Introduction
Triple negative breast cancer (TNBC) is a
heterogeneous disease, more common in young women, 15–20% of all
breast cancers (1). In 2011,
Lehmann classified 587 TNBC Gene expression profiling into six
subtypes: BL1, BL2, IM,M, MSL and LAR (2). In 2015, Burstein further used RNA and
DNA analysis to distinguish specific targets expressed by the
immune system to subdivide TNBC into Lar, MES, Blis, and Blia,
enabling specific targeted therapies through subtype molecular
expression differences (3). With
the development of modern biological science and technology, TNBC
typing has been refined gradually, to a certain extent, improve the
cure rate of clinical TNBC or prognosis of patients. At present,
anthracycline and platinum is still the main clinical treatment.
The survival rate of patients after clinical treatment is lower
than that of non-TNBC patients, and the postoperative prognosis is
poor, easy to relapse and metastasis (4,5).
Studying new treatment methods has become an urgent problem to be
solved in TNBC clinical practice. It may be a good idea to find
Chinese medicine with fewer side effects as an alternative or
complementary therapy for triple-negative breast cancer.
Ginger as a medicinal and edible Chinese medicine,
not only as spices and vegetables edible, medicinal history is very
long, in many classic prescriptions are recorded (6). Ginger is planted in all parts of
China. The medicinal part is the fresh rhizome of Zingiberaceae
plant ginger (7), which have the
effects of relieving cold, warming and stopping vomiting, resolving
phlegm and relieving cough. It contains gingerol, gingerol,
polyphenol, turmeric ketone and other bioactive components, which
have been reported to have anti-inflammatory and anti-tumor effects
(8–11), paclitaxel synergist has also been
studied to improve the clinical treatment of TNBC (12). Ginger's pharmacological effects and
edible characteristics highlight its different effects on cancer
and normal cells, which has broad prospects in clinical
application. However, the anti-tumor mechanism of ginger is still
unclear, and the potential biological pathways include but not
limited to cell cycle regulation, apoptosis and chromatin
regulation related to DNA damage (13–15).
With the rapid development of network technology and
bioinformatics, network pharmacology is becoming a new tool to
elucidate molecular mechanisms and pharmacological effects. It can
effectively establish a compound-protein/gene-disease network and
systematically explain the material basis and mechanism of
traditional Chinese medicine. Therefore, this study used network
pharmacology methods to predict the mechanism of ginger in the
treatment of triple-negative breast cancer and further explored the
possible molecular mechanism and pathway of ginger in the treatment
of triple-negative breast cancer through in vitro cell experiments.
In order to provide scientific reference for the clinical treatment
of triple negative breast cancer and the drug development of
ginger.
Materials and methods
Drugs
Dihydrocapsaicin (DHC) was purchased from Guangzhou
Qiyun Biotechnology (PCS0367) .
Cells
Triple negative breast cancer cell line MDA-MB-231
was purchased from Wuhan Fuheng Biological Co., Ltd.
Reagents
Reagents used in this study were Fetal bovine serum
(S-FBS-SA-015, SERANA company), DMEM high glucose medium (batch
number 12100, SOLAIBAO), thiazole blue powder (batch number
298-93-1, SOLAIBAO), RNA extraction kit (batch number ER501-01,
Beijing all-gold biological). A two-step fluorescence quantitative
PCR kit (batch number AUQ-01, Beijing all-gold biology) RIPA
tissue/cell lysate (batch number R0010, Suolaibao), BCA protein
detection kit (batch number PC0020, Suolaibao), CASP3 (batch number
ET1608-64, HUABIO), BAX (batch number T40051F, Abmart), β-actin
(batch number TA811000, ORIGENE), HRP-labeled rabbit anti-mouse
antibody (batch number WLA024a, ten thousand kinds of biology),
HRP-labeled goat anti-rabbit antibody (batch number HA1031,
HUABIO), 4× protein loading buffer (including DTT) (batch number
P1015, Suolaibao), skim milk powder (batch number D8340,
Suolaibao), ECL ultrasensitive chemiluminescence solution I (batch
number KF005. ECL Hypersensitive Chemiluminescence Solution II (lot
number KF001, Solebold).
Instruments
CO2 incubator (Binde, Germany),
biological inverted microscope (SOPTOP), high-speed refrigerated
centrifuge (Sigma, USA), one thousandth analytical balance
(Sartorius, Germany), multi-function microplate reader (Thermo),
PCR instrument (BD, USA), multi-function imager (analytikjena,
Germany), electrophoresis apparatus and membrane transfer
instrument (Beijing Liuyi Biotechnology Co., Ltd.), ultrasonic cell
disruptor (Ningbo Scientz Biotechnology Co., Ltd.) were used.
Methods
Ginger active ingredient collection
screening and target prediction
Ginger was used as the key word to collect the
active ingredients of ginger in TCMSP (16), BATMAN-TCM (17), HERB (18) database and literature search. The 3D
structure diagram of the ingredients was downloaded on PUBCHEM
[PubChem (nih.gov)] and uploaded to the Swiss ADME (http://www.swissadme.ch/) for prediction and screening
of pharmacokinetics (ADME) (19).
Ingredients that meet the following conditions are considered to be
active ingredients retained for subsequent experiments. 1 GI
absorption (gastrointestinal absorption) is shown as ‘high’ in the
pharmacokinetic parameters; 2 Druglikeness (drug-likeness
screening) in three or more consistent. Finally, the active
ingredient SDF format was uploaded to Pharmmapper (http://www.lilab-ecust.cn/pharmmapper/)
(20) for target prediction to
obtain the target of ginger.
Triple-negative breast cancer target
collection
Through the Genecards, OMIM, TTD, Drugbank, DisGeNET
and other disease databases, the disease targets were collected
with triple negative breast cancer as the keyword, and the triple
negative breast cancer related targets were obtained after deleting
the repeated targets. The data information was uploaded to
Cytoscape 3.7.1 software to draw the network diagram of
ginger-active ingredient-target-disease, and the Venn diagram was
obtained by intersecting the targets of ginger and triple negative
breast cancer.
Construction of Protein-Protein
Interaction (PPI) Network and Screening of Key Genes
The intersection target of ginger and triple
negative breast cancer was uploaded to the STRING database [STRING:
functional protein association networks (string-db.org)] for
protein-protein interaction. After selecting the confidence level
of 0.400, the tsv file was exported and the cytoNCA plug-in in
Cytoscape 3.7.1 was used to calculate betweenness centrality (BC),
closeness centrality (CC) and degree centrality (DC). Hub genes in
PPI protein interaction network were screened out.
GO analysis and KEGG analysis
Metascape (21) was
used to perform pathway enrichment analysis on the overlapping
targets of ginger and triple-negative breast cancer. The platform
integrates multiple authoritative functional databases such as GO
and KEGG, supports batch gene or annotation, enriches and analyzes
proteins. Metascape was updated once a month to ensure the
reliability of the data. The results were saved and visualized with
R software 3.6.1 and Cytoscape 3.7.1 was used to draw the network
diagram of ginger-key component-core target-pathway.
Molecular docking
In order to further determine the credibility of the
relationship between triple negative breast cancer targets and
ginger core components, molecular docking analysis was performed
between ginger active components and four important targets in PPI
network. From the Protein Database (PDB; http://www.rcsb.org/) Download protein crystal
structure in pdb format from the chemical composition database
[Pubchem; PubChem (nih.gov)] obtained the MOL2 structure file of
the active ingredients of ginger. Proteins prepare docking
macromolecules by dehydration, hydrogenation, calculation of
charge, and distribution of atomic types. Ligands were prepared by
hydrogenation, removing lone pair electrons and establishing
special rotatable bonds. AutoDock software is then used to set the
binding pocket for molecular docking, using the original ligand of
each target as a reference. Finally, each target protein was docked
to the ligand using AutoDock Vina and binding energy data were
obtained for analysis. Molecular docking score <-4.52 kJ/mol
indicates that the ligand and the target have binding activity,
score <-5.0 kJ/mol indicates good matching activity, score
<-7.0 kJ/mol indicates strong docking activity (22).
DHC inhibits proliferation of triple
negative breast cancer cells
MTT assay was used to determine the inhibitory
effect of ginger on the proliferation of human triple negative
breast cancer cells (MDA-MB-231, Zhongqiao Xinzhou, ZQ0118) at
different concentrations. Logarithmic phase cells were collected
and seeded into 96-well plates (5×103 cells/well) at 200
µl per well. After 24 h of culture, cells in each group of 6 wells
were treated with different concentrations of DHC solution (0, 100,
150, 200, 250 µmol/l) for 12,24,48 h. After that, 20 µl MTT (5
mg/ml) solution was added to the well, and the cell incubator
continued to incubate for 4 h. The supernatant was carefully
removed and 150 µl of dimethyl sulfoxide DMSO was added to each
well to dissolve the blue-violet methyl thiazoline crystal.
Finally, the absorbance (A) of the experimental hole was measured
at 490 nm using a microplate reader to calculate cell viability.
Cell viability (%)=[(experimental group ODs value-blank group ODw
value)/(control group ODc value-blank group ODw] ×100%, where ODw
is the absorbance of the blank group, ODc is the absorbance of the
control group, and ODs is the absorbance of the experimental group.
Cell proliferation inhibition rate=[(A control hole-A experimental
hole)/(A control hole-A blank hole)] ×100%.
DHC inhibits the migration of triple
negative breast cancer cells
MDA-MB-231 cells in the rapid growth phase were
collected to carry out the scratch test. Five straight lines were
drawn at the bottom of the six-well plate before the cell seed
plate to reduce the experimental error. When the cell fusion degree
≥90%, three straight wounds were drawn perpendicular to the bottom
of the six-hole plate. The injured monolayer was washed three times
with 1× PBS to remove cell debris, and incubated with ginger
administration group and blank control group for 24 h. Axivert
40CFL Zeiss microscope was used to capture photographs of wounds
and scratch areas at 0 and 24 h. By taking 0 h as 100% empty area,
the percentage of cell coverage area can be calculated by Image J
image analysis software. All experiments were performed in three
parts.
DHC increases caspase family CASP9
mRNA expression
When the cell seed plate grew to 70–80%, the drug
was administered. After 24 h, the cells were collected and RNA was
extracted using the RNA extraction kit. The RNA purity was measured
when the A260/A280 range was 1.8-2.0. The RNA purity standard can
be used for subsequent experiments. According to the instructions
of PerfectStart® Uni RT & qPCR Kit, the collected
RNA and the kit-related reagents were added to synthesize cDNA and
remove gDNA. After the cDNA was obtained, the upstream and
downstream primers were added and the qPCR SuperMix containing DNA
polymerase and nucleotide was configured with the kit. The
expression of CASP9(Forward primer: 5′-GCAGGCTCTGGATCTCGGC-3′;
Reverse primer: 5′-GCTGCTTGCCTGTTAGTTCGC-3′) mRNA was determined by
PCR, β-Actin (Forward primer: 5′-CGTTGACATCCGTAAAGACC-3′; Reverse
primer: Reverse: 5′-TAGAGCCACCAATCCACACA-3′) expression was used as
an internal reference to verify equal concentrations of cDNA in
each sample. All processes and experimental consumables need to be
sterile and enzyme-free. β-Actin expression was used as an internal
reference to verify equal concentrations of cDNA in each
sample.
DHC increases CASP3 and BAX protein
expression in triple negative breast cancer cells
The cells were treated when the cell seed plate grew
to 70–80%. After 24 h, the cells of each group were collected for
lysis and protein extraction. The protein concentration was
determined using the BCA protein assay kit and diluted to a uniform
level. The protein structure is destroyed by adding protein loading
buffer and high temperature boiling to denature the protein.
Configure 12% concentration separation gel, select 90 V constant
pressure running concentration gel, 120 V constant pressure running
separation gel to disperse the protein bands. At the end of
electrophoresis, the target protein and internal reference protein
at different sites were retained according to the color Marker
molecular weight cutting gel and transferred to PVDF membrane for
membrane transfer. After the end of the membrane at room
temperature, the membrane with 5% blocking site buffer (5% skim
milk + TBST) soaked gently shake 60 min to block protein
non-specific sites. Subsequently, the membrane was incubated with
primary antibodies, including caspase-3 antibody (Cell Signaling
Technology, CST #9662s; 1:1,000), caspase-9 antibody (CST #12827S;
1:1,000) CASPASE-8 Antibody (CST) #4790s; 1:1,000), overnight at
4°C. The second antibody was then added the next day and incubated
for 60 min. After washing the membrane, the strips are visualized
using Chemidoc TMXRS + (Bio-Rad, USA).
Statistical methods
The data were analyzed with GraphPad Prism 8.0.2,
and the data were expressed as mean ± SEM of three independent
biological replicates. The data between the two groups were
compared by student's t-test. Multi-group experimental data, after
one-way ANOVA, Dunnett method is used to compare multiple groups
with a single common group, and Tukey method is used to multiple
comparisons between different groups. IC50 value is calculated by
fitting MTT measurement results and expressed as average value, 95%
confidence interval (CI). And P<0.05 indicates that the
differences are statistically significant.
Results
Results of network pharmacology
analysis
Ginger active ingredients and
potential target screening prediction
From TCMSP and other databases and literature
search, 265 known components of ginger were found. After screening
and prediction by Swiss ADME database, there were 10 potential
active components of ginger, see Table
I. When the gastrointestinal absorption of ginger components is
strong and meets three or more of the five drug-like rules
including the Lipinski rule, it is considered to be a potential
active ingredient. The active ingredient SDF format was uploaded to
Pharmmapper for target prediction, and 53 targets of ginger were
obtained by deleting repeated targets.
 | Table I.Ginger potential effective ingredient
information. |
Table I.
Ginger potential effective ingredient
information.
|
|
|
| Drug-likeness |
|---|
|
|
|
|
|
|---|
| MOL ID | Ingredient
name | GI absorption | Lipinski | Ghose | Veber | Egan | Muegge |
|---|
| MOL002467 | 6-gingerol | High | Yes | Yes | Yes | Yes | Yes |
| MOL002495 | 6-shogaol | High | Yes | Yes | Yes | Yes | Yes |
| MOL002516 |
Vanillylacetone | High | Yes | Yes | Yes | Yes | No |
| MOL002459 | 10-gingerol | High | Yes | Yes | No | Yes | No |
| MOL008698 |
Dihydrocapsaicin | High | Yes | Yes | No | Yes | Yes |
| MOL000123 | Geraniol | High | Yes | No | Yes | Yes | No |
| MOL000122 | Eucalyptol | High | Yes | No | Yes | Yes | No |
| MOL000124 | Citral | High | Yes | No | Yes | Yes | No |
| MOL000198 | L-linalool | High | Yes | No | Yes | Yes | No |
| MOL003358 |
1,7-dihydroxyxanthone | High | Yes | Yes | Yes | Yes | Yes |
Targets of triple negative breast
cancer
Through the Genecards, OMIM, TTD, Drugbank, DisGeNET
and other disease databases, the disease targets were collected
with triple negative breast cancer as the keyword, and the triple
negative breast cancer related targets were obtained after deleting
the repeated targets. The intersection of ginger targets and
triple-negative breast cancer-related targets was taken in
Cytoscape 3.7.1 software to construct a ginger-active
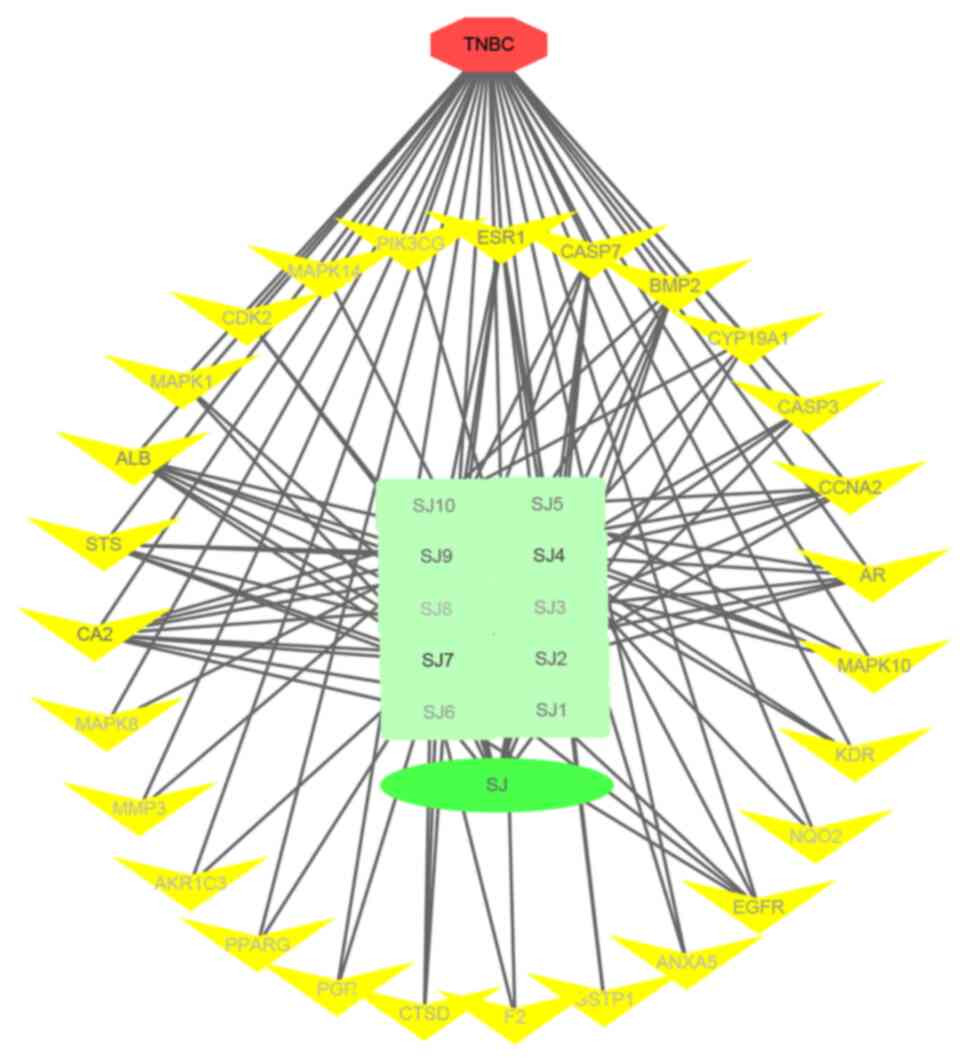
ingredient-target-disease network diagram, as shown in Fig. 1. Red is triple negative breast
cancer disease, green is ginger and its 10 selected active
ingredients, yellow is ginger and triple negative breast cancer
common targets a total of 27. The node label color in the figure
deepens as the degree value increases.
Ginger-triple negative breast cancer
network interaction and key gene screening
The intersection target of ginger and triple
negative breast cancer was imported into STRING database to obtain
the protein interaction relationship. The network data tsv file was
imported into Cytoscape 3.7.2 software to draw the protein
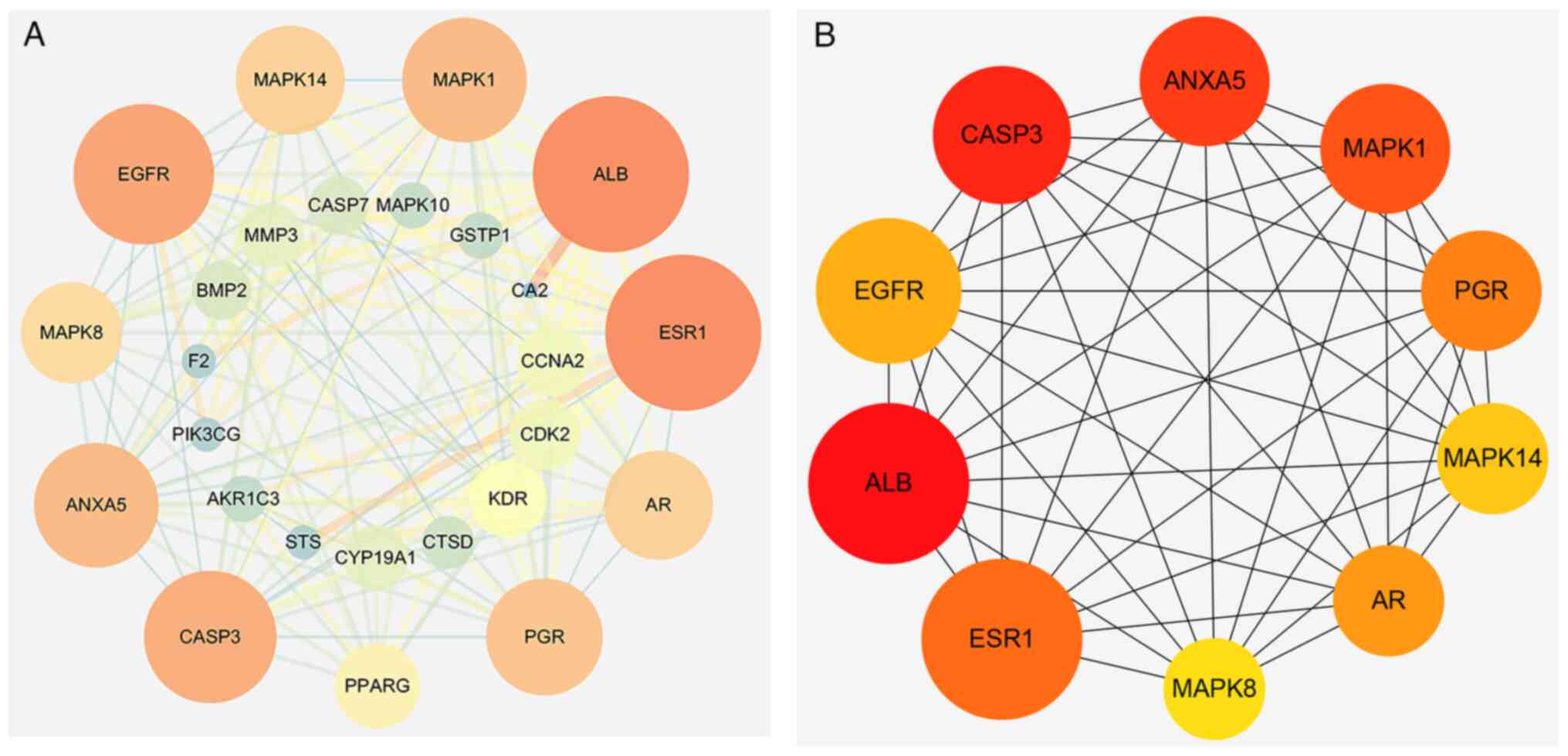
interaction network diagram, as shown in Fig. 2A. The network consisted of 26 nodes
(hidden unrelated node NQO2) and 144 edges (representing the
interaction between targets). The average node degree was 10.7, the
average local clustering coefficient was 0.737, and P<1.0×10-16,
indicating that the interaction between these proteins played an
important role in the network. Visualization in Cytoscape 3.7.1,
the greater the node Degree, the larger the node, the darker the
node color; the larger the edgeBetweenness (betweenness) of the
node connection, the thicker the line and the darker the color. The
cytoNCA plug-in in Cytoscape 3.7.1 was downloaded and screened by
Degree ≥10, Betweenness ≥10, Closeness ≥0.7. Finally, 10 core genes
were obtained as shown in Fig. 2B.
The core genes in the Uniprot database search to get Table II.
 | Table II.Core genes and details of
ginger-triple negative breast cancer intersection target
Protein-Protein Interaction network. |
Table II.
Core genes and details of
ginger-triple negative breast cancer intersection target
Protein-Protein Interaction network.
| Gene | Protein full
name | Degree | Betweenness | Closeness |
|---|
| ALB | Albumin | 21.0 | 87.5 | 0.86 |
| ESR1 | Estrogen
receptor | 21.0 | 68.2 | 0.86 |
| EGFR | Epidermal growth
factor receptor | 19.0 | 38.7 | 0.81 |
| CASP3 | Caspase-3 | 18.0 | 21.1 | 0.78 |
| ANXA5 | Annexin A5 | 17.0 | 20.1 | 0.76 |
| MAPK1 | Mitogen-activated
protein kinase 1 | 17.0 | 29.6 | 0.76 |
| PGR | Progesterone
receptor | 16.0 | 21.5 | 0.74 |
| MAPK14 | Mitogen-activated
protein kinase 14 | 15.0 | 21.9 | 0.71 |
| AR | Androgen
receptor | 15.0 | 23.7 | 0.71 |
| MAPK8 | Mitogen-activated
protein kinase 8 | 14.0 | 11.2 | 0.69 |
GO and KEGG analysis
GO functional enrichment analysis and KEGG pathway
analysis were performed on potential targets of ginger in the
treatment of triple negative breast cancer using Metascape
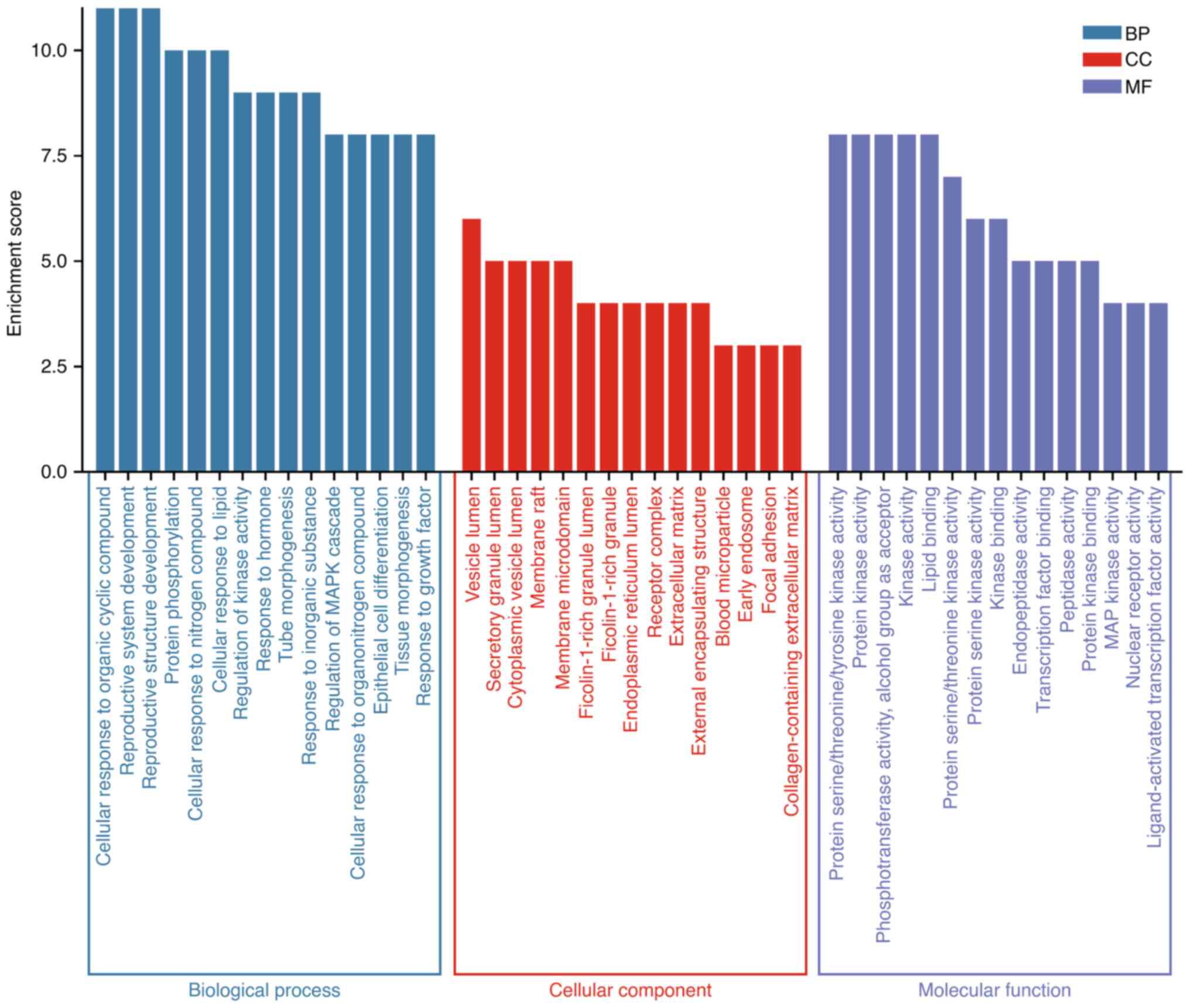
platform. Results A total of 343 GO terms were obtained, of which
287 were related to biological process (BP) (P<0.01), 18 to
cellular component (CC) (P<0.01) and 38 to molecular function
(MF) (P<0.01). Fig. 3 is drawn
for the first 18 GO entries according to the number of gene
enrichment, as shown in the figure. In the process of cell biology,
the function of ginger in the treatment of triple negative breast
cancer mainly focuses on the reaction of cells to organic cyclic
compounds, cell lipid reaction, cell reaction to nitrogen
compounds, reaction to inorganic substances, protein
phosphorylation, tube morphogenesis, hormone reaction, kinase
activity regulation and so on. The cell components were mainly
enriched in vesicle cavity, secretory granule cavity, cytoplasmic
vesicle cavity, membrane raft, membrane microdomain, granule cavity
rich in ficolin-1 (fibronectin), endoplasmic reticulum cavity,
extracellular matrix, etc. The molecular function part mainly
regulates protein kinase activity, phosphotransferase activity,
protein serine/threonine kinase activity, transcription factor
binding, peptidase activity, MAP kinase activity, nuclear receptor
activity, ligand-activated transcription factor activity, etc.
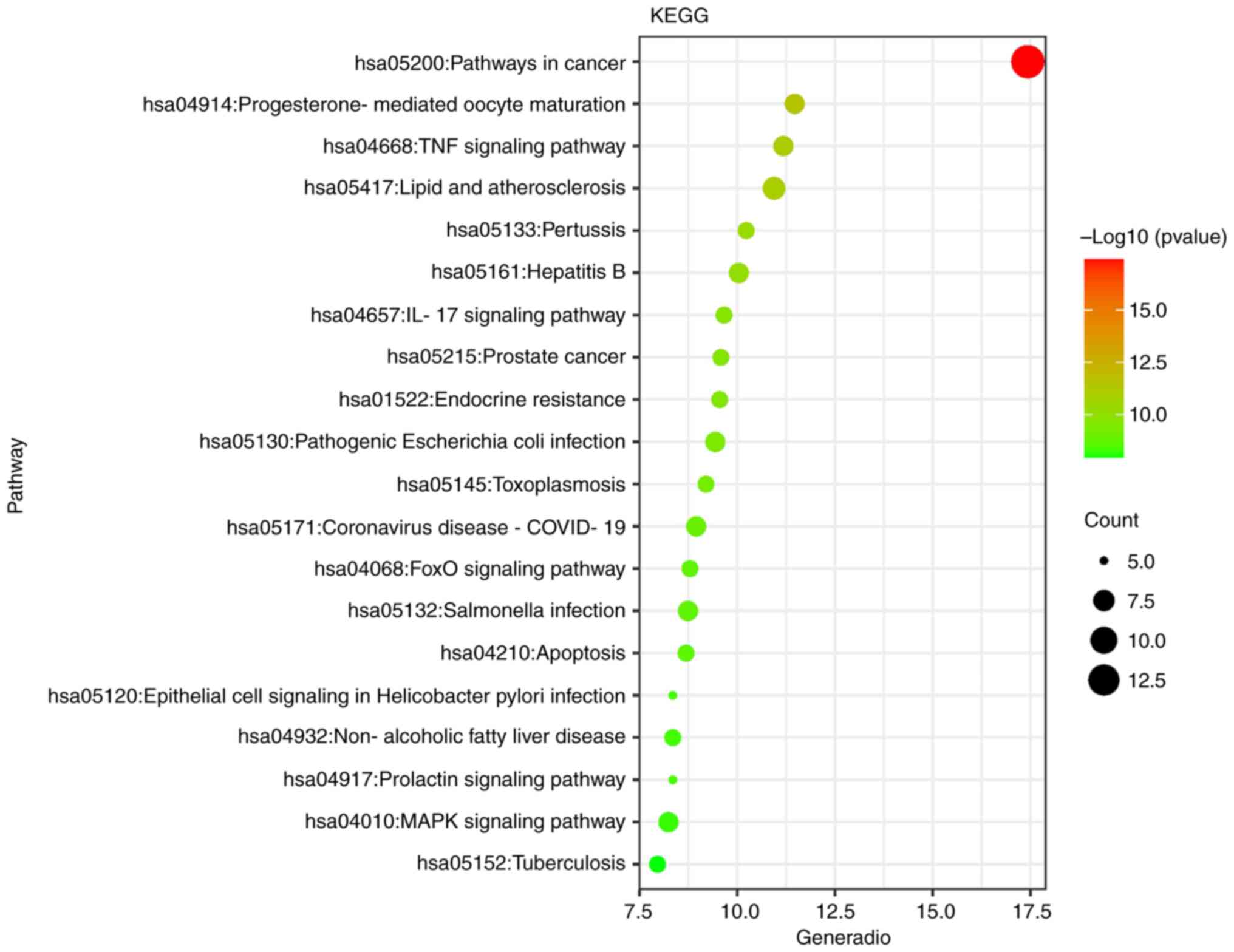
The KEGG enrichment analysis of ginger against
triple-negative breast cancer involved a total of 93 signaling
pathways, and a total of 93 pathways were screened under the
condition of P<0.01. It mainly involves TNF signaling pathway,
IL-17 signaling pathway, FoxO signaling pathway, prolactin
signaling pathway, MAPK signaling pathway, apoptosis, etc., and is
closely related to atherosclerosis, intestinal flora, breast
cancer, prostate cancer, pancreatic cancer, bladder cancer, gastric
cancer, liver cancer, colorectal cancer and other diseases,
indicating that there are common characteristics in the
pathogenesis of various malignant tumors. The first 20 functional
enrichment related pathways of ginger in the treatment of triple
negative breast cancer were drawn into a bubble diagram, as shown
in Fig. 4.
A series of data such as the active ingredients of
ginger, the common targets with triple-negative breast cancer, and
the top 20 KEGG signaling pathways were used to construct a
‘ginger-active ingredient-target-pathway’ network through Cytoscape
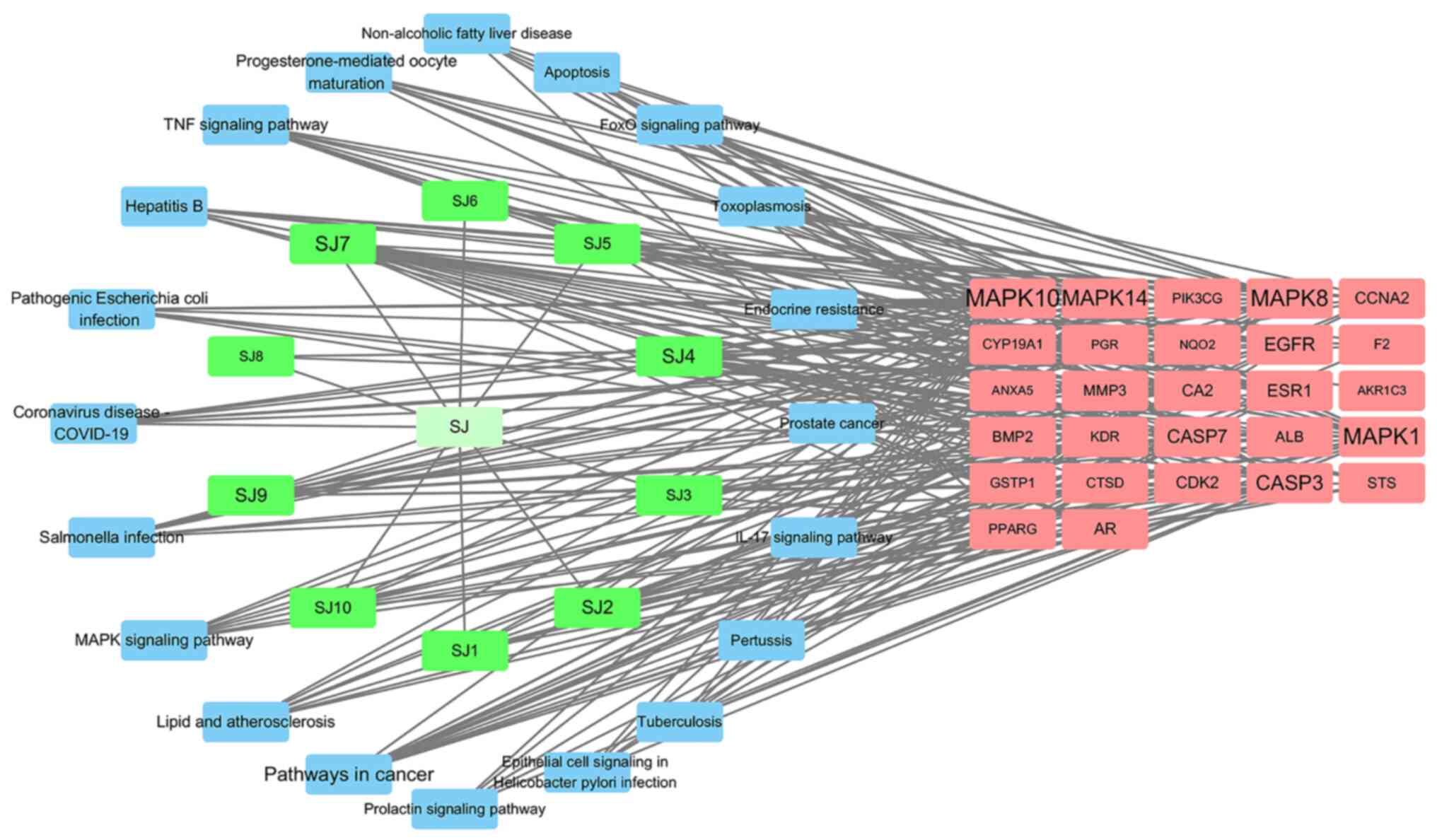
3.7.21. Fig. 5 shows the pathway of
ginger against triple-negative breast cancer. The network has 58
nodes and 223 edges, including 1 ginger drug node and 10 ginger
active ingredient nodes (green), 27 common targets of ginger and
triple-negative breast cancer (red). The top 20 KEGG signaling
pathways (blue), the greater the node degree value in the network,
the greater the role in the network, the greater the node label.
Ginger-active ingredient-target-pathway diagram clearly and
intuitively reflects that ginger inhibits triple negative milk by
acting on multiple targets of multiple components and multiple
pathways.
Molecular docking
Molecular docking was performed between 10-gingerol,
geraniol, citral, dihydrocapsaicin, L-linalool, 6-gingerol and the
top 6 core targets in the PPI network. The lower the binding energy
between the molecule and the target, the more stable the binding
between the ligand and the receptor protein. Generally, the binding
energy ≤-4.52 kcal/mol is used as the scoring standard, indicating
that there is binding activity between the ligand and the receptor
protein. When the binding energy is ≤-7.00 kcal/mol, it indicates
that there is a strong binding activity between the ligand and the
receptor protein (22–24). At the same time, the RMSD value of
the docking results is less than 2 Å, indicating that the docking
results deviate from the reference molecule to a low degree and the
docking results are reliable. The above 2.1.2,2.1.3 screened out
the core active ingredients of ginger in the treatment of triple
negative breast cancer and the target protein docking data are
shown in Table III.
 | Table III.Ginger core component and the core
target molecular docking results. |
Table III.
Ginger core component and the core
target molecular docking results.
| Active
ingredient | Target protein | PDB ID | Binding
energy/kcal·mol−1 | RMSD/Å |
|---|
| 10-Gingerol |
ALB | 6EZQ | −5.60 | 1.659 |
|
|
ESR1 | 6PSJ | −5.20 | 1.488 |
|
|
EGFR | 4RJ3 | −6.80 | 1.917 |
|
|
CASP3 | 7RN9 | −6.10 | 1.721 |
|
| ANXA5 | 6K25 | −6.50 | 1.605 |
|
| MAPK1 | 6G9J | −5.70 | 2.036 |
| Geraniol |
ALB | 6EZQ | −5.30 | 1.949 |
|
|
ESR1 | 6PSJ | −4.60 | 1.531 |
|
|
EGFR | 4RJ3 | −5.50 | 0.721 |
|
|
CASP3 | 7RN9 | −5.30 | 0.576 |
|
| ANXA5 | 6K25 | −5.50 | 1.387 |
|
| MAPK1 | 6G9J | −4.70 | 1.493 |
| Citral |
ALB | 6EZQ | −5.50 | 0.549 |
|
|
ESR1 | 6PSJ | −4.50 | 1.152 |
|
|
EGFR | 4RJ3 | −5.70 | 0.573 |
|
|
CASP3 | 7RN9 | −5.20 | 0.827 |
|
| ANXA5 | 6K25 | −4.90 | 0.792 |
|
| MAPK1 | 6G9J | −5.20 | 1.485 |
|
Dihydrocapsaicin |
ALB | 6EZQ | −6.80 | 0.992 |
|
|
ESR1 | 6PSJ | −6.90 | 1.102 |
|
|
EGFR | 4RJ3 | −7.70 | 1.777 |
|
|
CASP3 | 7RN9 | −7.20 | 1.554 |
|
| ANXA5 | 6K25 | −7.00 | 1.235 |
|
| MAPK1 | 6G9J | −5.90 | 0.789 |
| L-linalool |
ALB | 6EZQ | −5.50 | 1.762 |
|
|
ESR1 | 6PSJ | −4.70 | 1.179 |
|
|
EGFR | 4RJ3 | −5.80 | 1.107 |
|
|
CASP3 | 7RN9 | −4.90 | 1.041 |
|
| ANXA5 | 6K25 | −5.10 | 1.532 |
|
| MAPK1 | 6G9J | −4.70 | 0.814 |
| 6-Gingerol |
ALB | 6EZQ | −6.00 | 1.688 |
|
|
ESR1 | 6PSJ | −6.10 | 0.978 |
|
|
EGFR | 4RJ3 | −7.30 | 1.356 |
|
|
CASP3 | 7RN9 | −6.70 | 1.482 |
|
| ANXA5 | 6K25 | −5.90 | 0.927 |
|
| MAPK1 | 6G9J | −5.40 | 1.671 |
The results showed that all docking binding free
energies were less than −4.52 kcal·mol-1, in which the lowest
binding potential energy of dihydrocapsaicin and EGFR protein was
−7.70 kcal·mol-1, followed by the binding energy of 6-gingerol and
EGFR protein was −7.30 kcal·mol-1, and the binding energy of
dihydrocapsaicin and CASP3 protein was −7.20 kcal·mol-1, indicating
that dihydrocapsaicin and 6-gingerol had high affinity with the
core target, and the molecules could form stable conformation
through interaction. The molecular docking results of the above
three components with the lowest binding potential to their
proteins were selected for visualization. The three-dimensional
structure, two-dimensional structure diagram, detailed binding
sites between active components and targets and detailed binding
sites of each group were shown in Fig.
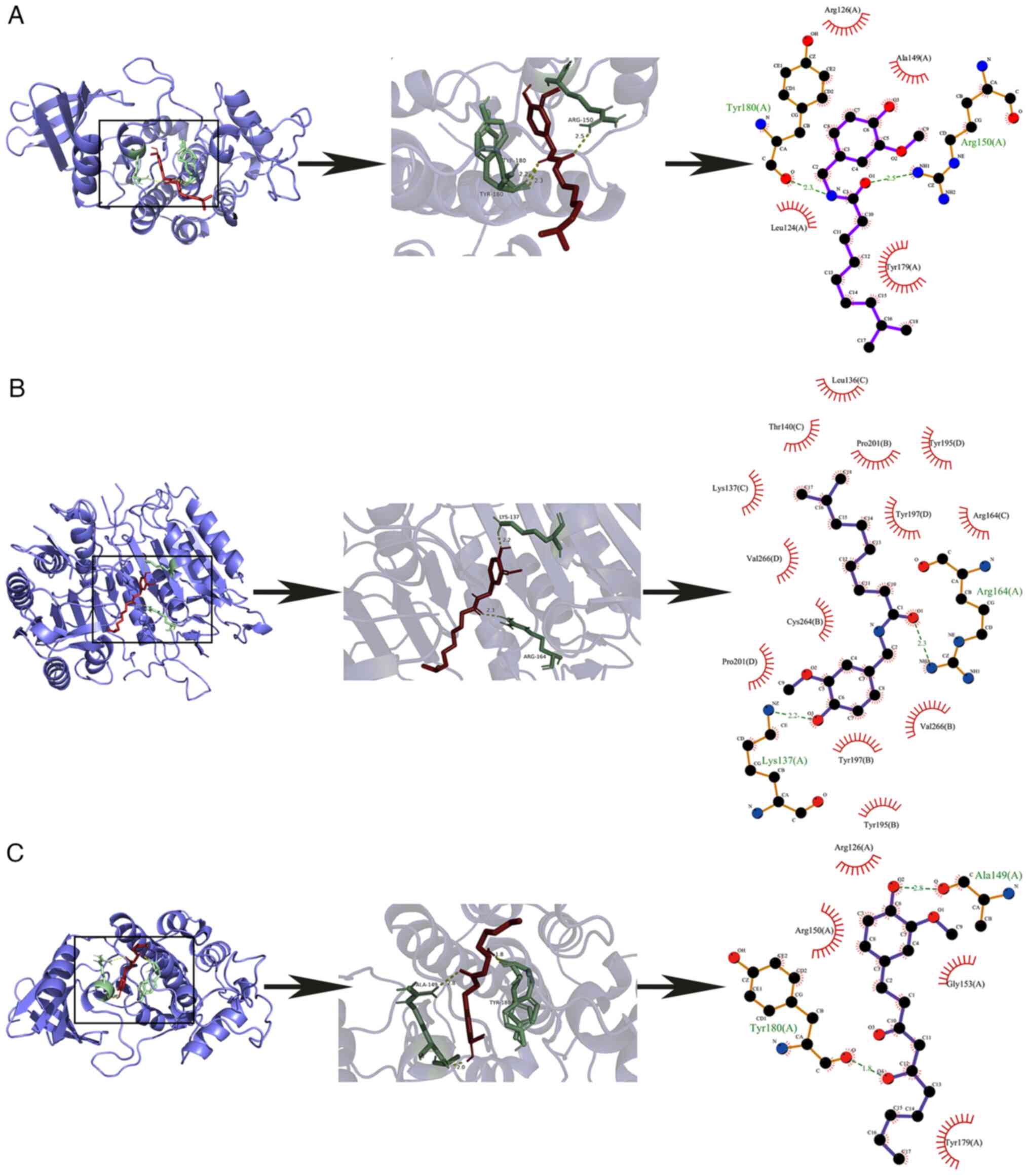
6. A, B, C purple structures are protein macromolecules; the
yellow dotted line is the hydrogen bond of the ligand small
molecule binding protein; the green structure is a protein-ligand
binding residue. Dihydrocapsaicin was linked to the amino acid
residues TYR-180 and ARG-150 of EGFR by hydrogen bonds with bond
lengths of 2.3 and 2.5, respectively. 6-Gingerol and EGFR amino
acid residues TYR-180, ALA-149 were linked by hydrogen bonds with
bond length of 1.0, 2.0, 2.8, respectively. Dihydrocapsaicin was
linked to the amino acid residues LYS-137 and ARG-164 of CASP3 by
hydrogen bonds with bond lengths of 2.2 and 2.3, respectively.
Further generating a 2D visualization, discovering
dihydrocapsaicin-EGFR formed hydrophobic effects with amino acids
such as Leu124, Tyr179 and Arg126. 6-Gingerol-EGFR forms
hydrophobic effects with amino acids such as Tyr179, Arg126 and
Arg150; Dihydrocapsaicin-CASP3 forms a hydrophobic effect with
amino acids such as Lys137, Pro201 and Cys264. Dihydrocapsaicin was
selected for subsequent cell experiments to verify the therapeutic
effect of ginger on triple negative breast cancer.
In vitro cell experiment results
Inhibitory effect of DHC on
proliferation of MDA-MB-231 cells
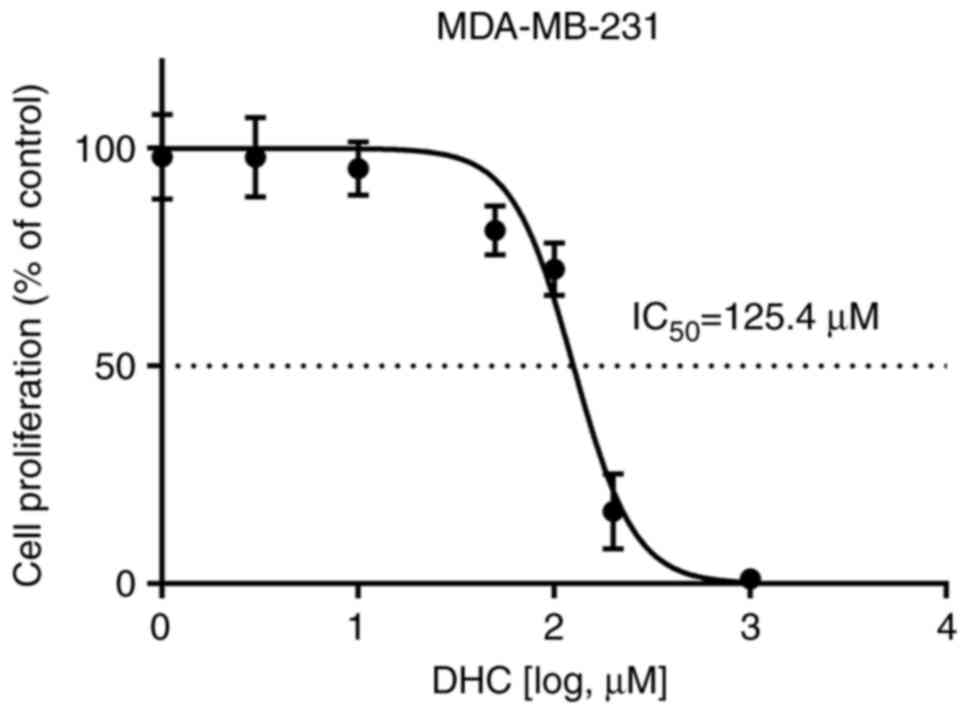
DHC had a significant inhibitory effect on the
proliferation of MDA-MB-231 cells (IC50: 125.4 µM,
95%CI: 114.1-137.3) (Fig. 7). With
the increase of DHC concentration, the inhibitory effect on the
proliferation of MDA-MB-231 cells was stronger. The effects of DHC
concentration of 100, 150, 200, 250 µmol/l cultured cells for 12,
24, 48 h were detected by MTT method, as shown in Table IV. Compared with the control group,
the DHC group had a certain inhibitory effect on the proliferation
of MDA-MB-231 cells. When the concentration of DHC was 150, 200,
250 µmol/l, the inhibitory effect was more obvious, and with the
prolongation of administration time, the inhibitory effect was
enhanced.
 | Table IV.Inhibitory effect of DHC on the
proliferation of MDA-MB-231 (x̄ ± s; n=6). |
Table IV.
Inhibitory effect of DHC on the
proliferation of MDA-MB-231 (x̄ ± s; n=6).
|
|
| Inhibitory effect,
% |
|---|
|
|
|
|
|---|
| Group | Dosage | 12 | 24 | 48 |
|---|
| Control | - | 0 | 0 | 0 |
| DHC | 100 µmol/l |
18.1±2.3a |
16.0±5.3a |
1.6±3.6a |
|
| 150 µmol/l |
40.9±7.7a |
67.2±4.1a |
52.3±6.9a |
|
| 200 µmol/l |
63.5±4.6a |
81.5±1.5a |
96.9±1.0a |
|
| 250 µmol/l |
76.2±4.4a |
95.8±1.8a |
99.2±0.3a |
Inhibitory effect of DHC on MDA-MB-231
cell migration
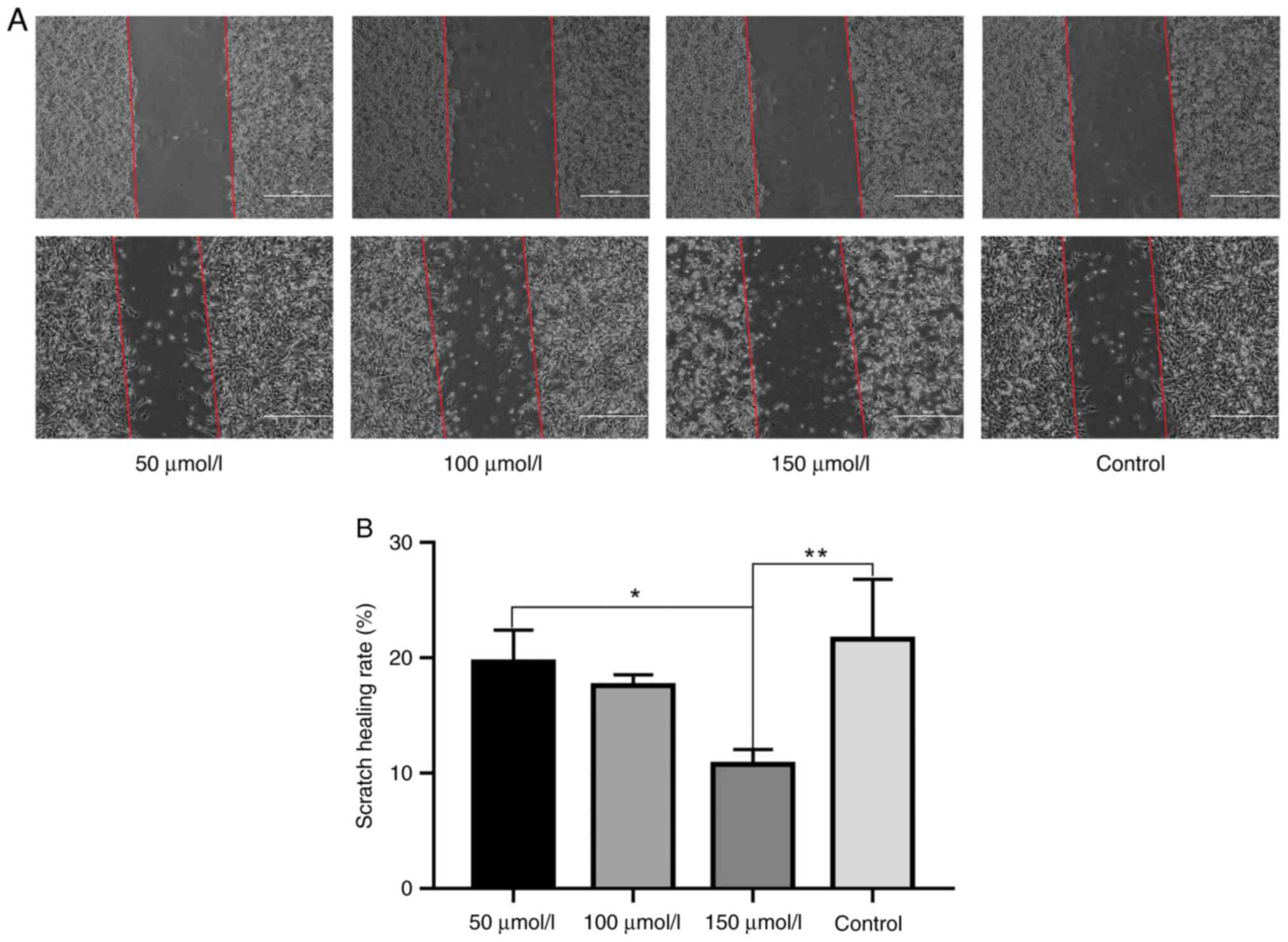
The MDA-MB-231 cells were treated with drugs after
scratching. Compared with the blank group, the cell morphology of
the high-dose DHC group changed significantly, and the cell scratch
wound healing ability was significantly reduced (P<0.01). The
cell scratch wound healing ability of high dose DHC group was
significantly lower than that of low dose DHC group (P<0.05).
The low dose DHC group and middle-dose DHC group cell migration
ability has a downward trend, but compared with the blank group,
the difference is not obvious, see Fig.
8A and B.
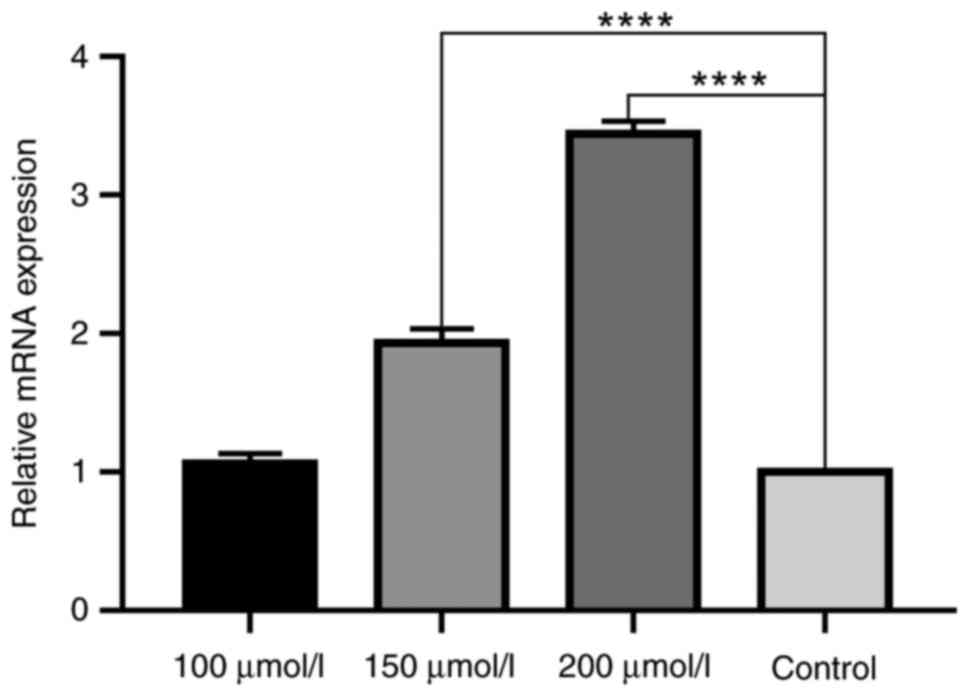
Effect of DHC on CASP9 mRNA expression
in MDA-MB-231 cells
The effect of DHC on the expression of CASP9 in
MDA-MB-231 triple negative breast cancer cells was detected by PCR.
It was found that the expression of CASP9 in each dose group of DHC
was increased to a certain extent compared with the solvent control
group. There was no significant difference between the low dose
group of DHC and the control group. The middle dose group and the
high dose group of DHC were significantly different from the
control group (P<0.05), as shown in Fig. 9.
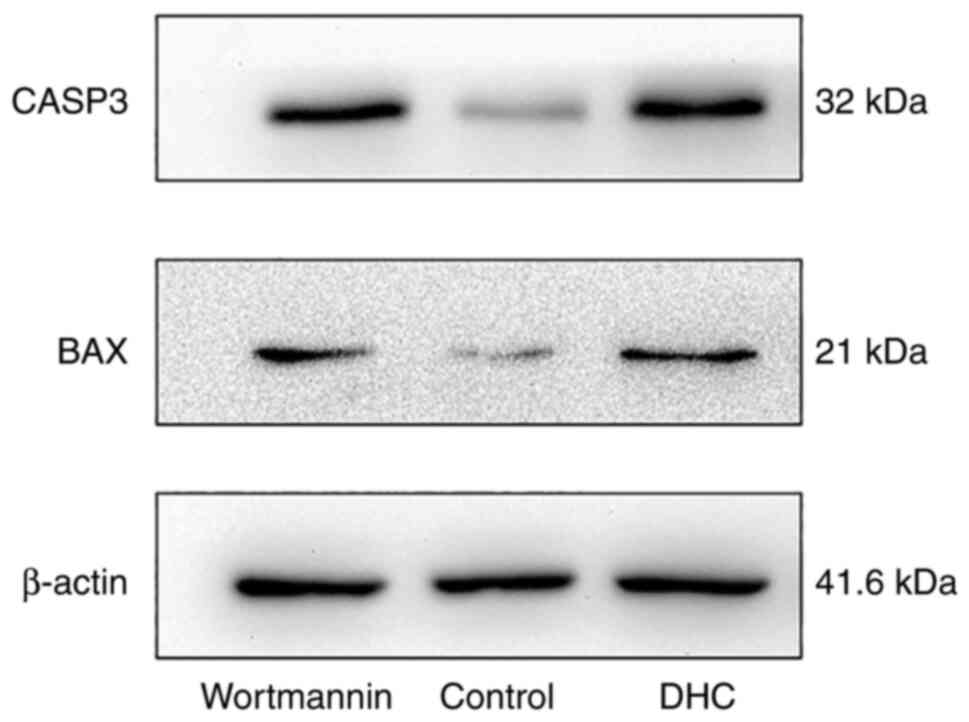
Effect of DHC on CASP3 and BAX protein
expression in MDA-MB-231 cells
Based on the above network pharmacology research,
the apoptosis pathway was selected to verify the effect of ginger
on MDA-MB-231 triple negative breast cancer cells by WB experiment.
Compared with the normal group, the expression of CASP3 protein and
BAX protein in DHC group and PI3K/AKT pathway inhibitor Wortmannin
group increased significantly (P<0.05), as shown in Table V and Fig. 10.
 | Table V.Effects of DHC on CASP3 and BAX
protein expression in MDA-MB-231 (x̄ ± s; n=3). |
Table V.
Effects of DHC on CASP3 and BAX
protein expression in MDA-MB-231 (x̄ ± s; n=3).
| Group | Dosage | CASP3/β-actin | BAX/β-actin |
|---|
| Wortmannin | 1 µmol/l |
1.02±0.18a |
1.00±0.36a |
| Control | - | 0.38±0.01 | 0.55±0.22 |
| DHC | 200 µmol/l |
1.00±0.01a |
1.07±0.25a |
Discussion
Breast cancer accounts for 31% of confirmed cancer
cases in women in 2022, far higher than other common cancer
diseases such as lung cancer and colorectal cancer, and is a major
public health problem that threatens women's health (25). Triple negative breast cancer (TNBC)
is a subtype of breast cancer that does not express estrogen
receptor (ER) and progesterone receptor (PR), and does not
overexpress human epidermal growth factor receptor 2 (ERBB2) of
TNBC accounts for 15–20% of all breast cancers (26,27).
At the molecular level, it is a highly heterogeneous disease.
According to the gene expression profile, TNBC has six different
subtypes, each subtype has unique gene expression characteristics.
The clinical significance of these subtype differences is still
under study (2). TNBC is also
considered to be one of the most aggressive types of breast cancer,
with rapid progression and low survival rate, mostly in young women
(2,21,27).
Compared with hormone receptor positive or ERBB2 positive subtypes,
TNBC is more aggressive in clinical behavior and lacks molecular
targets for treatment. Patients cannot use traditional hormone or
ERBB2 targeted therapy. Chemotherapy is the main treatment option,
and systemic chemotherapy has acute and chronic toxicity, which can
cause nausea, vomiting and other adverse reactions. At the same
time, TNBC has shorter progression-free survival, worse prognosis,
higher recurrence, distant metastasis and mortality, especially in
the first 5 years after diagnosis (21,28,29).
Therefore, it is necessary to optimize current treatment strategies
for triple-negative breast cancer patients.
Clinically many difficult ‘Miscellaneous’ use of
modern medical methods cannot solve the use of traditional Chinese
medicine may be relatively large breakthrough. A tumor is a mass of
lumps formed by abnormal proliferation of local tissue cells.
Traditional Chinese medicine attributes this morphological feature
to ‘accumulation’. ‘Classic on medical problems. Fifty-five Classic
on medical problems’: ‘Accumulation, Yin Qi also’ (30). Sui dynasty ‘Classic on medical
problems. Fifty-five Classic on medical problems’: ‘Accumulators,
born from the cold inside’ (31).
The close relationship between ‘accumulation’ and ‘cold’ can be
seen in ancient Chinese classic medical books. Modern
pharmacological studies have shown that aconite, cinnamon and
ginger can regulate the expression of oncogenes, induce tumor cell
apoptosis, inhibit tumor cell metastasis, block tumor cell cycle
and other anti-tumor ways (31,32).
Ginger is a typical edible traditional Chinese medicine. It has
been used for thousands of years as medicine and condiment.
Ginger has the effect of relieving cold, warming and
stopping vomiting. Pharmacological effects include protecting liver
and gallbladder, regulating immune system, anti-inflammatory and
anti-allergic, anti-pathogenic microorganisms, anti-tumor and so
on. The anti-tumor effect of ginger has been reported in many ways.
Ling Zhou found that 6-shogaol inhibited the activation of
TLR4/NF-κB signaling pathway, affected NF-κB signaling and
down-regulated COX-2, cyclinD1, Bcl-2, MMP-9 and other key
cytokines, thereby enhancing the anti-pancreatic cancer activity
with gemcitabine (33). Kaewtunjai
found that 60 days after lung cancer A549 cells were treated with a
cytotoxic dose of crude ginger extract (ZOE), the protein
expression of telomerase reverse transcriptase (hTERT) decreased,
resulting in telomere shortening and cell senescence, thereby
achieving the effect of crude ginger extract (ZOE) in inhibiting
the activity and proliferation of lung cancer cells (34).
Ginger has many components and pharmacological
activities. This study explored the mechanism of ginger in the
treatment of triple negative breast by combining network
pharmacology and in vitro cell experiments. After screening
through database and literature search, ten potential active
ingredients of ginger, 10-gingerol, geraniol, citral,
dihydrocapsaicin, L-linalool, 6-gingerol, 1,7-dihydroxyxanthone,
eucalyptol, 6-shogaol and zingerone, were obtained. These active
ingredients have been reported to have anti-tumor effects.
10-gingerol was made into a loaded nanoemulsion to improve
bioavailability. It was found to be cytotoxic to mouse and human
TNBC cell lines 4T1 and MDA-MB-231 cells, arresting the cell cycle
in the sub-G0 phase and inducing apoptosis (35). Liany used 6-gingerol-derived
semi-synthetic compound SSi6 in a preclinical xenograft model to
demonstrate that SSi6, as a single drug, has good anti-tumor
activity in vivo without obvious side effects and can block
the typical visceral organ metastasis of breast cancer, lymph node
metastasis to the lungs and multiple organs (36). These documents and research results
provide the basis and important reference for this study. In
particular, network pharmacological docking results suggested that
DHC binding activity to TNBC target proteins was particularly good,
so we chose the DHC as the object of further study.
PI3K/AKT signaling pathway is a star signaling
pathway in cancer research. It plays an important role in
regulating various cell functions (including metabolism, growth,
proliferation, apoptosis, transcription and protein synthesis).
PI3K, AKT and PTEN are important node proteins in the pathway and
important factors regulating intracellular effects of downstream
signaling pathways. The results of cell experiments showed that DHC
group and PI3K/AKT inhibitor Wortmannin group could induce
apoptosis and increase the protein expression of CASP3 and BAX.
After the intervention of DHC and Wortmannin, the expression of
apoptotic proteins increased. DHC may participate in the regulation
of PI3K/AKT signaling pathway by inhibiting the expression of PI3K
and AKT proteins or promoting the expression of PTEN protein. The
results of MTT assay showed that DHC had a good inhibitory effect
on the proliferation of MDA-MB-231 cells at a dose concentration of
100, 150, 200 and 250 µmol/l. When the dose of DHC was 200 and 250
µmol/l, it showed dose-dependent and time-dependent. After 24 days
of administration, the inhibition rate reached 80% or more, and the
cells died in a large area. The results of cell scratch test showed
that when the dose of DHC was 150 µmol/l, the cell migration was
significantly inhibited. When the dose of DHC was 200 and 250
µmol/l, the scratch results of large area apoptosis could not be
counted. The results of rt-PCR showed that DHC at 150 and 200
µmol/l could significantly reduce the mRNA expression of CASP9 in
MDA-MB-231 cells.
The mechanism of DHC in treating triple negative
breast cancer is closely related to Caspase family. The molecular
docking results also confirmed the close relationship between the
mechanism of DHC in the treatment of triple negative breast cancer
and the Caspase family. The docking binding energy of CASP3 with
the six core components of ginger was less than −4.25, and the
average binding energy was −5.9, indicating that CASP3 was stably
bound to the active ingredients of ginger.
This study explored the mechanism of ginger in the
treatment of triple negative breast cancer by network pharmacology,
molecular docking and in vitro cell experiments. The results
showed that dihydrocapsaicin could inhibit the proliferation and
migration of triple negative breast cancer MDA-MB-231 cells, and
up-regulate the mRNA expression of Caspase family CASP9 and the
protein expression of CASP3 and BAX. We will further explore other
molecular mechanisms of DHC in the treatment of TNBC, including the
use of different experimental methods such as flow cytometry to
detect apoptosis, WB to detect the expression of signal protein in
the treatment of TNBC pathway by DHC, and animal experiments to
verify the therapeutic effect of DHC on TNBC in vivo. At the
same time, other active components of ginger, such as 6-gingerol,
were selected for the pharmacodynamic experiment to explore its
therapeutic effect on TNBC. This study provides a direction for the
treatment of triple negative breast cancer, and also provides an
idea for the drug development of ginger.
Acknowledgements
The authors would like to thank Dr Lai Chen (Chinese
and Western Cancer Center, Jiangxi, China) and Mr Xu Lei (The
Laboratory Animal Science and Technology Center, Jiangxi, China)
for providing suggestions on the experimental design, and Ms Zhang
Hong (School of Pharmacy, Jiangxi University of Chinese Medicine,
Jiangxi, China) for providing suggestions on the response
reviewers.
Funding
The present study was supported by the Science and Technology
Research Project of Jiangxi Provincial Department of Education
(grant nos. GJJ180661, GJJ211240 and GJJ211210).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL, KS conceived and designed the study and helped
to draft the manuscript. LL and YC are responsible for the
collection of data. TH and LL participated in the statistical
analyses. QM and YH made the figures, participated in data
analysis, and were the main contributors to writing the manuscript.
All authors read and approved the final version of the manuscript.
QM and YH confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal studies were approved by the Committee on the
Ethics of Animal Experiments of the Jiangxi University of Chinese
Medicine (approval no. JZSYDWLL20200105).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burstein MD, Tsimelzon A, Poage GM,
Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK,
Hilsenbeck SG, Chang JC, et al: Comprehensive genomic analysis
identifies novel subtypes and targets of triple-negative breast
cancer. Clin Cancer Res. 21:1688–1698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma P: Biology and management of
patients with triple-negative breast cancer. Oncologist.
21:1050–1062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu J, Xue X, Hu C, Xu H, Kou D, Li R and
Li M: Comparison of clinicopathological features and prognosis in
triple-negative and non-triple negative breast cancer. J Cancer.
7:167–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J, Jiang S, Wang Y, Fan J, Liu X, Yan
L, Wang Z, Kuang YH, Wang DQ and Luo W: Herbal textual research and
quality evaluation of ginger in classical prescriptions. Chin J Exp
Formulaol. 28:27–37. 2022.
|
|
7
|
He P and Zhong L: Research progress of
dried ginger, ginger and ginger juice. Chin J Exp Formulaol.
22:219–223. 2016.
|
|
8
|
Qi XG, Xiang H, Hao J, Yang P, Xing X and
Yang X: Mechanism of curcumin inhibiting proliferation and invasion
of breast cancer cells. Chin J Cancer Prev Treat. 25:1211–1216.
2018.
|
|
9
|
Prasad S and Tyagi AK: Ginger and its
constituents: Role in prevention and treatment of gastrointestinal
cancer. Gastroenterol Res Pract. 2015:1429792015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semwal RB, Semwal DK, Combrinck S and
Viljoen AM: Gingerols and shogaols: Important nutraceutical
principles from ginger. Phytochemistry. 117:554–568. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahomoodally MF, Aumeeruddy MZ, Rengasamy
KRR, Roshan S, Hammad S, Pandohee J, Hu X and Zengin G: Ginger and
its active compounds in cancer therapy: From folk uses to
nano-therapeutic applications. Semin Cancer Biol. 69:140–149. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang Y, Wu G, Luo T, Xie H, Zuo Q, Huang
P, Li H, Chen L, Lu H and Chen Q: 10-gingerol enhances the effect
of taxol in triple-negative breast cancer via targeting ADRB2
signaling. Drug Des Devel Ther. 17:129–142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastogi N, Duggal S, Singh SK, Porwal K,
Srivastava VK, Maurya R, Bhatt ML and Mishra DP: Proteasome
inhibition mediates p53 reactivation and anti-cancer activity of
6-gingerol in cervical cancer cells. Oncotarget. 6:43310–43325.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Y, Chen X, Luo L, Zhang Q, Gao C,
Zhuang X, Yuan S and Qiao T: [6]-Gingerol enhances the
radiosensitivity of gastric cancer via G2/M phase arrest and
apoptosis induction. Oncol Rep. 39:2252–2260. 2018.PubMed/NCBI
|
|
15
|
Jagetia GC, Baliga MS, Venkatesh P and
Ulloor JN: Influence of ginger rhizome (Zingiber officinale Rosc)
on survival, glutathione and lipid peroxidation in mice after
whole-body exposure to gamma radiation. Radiat Res. 160:584–592.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminformatics. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Guo F, Wang Y, Li C, Zhang X, Li H,
Diao L, Gu J, Wang W, Li D and He F: BATMAN-TCM: A bioinformatics
analysis tool for molecular mechANism of traditional Chinese
medicine. Sci Rep. 6:211462016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang S, Dong L, Liu L, Guo J, Zhao L,
Zhang J, Bu D, Liu X, Huo P, Cao W, et al: HERB: A high-throughput
experiment- and reference-guided database of traditional Chinese
medicine. Nucleic Acids Res. 49:D1197–D1206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daina A, Michielin O and Zoete V:
SwissADME: A free web tool to evaluate pharmacokinetics,
drug-likeness and medicinal chemistry friendliness of small
molecules. Sci Rep. 7:427172017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu
X, Lai L, Pei J and Li H: PharmMapper 2017 update: A web server for
potential drug target identification with a comprehensive target
pharmacophore database. Nucleic Acids Res. 45:W356–W360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9 Suppl 2 (Suppl 2):S73–S81.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsin KY, Ghosh S and Kitano H: Combining
machine learning systems and multiple docking simulation packages
to improve docking prediction reliability for network pharmacology.
PLoS One. 8:e839222013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan SA and Lee TKW: Investigations of
nitazoxanide molecular targets and pathways for the treatment of
hepatocellular carcinoma using network pharmacology and molecular
docking. Front Pharmacol. 13:9681482022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saraswati S, Alhaider A, Abdelgadir AM,
Tanwer P and Korashy HM: Phloretin attenuates STAT-3 activity and
overcomes sorafenib resistance targeting SHP-1-mediated inhibition
of STAT3 and Akt/VEGFR2 pathway in hepatocellular carcinoma. Cell
Commun Signal. 17:1272019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Classic on Medical Problems. Beijing:
People's Health Publishing House; 2013
|
|
31
|
Yuanfang C: Nest Cube. On the etiology of
diseases Beijing: People's Health Publishing House; 1983
|
|
32
|
Lei D and Liu Z: Research progress on
anti-tumor effect of traditional Chinese medicine for warming
middle and dispelling cold. Shi Zhenguo Medicine. 32:170–173.
2021.
|
|
33
|
Zhou L, Qi L, Jiang L, Zhou P, Ma J, Xu X
and Li P: Antitumor activity of gemcitabine can be potentiated in
pancreatic cancer through modulation of TLR4/NF-κB signaling by
6-shogaol. AAPS J. 16:246–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaewtunjai N, Wongpoomchai R, Imsumran A,
Pompimon W, Athipornchai A, Suksamrarn A, Lee TR and
Tuntiwechapikul W: Ginger extract promotes telomere shortening and
cellular senescence in A549 lung cancer cells. ACS Omega.
3:18572–18581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zanesco-Fontes I, Silva ACL, da Silva PB,
Duarte JL, Di Filippo LD, Chorilli M, Cominetti MR and Martin ACBM:
[10]-Gingerol-loaded nanoemulsion and its biological effects on
triple-negative breast cancer cells. AAPS PharmSciTech. 22:1572021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luna-Dulcey L, Almada da Silva J,
Jimenez-Renard V, Caleiras E, Mouron S, Quintela-Fandino M and
Cominetti MR: [6]-Gingerol-derived semi-synthetic compound SSi6
inhibits tumor growth and metastatic dissemination in
triple-negative breast cancer xenograft models. Cancers (Basel).
13:28552021. View Article : Google Scholar : PubMed/NCBI
|