Introduction
Renal cell carcinoma (RCC) is the most common
malignancy of all functional renal tissues, except for the renal
urothelium. There are three subtypes of RCC: Clear cell RCC (ccRCC)
(75–80%), papillary RCC (10–15%) and chromophobe RCC (5–10%)
(1). ccRCC is the most common type
of renal malignancy and it accounts for the majority of renal
cancer-associated deaths. As the most common and lethal kidney
tumor, ccRCC incidence is increasing and the prognosis is poor
(2). Surgical removal is still the
primary treatment option for ccRCC due to resistance to traditional
chemotherapy and radiotherapy strategies (3). As there are few useful biomarkers and
treatments for ccRCC, a poor prognosis and high mortality rates are
common in patients at an advanced stage. However, the molecular
mechanisms underlying ccRCC tumorigenesis remain unclear, and new
therapies for ccRCC are difficult to develop (4). There is a requirement for researchers
to determine the molecular mechanisms underlying the tumorigenesis
of renal cancer and to engineer new therapeutics, including those
aimed at specific molecular targets, in order to decrease the
mortality rate associated with renal cancer.
Circular RNAs (circRNAs) are a family of non-coding
RNAs (ncRNAs) that are circularized by joining the RNA 3′ and 5′
ends to form a circular structure (5). There are different types of circRNAs,
including exonic, intronic and intergenic circRNA. Changes in
circRNA expression induce the expression of tumor-related genes,
which affect the occurrence and development of tumors (6,7).
circRNAs have three main functions: i) Serving as microRNA
(miR/miRNA) sponges to sequester and inhibit miRNA activity; ii)
acting as regulators that interact with proteins; iii) serving as
templates for protein synthesis (8). circRNAs play an important role in the
development and progression of human tumors, according to
accumulating evidence (9–11).
WW and C2 domain-containing protein 3 (WWC3) is a
member of the WWC scaffold protein family, with involvement in
cellular transport processes that are necessary the migration,
polarity and synaptic signaling of cells (12,13).
circRNAs may be potential regulators at the RNA level, and the
abnormal expression of circRNAs may be associated with specific
human diseases, especially cancer. WWC3 is a tumor suppressor that
is downregulated in a number of malignancies, such as colorectal
carcinoma, lung cancer and osteosarcoma (13–15).
An association has been found between WWC3 downregulation and a
poor prognosis in patients with cancer (16). A recent study has shown that linear
WWC3 is associated with a good prognosis, and that it inhibits
breast cancer cell growth and metastasis (17). However, the circular form of WWC3
(circWWC3) dominates and exhibits oncogenic functions in breast
cancer, suggesting that circWWC3 competes with linear WWC3 to
promote the progression of breast cancer (18).
circRNAs act as essential regulators of RCC
tumorigenesis. Wang et al (19) revealed that hsa-circ_0035483 is
highly expressed in RCC and enhances gemcitabine resistance by
regulating the hsa-miR-335/CCNB1 signaling pathway. In RCC, ZEB2
expression is upregulated by circPCNXL2 and downregulated by
miR-153 (20). The derivation of
circPCNXL2 (circ_406752) is the PCNXL2 gene and the circRNA acts to
augment in vitro and in vivo tumor growth, while
miR-153 can reverse its effect. Some circRNA genome-wide
transcriptional profiles have been reported in RCC (19). Using a circRNA microarray, the study
by Ma et al (21) showed the
aberrant expression of 542 circRNAs in ccRCC. Among these circRNAs,
324 exhibited significant downregulation, whereas 218 exhibited
significant upregulation in ccRCC. The majority of circRNAs enhance
RCC development and progression via circRNA-miRNA-mRNA
interactions. Some circRNAs can also be used as prognostic
biomarkers. For example, circ-ABCB10 promotes RCC cell growth and
suppresses apoptosis in vitro (22).
While a number of advances have been made in circRNA
biological research, the exact effect on patient prognosis and the
regulation of circRNA function remain largely unclear. The present
study aimed to predict the survival of patients with RCC by
analyzing circWWC3 expression data and demographic and clinical
variables. Therefore, the study was conducted on patients who had
previously undergone kidney cancer resection and were undergoing
follow-up visits. The study further aimed to identify a better
prognostic molecular marker through a retrospective analysis of
existing data.
Patients and methods
Ethical statement
Ethical approval was obtained from the Ethics
Committee of The Fourth Hospital of Hebei Medical University
(Shijiazhuang, China; approval no. 202159). This study conformed to
the Declaration of Helsinki. Patients were followed up according to
national clinical guidelines. All patients provided written
informed consent.
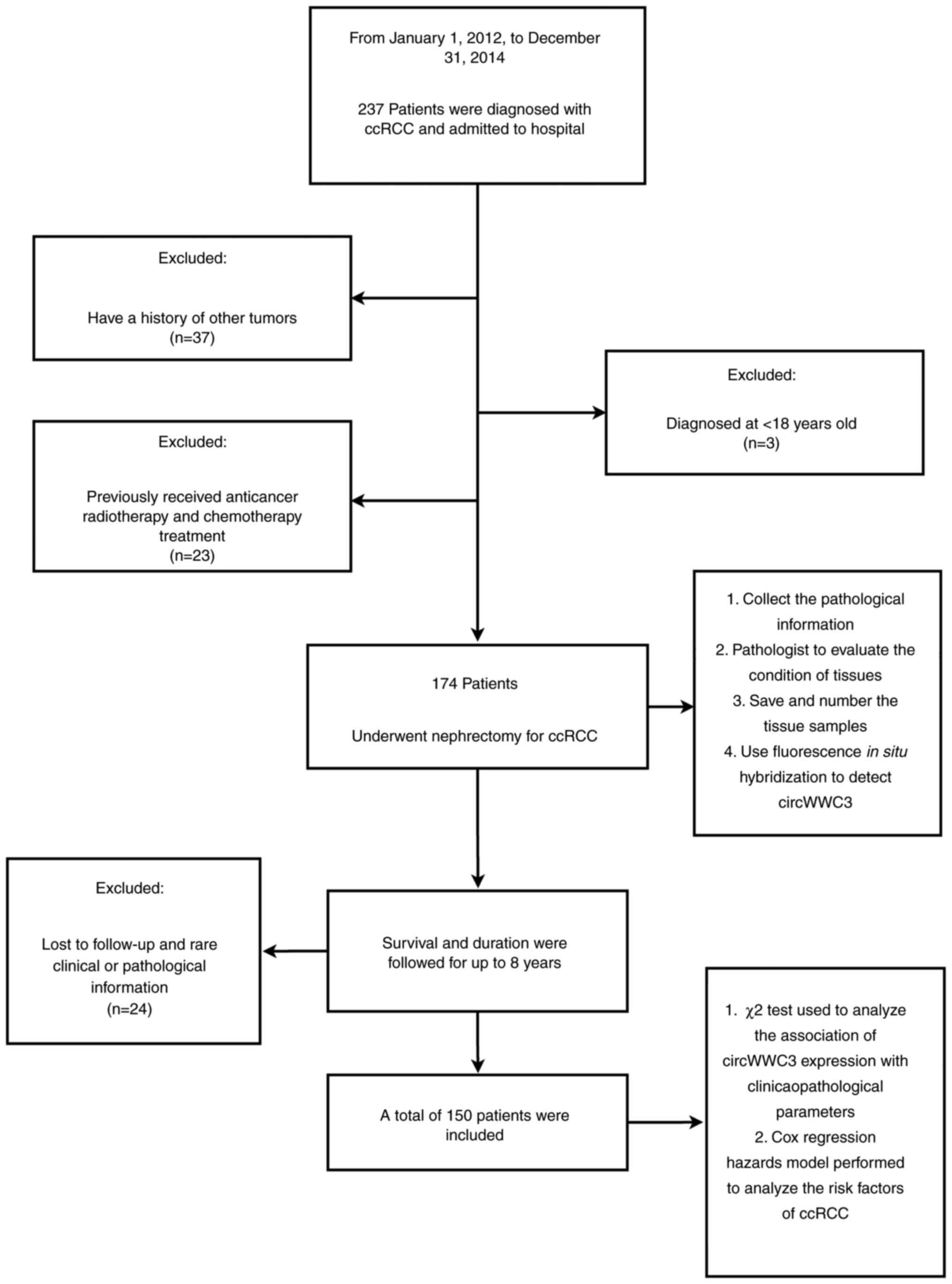
Patients' inclusion and exclusion
criteria
Patients who underwent radical nephrectomy in The
Fourth Hospital of Hebei Medical University with a pathological
diagnosis of ccRCC and who had both tumor and normal renal tissues
available after surgery were included. All patients needed to be
available for regular follow-up for ≥8 years. None of the included
patients had received anticancer radiotherapy or chemotherapy
before surgery. Other exclusion criteria were as follows: i) A
history of other tumors (benign or malignant); ii) an age of <18
years; iii) rare pathological features; iv) data with incomplete
information; and v) death from other causes. A flow chart
illustrating the inclusion and exclusion criteria is shown in
Fig. 1.
Collection of excised tissue
samples
All kidney cancer tissue samples (ccRCC) and
adjacent morphologically normal renal cortex (adjacent tissues)
samples were collected from patients with ccRCC who met the
inclusion criteria and underwent renal surgical resection at the
Fourth Affiliated Hospital of Hebei Medical University between
January 1, 2012, and February 31, 2014.
All samples were collected during surgical resection
and either snap frozen in liquid nitrogen and stored at −80°C or
fixed in formalin and embedded in paraffin. After fixing the kidney
tissue with 4% paraformaldehyde at room temperature for 24 h, it
was dehydrated, embedded in paraffin, sectioned (4 µm) and
subjected to hematoxylin and eosin (HE) staining. Formalin-fixed
paraffin-embedded slides was stained with hematoxylin for 10 min
and 1% eosin for 30 sec at room temperature. Using an optical
microscope, the pathological changes in the kidney tissue after HE
staining were examined. According to the Fuhrman grade (23) and tumor condition, pathologists in
the Department of Pathology performed microscopic pathological
observations of the tissue samples of all patients and recorded all
pathological information.
All tissue samples were numbered for subsequent
molecular histological examination and follow-up.
Fluorescence in situ hybridization
(FISH)
circWWC3 expression in ccRCC and normal tissues was
detected using FISH. FISH was performed with probes specific for
circWWC3 sequences. The Biotin-labeled circWWC3 probes were
purchased from Qiagen GmbH. The probe sequences were as follows:
5′BiosG/TCAATGGCTTTGTTATCCTCTTTCT/3′Bio. The paraffin slides of
tissue were first deparaffinized in xylene and ethanol solutions.
Following prehybridization in PBS with 0.5% Triton X-100, the cells
were hybridized with the aforementioned probes overnight at 37°C.
The fluorescence signal of circWWC3 was detected by
Cy5-Streptavidin Conjugate (ZyMAXTM Grade; Invitrogen; Thermo
Fisher Scientific, Inc.). The nuclei were counterstained with
4′,6-diamidino-2-phenylindole (DAPI) and the images were taken
under a ZEISS LSM 710 confocal microscope (Zeiss AG). Based on the
cytoplasmic expression intensity of circWWC3, tumor tissue samples
were classified as follows: Negative or weak expression in most
cells was defined as the negative group, weak expression in most
cells or moderate expression in <50% of cells was defined as the
low expression group, and moderate to strong expression in most
cells was defined as the high expression group. Fluorescence
intensity measurements were performed using ImageJ (National
Institutes of Health). A mean fluorescence intensity (MFI) value of
>90 was defined as high expression, a value of <70 was
defined as low expression, and MFI values between these thresholds
were defined as moderate expression. All of the results of the 150
tumor and normal tissue samples were recorded in a table for
association and survival analysis.
Clinical information collection and
patient follow-up
The admission and pathological information of all
patients was collected. Clinical data collected on admission
included age, sex, tumor stage (T stage) (24), Fuhrman grade, circWWC3 expression in
tumor samples and tumor size. Due to the limition of sample size, T
stage was classified into T1/T2 and T3/T4. All patients were
followed up for 8 years after discharge. Patients were followed up
via telephone every year. Meanwhile, the patients' survival status
and time were recorded for survival analysis.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows (version 26.0; IBM Corp.). Clinicopathological prognostic
factors were compared with regard to circWWC3 expression using the
χ2 test. Mean fluorescence intensity was analyzed using
one-way ANOVA, followed by SNK-q test. Univariate and multivariate
analyses were performed using a Cox regression hazard model.
Kaplan-Meier curves were created and log-rank tests were used for
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic characteristics
The present study included 150 patients; 48.7% were
women and 51.3% were men. Patients with ccRCC whose tumor diameter
was measured after resection were divided into two groups; one
group had tumor diameters ≥4 cm and the other had tumor diameters
<4 cm. The results of all followed up patients were classified
according to the pathologist's examination report. Pathological
grades I and II were considered to be the low differentiation
group, and patients with grades III and IV were considered to be
the high differentiation group.
circWWC3 expression in para-cancer and
cancer tissue samples
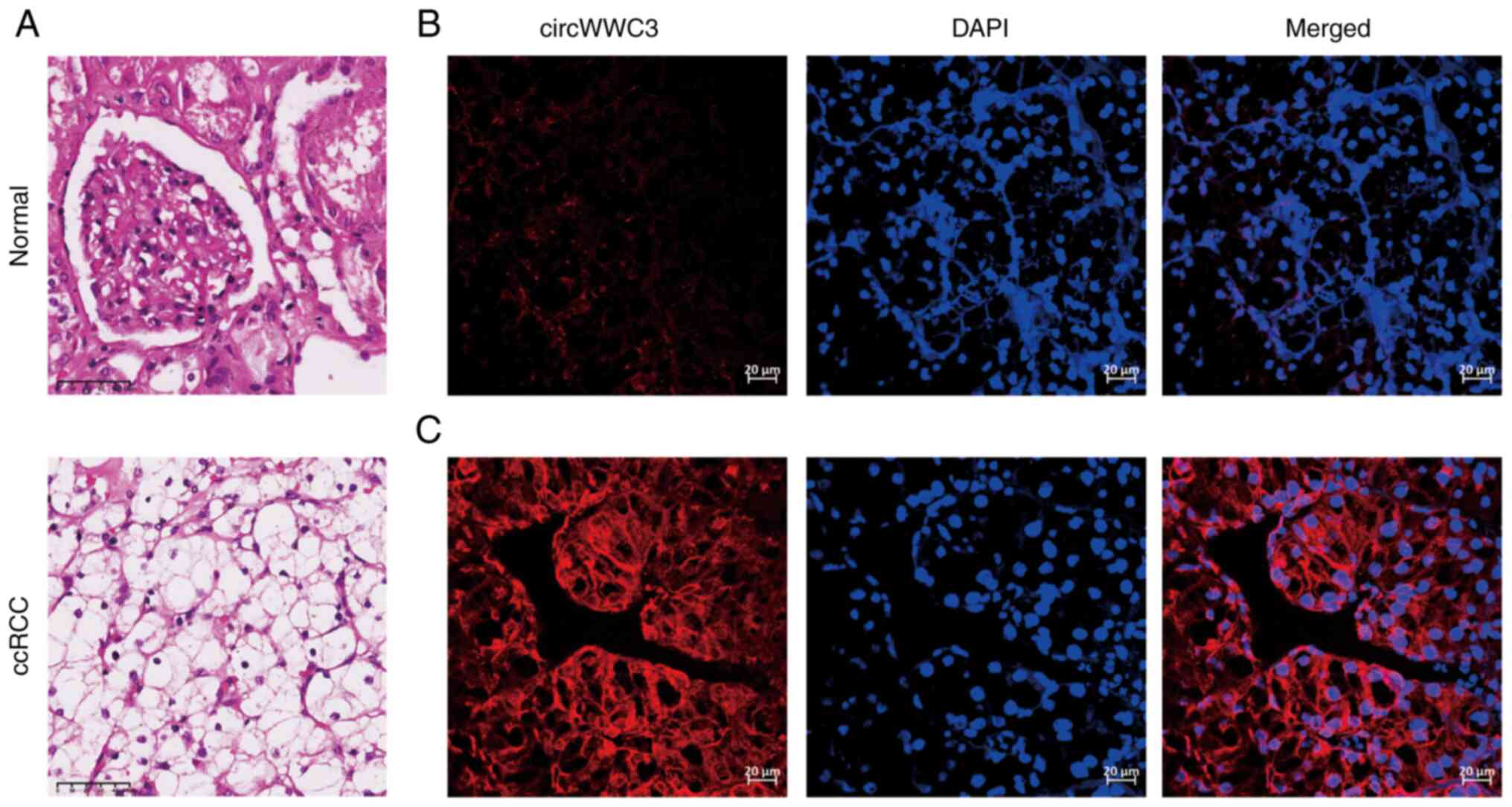
circWWC3 expression in ccRCC tissues was examined
using FISH in 150 tumor and adjacent normal tissues (Fig. 2). Using FISH, DAPI staining of the
nuclei was indicated in blue and circWWC3 staining of the cytoplasm
was indicated in red. circWWC3 and DAPI together were indicated by
a purple color in merged images. circWWC3 expression was higher in
all the examined tumor samples compared with that in the normal
samples using ImageJ. Subsequently, the 150 ccRCC tissue samples
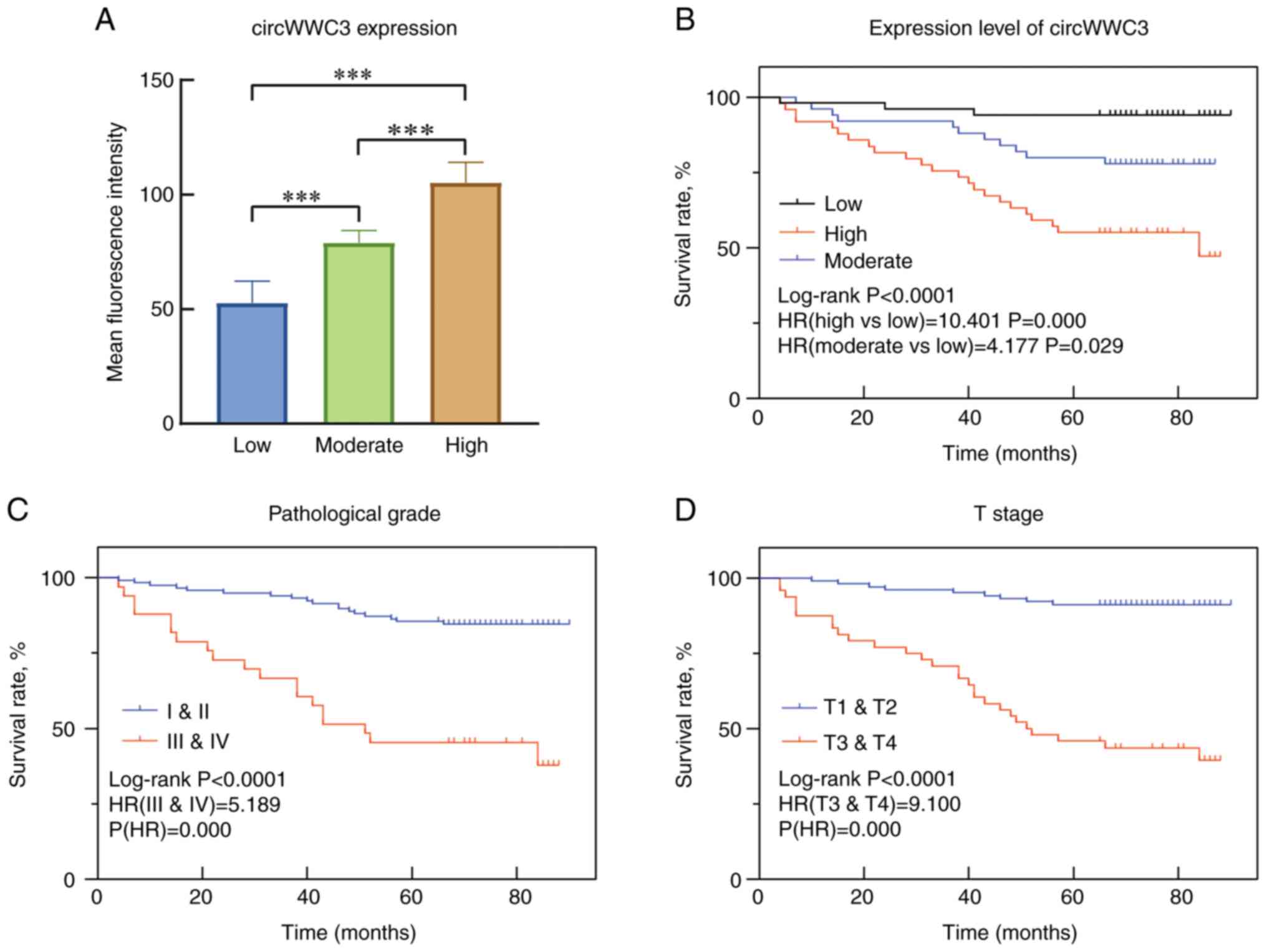
were divided into three circWWC3 expression levels based on their
mean fluorescence intensity: Low, moderate and high (Fig. 3A). Among all the followed up
patients, 51 had low circWWC3 expression, 50 had moderate circWWC3
expression and 49 had high circWWC3 expression.
Associations between circWWC3
expression and clinicopathological parameters
Associations between circWWC3 expression and other
clinicopathological parameters were evaluated using the
χ2 test (Table I). The
results revealed that tumor samples with high circWWC3 expression
were significantly associated with advanced tumor stage, including
T stage (P=0.005) and pathological grade (P=0.033). There were no
associations between expression level and age, sex or tumor
diameter.
 | Table I.Association between circWWC3
expression and the demographic and clinicopathological parameters
of patients with primary clear cell carcinoma of the kidney. |
Table I.
Association between circWWC3
expression and the demographic and clinicopathological parameters
of patients with primary clear cell carcinoma of the kidney.
|
| circWWC3, n (%) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Low | Moderate | High | Total no. | χ2 | P-value |
|---|
| Age, years |
|
|
|
| 1.535 | 0.464 |
|
<60 | 31 (36.9) | 29 (34.5) | 24 (28.6) | 84 |
|
|
| ≥60 | 20 (30.3) | 21 (31.8) | 25 (37.9) | 66 |
|
|
| Sex |
|
|
|
| 0.817 | 0.665 |
|
Female | 25 (34.2) | 22 (30.1) | 26 (35.6) | 73 |
|
|
|
Male | 26 (33.8) | 28 (36.4) | 23 (29.9) | 77 |
|
|
| Tumor diameter,
cm |
|
|
|
| 3.13 | 0.209 |
|
<4 | 32 (35.2) | 34 (37.4) | 25 (27.5) | 91 |
|
|
| ≥4 | 19 (32.2) | 16 (27.1) | 24 (40.7) | 59 |
|
|
| Pathological
grade |
|
|
|
| 6.835 | 0.033 |
|
I/II | 43 (36.8) | 42 (35.9) | 32 (27.4) | 117 |
|
|
|
III/IV | 8 (24.2) | 8 (24.2) | 17 (51.5) | 33 |
|
|
| T stage |
|
|
|
| 10.459 | 0.005 |
| T1 and
T2 | 41 (40.2) | 36 (35.3) | 25 (24.5) | 102 |
|
|
| T3 and
T4 | 10 (20.8) | 14 (29.2) | 24 (50.0) | 48 |
|
|
Survival analyses
The results of the univariate and multivariate Cox
regression and prognostic factor analysis with regard to mortality
based on the final Cox model are shown in Table II. Univariate survival analyses
were employed to determine the differences between patients with
ccRCC with different circWWC3 expression levels. The possible
prognostic factors for patients with ccRCC who were included in the
univariate analysis were determined to be the pathological grade of
the tumor (P<0.001), T stage (P<0.001) and the circWWC3
expression level (P<0.05). In the multivariate analysis, the
pathological grade of the tumor (P<0.05), T stage (P<0.001)
and the circWWC3 expression level (P<0.05) were determined to be
independent risk factors. The results showed that, in the
univariate analysis, moderate circWWC3 expression increased the
risk of ccRCC-related mortality by 4.177 (95% CI, 1.162-15.010; P =
0.029) compared with low circWWC3 expression. Furthermore, high
circWWC3 expression increased the risk of ccRCC-related mortality
by 10.401 (95% CI, 3.113-34.750; P<0.001). A Kaplan-Meier plot
of mortality was constructed to illustrate the differences between
the groups (Fig. 3B-D). The
pathological grade of the tumor and T stage were also incorporated
into the multivariate Cox proportional hazards model (grades
III/IV: HR, 5.189; 95% CI, 2.715-9.920; P<0.0001; stage T3/T4:
HR, 9.100; 95% CI, 4.281-19.343; P<0.0001). circWWC3 expression
was shown to be an independent risk factor upon multivariate
analysis (moderate: HR, 3.918; 95% CI, 1.084-14.153; P=0.037; high:
HR, 6.216; 95% CI, 1.830-21.117; P=0.003).
 | Table II.Univariate and multivariate analysis
of overall survival in patients with primary clear cell carcinoma
of the kidney. |
Table II.
Univariate and multivariate analysis
of overall survival in patients with primary clear cell carcinoma
of the kidney.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<60 | 1.000 | - | - | - | - | - |
|
≥60 | 1.597 | 0.836–3.051 | 0.156 | - | - | - |
| Sex |
|
|
|
|
|
|
|
Female | 1.000 | - | - | - | - | - |
|
Male | 1.011 | 0.530–1.926 | 0.974 | - | - | - |
| Tumor diameter,
cm |
|
|
|
|
|
|
|
<4 | 1.000 | - | - | - | - | - |
| ≥4 | 1.772 | 0.902–3.275 | 0.100 | - | - | - |
| Pathological
grade |
|
|
|
|
|
|
|
I/II | 1.000 | - | - | 1.000 | - | - |
|
III/IV | 5.189 | 2.715–9.920 | <0.001 | 2.318 | 1.125–4.775 | 0.023 |
| T stage |
|
|
|
|
|
|
|
T1/T2 | 1.000 | - | - | 1.000 | - | - |
|
T3/T4 | 9.100 | 4.281–19.343 | <0.001 | 5.117 | 2.220–11.800 | <0.001 |
| circWWC3
expression |
|
|
|
|
|
|
|
Low | 1.000 | - | - | 1.000 | - | - |
|
Moderate | 4.177 | 1.162–15.010 | 0.029 | 3.918 | 1.084–14.153 | 0.037 |
|
High | 10.401 | 3.113–34.750 | <0.001 | 6.216 | 1.830–21.117 | 0.003 |
Overall, the results showed that patients with high
circWWC3 expression in cancer tissues had a shorter survival time
(P<0.05), suggesting that circWWC3 was negatively associated
with patient prognosis. The analysis revealed that circWWCC3 was an
independent prognostic risk factor for ccRCC. In addition,
statistical analysis showed that the pathological grade of the
tumor (P<0.005) and T stage (P<0.005) were risk factors for
the prognosis of patients with ccRCC.
Discussion
ccRCC is the most common type of renal cancer among
adults. The survival rate of patients with ccRCC remains
unsatisfactory, as they present with insidious symptoms during the
earliest stages and exhibit little sensitivity to chemotherapy and
radiotherapy (25). The outcome of
patients with ccRCC depends on several factors, including the type
of surgery, the treatment method and the genetic heterogeneity of
the disease. Of these factors, surgery and treatment methods can be
controlled; however, genetic heterogeneity cannot (26). Recent studies have reported the role
of circRNA in ccRCC. For example, Gui et al (27) reported that circCHST15 promotes
ccRCC cell proliferation and metastasis through the
miR-125a-5p/EIF4EBP1 axis, Zhang et al (28) proposed the circME1/ME1 pathway as
involved in ccRCC progression and sunitinib resistance development
(29) and Wang et al
(29) presented circDVL1 as
exerting a tumor-suppressive function during ccRCC progression
through the circDVL1/miR-412-3p/PCDH7 axis. The upregulation of
circWWC3 expression was observed in ccRCC tissues in the present
study, and patients with high circWWC3 expression levels exhibited
worse overall survival OS times.
In the present study, circWWC3 expression was higher
in ccRCC tissues than in normal renal tissues. circWWC3 was
demonstrated to predict survival in patients with ccRCC and may
prove to be a new prognostic biomarker. Univariate regression
analysis showed that T stage, Fuhrman stage and circWWC3 expression
levels affected patient survival. The follow-up assessment for
circWWC3 may be used as an index for monitoring patients, which can
be an invaluable reference for medical practice. circWWC3 has seven
open reading frames, and 76 RNA-binding protein sites and 40
microRNA response elements can be observed in the circWWC3
structure according to the Cancer-Specific CircRNA Database
(http://gb.whu.edu.cn/CSCD/).
Currently, ccRCC transmission and recurrence risk
assessment are limited to the tumor stage, pathological
classification and clinical characteristics (30,31).
These shortcomings and lack of risk factors may lead to an
unsatisfactory prognosis prediction. The study of effective
postoperative adjuvant therapy is also necessary, as ccRCC is not
sensitive to chemotherapy and radiotherapy. circRNAs can serve as a
novel and attractive class of ncRNA biomarkers for liquid biopsy
due to their resistance to RNase R digestion and stability within
the blood circulation (32). Body
fluids contain abundant circRNAs, which can be detected using
reverse transcription-polymerase chain reaction assays, which are
relatively inexpensive. circRNAs have been shown to be abnormally
expressed in hepatocellular carcinoma, lung cancer and breast
cancer, to have higher disease specificity and to exhibit clinical
relevance in clinical RCC samples, making them ideal candidates for
RCC diagnosis (19,33,34).
In clinical practice, when conducting a pathological
diagnosis of a renal tumor biopsy, it is often impossible to obtain
sufficient tissue for a definitive pathological diagnosis. However,
if the circWWC3 expression level in tissues is measured during this
process, it will likely help pathologists diagnose tissues and
improve diagnostic accuracy. The circWWC3 expression level is
associated with pathological grade and T stage. High circWWC3
indicates a higher pathological grade and a greater probility of
T3/T4 stage disease. However, there were a limited number of
samples in the present study, and the inclusion of further patient
samples is required in future investigations to study the
association between circWWC3 and T stage progression. In addition,
circWWC3 expression is significantly associated with the overall
survival for patients with ccRCC; therefore, it can also be used as
a prognostic indicator.
In conclusion, the present clinical study was
conducted to evaluate the prognostic value of circWWC3 in patients
with ccRCC. It was observed that circWWC3 could be a novel
prognostic biomarker due to the longer survival time of patients
with low circWWC3 expression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XN provided all patient information and experimental
results, revised the manuscript critically and given final approval
of the version to be published. HW and XM performed the statistical
analysis and wrote the manuscript. SL performed the statistical
analysis and plotted the tables and images. All the authors have
read and approved the final manuscript. HW and XN confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of the Fourth Hospital of Hebei Medical University
(Shijiazhuang, China). All participants provided written informed
consent before inclusion in the study.
Patient consent for publication
The patients provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yicong Y, Wang Y, Denglong W and Baoying
H: Increased CDC6 expression associated with poor prognosis in
patients with clear cell renal cell carcinoma. Front Oncol.
11:6664182021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jing J, Zhao X, Wang J and Li T: Potential
diagnostic and prognostic value and regulatory relationship of long
noncoding RNA CCAT1 and miR-130a-3p in clear cell renal cell
carcinoma. Cancer Cell Int. 21:682021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao C, Ma Q, Huang X, Li A, Liu J, Ye J
and Gui Y: Targeted demethylation of the PLOD2 mRNA inhibits the
proliferation and migration of renal cell carcinoma. Front Mol
Biosci. 8:6756832021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu G, Wang Q, Xu Y, Li J, Zhang H, Qi G
and Xia Q: Targeting the transcription factor receptor LXR to treat
clear cell renal cell carcinoma: Agonist or inverse agonist? Cell
Death Dis. 10:4162019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui C, Yang J, Li X, Liu D, Fu L and Wang
X: Functions and mechanisms of circular RNAs in cancer radiotherapy
and chemotherapy resistance. Mol Cancer. 19:582020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao B, Zhang Q, Yang Z, An F, Nie H, Wang
H, Yang C, Sun J, Chen K, Zhou J, et al: CircEZH2/miR-133b/IGF2BP2
aggravates colorectal cancer progression via enhancing the
stability of m6A-modified CREB1 mRNA. Mol Cancer.
21:1402022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Yang T and Xiao J: Circular RNAs:
Promising biomarkers for human diseases. EBioMedicine. 34:267–274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei W, Ji L, Duan W and Zhu J: Circular
RNA circ_0081001 knockdown enhances methotrexate sensitivity in
osteosarcoma cells by regulating miR-494-3p/TGM2 axis. J Orthop
Surg Res. 16:502021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Chou X, Zhuang M, Zhu C, Hu Y,
Cheng D and Liu Z: circKMT2D contributes to H2O2-attenuated
osteosarcoma progression via the miR-210/autophagy pathway. Exp
Ther Med. 20:652020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beilerli A, Gareev I, Beylerli O, Yang G,
Pavlov V, Aliev G and Ahmad A: Circular RNAs as biomarkers and
therapeutic targets in cancer. Semin Cancer Biol. 83:242–252. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y,
Huang K, Wang G, Wang J, Ma J, et al: Circular RNA circTADA2A
promotes osteosarcoma progression and metastasis by sponging
miR-203a-3p and regulating CREB3 expression. Mol Cancer. 18:732019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rong X, Han Q, Lin X, Kremerskothen J and
Wang E: FRMPD1 activates the Hippo pathway via interaction with
WWC3 to suppress the proliferation and invasiveness of lung cancer
cells. Cancer Manag Res. 11:3395–3410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Q, Rong X, Wang E and Liu S: WW and
C2 domain-containing protein-3 promoted EBSS-induced
apoptosis through inhibiting autophagy in non-small cell lung
cancer cells. J Thorac Dis. 12:4205–4215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Zhang J, Zheng T, Mou X and Xin W:
Circ_WWC3 overexpression decelerates the progression of
osteosarcoma by regulating miR-421/PDE7B axis. Open Life Sci.
16:229–241. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C and Li X: Antitumor Activity of
lncRNA NBAT-1 via Inhibition of miR-4504 to Target to WWC3 in
oxaliplatin-resistant colorectal carcinoma. J Healthc Eng.
2022:91215542022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Jiang M, Yao Y and Cai Z: WWC3
inhibits glioma cell proliferation through suppressing the
Wnt/β-catenin signaling pathway. DNA Cell Biol. 37:31–37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng L, Liu S, Liu F and Sang M, Ju Y, Fan
X, Gu L, Li Z, Geng C and Sang M: ZEB1-mediated transcriptional
upregulation of circWWC3 promotes breast cancer progression through
activating Ras signaling pathway. Mol Ther Nucleic Acids.
22:124–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou F, Wang D, Wei W, Chen H, Shi H, Zhou
N, Wu L and Peng R: Comprehensive profiling of circular RNA
expressions reveals potential diagnostic and prognostic biomarkers
in multiple myeloma. BMC Cancer. 20:402020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Zhang Y, Wang P, Fu X and Lin W:
Circular RNAs in renal cell carcinoma: implications for
tumorigenesis, diagnosis, and therapy. Mol Cancer. 19:1492020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Zou X, Zou J and Zhang G:
Functions of circular RNAs in bladder, prostate and renal cell
cancer (Review). Mol Med Rep. 23:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma C, Qin J, Zhang J, Wang X, Wu D and Li
X: Construction and analysis of circular RNA molecular regulatory
networks in clear cell renal cell carcinoma. Mol Med Rep.
21:141–150. 2020.PubMed/NCBI
|
|
22
|
Feng W, Guo R, Zhang D and Zhang R:
Circ-ABCB10 knockdown inhibits the malignant progression of
cervical cancer through microRNA-128-3p/ZEB1 axis. Biol Proced
Online. 23:172021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erdoğan F, Demirel A and Polat O:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Int J Clin Pract. 58:333–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delahunt B, Eble JN, Egevad L and
Samaratunga H: Grading of renal cell carcinoma. Histopathology.
74:4–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Deng X and Zhang J: Identification
of dysregulated serum miR-508-3p and miR-885-5p as potential
diagnostic biomarkers of clear cell renal carcinoma. Mol Med Rep.
20:5075–5083. 2019.PubMed/NCBI
|
|
26
|
El-Deiry WS, Taylor B and Neal JW: Tumor
evolution, heterogeneity, and therapy for our patients with
advanced cancer: How far have we come? Am Soc Clin Oncol Educ Book.
37:e8–e15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gui CP, Liao B, Luo CG, Chen YH, Tan L,
Tang YM, Li JY, Hou Y, Song HD, Lin HS, et al: circCHST15 is a
novel prognostic biomarker that promotes clear cell renal cell
carcinoma cell proliferation and metastasis through the
miR-125a-5p/EIF4EBP1 axis. Mol Cancer. 20:1692021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang MX, Wang JL, Mo CQ, Mao XP, Feng ZH,
Li JY, Lin HS, Song HD, Xu QH, Wang YH, et al: CircME1 promotes
aerobic glycolysis and sunitinib resistance of clear cell renal
cell carcinoma through cis-regulation of ME1. Oncogene.
41:3979–3990. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang Y, Su X, Qiu Q, Yuan Y, Weng
C, Zou S, Tian Y, Han W, Liu P, et al: Circular RNA circDVL1
inhibits clear cell renal cell carcinoma progression through the
miR-412-3p/PCDH7 axis. Int J Biol Sci. 18:1491–1507. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Downs TM, Schultzel M, Shi H, Sanders C,
Tahir Z and Sadler GR: Renal cell carcinoma: Risk assessment and
prognostic factors for newly diagnosed patients. Crit Rev Oncol
Hematol. 70:59–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miller K, Bergmann L, Doehn C, Grünwald V,
Gschwend JE, Ivanyi P and Kuczyk MA: Interdisciplinary
recommendations for the treatment of advanced renal cell carcinoma.
Aktuel Urol. 53:403–415. 2022.(In German). PubMed/NCBI
|
|
32
|
Babin L, Andraos E, Fuchs S, Pyronnet S,
Brunet E and Meggetto F: From circRNAs to fusion circRNAs in
hematological malignancies. JCI Insight. 6:e1515132021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang G, Liang M, Liu H, Huang J, Li P,
Wang C, Zhang Y, Lin Y and Jiang X: CircRNA hsa_circRNA_104348
promotes hepatocellular carcinoma progression through modulating
miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell
Death Dis. 11:10652020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng X, Huang M, Xing L, Yang R, Wang X,
Jiang R, Zhang L and Chen J: The circRNA circSEPT9 mediated by E2F1
and EIF4A3 facilitates the carcinogenesis and development of
triple-negative breast cancer. Mol Cancer. 19:732020. View Article : Google Scholar : PubMed/NCBI
|