Introduction
Approximately 500,000 new cases and 200,000 deaths
from bladder cancer occur worldwide each year. The incidence of
bladder cancer has gradually increased, causing a great burden on
human health (1–3). Bladder urothelial carcinoma (BLCA) is
the most common subtype of bladder cancer, accounting for >95%
of cases (4). Although early BLCA
diagnosis and treatment options have rapidly improved, the
prognosis of patients with metastatic BLCA remains poor, with a
5-year survival rate of ~50% (2).
In the past few decades, numerous studies have aimed to reveal the
cause of BLCA pathogenesis; however, the underlying mechanisms are
still unclear (5–7). Thus, more valuable markers for the
prognosis and effective targets for BLCA therapy are urgently
needed.
Chromobox (CBX) family members participate in
chromosomal modification, transcriptional repression, cell
differentiation and senescence (8),
and eight CBX proteins have been described to date. These proteins
are divided into two groups, including the Pc (containing
N-terminal chromodomain) group and the HP1 (containing C-terminal
and N-terminal chromodomains) group (9). Additionally, a recent study confirmed
that CBXs are associated with a number of tumors (10). For example, CBX2 is significantly
associated with colorectal cancer (CRC) prognosis because of its
regulatory function in the apoptosis and proliferation of CRC cells
(11). Furthermore, CBX4 can
predict a worse prognosis in patients with hepatocellular carcinoma
by promoting tumor angiogenesis (12) and CBX7 can suppress bladder cancer
progression by modulating aldo-keto reductase family 1 member
B10/ERK signaling (13).
Nevertheless, whether the other CBXs participate in BLCA occurrence
remains unknown. In the current study, the expression patterns and
genetic alterations of CBXs and their association with the
clinicopathological parameters and infiltration of immune cells in
BLCA were analyzed using online public databases. The present
results showed that CBXs participated significantly in BLCA
pathogenesis and progression, and may be used as potential clinical
therapeutic targets for BLCA therapy.
Materials and methods
UALCAN
UALCAN (http://ualcan.path.uab.edu) is a comprehensive cancer
data website based on The Cancer Genome Atlas (TCGA) database
(14,15). UALCAN was used to obtain data
(TCGA-BLCA) on CBX mRNA expression and promoter methylation levels
in BLCA tissues and normal tissues (from healthy controls), as well
as the association between CBX expression and clinicopathological
parameters. The beta value (percentage of methylation signal
strength) is the most commonly used quantitative method for
methylation levels. Differences in CBX mRNA expression and promoter
methylation levels compared with the corresponding controls (from
healthy controls) were analyzed by Welch's T-test, and P<0.05
was considered to indicate a statistically significant difference.
Differences in CBX expression were compared among individual cancer
stages by one-way ANOVA followed by the Dunnett's post hoc multiple
comparisons test, and P<0.05 was considered to indicate a
statistically significant difference.
ONCOMINE
Expression analyses were conducted using ONCOMINE
(www.oncomine.org). An unpaired t-test was used to
examine differential expression between the cancer and normal
groups (from healthy controls), and the error detection rate was
used as a correction measure of significance (16). Fold change and cut-off P-values were
set as 1.5 and 0.01, respectively.
Cell culture
The SV-HUC-1 human ureteral epithelial immortalized
cell line was obtained from the American Type Culture Collection
and cultured in f12k (iCell Bioscience, Inc.) media with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with
5% CO2 in humidified air. The 5637 bladder cancer cell
line was obtained from the American Type Culture Collection and
cultured in 1640 media (Gibco; Thermo Fisher Scientific, Inc.) with
10% fetal bovine serum at 37°C with 5% CO2 in humidified
air. The UMUC3 bladder cancer cell line was obtained from the
American Type Culture Collection and cultured in 1640 media with
10% fetal bovine serum at 37°C with 5% CO2 in humidified
air. Media were not supplemented with antibiotics. The culture
medium was replaced every 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA (from SV-HUC-1, 5637 and UMUC3 cells) was
isolated using the total RNA extraction kit (Takara Bio, Inc.). The
Bestar™ qPCR RT Kit (Takara Bio, Inc.) was used to synthesize cDNA
from isolated total RNA, according to the manufacturer's protocol.
Samples were processed in the Applied Biosystems 7500 Real-Time PCR
System using TB Green Premix Ex Taq II (cat. no. RR820A; Takara
Bio, Inc.). The reaction steps were as follows: i)
Pre-denaturation, 95°C for 30 sec; and ii) PCR reaction (40
cycles), 95°C for 5 sec, 60°C for 30 sec. The primer sequences for
β-actin and CBXs are shown in Table
I. Cycle threshold values were collected to calculate the
relative expression of target genes, using the 2−ΔΔCq
method of quantification (17).
 | Table I.Primer sequences of CBX family
members. |
Table I.
Primer sequences of CBX family
members.
| Gene | Species | Forward | Reverse |
|---|
| β-actin | Human |
5-TCTCCCAAGTCCACACAGG-3 |
5-GGCACGAAGGCTCATCA-3 |
| CBX1 | Human |
5-GAGCCGGAGCGGATTATTGG-3 |
5-GGGCACTTGACATTGGCTTC-3 |
| CBX2 | Human |
5-TCCTGGCCTTCCAGAAGAAG-3 |
5-CAGGAGGACATGGCAGTGAG-3 |
| CBX3 | Human |
5-TTGAAGAGGCAGAGCCTGAA-3 |
5-AGGTTCCCAAGTATTGTCAGC-3 |
| CBX4 | Human |
5-TGATCGCCTTCCAGAACAGG-3 |
5-TCAGTGGAGGAGTCCTGGAG-3 |
| CBX6 | Human |
5-CGAAAGGGACGCATCGAGTA-3 |
5-GCGAGTCCAGGATGTTCTCC-3 |
| CBX7 | Human |
5-TGCGGAAGGGTAAAGTCGAG-3 |
5-TCCTCCTTCTCCTCGTAGGC-3 |
| CBX8 | Human |
5-GATCCCTGTGGCCAGAATCC-3 |
5-GTGACCACCACCTTCTCCAG-3 |
Western blotting
SV-HUC-1, 5637 and UMUC3 cells were lysed in NP-40
lysis buffer (cat. no. P0013F; Beyotime Institute of Biotechnology)
containing protease inhibitors (Beyotime Institute of
Biotechnology). After a 15 min of centrifugation at 15,000 × g at
4°C, the supernatants were collected and protein concentration was
assessed using a BCA assay (Thermo Fisher Scientific, Inc.)
analyzed in a Multiskan full-wavelength microplate reader (Thermo
Fisher Scientific, Inc.). After separation by SDS-PAGE on 10% gels
(40 µg protein loaded per lane), proteins were transferred to a
PVDF membrane and blocked for 1 h using 5% non-fat dry milk at room
temperature. Subsequently, membranes were incubated with primary
antibodies overnight at 4°C, and then with the secondary antibody
for 1 h at room temperature after three washes in TBS-Tween
(containing 0.1% Tween). Primary antibodies were used at the
following dilutions: CBX1 (cat. no. 10241-2-AP; Proteintech Group,
Inc.) at 1:2,000, CBX2 (cat. no. 15579-1-AP; Proteintech Group,
Inc.) at 1:3,000 and CBX7 (cat. no. 26278-1-AP; Proteintech Group,
Inc.) at 1:1,000, and GAPDH (cat. no. 2118; Cell Signaling
Technology, Inc.) at 1:2,000. The secondary antibody was HRP-linked
anti-rabbit IgG (1:1,000; cat. no. 7074; Cell Signaling Technology,
Inc.). The protein bands were visualized and semi-quantified using
the Gel DOC XR Gel Imaging System and Image lab v5.2 software
(Bio-Rad Laboratories, Inc.), respectively.
Human protein atlas
(HPA). The HPA (https://www.proteinatlas.org) was used to collect data
about protein levels and distribution in BLCA and normal urothelial
tissue immunohistochemistry (18).
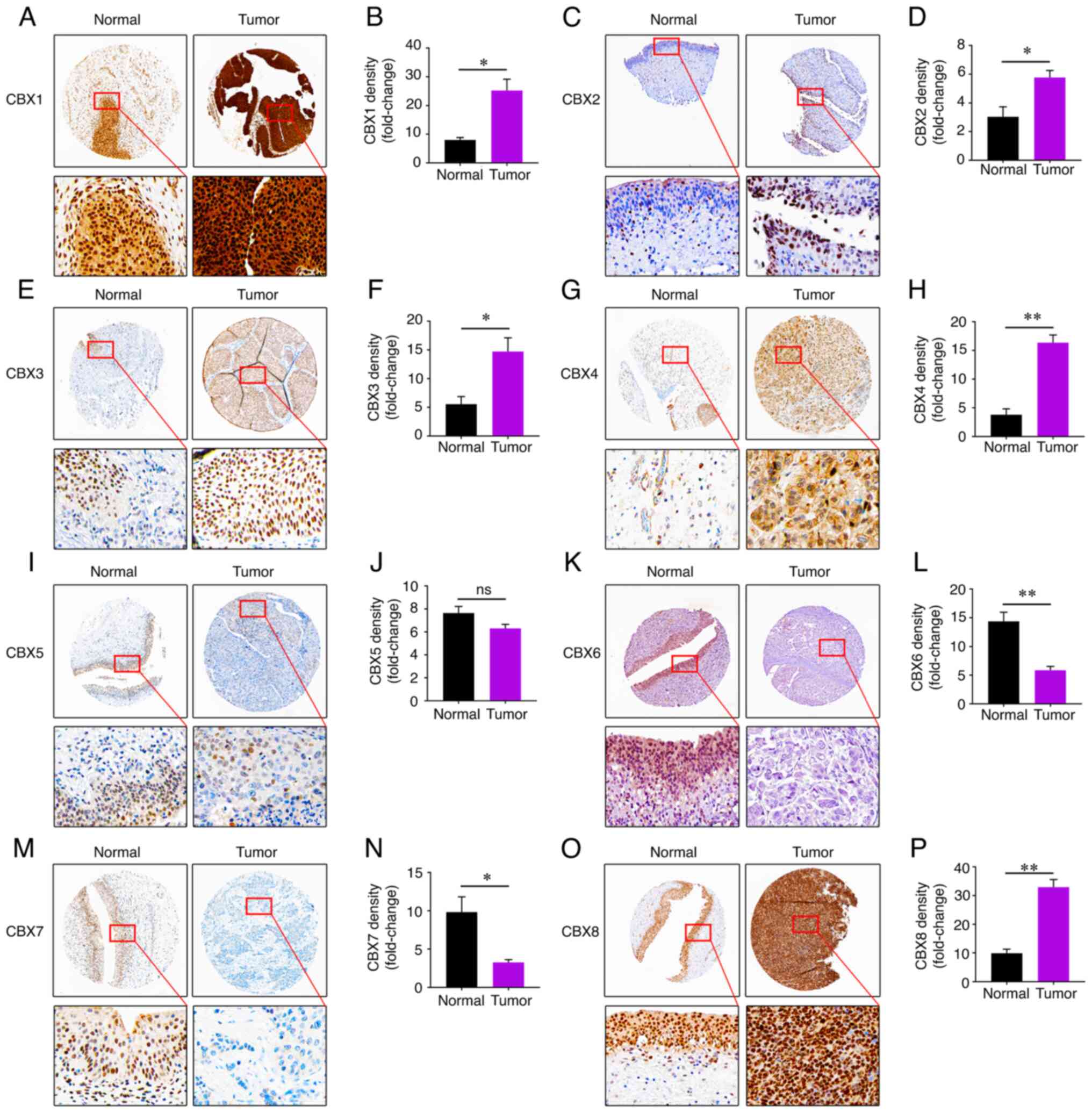
The immunohistochemical images of the expression of CBXs in BLCA
and normal tissues (from healthy controls) were extracted from the
HPA.
Survival analysis
To investigate the prognostic value of CBXs, patient
data from the Kaplan-Meier (KM) plotter (http://kmplot.com; dataset name, BLCA; mRNA; 0.05
significance cut-off value) were classified into two groups
according to the median expression value of CBX, and the
practicality in determining patient prognosis was evaluated. A
number of R (version 3.6.1) packages were used for statistical
computations and for generating the output graphs (19–22).
The ‘survival’ package was used for univariate and multivariate KM
analyses (21). Survival curve
plots were generated using the ‘survplot’ package. The ‘XML’ and
‘rjson’ R packages were used to load configuration files, the
‘RODBC’ package was used to communicate with the database, and the
‘ggplot2’ package was used to visualize the results (19,20,22).
The significance was computed using the Cox-Mantel test to compare
two cohorts. The difference between cohorts was numerically
characterized by the hazard rate (HR) based on the differential
descent rate of the two cohorts. Since the HR is a comparison to
the baseline by definition, a relative two-fold drop in one cohort
equals a half-fold drop in the other cohorts. Thus, depending on
the context, an HR of 2 equals an HR of 0.5. As it is easier to
understand an HR above 1 in most cases, an option to invert all HR
values below 1 was implemented (23,24).
P<0.05 was considered to indicate a statistically significant
difference.
Gene expression profiling interactive
analysis (GEPIA)
GEPIA (http://gepia.cancer-pku.cn/index.html) is a website
for gene expression profiling analysis. GEPIA generates a list of
genes with similar expression patterns ranked by Pearson
correlation coefficient (25).
Here, GEPIA was used to select the first 20 genes in BLCA that were
similar to the CBX family; a total of 129 genes were identified for
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) analysis (26).
Metascape
The Metascape database (http://metascape.org) was used for enrichment
analysis. The express analysis module in Metascape was used for GO
and KEGG analysis of CBX and similar genes (26). The relationship between physical
proteins captured in BioGrid is the main data source of Metascape
(27). Metascape automatically
extracts protein-protein interaction networks from these
candidates. Then, for each connected network component, the MCODE
algorithm is applied to identify the complex of dense connections
(28), and Cytoscape is used for
visualization (29).
cBioPortal
cBioPortal (www.cbioportal.org) is an online open access website
resource for analyzing cancer genomics data (30). In the present study, gene mutations
of CBXs in BLCA were analyzed using this resource.
Tumor immune estimation resource
(TIMER)
TIMER (https://cistrome.shinyapps.io/timer/) is a website for
analyzing immune cell infiltration into tumor tissues (31). The present study used the Gene and
Survival modules in TIMER to analyze the link between the
expression of CBXs and immune cell infiltration, as well as the
association of immune cell infiltration with the clinical outcome
of patients with BLCA. The ‘Gene’ module was used to explore the
correlation between CBXs and the level of immune infiltration in
BLCA. Scatterplots were generated, showing the purity-corrected
partial Spearman's ρ value and statistical significance. The
‘DiffExp’ module allows the study of differential expression
between BLCA tumor and adjacent normal tissues (adjacent tissues
from the same patients) for the CBX genes across the BLCA dataset
from TCGA. The Cox proportional hazard model (‘survival’ module)
was used to explore the clinical relevance of BLCA tumor immune
cell subsets and CBX expression levels. The distributions of gene
expression levels are displayed using box plots, with the
statistical significance of differential expression evaluated using
the Wilcoxon test. Up or downregulated genes in tumors compared
with in normal tissues (adjacent tissues from the same patients)
can be identified for each cancer type.
Statistical analysis
All collected data were analyzed using SPSS (version
20.0; IBM Corp.) and are expressed as the mean ± SEM. In
vitro experiments were repeated 33 times. The
difference between indicated groups was evaluated by Student's
t-test, Welch's T-test, Wilcoxon test or ANOVA. Unpaired Student's
t-test (parametric) or Wilcoxon signed-rank test (non-parametric)
were used to compare the datasets of two groups. One-way ANOVA
(parametric) were used to compare the datasets of multiple groups.
If three datasets were analyzed, the LSD post hoc multiple
comparisons test was performed following ANOVA; otherwise, the
Dunnett's post hoc multiple comparisons test was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of CBXs and methylation of
their promoters in patients with BLCA
The UALCAN, TIMER and ONCOMINE databases were used
to evaluate the expression of CBXs in patients with BLCA. Compared
with in normal bladder mucosa tissues, BLCA tissues exhibited
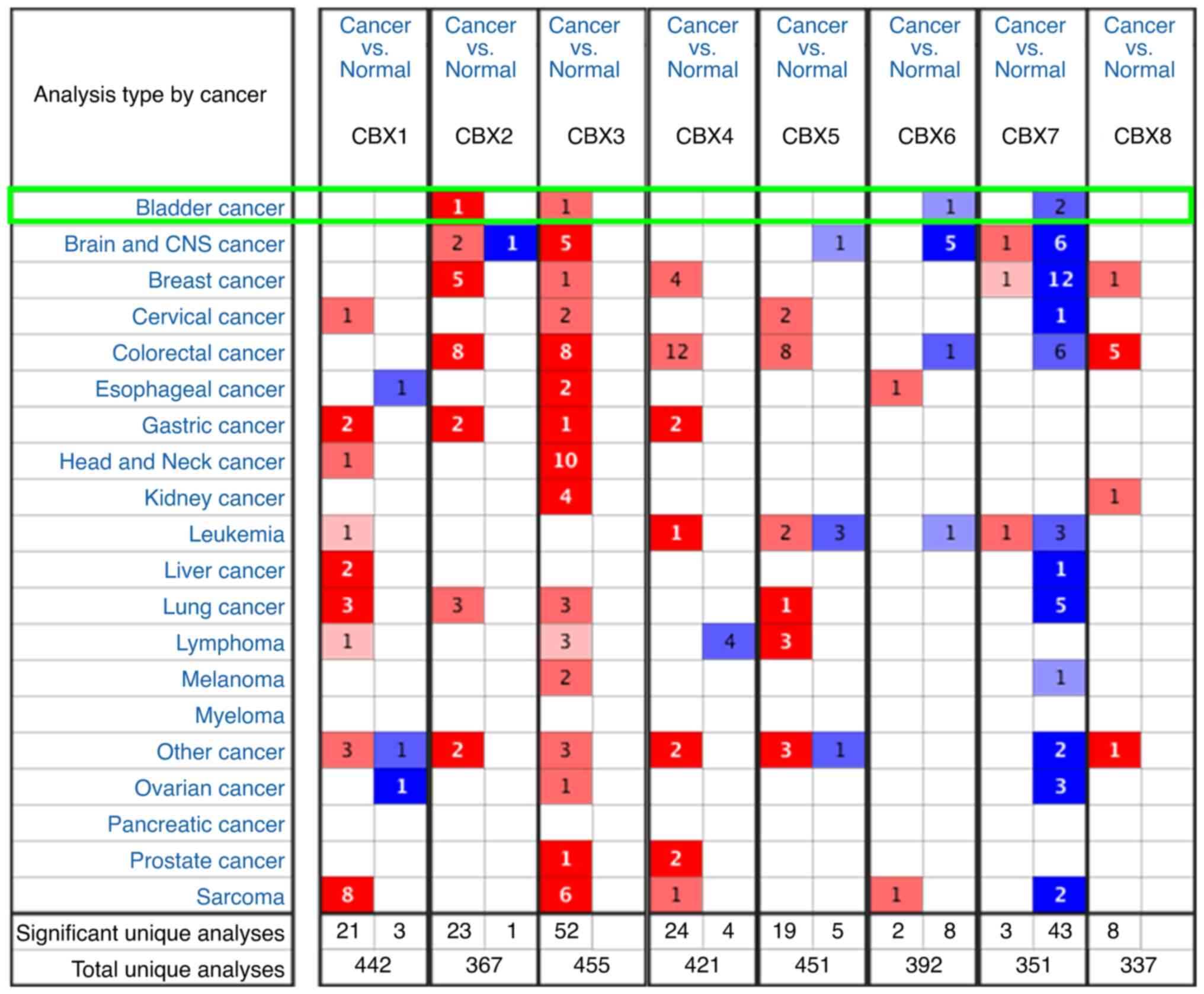
markedly elevated expression of CBX1 (Figs. 1A and 2), CBX2 (Figs.
1B, 2 and 3), CBX3 (Figs.
1C, 2 and 3), CBX4 (Figs.
1D and 2) and CBX8 (Figs. 1H and 2), and reduced expression of CBX6
(Figs. 1F, 2 and 3)
and CBX7 (Figs. 1G, 2 and 3).
However, no significant difference was detected for CBX5 (Figs. 1E, 2
and 3) between BLCA and normal
bladder mucosa tissues. Moreover, the expression of CBXs in normal
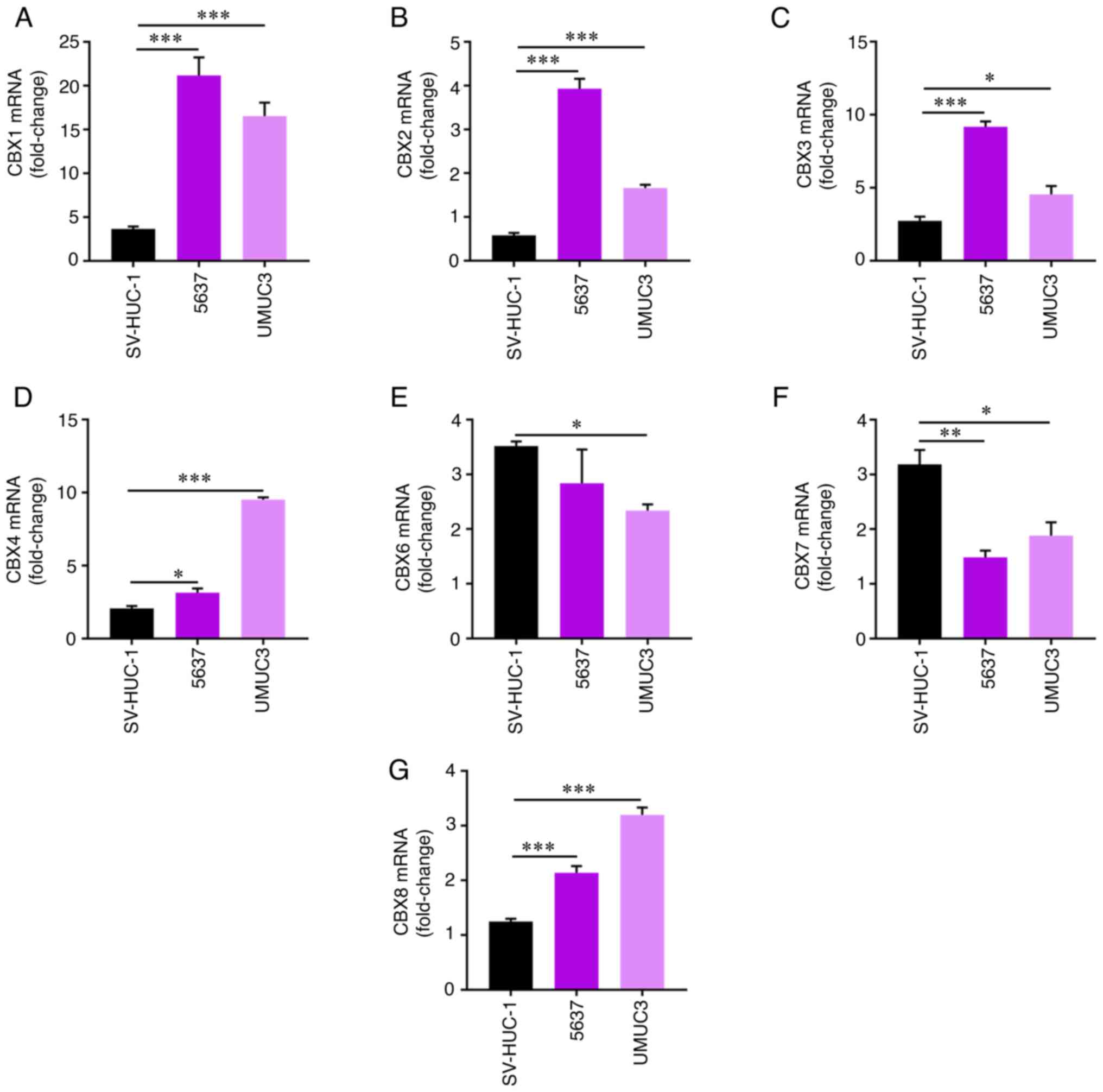
SV-HUC-1 bladder cells, and 5637 and UMUC3 bladder tumor cells was
evaluated by RT-qPCR. Consistent with the aforementioned results,
elevated expression levels of CBX1 (Fig. 4A), CBX2 (Fig. 4B), CBX3 (Fig. 4C), CBX4 (Fig. 4D) and CBX8 (Fig. 4G), and reduced expression levels of
CBX6 (Fig. 4E) and CBX7 (Fig. 4F) were detected in BLCA cells
compared with in normal SV-HUC-1 cells. These results were also
supported by the expression of CBXs at the protein level determined
using the HPA (Fig. 5).
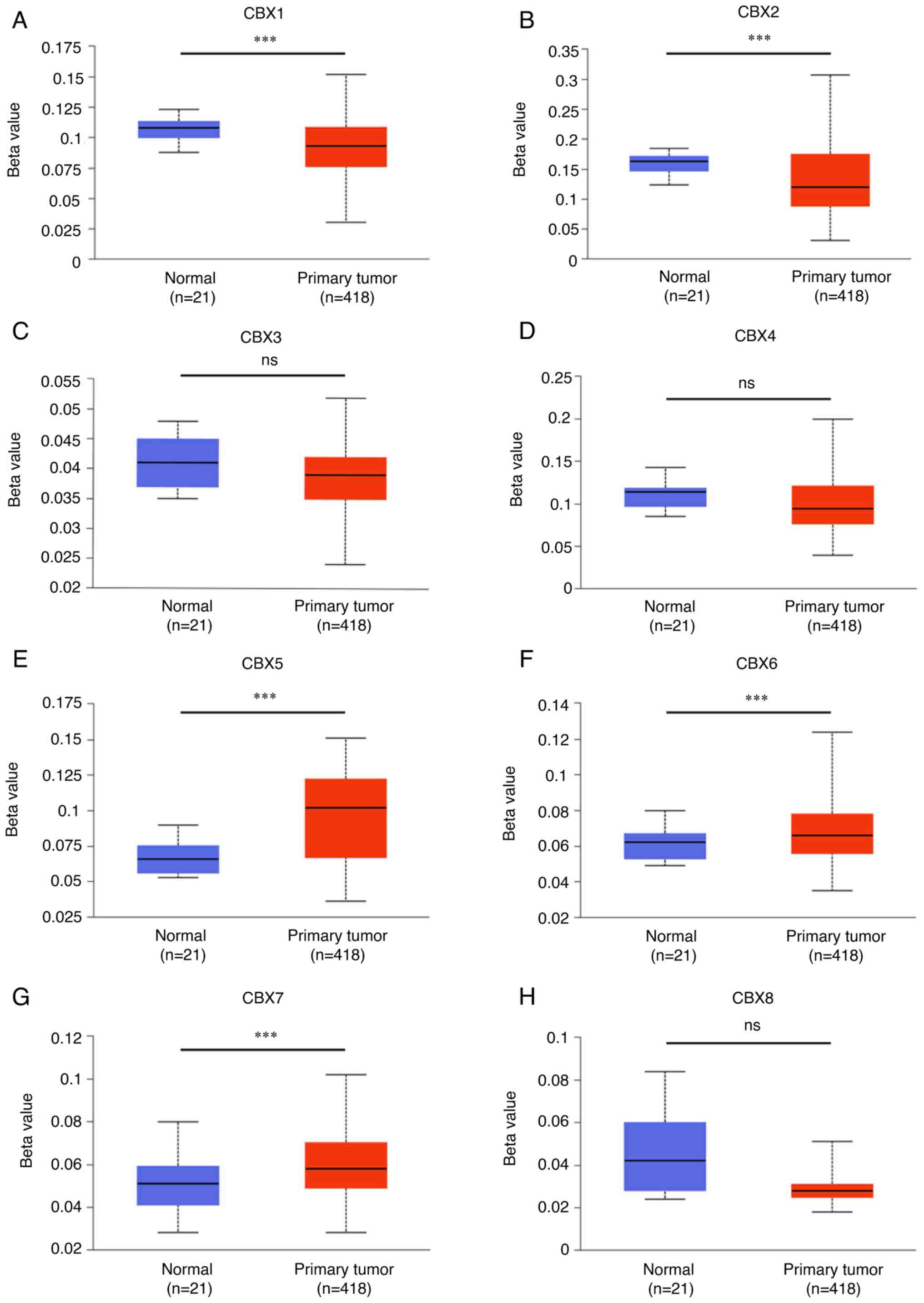
Additionally, promoter methylation was associated with the
progression of bladder tumors. Hypermethylation was detected in the
promoters of CBX5 (Fig. 6E), CBX6
(Fig. 6F) and CBX7 (Fig. 6G), whereas hypomethylation was
identified in the promoters of CBX1 (Fig. 6A) and CBX2 (Fig. 6B).
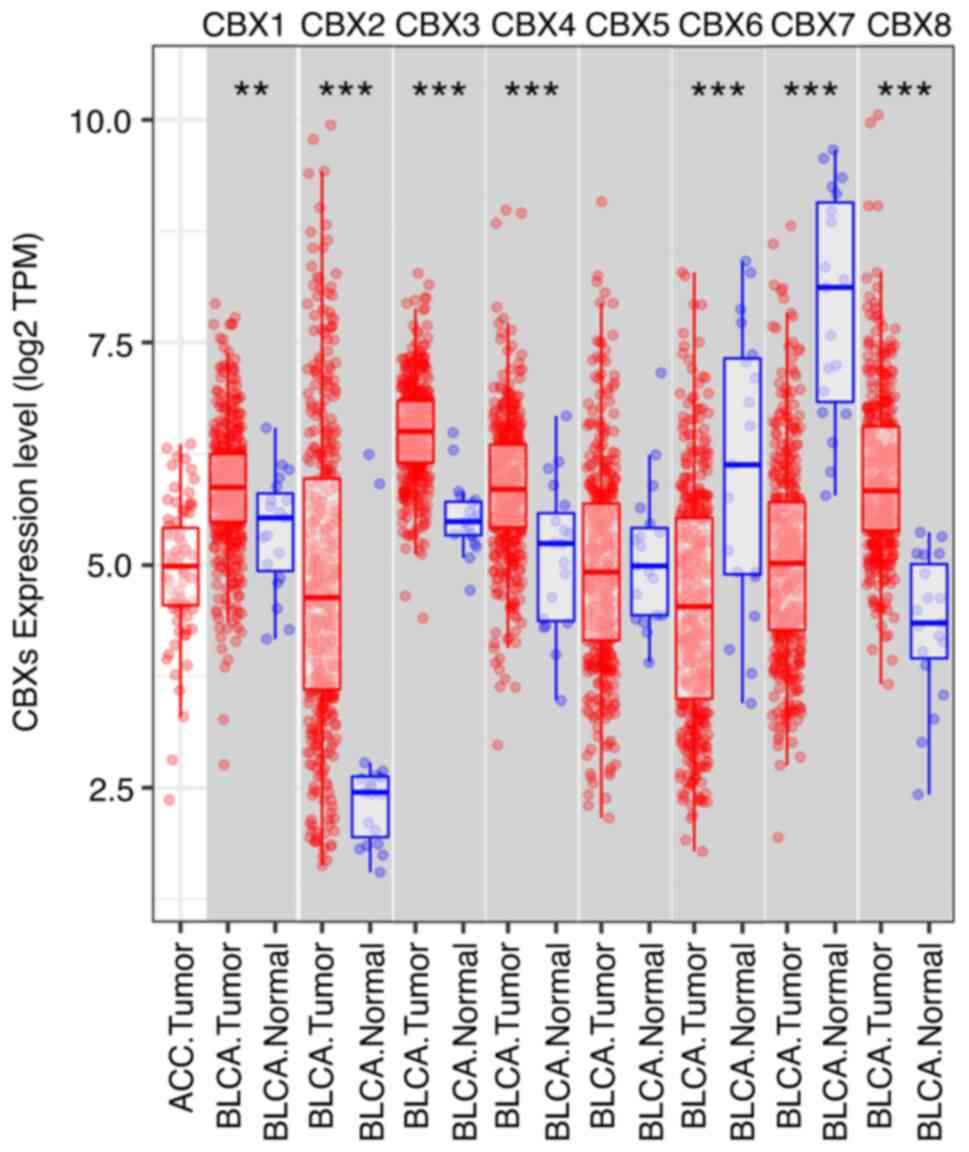 | Figure 2.mRNA expression levels of CBXs in
BLCA, as determined using TIMER. The mRNA expression levels of
CBX1, CBX2, CBX3, CBX4 and CBX8 were increased in BLCA tissues
compared with in normal (adjacent tissues from the same patients)
bladder tissues, whereas CBX6 and CBX7 were reduced. CBX5
expression was unaltered. Distributions of gene expression levels
are displayed using box plots, with the statistical significance of
differential expression evaluated by the Wilcoxon signed-rank test.
TPM represent the relative abundance of a transcript among a
population of sequenced transcripts. **P<0.01, ***P<0.001.
ns, no significance; TPM, transcripts per million; BLCA, bladder
urothelial carcinoma; CBX, chromobox; TIMER, Tumor Immune
Estimation Resource. |
Association between CBXs and the
cancer stage of patients with BLCA
The cancer stage was negatively associated with the
expression of CBX6 (Fig. 7F) and
CBX7 (Fig. 7G), whereas it was
positively associated with CBX1 (Fig.
7A), CBX2 (Fig. 7B), CBX3
(Fig. 7C), CBX4 (Fig. 7D) and CBX8 (Fig. 7H). Notably, no association was
identified between CBX5 (Fig. 7E)
expression and the cancer stages of patients. Hence, it was
indicated that CBX1, CBX2, CBX3, CBX4, CBX6, CBX7 and CBX8
expression levels were closely associated the stages of patients
with BLCA.
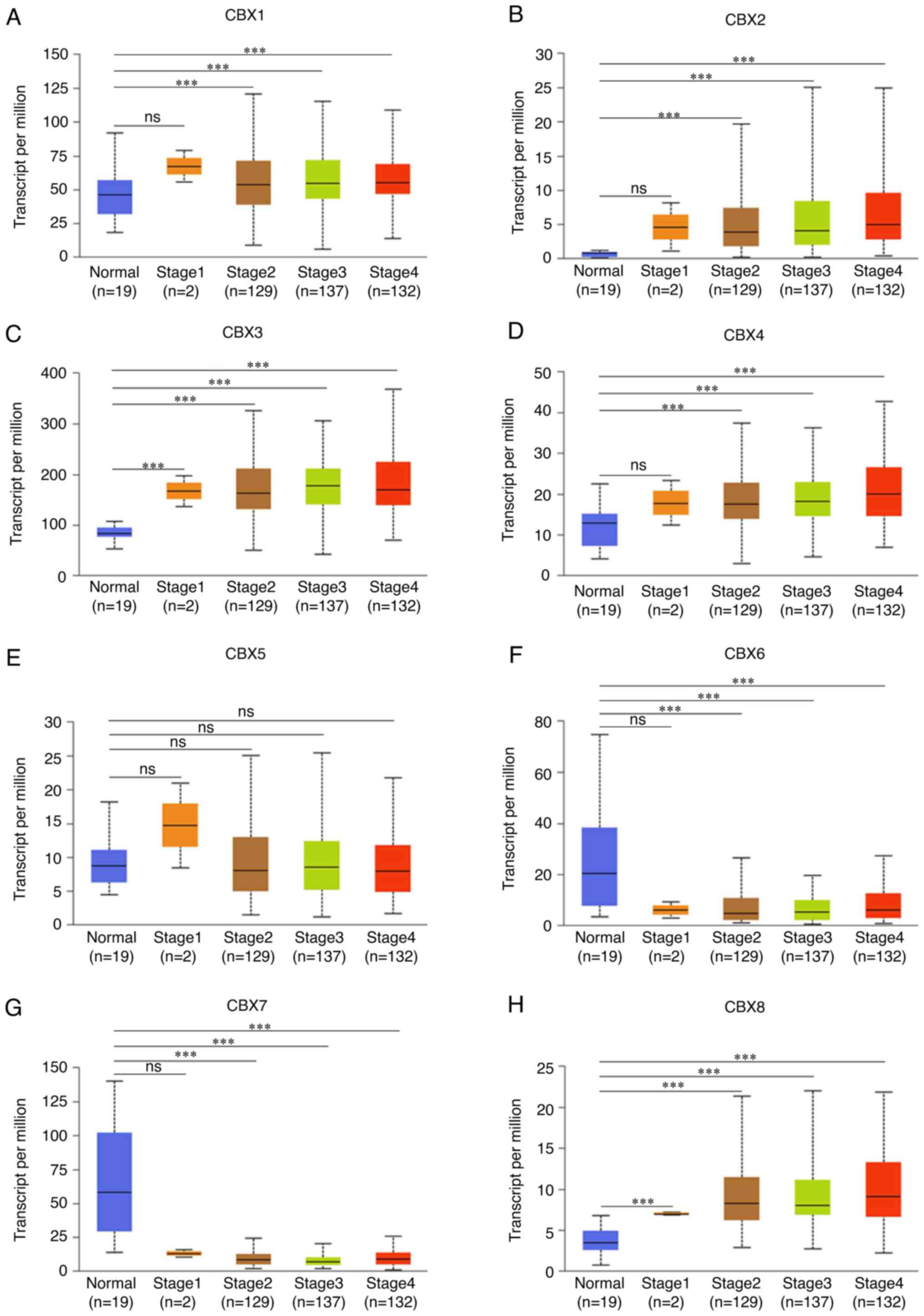 | Figure 7.Association between mRNA expression
levels of CBXs and tumor stages in patients with bladder urothelial
carcinoma using UALCAN. The cancer stage was positively associated
with the expression of (A) CBX1, (B) CBX2, (C) CBX3 and (D) CBX4,
whereas it was not related to (E) CBX5. The cancer stage was
negatively associated with the expression of (F) CBX6 and (G) CBX7,
but it was positively associated with the expression of (H) CBX8.
Stage 1, noninvasive papillary carcinoma, carcinoma in situ
and tumor invades subepithelial connective tissue; Stage 2, tumor
invades muscle; Stage 3, tumor invades perivesical tissue; Stage 4,
tumor invades prostate, uterus, vagina, pelvic wall or abdominal
wall. The difference among groups was evaluated by one-way ANOVA
followed by the Dunnett's post hoc multiple comparisons test.
***P<0.001. ns, no significance; CBX, chromobox. |
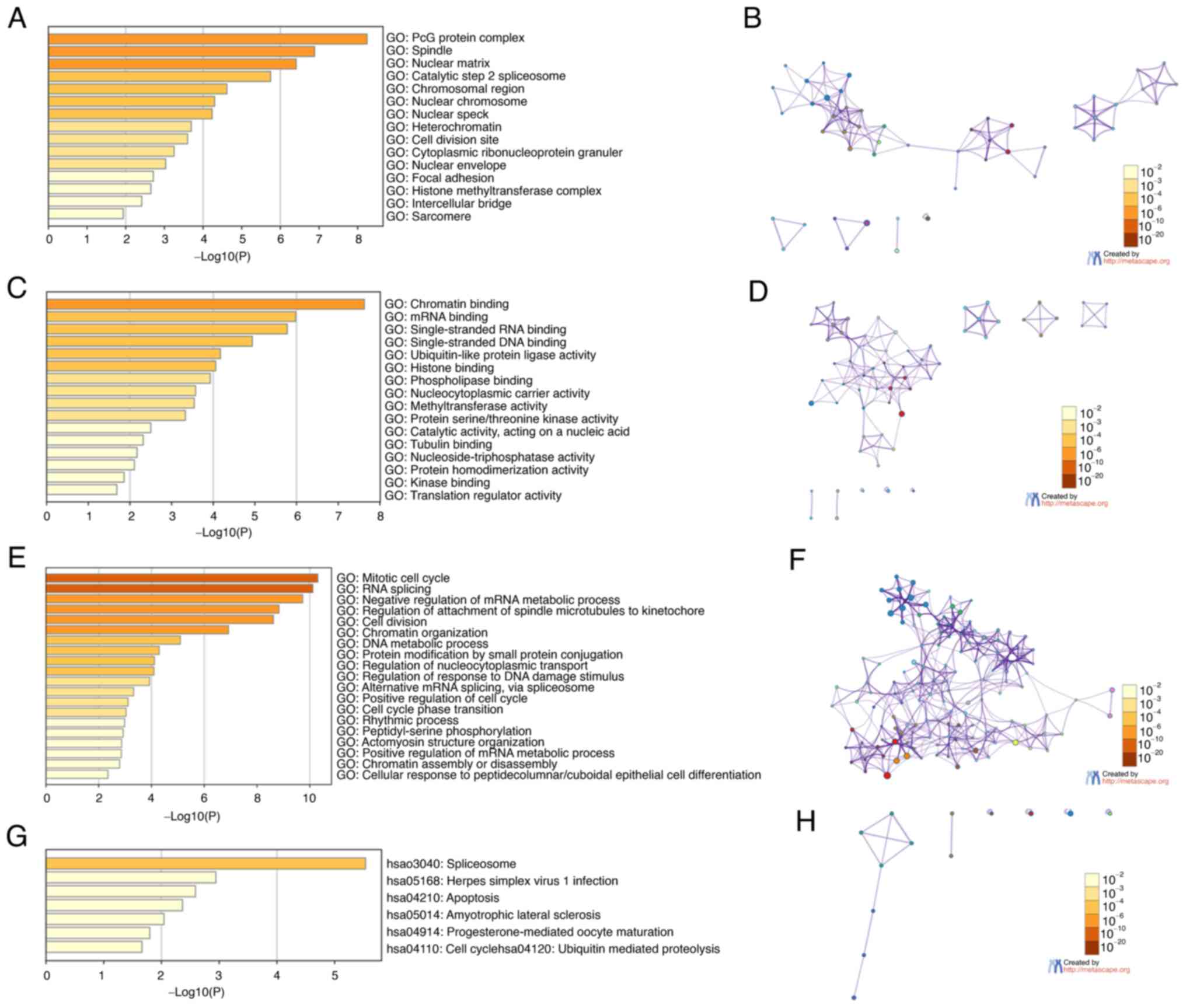
Network and functional enrichment
analyses of the CBX family and their neighboring genes in patients
with BLCA
The biological classification of CBXs was
investigated via pathway and function enrichment analyses using
Metascape. The GO and KEGG analyses predicted the functions of CBX
family genes according to the enrichment of CBXs. The enrichment
items were divided into four functional groups: Cellular components
(Fig. 8A and B), molecular
functions (Fig. 8C and D),
biological processes (Fig. 8E and
F), and KEGG pathways (Fig. 8G and
H). CBX and their related genes were enriched for: The ‘mitotic
cell cycle’, ‘RNA splicing’, ‘cell division’, ‘PcG protein
complex’, ‘chromatin binding’, ‘nuclear matrix’, ‘single-stranded
RNA binding’, ‘single-stranded DNA binding’, ‘nuclear chromosome’,
‘spliceosome’, ‘herpes simplex virus 1 infection’, ‘apoptosis’ and
‘amyotrophic lateral sclerosis’.
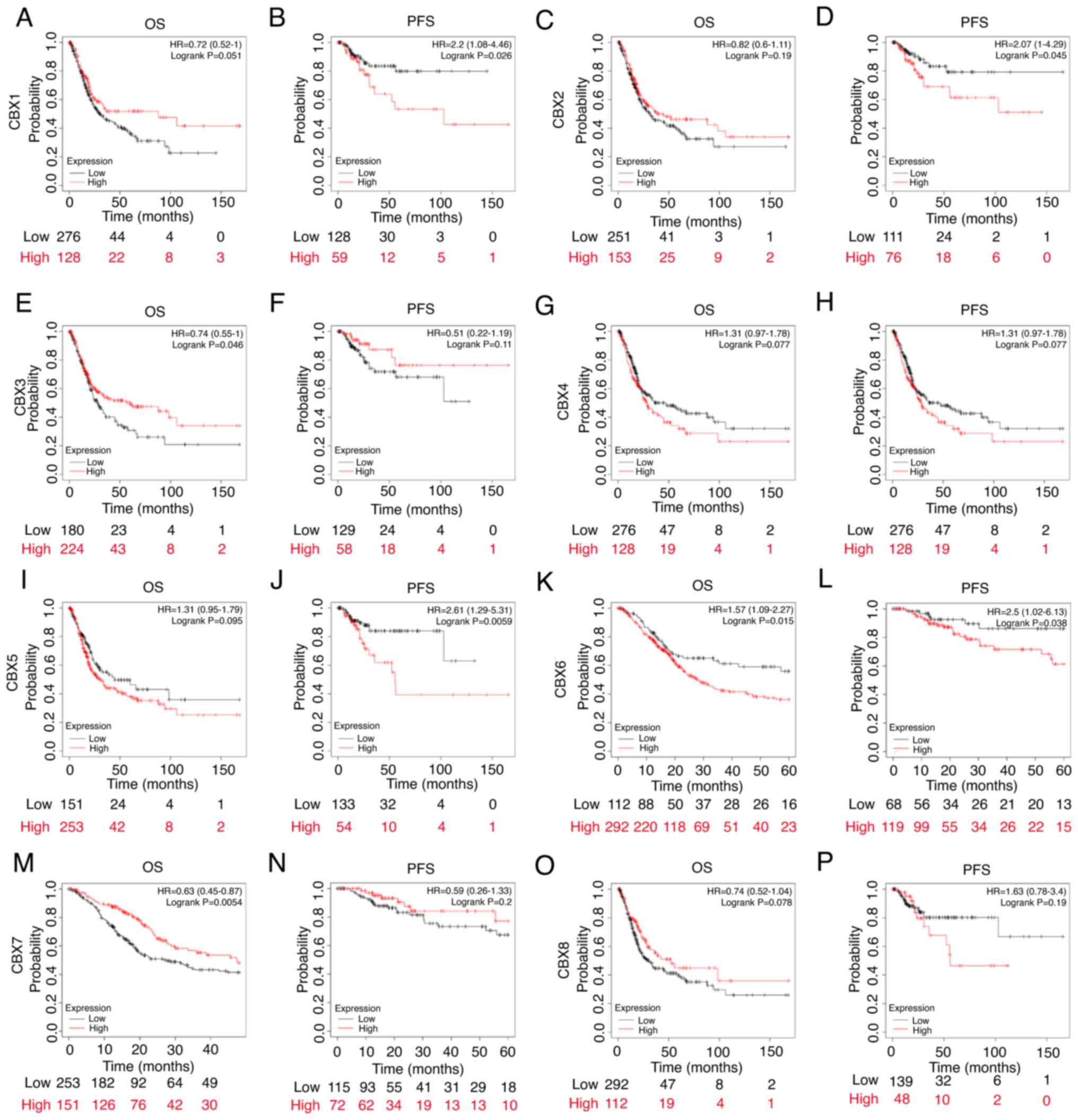
Value of CBXs for the prognosis of
patients with BLCA
BLCA patients were ranked according to CBXs
expression. Half of the patients with high CBXs expression were
included in the high expression group, and the second half of the
patients with low CBXs expression were included in the low
expression group. Patients with high expression of CBX1 (Fig. 9B), CBX2 (Fig. 9D), CBX5 (Fig. 9J) and CBX6 (Fig. 9L) exhibited poor progression-free
survival (PFS), whereas patients with low expression of CBX3
(Fig. 9E) and CBX7 (Fig. 9M) exhibited poor overall survival
(OS). Patients with high expression of CBX6 (Fig. 9K) exhibited poor OS. These results
suggested that CBX1, CBX2 and CBX7 expression levels were closely
related to the prognosis of patients with BLCA. There was no
significant difference in OS between patients with high and low
expression of CBX1 (Fig. 9A), CBX2
(Fig. 9C), CBX4 (Fig. 9G), CBX5 (Fig. 9I) and CBX8 (Fig. 9O). Additionally, there was no
significant difference in PFS between patients with high and low
expression of CBX3 (Fig. 9F), CBX4
(Fig. 9H), CBX7 (Fig. 9N) and CBX8 (Fig. 9P).
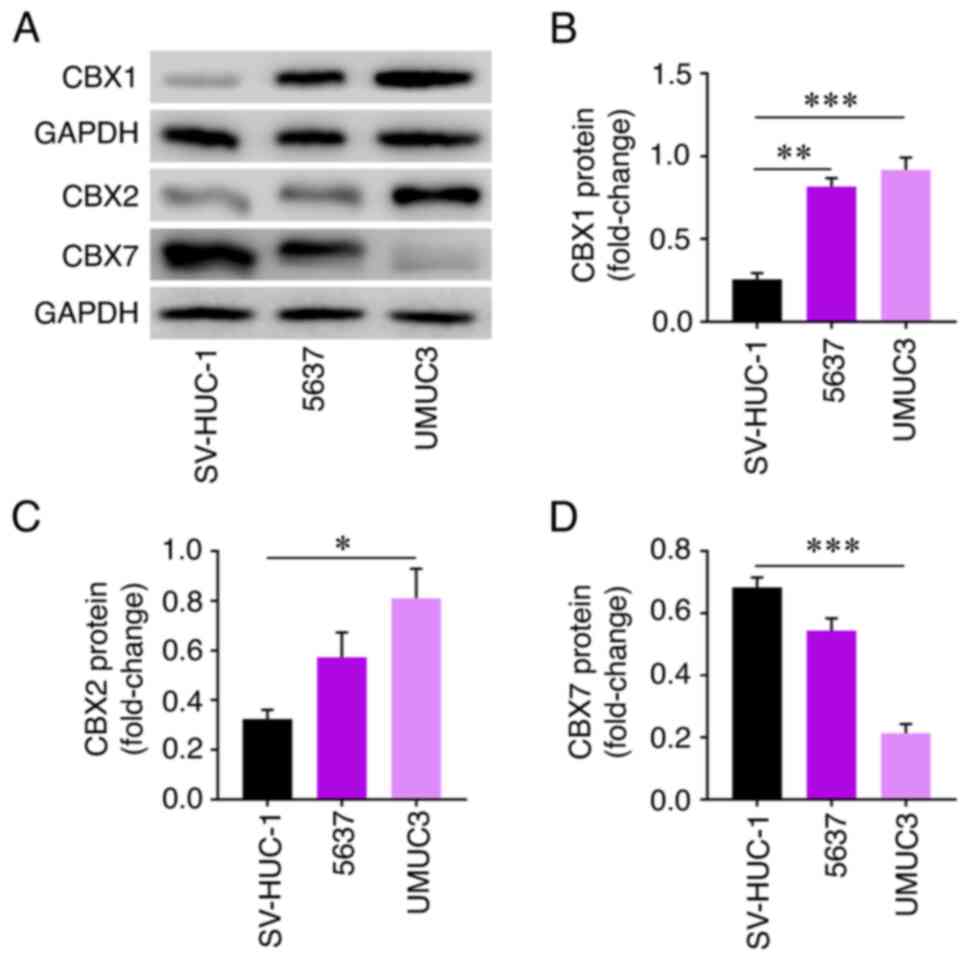
Protein levels of CBXs with prognostic
value in different cell lines
The protein expression levels of CBX1 were
significantly increased in 5637 and UMUC3 cells compared with in
SV-HUC-1 cells (Fig. 10B).
Similarly, the protein expression levels of CBX2 were significantly
increased in UMUC3 cells compared with in SV-HUC-1 cells (Fig. 10C). By contrast, the protein
expression levels of CBX7 were significantly decreased in UMUC3
cells compared with in SV-HUC-1 cells (Fig. 10D).
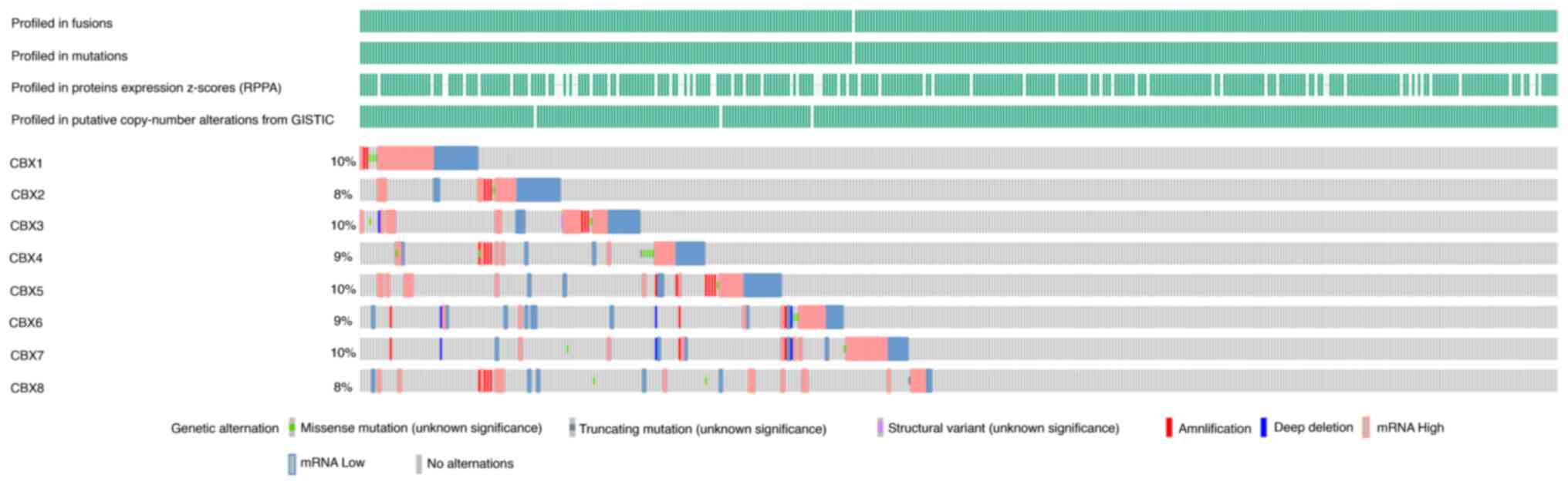
Genetic alterations of CBXs in
patients with BLCA
The genetic alterations of CBXs in patients with
BLCA were analyzed using the cBioPortal online tool. The results
indicated that generally, 48% of patients with BLCA exhibited
CBX1-8 genetic alterations. The OncoPrints include missense
mutations, truncating mutations, structural variants,
amplification, deep deletion, and abnormal expressions. The genetic
alteration rates for CBX1-8 were 10, 8, 10, 9, 10, 9, 10 and 8%,
respectively (Fig. 11).
Infiltration of immune cells in
patients with BLCA
Positive correlations were observed between CBX1 and
CBX3 expression and the infiltration of dendritic cells,
neutrophils, macrophages and CD8+ T cells (Fig. 12A and C); CBX2 was positively
correlated with macrophage infiltration (Fig. 12B); CBX4 was positively correlated
with neutrophil, macrophage and B cell infiltration (Fig. 12D); CBX5 was positively correlated
with the infiltration of dendritic cells, neutrophils, macrophages,
CD4+ and CD8+ T cells (Fig. 12E); CBX6 was positively correlated
with macrophage, CD4+ T cell, CD8+ T cell and
B cell infiltration (Fig. 12F);
CBX7 was positively correlated with the infiltration of
macrophages, CD4+ T cells and B cells (Fig. 12G); and CBX8 was positively
correlated with macrophage infiltration (Fig. 12H). Meanwhile, negative
correlations were observed between CBX7 expression and the
infiltration of dendritic cells and CD8+ T cells
(Fig. 12G); and CBX8 with
dendritic cell and neutrophil infiltration (Fig. 12H). The present results also found
that macrophages, CBX6 and CBX7 were associated with the clinical
outcome of patients with BLCA (Table
II). However, all correlation values were <0.3 or >-0.3,
which indicates that they were weak.
 | Figure 12.Correlation between differentially
expressed CBXs and infiltration of immune cells using TIMER. The
expression of (A) CBX1 was positively related to tumor purity and
the infiltration of CD8+ T cells, macrophages,
neutrophils and dendritic cells; (B) CBX2 expression was positively
related to tumor purity and the infiltration of macrophages; (C)
CBX3 expression was positively related to the infiltration of
CD8+ T cells, macrophages, neutrophils and dendritic
cells; (D) CBX4 expression was positively related to the
infiltration of B cells, macrophages and neutrophils; (E) CBX5
expression was positively related to tumor purity and the
infiltration of CD8+ T cells, CD4+ T cells,
macrophages, neutrophils and dendritic cells; (F) CBX6 expression
was positively related to the infiltration of B cells,
CD8+ T cells and macrophages; (G) CBX7 expression was
positively related to the infiltration of B cells, CD4+
T cells, macrophages, and negatively associated with the
infiltration of CD8+ T cells and dendritic cells; (H)
CBX8 expression was positively associated with tumor purity, the
infiltration of macrophages and negatively associated with the
infiltration of neutrophils and dendritic cells. TPM, transcripts
per million; CBX, chromobox; TIMER, Tumor Immune Estimation
Resource. |
 | Table II.Cox proportional hazard model of CBXs
and six tumor-infiltrating immune cells in BLCA performed using
TIMER. |
Table II.
Cox proportional hazard model of CBXs
and six tumor-infiltrating immune cells in BLCA performed using
TIMER.
| Variables | Coefficient | HR | HR 95%CI_l | 95%CI_u | P-value |
|---|
| B_cell | −2.589 | 0.075 | 0.004 | 1.575 | 0.095 |
| CD8_T cell | 1.139 | 3.124 | 0.199 | 48.947 | 0.417 |
| CD4_T cell | −0.446 | 0.640 | 0.016 | 26.018 | 0.813 |
| Macrophage | 5.128 | 168.639 | 18.450 | 1541.408 |
<0.001a |
| Neutrophil | −4.449 | 0.012 | 0.000 | 1.659 | 0.078 |
| Dendritic | 0.046 | 1.047 | 0.224 | 4.895 | 0.953 |
| CBX1 | −0.246 | 0.782 | 0.582 | 1.050 | 0.102 |
| CBX2 | 0.053 | 1.054 | 0.899 | 1.236 | 0.516 |
| CXB3 | −0.111 | 0.895 | 0.613 | 1.306 | 0.564 |
| CBX4 | 0.225 | 1.252 | 0.915 | 1.714 | 0.160 |
| CBX5 | 0.177 | 1.194 | 0.956 | 1.491 | 0.118 |
| CBX6 | 0.201 | 1.223 | 1.019 | 1.467 | 0.031b |
| CBX7 | −0.346 | 0.708 | 0.569 | 0.881 | 0.002c |
| CBX8 | −0.216 | 0.806 | 0.589 | 1.102 | 0.177 |
Discussion
BLCA progression is considered a long-term,
multi-step process. Besides genetics, studies have also indicated
that epigenetic regulation participates in BLCA progression
(32,33). CBX family proteins are important
components of epigenetic regulatory complexes and are involved in
chromatin modification, and the pathogenesis and progression of a
number of tumors (10).
Nevertheless, the function of CBXs in BLCA development and patient
prognosis remains unclear. Herein, the present study analyzed the
expression, the prognostic value and mutation of CBXs in BLCA
samples, and showed that seven CBXs were differentially expressed
in BLCA samples. Furthermore, the expression of CBX1, CBX2 and CBX7
was associated with the prognosis of patients with BLCA. Besides,
the expression of six CBXs was related to the cancer stages of
BLCA. In addition, significant associations between CBX expression
and the infiltration of six immune cells and the prognosis of
patients with BLCA were observed. Moreover, abnormal expression of
CBXs in BLCA could be caused by promoter methylation and genetic
mutations. To the best of our knowledge, this was the first study
to comprehensively analyze the significance of CBXs in BLCA
progression.
A number of studies have demonstrated high CBX1
expression in numerous tumor tissues, such as gastric carcinoma
(34), castration-resistant
prostate cancer (PCa) (35),
hepatocellular carcinoma (36),
ovarian carcinoma (37) and
pituitary carcinoma (38). In PCa,
CBX1 overexpression has been reported to be involved in
trimethylation levels of histone H3K9 and higher Gleason score.
Impairment of CBX1 expression can reduce androgen receptor
expression, inhibiting the proliferation of PCa cells (35). CBX1 also has reduced expression in
renal clear cell carcinoma, and is related to tumor grade, tumor
stage and patient prognosis (39).
Nevertheless, the role of CBX1 in BLCA is poorly understood.
Herein, the present study found that CBX1 expression was increased
in BLCA tissues. Also, elevated CBX1 expression was related to
patient tumor stages. Hence, the present results indicated that
CBX1 may serve an oncogenic role in BLCA.
Previous studies have demonstrated that CBX2 has
higher expression in various tumors than in normal tissues
(40–42). For example, Hu et al
(41) reported that CBX2 and
enhancer of zeste homolog 2 (EZH2) synergistically promoted lung
cancer progression, combining with promoters of the PPAR signaling
pathway and tumor suppressor genes, cooperatively or individually,
to downregulate their expression. Moreover, CBX2 overexpression can
promote breast cancer progression by activating the PI3K/AKT
signaling pathway (42).
Additionally, cancer susceptibility candidate 9 promotes bladder
cancer progression by regulating CBX2 (43). The present study detected
significantly elevated CBX2 expression in BLCA and a connection
with tumor stages. CBX2 overexpression was closely associated with
poor PFS in patients with BLCA, suggesting the promotive effect of
CBX2 on BLCA.
Significant CBX3 upregulation has been found in
various tumors, such as PCa (44),
gastric cancer (45) and lung
cancer (46,47). Smoking-related upregulation of CBX3
promotes lung adenocarcinoma progression by forming a complex with
tripartite motif-containing (TRIM)28, TRIM24 and RBBP4, suppressing
Rho GTPase-activating protein 24 expression and increasing active
Rac1 in lung adenocarcinoma cells (47). Huang et al (48) showed that LINC00857 is involved in
the proliferation and development of diffuse large B-cell lymphoma
by regulating the microRNA370-3p/CBX3 axis. However, another study
has shown that the long non-coding RNA, LINC00998, can inhibit the
development of malignant glioma by regulating the c-Met/Akt/mTOR
signaling pathway to prevent CBX3 ubiquitination and degradation
(49). These studies suggested that
CBX3 could be an oncogene or a tumor suppressor gene, depending on
the tumor type. Herein, CBX3 expression was significantly increased
in BLCA tissues and was associated with tumor stages. However, low
expression of CBX3 was significantly related to poorer OS in
patients with BLCA, which is in contradiction with the high
expression of CBX3 observed in BLCA. Therefore, the role of CBX3 in
BLCA is not clear.
CBX4 is upregulated in various human tumors, such as
osteosarcoma (50), lung
adenocarcinoma (51) and breast
cancer (52). Lin et al
(45) showed that patients with
gastric cancer and with high CBX4 expression had poor prognoses.
Wang et al (50) reported
that CBX4 overexpression in osteosarcoma promoted tumor metastasis
by transcriptionally upregulating RUNX2 and recruiting GCN5 to the
RUNX2 promoter. Overexpressed CBX4 can promote cancer cell
migration by regulating CDK2, cyclin E, MMP2, MMP9 and CXCR4
(51). However, Wang et al
(53) reported that CBX4 inhibited
CRC migration, invasion and metastasis by suppressing RUNX2
expression. Herein, the present study found that CBX4 expression
was higher in BLCA tissues, and CBX4 was related to tumor stages,
suggesting that it plays a pro-tumor role in BLCA.
CBX5 is expressed in various types of cancer,
including renal and gastric cancer (54). Sun et al (55) reported that LINC02381 can promote
CBX5 transcription through the interaction with CEBPβ, exerting a
tumorigenic effect on glioma cells. Nevertheless, the present
results suggested that CBX5 might not affect BLCA.
Conflicting roles of CBX6 have been found in tumors.
For example, CBX6 overexpression can promote liver cancer
progression by regulating the NF-κB/MAPK pathway (56). By contrast, Deng et al
(57) reported that CBX6 was
negatively regulated by EZH2 and exerted a tumor suppressor role in
breast cancer; CBX6 was shown to inhibit breast cancer development
by interacting with EZH2. Additionally, Sakai et al
(58) reported that CBX6
proteasomal degradation promoted MMP-2 expression to induce
mesothelioma progression. In the present study, CBX6 expression was
associated with BLCA stage. Overall, the current findings suggested
that CBX6 plays an anti-tumor role in BLCA.
Notably, CBX7 shows opposing effects in different
tumors (59,60). Previous studies have shown that CBX7
is decreased in bladder cancer, cervical cancer and CRC, and its
downregulation is related to poor survival in patients with cancer.
These results suggested that CBX7 has a tumor suppressor role in
these cancers (13,61–63).
Conversely, CBX7 is overexpressed in ovarian cancer and PCa, and is
related to poor prognosis, suggesting that increased CBX7
expression exhibits a cancer-promoting role in these tumors
(64,65). The present study demonstrated that
CBX7 levels were significantly reduced in BLCA tissues and that low
CBX7 expression was positively related to the cancer stage of
patients with BLCA. Furthermore, reduced levels of CBX7 were
associated with poor prognoses, suggesting that it exerts an
anti-tumor role in BLCA.
A number of studies have demonstrated that CBX8
participates in different types of cancer. Zhang et al
(66) reported that CBX8
overexpression promoted hepatocellular carcinoma progression by
upregulating the AKT/β-catenin pathway. Additionally, Zeng et
al (67) showed that CBX8
accelerated BLCA progression by downregulating PRDM1, thus
promoting c-FOS expression. The present study found that CBX8 was
significantly increased in BLCA tissues, and its overexpression was
related to the tumor stage of patients, suggesting it exerts a
tumorigenic role in BLCA.
CBX family members participate in chromosomal
modification, transcriptional repression, cell differentiation and
senescence (8). Previous studies
have confirmed that CBXs are associated with a number of tumors
(10,37,41,47).
Herein, the present study performed GO functional and KEGG pathway
enrichment analyses for CBX family members and their related genes.
The results revealed that CBX and their related genes were enriched
in the ‘mitotic cell cycle’, ‘RNA splicing’ and ‘cell division’.
These results suggested that CBX might affect the pathogenesis of
BLCA by participating in these biological processes. Previous
studies have indicated that the infiltration of immune cells into
tumors can affect the efficacy of immunotherapy and thus tumor
progression in patients (68–71).
The present study showed that the expression of CBXs was
significantly associated with the infiltration of immune cells,
including dendritic cells, neutrophils, macrophages, B cells,
CD4+ T cells and CD8+ T cells. These results
suggested that CBXs might affect the immune infiltration status of
patients with BLCA. In summary, CBX1, CBX2, CBX3, CBX4 and CBX8
might promote BLCA progression, whereas CBX6 and CBX7 might exert a
suppressive role. Although further investigations are needed to
verify the results, the present study provides a theoretical basis
for discovering new targets and prognostic markers for BLCA
therapy.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82060007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LBZ, BF and RFC designed the study and wrote the
article. XQL, XM, LML, MCJ, KHW, YFL, QQZ and CY performed the
literature search and data analysis. LBZ and XM confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richters A, Aben KKH and Kiemeney LALM:
The global burden of urinary bladder cancer: An update. World J
Urol. 38:1895–1904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel VG, Oh WK and Galsky MD: Treatment
of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J
Clin. 70:404–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seidl C: Targets for therapy of bladder
cancer. Semin Nucl Med. 50:162–170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobatake K, Ikeda KI, Nakata Y, Yamasaki
N, Ueda T, Kanai A, Sentani K, Sera Y, Hayashi T, Koizumi M, et al:
Kdm6a deficiency activates inflammatory pathways, promotes M2
macrophage polarization, and causes bladder cancer in cooperation
with p53 dysfunction. Clin Cancer Res. 26:2065–2079. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao L, Mu X, Chen H, Jin D, Zhang R, Zhao
Y, Fan J, Cao M and Zhou Z: FTO modifies the m6A level of MALAT and
promotes bladder cancer progression. Clin Transl Med. 11:e3102021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Zhang Z, Chen H, Bao W, Kuang X,
Zhou P, Gao Z, Li D, Xie X, Yang C, et al: SBSN drives bladder
cancer metastasis via EGFR/SRC/STAT3 signalling. Br J Cancer.
127:211–222. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gil J and O'Loghlen A: PRC1 complex
diversity: Where is it taking us? Trends Cell Biol. 24:632–641.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wotton D and Merrill JC: Pc2 and
SUMOylation. Biochem Soc Trans. 35:1401–1404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Wijnen AJ, Bagheri L, Badreldin AA,
Larson AN, Dudakovic A, Thaler R, Paradise CR and Wu Z: Biological
functions of chromobox (CBX) proteins in stem cell self-renewal,
lineage-commitment, cancer and development. Bone. 143:1156592021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou H, Xiong Y, Liu Z, Hou S and Zhou T:
Expression and prognostic significance of CBX2 in colorectal
cancer: Database mining for CBX family members in malignancies and
vitro analyses. Cancer Cell Int. 21:4022021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Xu Y, Long XD, Wang W, Jiao HK, Mei
Z, Yin QQ, Ma LN, Zhou AW, Wang LS, et al: Cbx4 governs HIF-1α to
potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3
ligase activity. Cancer Cell. 25:118–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Z, Yan Y, Zhu Z, Liu J, He X,
Dalangood S, Li M, Tan M, Cai J, Tang P, et al: CBX7 suppresses
urinary bladder cancer progression via modulating AKR1B10-ERK
signaling. Cell Death Dis. 12:5372021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asplund A, Edqvist PHD, Schwenk JM and
Pontén F: Antibodies for profiling the human proteome-the human
protein atlas as a resource for cancer research. Proteomics.
12:2067–2077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Survplot. URL. http://www.cbs.dtu.dk/~eklund/survplot2021 07 07
|
|
20
|
BeeSwarm. URL. http://www.cbs.dtu.dk/~eklund/beeswarm/2021 07 07
|
|
21
|
Therneau TM and Grambsch PM: Modeling
survival data: Extending the Cox model. New York, NY:
Springer-Verlag; 2000, View Article : Google Scholar
|
|
22
|
Wickham H: ggplot2. Elegant graphics for
data analysis. New York: Springer-Verlag; 2009
|
|
23
|
Wu W, Jia G, Chen L, Liu H and Xia S:
Analysis of the expression and prognostic value of annexin family
proteins in bladder cancer. Front Genet. 12:7316252021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lánczky A and Győrffy B: Web-based
survival analysis tool tailored for medical research (KMplot):
Development and implementation. J Med Internet Res. 23:e276332021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chatr-Aryamontri A, Oughtred R, Boucher L,
Rust J, Chang C, Kolas NK, O'Donnell L, Oster S, Theesfeld C,
Sellam A, et al: The BioGRID interaction database: 2017 Update.
Nucleic Acids Res. 45(D1): D369–D379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bader GD and Hogue CWV: An automated
method for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thy S, Hommel A, Meneceur S, Bartkowiak
AL, Schulz WA, Niegisch G and Hoffmann MJ: Epigenetic treatment of
urothelial carcinoma cells sensitizes to cisplatin chemotherapy and
PARP inhibitor treatment. Cancers (Basel). 13:13762021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harsanyi S, Novakova ZV, Bevizova K,
Danisovic L and Ziaran S: Biomarkers of bladder cancer: Cell-free
DNA, epigenetic modifications and non-coding RNAs. Int J Mol Sci.
23:132062022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He M, Yue L, Wang H, Yu F, Yu M, Ni P,
Zhang K, Chen S, Duan G and Zhang R: Evaluation of the prognostic
value of CBXs in gastric cancer patients. Sci Rep. 11:123752021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiota M, Song Y, Yokomizo A, Tada Y,
Kuroiwa K, Eto M, Oda Y, Inokuchi J, Uchiumi T, Fujimoto N, et al:
Human heterochromatin protein 1 isoform HP1beta enhances androgen
receptor activity and is implicated in prostate cancer growth.
Endocr Relat Cancer. 17:455–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YF, Pan YH, Tian QH, Wu DC and Su SG:
CBX1 indicates poor outcomes and exerts oncogenic activity in
hepatocellular carcinoma. Transl Oncol. 11:1110–1118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu K, Yao L, Xu Z, Yan Y and Li J:
Prognostic value and therapeutic potential of CBX family members in
ovarian cancer. Front Cell Dev Biol. 10:8323542022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu A, Zhang Y, Zhao X, Li J and Ying Y:
CBX1 is a direct target of miR-205-5p and contributes to the
progression of pituitary tumor. Pharmazie. 74:154–156.
2019.PubMed/NCBI
|
|
39
|
Zhu Y, Pu Z, Li Z, Lin Y, Li N and Peng F:
Comprehensive analysis of the expression and prognosis value of
chromobox family members in clear cell renal cell carcinoma. Front
Oncol. 11:7005282021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clermont PL, Sun L, Crea F, Thu KL, Zhang
A, Parolia A, Lam WL and Helgason CD: Genotranscriptomic
meta-analysis of the polycomb gene CBX2 in human cancers: Initial
evidence of an oncogenic role. Br J Cancer. 111:1663–1672. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu FF, Chen H, Duan Y, Lan B, Liu CJ, Hu
H, Dong X, Zhang Q, Cheng YM, Liu M, et al: CBX2 and EZH2
cooperatively promote the growth and metastasis of lung
adenocarcinoma. Mol Ther Nucleic Acids. 27:670–684. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng S, Lv P, Su J, Miao K, Xu H and Li
M: Overexpression of CBX2 in breast cancer promotes tumor
progression through the PI3K/AKT signaling pathway. Am J Transl
Res. 11:1668–1682. 2019.PubMed/NCBI
|
|
43
|
Huo W, Tan D and Chen Q: CASC9 facilitates
cell proliferation in bladder cancer by regulating CBX2 expression.
Nephron. 144:388–399. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen LY, Cheng CS, Qu C, Wang P, Chen H,
Meng ZQ and Chen Z: Overexpression of CBX3 in pancreatic
adenocarcinoma promotes cell cycle transition-associated tumor
progression. Int J Mol Sci. 19:17682018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin H, Lian J, Xia L, Guan G and You J:
CBX3 promotes gastric cancer progression and affects factors
related to immunotherapeutic responses. Cancer Manag Res.
12:10113–10125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alam H, Li N, Dhar SS, Wu SJ, Lv J, Chen
K, Flores ER, Baseler L and Lee MG: HP1γ promotes lung
adenocarcinoma by downregulating the transcription-repressive
regulators NCOR2 and ZBTB7A. Cancer Res. 78:3834–3848. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin X, Zhang B, Zhang H and Yu H:
Smoking-associated upregulation of CBX3 suppresses ARHGAP24
expression to activate Rac1 signaling and promote tumor progression
in lung adenocarcinoma. Oncogene. 41:538–549. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang Y, Lin Y, Song X and Wu D: LINC00857
contributes to proliferation and lymphomagenesis by regulating
miR-370-3p/CBX3 axis in diffuse large B-cell lymphoma.
Carcinogenesis. 42:733–741. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cai H, Yu Y, Ni X, Li C, Hu Y, Wang J,
Chen F, Xi S and Chen Z: LncRNA LINC00998 inhibits the malignant
glioma phenotype via the CBX3-mediated c-Met/Akt/mTOR axis. Cell
Death Dis. 11:10322020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang X, Qin G, Liang X, Wang W, Wang Z,
Liao D, Zhong L, Zhang R, Zeng YX, Wu Y and Kang T: Targeting the
CK1α/CBX4 axis for metastasis in osteosarcoma. Nat Commun.
11:11412020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu C, Zhang Q, Tang Q, Zhou H, Liu W,
Huang J, Liu Y, Wang Q, Zhang J, Zhou M, et al: CBX4 promotes the
proliferation and metastasis via regulating BMI-1 in lung cancer. J
Cell Mol Med. 24:618–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng JS, Zhang ZD, Pei L, Bai ZZ, Yang Y,
Yang H and Tian QH: CBX4 exhibits oncogenic activities in breast
cancer via Notch1 signaling. Int J Biochem Cell Biol. 95:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang X, Li L, Wu Y, Zhang R, Zhang M, Liao
D, Wang G, Qin G, Xu RH and Kang T: CBX4 suppresses metastasis via
recruitment of HDAC3 to the Runx2 promoter in colorectal carcinoma.
Cancer Res. 76:7277–7289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu C and Zhang J: Long non-conding RNA
LOXL1-AS1 sponges miR-589-5p to up-regulate CBX5 expression in
renal cell carcinoma. Biosci Rep. 40:BSR202002122020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun Y, Wang X and Bu X: LINC02381
contributes to cell proliferation and hinders cell apoptosis in
glioma by transcriptionally enhancing CBX5. Brain Res Bull.
176:121–129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zheng H, Jiang WH, Tian T, Tan HS, Chen Y,
Qiao GL, Han J, Huang SY, Yang Y, Li S, et al: CBX6 overexpression
contributes to tumor progression and is predictive of a poor
prognosis in hepatocellular carcinoma. Oncotarget. 8:18872–18884.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Deng H, Guan X, Gong L, Zeng J, Zhang H,
Chen MY and Li G: CBX6 is negatively regulated by EZH2 and plays a
potential tumor suppressor role in breast cancer. Sci Rep.
9:1972019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sakai K, Nishiuchi T, Tange S, Suzuki Y,
Yano S, Terashima M, Suzuki T and Matsumoto K: Proteasomal
degradation of polycomb-group protein CBX6 confers MMP-2 expression
essential for mesothelioma invasion. Sci Rep. 10:166782020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pallante P, Forzati F, Federico A, Arra C
and Fusco A: Polycomb protein family member CBX7 plays a critical
role in cancer progression. Am J Cancer Res. 5:1594–1601.
2015.PubMed/NCBI
|
|
60
|
Li J, Ouyang T, Li M, Hong T, Alriashy M,
Meng W and Zhang N: CBX7 is dualistic in cancer progression based
on its function and molecular interactions. Front Genet.
12:7407942021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang Z, Liu J, Yang J, Yan Y, Yang C, He
X, Huang R, Tan M, Wu D, Yan J and Shen B: PDE4B induces
epithelial-to-mesenchymal transition in bladder cancer cells and is
transcriptionally suppressed by CBX7. Front Cell Dev Biol.
9:7830502021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li R, Yan Q, Tian P, Wang Y, Wang J, Tao
N, Ning L, Lin X, Ding L, Liu J and Ma C: CBX7 inhibits cell growth
and motility and induces apoptosis in cervical cancer cells. Mol
Ther Oncolytics. 15:108–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pallante P, Terracciano L, Carafa V,
Schneider S, Zlobec I, Lugli A, Bianco M, Ferraro A, Sacchetti S,
Troncone G, et al: The loss of the CBX7 gene expression represents
an adverse prognostic marker for survival of colon carcinoma
patients. Eur J Cancer. 46:2304–2313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gong L, Tang Y, Jiang L, Tang W and Luo S:
Regulation of circGOLPH3 and its binding protein CBX7 on the
proliferation and apoptosis of prostate cancer cells. Biosci Rep.
40:BSR202009362020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shinjo K, Yamashita Y, Yamamoto E,
Akatsuka S, Uno N, Kamiya A, Niimi K, Sakaguchi Y, Nagasaka T,
Takahashi T, et al: Expression of chromobox homolog 7 (CBX7) is
associated with poor prognosis in ovarian clear cell adenocarcinoma
via TRAIL-induced apoptotic pathway regulation. Int J Cancer.
135:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang CZ, Chen SL, Wang CH, He YF, Yang X,
Xie D and Yun JP: CBX8 exhibits oncogenic activity via
AKT/β-catenin activation in hepatocellular carcinoma. Cancer Res.
78:51–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zeng F, Luo L, Li D, Guo J and Guo M:
KPNA2 interaction with CBX8 contributes to the development and
progression of bladder cancer by mediating the PRDM1/c-FOS pathway.
J Transl Med. 19:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Baci D, Bosi A, Gallazzi M, Rizzi M,
Noonan DM, Poggi A, Bruno A and Mortara L: The Ovarian cancer tumor
immune microenvironment (TIME) as target for therapy: A focus on
innate immunity cells as therapeutic effectors. Int J Mol Sci.
21:31252020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rohaan MW, van den Berg JH, Kvistborg P
and Haanen JBAG: Adoptive transfer of tumor-infiltrating
lymphocytes in melanoma: A viable treatment option. J Immunother
Cancer. 6:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Okła K, Farber DL and Zou W:
Tissue-resident memory T cells in tumor immunity and immunotherapy.
J Exp Med. 218:e202016052021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17:1742016. View Article : Google Scholar : PubMed/NCBI
|