: The correlation
with clinicopathological factors and human papillomavirus
infection. Virchows Arch. 478:219–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szymonowicz KA and Chen J: Biological and
clinical aspects of HPV-related cancers. Cancer Biol Med.
17:864–878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang C, Fischer-Kešo R, Schlechter T,
Ströbel P, Marx A and Hofmann I: Plakophilin 1-deficient cells
upregulate SPOCK1: Implications for prostate cancer progression.
Tumour Biol. 36:9567–9577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang B, Tang D, Liu Z, Wang Q, Xue S, Zhao
Z, Feng D, Sheng C, Li J and Zhou Z: LINC00958 promotes
proliferation, migration, invasion, and epithelial-mesenchymal
transition of oesophageal squamous cell carcinoma cells. PLoS One.
16:e02517972021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alaibac M: Targeting DSG3: From pemphigus
to squamous cell carcinoma. Expert Opin Ther Targets. 17:477–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen YJ, Lee LY, Chao YK, Chang JT, Lu YC,
Li HF, Chiu CC, Li YC, Li YL, Chiou JF and Cheng AJ: DSG3
facilitates cancer cell growth and invasion through the
DSG3-plakoglobin-TCF/LEF-Myc/cyclin D1/MMP signaling pathway. PLoS
One. 8:e640882013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin MG and Hurley JH: Structure and
function of the ULK1 complex in autophagy. Curr Opin Cell Biol.
39:61–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang
D, Li T, Wang CZ, Tan YX, Ding J, et al: ADRB2 signaling promotes
HCC progression and sorafenib resistance by inhibiting autophagic
degradation of HIF1α. J Hepatol. 65:314–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu
JM, Nie L, Chen Y, Wang YC, Liu C, et al: PD-L1-mediated gasdermin
C expression switches apoptosis to pyroptosis in cancer cells and
facilitates tumour necrosis. Nat Cell Biol. 22:1264–1275. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiao Y, Wang L, Lu L, Liu J, Li X, Zhao H,
Hou Z and Zheng B: The role of caspase-4 and NLRP1 in MCF7 cell
pyroptosis induced by hUCMSC-secreted factors. Stem Cells Int.
2020:88671152020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rathinam VAK, Zhao Y and Shao F: Innate
immunity to intracellular LPS. Nat Immunol. 20:527–533. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang SH, Cui LG, Su XL, Komal S, Ni RC,
Zang MX, Zhang LR and Han SN: GSK-3β-mediated activation of NLRP3
inflammasome leads to pyroptosis and apoptosis of rat
cardiomyocytes and fibroblasts. Eur J Pharmacol. 920:1748302022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heidegger I, Borena W and Pichler R: The
role of human papilloma virus in urological malignancies.
Anticancer Res. 35:2513–2519. 2015.PubMed/NCBI
|
|
53
|
Badarni M, Prasad M, Balaban N, Zorea J,
Yegodayev KM, Joshua BZ, Dinur AB, Grénman R, Rotblat B, Cohen L
and Elkabets M: Repression of AXL expression by AP-1/JNK blockage
overcomes resistance to PI3Ka therapy. JCI Insight. 5:e1253412019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu CY, Li F, Zeng Y, Tang MZ, Huang Y, Li
JT and Zhong RG: Infection and integration of high-risk human
papillomavirus in HPV-associated cancer cells. Med Oncol.
32:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liao X, Gao Y, Liu J, Tao L, Xie J, Gu Y,
Liu T, Wang D, Xie D and Mo S: Combination of tanshinone IIA and
cisplatin inhibits esophageal cancer by downregulating
NF-κB/COX-2/VEGF pathway. Front Oncol. 10:17562020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mattoscio D, Casadio C, Miccolo C, Maffini
F, Raimondi A, Tacchetti C, Gheit T, Tagliabue M, Galimberti VE, De
Lorenzi F, et al: Autophagy regulates UBC9 levels during
viral-mediated tumorigenesis. PLoS Pathog. 13:e10062622017.
View Article : Google Scholar : PubMed/NCBI
|