Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the common malignant tumors threatening human health in China
(1). Due to the lack of specific
symptoms in early esophageal cancer and the low rate of gastroscopy
in China, most patients lose the opportunity for surgery at the
time of diagnosis, and the 5-year survival rate is poor (2). With the major breakthrough of
immunotherapy in the treatment of cancer, esophageal cancer therapy
has entered a new era. The significant efficacy of immunotherapy
was demonstrated in the second-line treatment of esophageal cancer
in several studies (3–6). Moreover, the results of other studies
(7–10) further indicated that first-line
immunotherapy combined with chemotherapy could significantly
prolong survival time in patients with metastatic esophageal
cancer, providing a strong basis for the use of first-line
immunotherapy for esophageal cancer. However, there are still a
considerable number of patients with primary or acquired
resistance. To date, certain biomarkers, such as programmed cell
death-ligand 1 (PDL-1), mismatch repair defects (MMR) and tumor
mutation burden (TMB), have been frequently used to select the
population that would best benefit from treatment (11–13).
However, they are not ideal biomarkers owing to the differences in
detection platforms and cutoff values, as well as the lack of
sufficient tumor tissues to perform testing. There is therefore an
urgent clinical need to explore biomarkers related to the efficacy
of tumor immunotherapy.
A certain degree of malnutrition is present in a
considerable number of patients with esophageal cancer. In recent
years, more studies (14,15) have emphasized the importance of the
nutritional and immune statuses in patients with tumors, indicating
that they play an important role in tumorigenesis, evolution and
prognosis. In clinical practice, albumin (ALB), body mass index
(BMI) and total lymphocyte count are often adopted to evaluate the
nutritional status of patients with tumors. Buzby et al
(16) first put forward the concept
of the prognostic nutritional index (PNI), which is obtained by
combining serum ALB level and peripheral blood lymphocyte count,
and can to some extent reflect the nutritional and immune statuses
of patients with tumors. Some studies (17–19)
have reported that PNI is closely associated with the response and
survival prognosis of esophageal cancer, metastatic non-small cell
lung cancer and colorectal cancer. In addition, since PNI has the
characteristic of being non-invasive and can be dynamically
monitored, it has a potential clinical application value. However,
whether nutritional indicators can predict the therapeutic efficacy
and prognosis of patients with metastatic ESCC treated with
immunotherapy is still unknown. Therefore, the present study
explores the association between nutritional indicators and the
efficacy and prognostic value of immunotherapy in patients with
metastatic ESCC, in order to further guide clinical practice.
Patients and methods
Patient selection
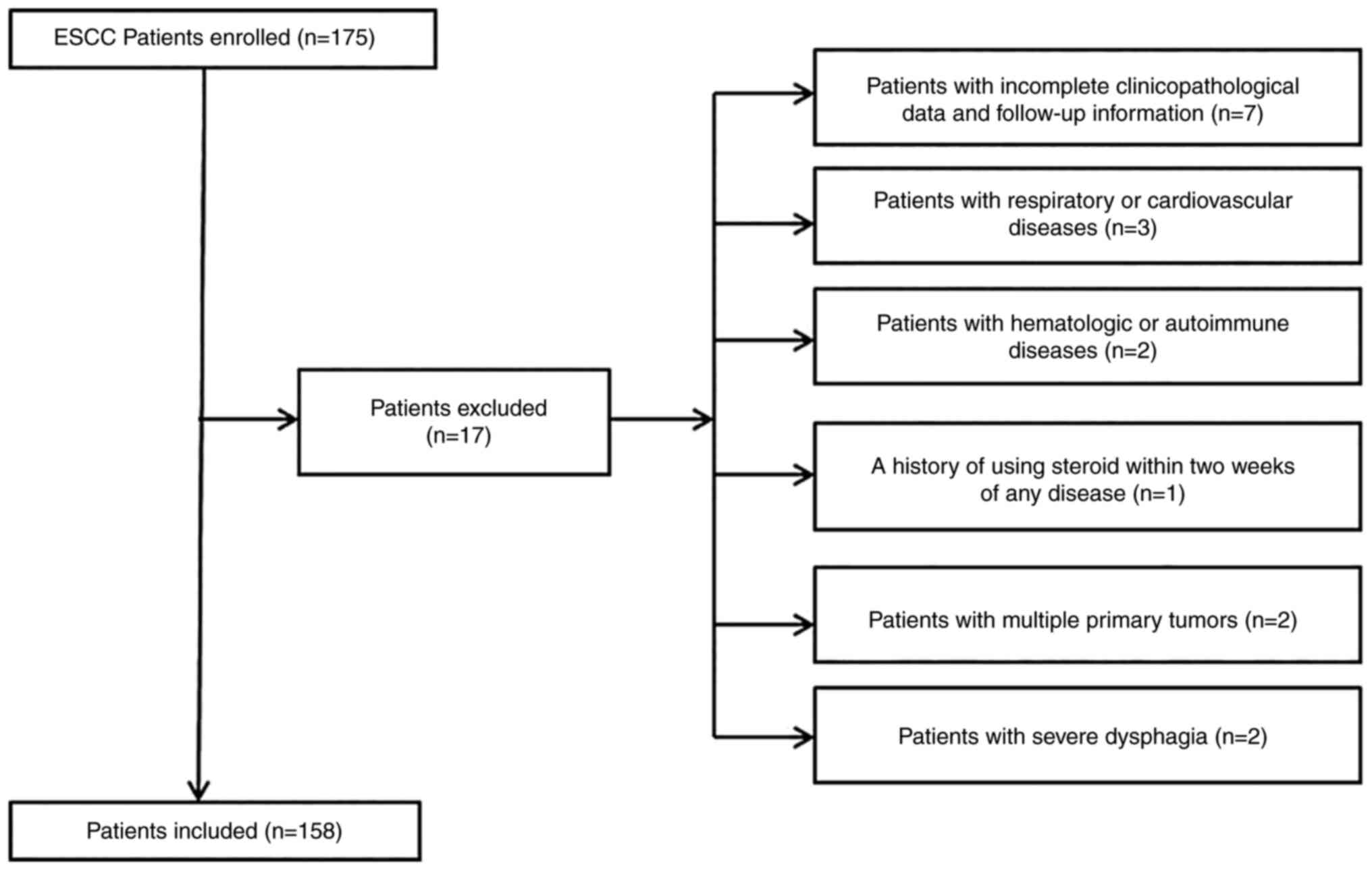
The clinical data of 158 patients with metastatic
ESCC treated with camrelizumab in The Affiliated Xinghua People's
Hospital, Medical School of Yangzhou University (Xinghua, China)
between September 2019 and July 2022 was collected. The inclusion
criteria were as follows: i) Patients >18 years old; ii)
patients who were pathologically diagnosed with ESCC; iii) patients
who were treated with at least 3 cycles of camrelizumab; iv) an
Eastern Cooperative Oncology Group Performance Status (20) score of 0–2; v) patients with
complete evaluable imaging data and peripheral hematological
parameters (peripheral lymphocyte count and serum ALB level) before
treatment; and vi) patients at clinical stage IV, according to the
eighth edition of the American Joint Committee on Cancer staging
(21) manual. The exclusion
criteria were as follows: i) Patients with incomplete
clinicopathological data and follow-up information; ii) patients
with hematological or autoimmune diseases; iii) patients with
respiratory or cardiovascular diseases; iv) patients with a history
of using steroid within 2 weeks of any disease; v) patients with
multiple primary tumors; and vi) patients with severe dysphagia
before treatment. A total of 17 patients were excluded and 158
patients were eventually enrolled. The whole enrollment process is
presented in Fig. 1. The present
study was approved by the Ethics Committee (protocol number:
JSXHRYLL-NK-201901) of the Medical School of Yangzhou University
(Xinghua, China). The last follow-up was performed on July 20,
2022.
Evaluation of efficacy and definition
of PNI and BMI
A low-dose computed tomography scan and barium enema
examination were performed before treatment and every 8 weeks after
treatment. The Response Evaluation Criteria in Solid Tumors (RECIST
version 1.1) (22) were adopted to
evaluate response and efficacy. Three radiologists were asked to
conduct efficacy evaluations for every patient. The primary
endpoint of the study was OS time, which was defined as the time
from initial treatment to death from any cause. For patients who
had been lost to follow-up before death, the last follow-up time
was considered as the equivalent to time of death. The secondary
endpoint was PFS time, which was defined as the time from the start
of treatment to disease progression or death from any cause.
Immune-related adverse events (irAEs) were diagnosed using clinical
practice guidelines (23).
PNI was defined as serum ALB (g/l) plus five times
the total count of peripheral blood lymphocytes (109/l).
According to the Guidelines for the Prevention and Control of
Overweight and Obesity in Chinese Adults (24), BMI is defined as the weight before
treatment divided by the square of the height, namely weight
(kg)/height (m2). A Beckman AU Series AU5800 instrument
(Beckman Coulter, Inc.) and a Xisen Meikang XN9100 blood analysis
instrument [Xisen Meikang Medical Electronics (Shanghai) Co. Ltd.]
were used to perform peripheral blood testing. In order to ensure
the accuracy of the ALB level and platelet count, internal quality
control was performed once a day and the external quality
assessment of Jiangsu Province Clinical Examination Center and the
National Health Commission Clinical Inspection Center was
participated in every half a year.
Statistical analysis
The optimal cut-off values for PNI and ALB were
determined by receiver operating characteristic (ROC) curve. The
χ2 test was used to analyze the clinicopathological data
between high and low PNI/ALB/BMI groups. Survival analyses between
groups were performed using the Kaplan-Meier method and the
log-rank test. Univariate and multivariate Cox proportional hazard
models were used to evaluate the prognostic value of related
variables. All statistical analyses were two-sided probability
tests (α=0.05) and P<0.05 was considered to indicate a
statistically significant difference. The statistical analyses were
performed using IBM SPSS Statistic 26.0 (IBM Corp.).
Results
Patient characteristics
A total of 158 patients with metastatic ESCC
receiving camrelizumab treatment were enrolled in this study. The
median age at the time of diagnosis was 67 years (range, 52–87
years). The proportion of male patients was 63.9%. Patients with a
drinking history accounted for 60.1% of all participants. A total
of 50 patients (31.6%) had received first-line camrelizumab plus
paclitaxel and cisplatin treatment. A total of 108 patients (68.4%)
had received at least first-line treatment with paclitaxel plus
cisplatin. Monotherapy was administered to 38.6% of the patients
and combined chemotherapy was administered to 61.4% of the
patients. The proportion of well-differentiated, moderately
differentiated and poorly differentiated ESCC was 18.5, 51.3 and
32.3%, respectively. All patients were of clinical stage IV. The
median PNI and ALB values before treatment were 44.3 (range,
34.5-55.4) and 39.3 g/l (range, 30.5-47.6 g/l), respectively. By
July 2022, the median follow-up time was 11.1 months (range,
2.0-26.2 months).
Determination of optimal cut-off
values for PNI, ALB and BMI
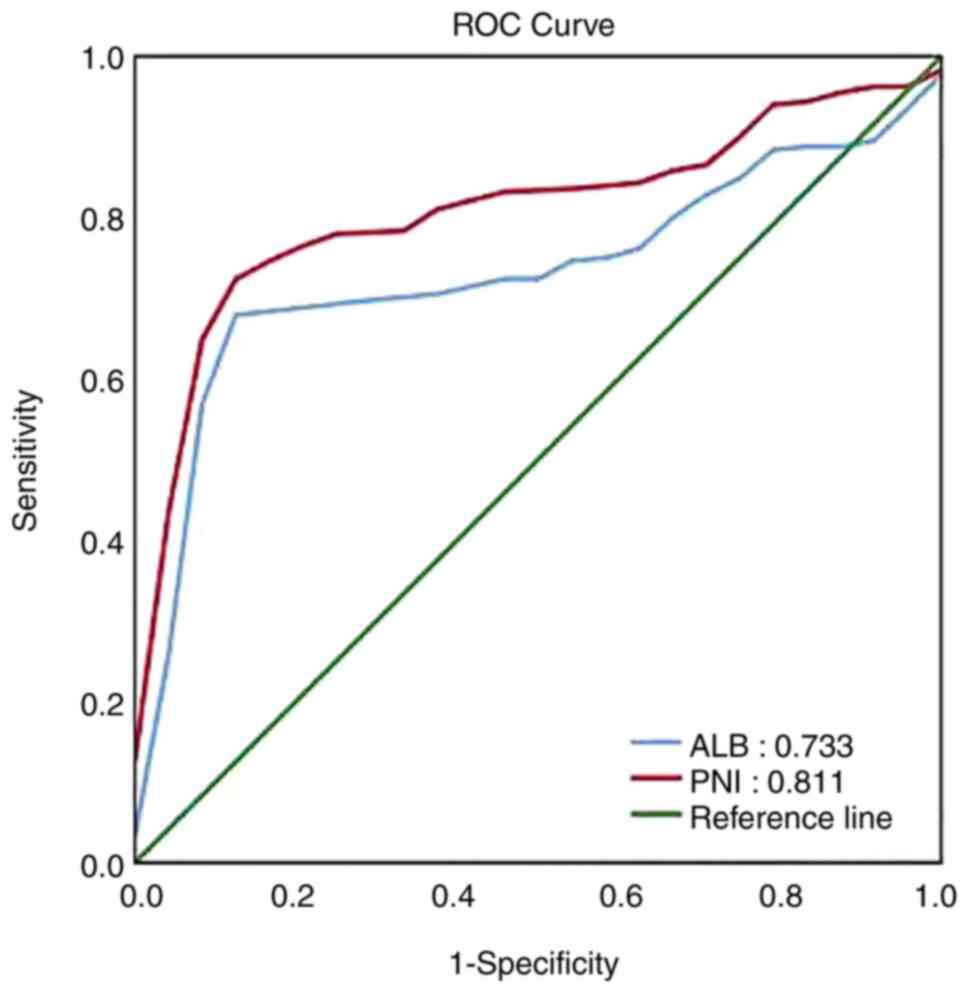
As shown in Fig. 2,
the area under the ROC curve for PNI and ALB was 0.811 and 0.733,
respectively. The optimal cut-off values for PNI and ALB, which
were calculated by ROC curve, were 41.35 and 36.8 g/l,
respectively. Since the normal range of recognized BMI is 18.5-23.9
kg/m2, the cut-off value for BMI was set at the normal
lower limit (18.5 kg/m2). Patients were separately
divided into high and low PNI/ALB/BMI groups based on the optimal
cut-off values.
Associations between the
clinicopathological parameters and PNI, ALB and BMI
The associations between the clinicopathological
parameters of the patients and the PNI, ALB and BMI values are
presented in Table I. PNI, ALB and
BMI before treatment were significantly associated with the
treatment regimen (P=0.001, P=0.002 and P=0.002, respectively).
There were no significant differences with regard to the
associations between PNI, ALB and BMI before treatment and age,
sex, drinking history, ECOG PS, therapy line, differentiation and
esophageal cancer location.
 | Table I.Associations between PNI, ALB and BMI
and the clinical characteristics of the patients with metastatic
esophageal squamous cell carcinoma. |
Table I.
Associations between PNI, ALB and BMI
and the clinical characteristics of the patients with metastatic
esophageal squamous cell carcinoma.
|
|
| PNI | ALB, g/l | BMI,
kg/m2 |
|---|
|
|
|
|
|
|
|---|
| Variable | Cases, n | >41.35, n | ≤41.35, n | P-value | >36.8, n | ≤36.8, n | P-value | ≥18.5, n | <18.5, n | P-value |
|---|
| Total patients | 158 | 97 | 61 |
| 96 | 62 |
| 87 | 71 |
|
| Age, years |
|
|
| 0.923 |
|
| 0.769 |
|
| 0.686 |
|
>65 | 94 | 58 | 36 |
| 58 | 36 |
| 53 | 41 |
|
|
≤65 | 64 | 39 | 25 |
| 38 | 26 |
| 34 | 30 |
|
| Sex |
|
|
| 0.306 |
|
| 0.643 |
|
| 0.229 |
|
Male | 101 | 59 | 42 |
| 60 | 41 |
| 52 | 49 |
|
|
Female | 57 | 38 | 19 |
| 36 | 21 |
| 35 | 22 |
|
| Drinking
history |
|
|
| 0.659 |
|
| 0.926 |
|
| 0.451 |
|
Yes | 95 | 57 | 38 |
| 58 | 37 |
| 50 | 45 |
|
| No | 63 | 40 | 23 |
| 38 | 25 |
| 37 | 26 |
|
| ECOG PS |
|
|
| 0.283 |
|
| 0.225 |
|
| 0.641 |
| 0 | 68 | 45 | 23 |
| 45 | 23 |
| 36 | 32 |
|
|
1-2 | 90 | 52 | 38 |
| 51 | 39 |
| 51 | 39 |
|
| Therapy lines,
n |
|
|
| 0.246 |
|
| 0.57 |
|
| 0.598 |
| 1 | 50 | 34 | 16 |
| 32 | 18 |
| 26 | 24 |
|
| ≥2 | 108 | 63 | 45 |
| 64 | 44 |
| 61 | 47 |
|
| Regimen |
|
|
| 0.001 |
|
| 0.002 |
|
| 0.002 |
|
Monotherapy | 61 | 26 | 35 |
| 28 | 33 |
| 24 | 37 |
|
|
Combination therapy | 97 | 71 | 26 |
| 68 | 29 |
| 63 | 34 |
|
|
Differentiation |
|
|
| 0.649 |
|
| 0.511 |
|
| 0.402 |
|
Well | 26 | 14 | 12 |
| 14 | 12 |
| 13 | 13 |
|
|
Moderate | 81 | 50 | 31 |
| 48 | 33 |
| 42 | 39 |
|
|
Poor | 51 | 33 | 18 |
| 34 | 17 |
| 32 | 19 |
|
| Esophageal cancer
location |
|
|
| 0.349 |
|
| 0.109 |
|
| 0.515 |
|
Upper | 29 | 16 | 13 |
| 14 | 15 |
| 18 | 11 |
|
|
Middle | 77 | 45 | 32 |
| 45 | 32 |
| 39 | 38 |
|
|
Lower | 52 | 36 | 16 |
| 37 | 15 |
| 30 | 22 |
|
Survival analyses
Survival curves for PFS and OS were plotted using
the Kaplan-Meier method, and the log-rank test was used to compare
the differences between the groups. The PFS and OS times in the
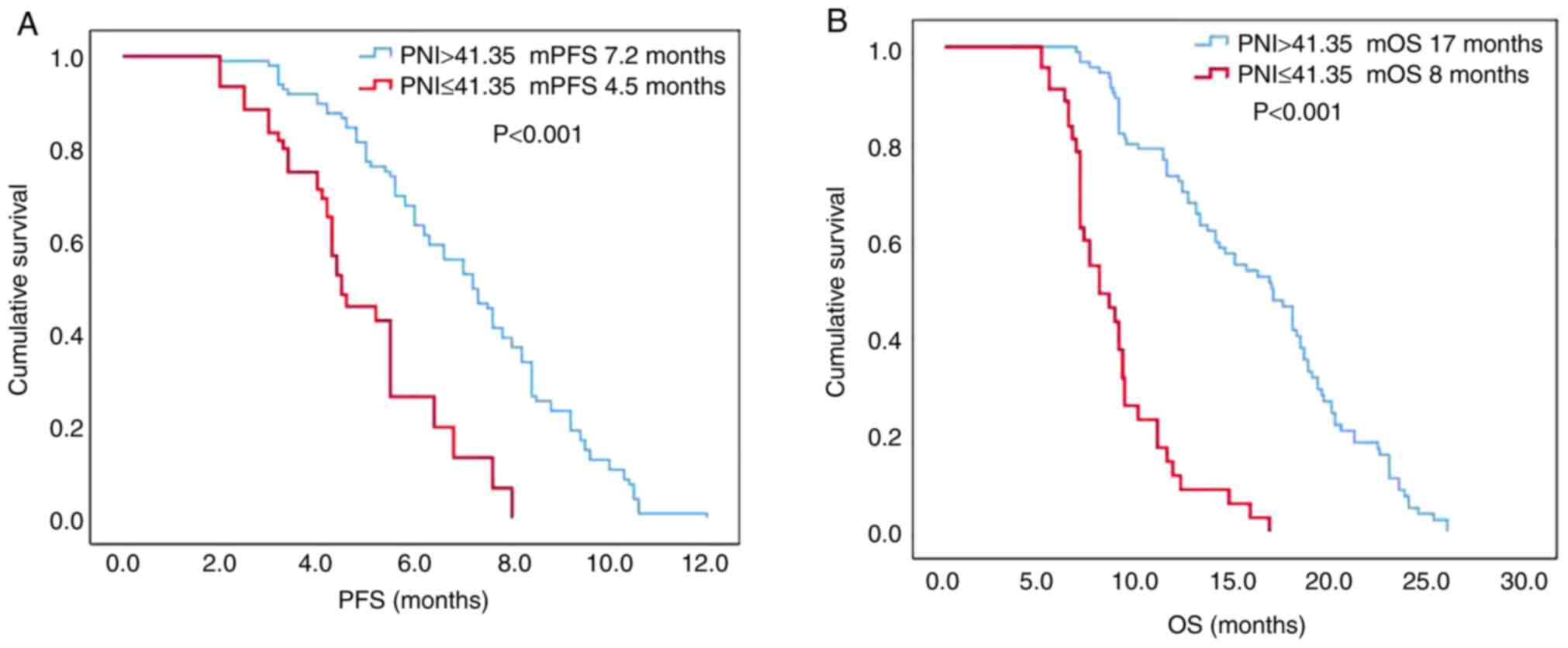
high PNI group before treatment were significantly longer than
those in the low PNI group [median (m)PFS: 7.2 vs. 4.5 months,
respectively; P<0.001; mOS: 17 vs. 8 months, respectively;
P<0.001; Fig. 3A and B). The PFS
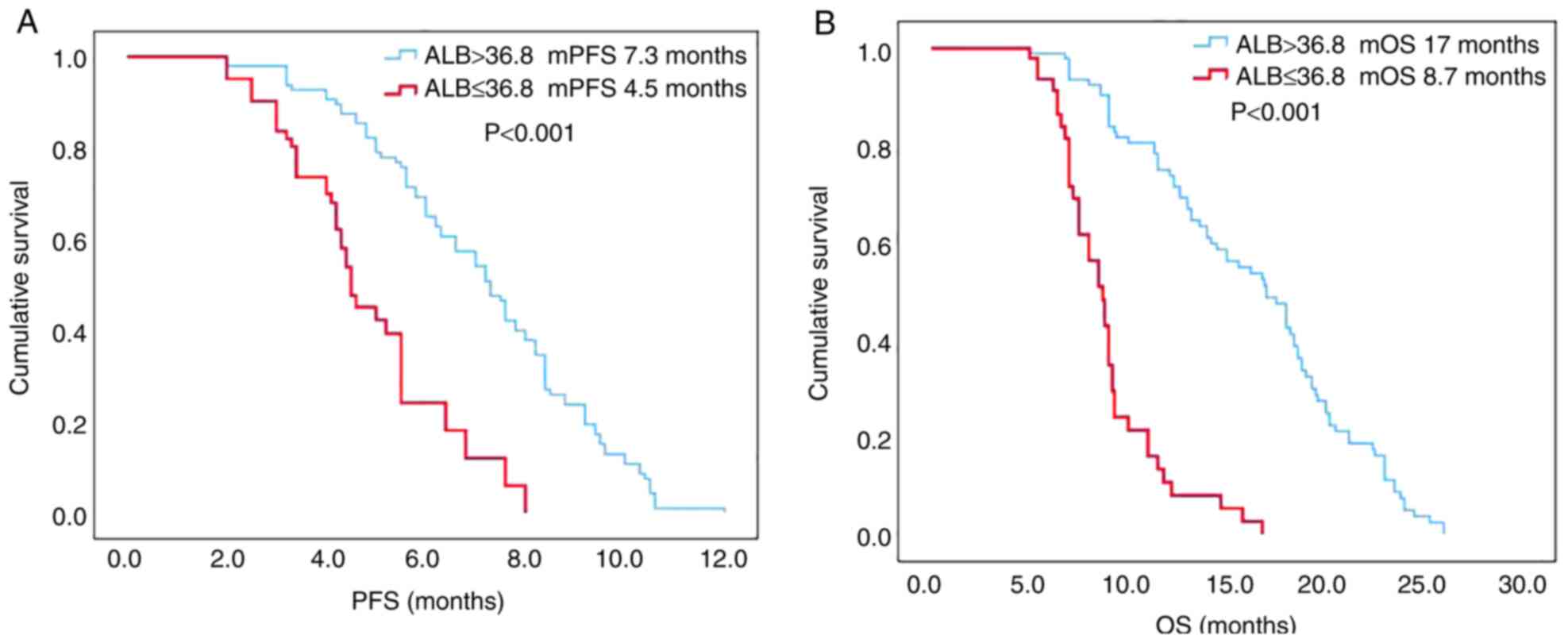
and OS times in the high ALB group before treatment were
significantly longer than those in the low ALB group (mPFS: 7.3 vs.
4.5 months, respectively; P<0.001; mOS: 17 vs. 8.7 months,
respectively; P<0.001; Fig. 4A and
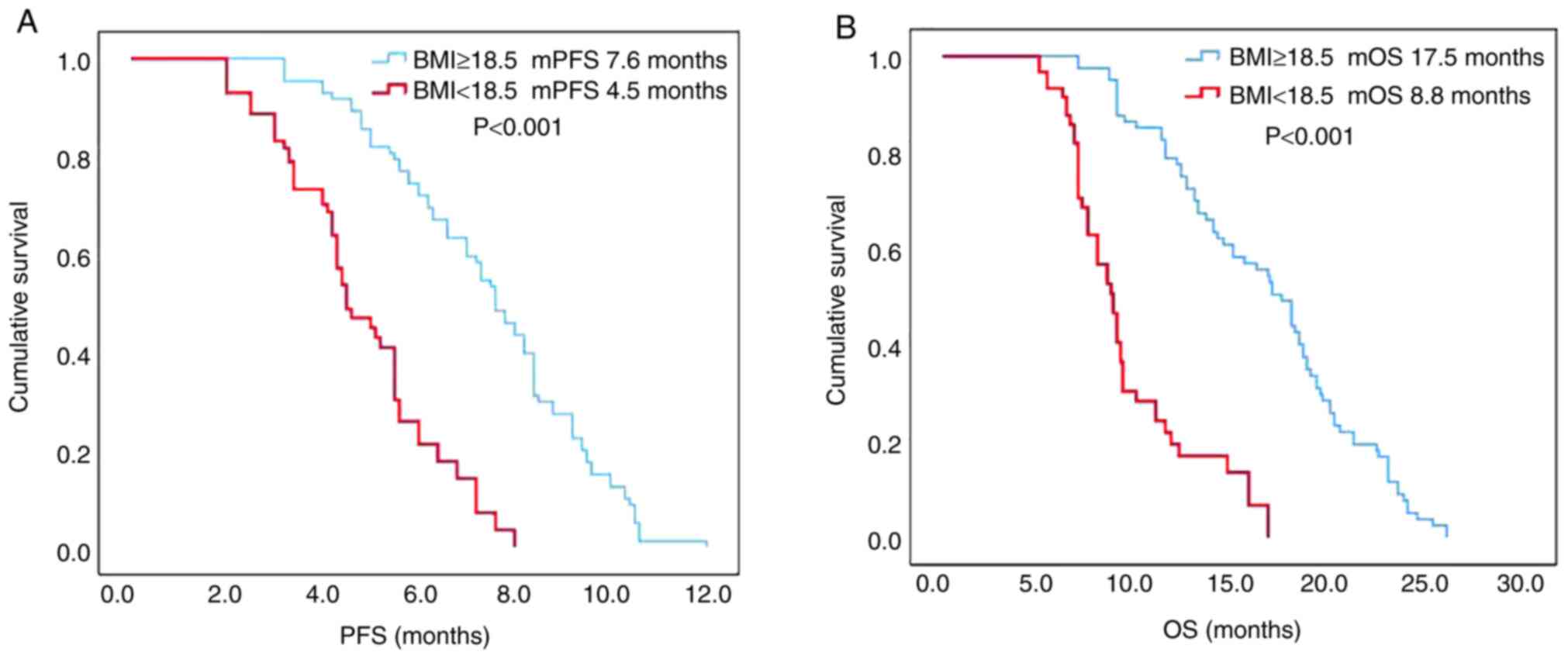
B). The PFS and OS times in the high BMI group before treatment
were significantly longer than those in the low BMI group (mPFS:
7.6 vs. 4.5 months, respectively; P<0.001; mOS: 17.5 vs. 8.8
months, respectively; P<0.001; Fig.
5A and B).
Univariate and multivariate
analyses
The prognostic values of PNI, ALB and BMI were
further evaluated by Cox proportional hazards model. The results of
the univariate and multivariate analyses of PFS and OS are shown in
Tables II and III, respectively. Univariate analyses
indicated that the high PNI, ALB and BMI groups before treatment
were associated with longer PFS and OS times (all P<0.001). To
avoid the multicollinearity among PNI, ALB and BMI, in the
multivariate analyses, three independent Cox models were separately
constructed. Each model included only one of the three variables.
The multivariate analyses indicated that PNI, ALB and BMI were
independent prognostic factors (all P<0.001) for survival
outcomes in patients with metastatic ESCC treated with
camrelizumab.
 | Table II.Univariate and multivariate analyses
of progression-free survival in patients with metastatic esophageal
squamous cell carcinoma treated with camrelizumab. |
Table II.
Univariate and multivariate analyses
of progression-free survival in patients with metastatic esophageal
squamous cell carcinoma treated with camrelizumab.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>65 vs. ≤65
years) | 0.975
(0.690–1.376) | 0.884 |
|
|
| Sex (male vs.
female) | 1.067
(0.747–1.522) | 0.722 |
|
|
| Drinking history
(yes vs. no) | 1.107
(0.778–1.575) | 0.572 |
|
|
| ECOG PS (0 vs.
1–2) | 0.925
(0.653–1.311) | 0.663 |
|
|
| Therapy lines (1
vs. ≥2) | 0.997
(0.697–1.426) | 0.988 |
|
|
| Regimen
(monotherapy vs. combination therapy) | 0.839
(0.585–1.204) | 0.342 |
|
|
| Differentiation
(well vs. moderate vs. poor) | 1.026
(0.788–1.335) | 0.850 |
|
|
| Tumor location
(upper vs. middle vs. lower) | 1.044
(0.819–1.331) | 0.725 |
|
|
| PNI (>41.35 vs.
≤41.35) | 3.294
(2.114–5.133) | <0.001 | 3.599
(2.233–5.800) | <0.001 |
| ALB (>36.8 vs.
≤36.8) | 3.615
(2.323–5.627) | <0.001 | 4.148
(2.564–6.711) | <0.001 |
| BMI (≥18.5 vs.
<18.5) | 4.564
(2.938–7.088) | <0.001 | 5.623
(3.419–9.249) | <0.001 |
 | Table III.Univariate and multivariate analyses
of overall survival in patients with metastatic esophageal squamous
cell carcinoma treated with camrelizumab. |
Table III.
Univariate and multivariate analyses
of overall survival in patients with metastatic esophageal squamous
cell carcinoma treated with camrelizumab.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>65 vs. ≤65
years) | 1.209
(0.842–1.735) | 0.304 |
|
|
| Sex (male vs.
female) | 1.278
(0.885–1.845) | 0.192 |
|
|
| Drinking history
(yes vs. no) | 1.285
(0.890–1.854) | 0.180 |
|
|
| ECOG PS (0 vs.
1–2) | 0.999
(0.695–1.438) | 0.998 |
|
|
| Therapy lines (1
vs. ≥2) | 1.258
(0.853–1.854) | 0.247 |
|
|
| Regimen
(monotherapy vs. combination therapy) | 0.821
(0.562–1.199) | 0.307 |
|
|
| Differentiation
(well vs. moderate vs. poor) | 1.091
(0.831–1.434) | 0.530 |
|
|
| Tumor location
(upper vs. middle vs. lower) | 0.957
(0.739–1.239) | 0.739 |
|
|
| PNI (>41.35 vs.
≤41.35) | 6.168
(3.862–9.848) | <0.001 | 7.605
(4.460–12.969) | <0.001 |
| ALB (>36.8 vs.
≤36.8) | 6.406
(3.996–10.271) | <0.001 | 7.852
(4.616–13.358) | <0.001 |
| BMI (≥18.5 vs.
<18.5) | 5.499
(3.438–8.797) | <0.001 | 7.915
(4.597–13.626) | <0.001 |
Analyses of immune-related adverse
events
irAEs occurred in 141 participants (89.2%).
Categories and grades of irAEs for the different treatment regimens
are displayed in Table IV.
Reactive cutaneous capillary endothelial proliferation was one of
the most common irAEs. Grade 3–4 irAEs were rare in the
camrelizumab group, while in the camrelizumab plus chemotherapy
group, grade 3–4 adverse events such as fatigue, thrombocytopenia,
anemia and leukopenia were markedly increased, which is likely
related to the chemotherapy. Most of the irAEs were mild and
controllable in the two groups. There were no irAEs leading to
treatment termination or death.
 | Table IV.Summary of immune-related adverse
events (n=158). |
Table IV.
Summary of immune-related adverse
events (n=158).
|
| Camrelizumab +
chemotherapy (n=97) | Camrelizumab
(n=61) |
|---|
|
|
|
|
|---|
| irAE category | Any grade | Grade 3–4 | Any grade | Grade 3–4 |
|---|
| RCCEP | 80 (82.5) | 10 (10.3) | 51 (83.6) | 3 (4.9) |
| ALT increase | 32 (33.0) | 4 (4.1) | 9 (14.8) | 0 (0.0) |
| AST increase | 33 (34.0) | 6 (6.2) | 8 (13.1) | 0 (0.0) |
| Increased blood
bilirubin | 12 (12.4) | 2 (2.1) | 3 (4.9) | 0 (0.0) |
| Hypothyroidism | 11 (11.3) | 0 (0.0) | 7 (11.5) | 0 (0.0) |
| Fatigue | 31 (32.0) | 0 (0.0) | 5 (8.2) | 0 (0.0) |
| Anemia | 69 (71.1) | 18 (18.6) | 8 (13.1) | 0 (0.0) |
| Proteinuria | 6 (6.2) | 0 (0.0) | 2 (3.3) | 0 (0.0) |
| Leukopenia | 73 (75.3) | 35 (36.1) | 6 (9.8) | 0 (0.0) |
|
Thrombocytopenia | 31 (32.0) | 17 (17.5) | 0 (0.0) | 0 (0.0) |
| Rash | 13 (13.4) | 3 (3.1) | 4 (6.6) | 0 (0.0) |
Discussion
Previously, the main treatments for patients with
esophageal cancer such as surgery, chemoradiotherapy and molecular
targeted therapy failed to significantly prolong survival time
(25). Recently, immunotherapy has
achieved marked efficacy in such patients and has become one of the
standard treatments for esophageal cancer. Some studies have
demonstrated the sustained response and long-term survival benefits
of immunotherapy in advanced oesophageal cancer, metastatic
non-small-cell lung cancer and microsatellite-instability-high
advanced colorectal cancer (6,11,12).
However, only a small proportion of patients benefit from this
treatment, and knowing how to identify the populations that would
receive the greatest benefit is still an urgent problem to be
solved. Therefore, it is of great clinical importance to explore
biomarkers that predict the best efficacy of immunotherapy. In
addition, more than half of all malignant tumor patients exhibit
nutritional risk at the time of diagnosis and subsequent treatment
(26). Pan et al (26) reported that malnutrition and
nutritional risk were common problems affecting the efficacy of
treatment for patients with cancer in China during hospitalization.
A retrospective analysis of 158 patients with metastatic ESCC
treated with camrelizumab was conducted in the present study. It
was found that low PNI, ALB and BMI values were independent risk
factors for survival outcomes in patients with metastatic ESCC who
underwent treatment with camrelizumab.
Although the tumor microenvironment is crucial in
the selection of immunotherapy biomarkers, other factors of the
host, especially nutrition and immune status, cannot be ignored.
The level of serum ALB is a common biomarker to evaluate the
nutritional status of a patient (27). Low ALB level reflects the poor
nutritional status of the body, weakens the body's cellular
immunity, humoral immunity and other defense mechanisms, and is
associated with a poor prognosis in patients with tumors (28). ALB is produced by hepatocytes and
regulated by various pro-inflammatory cytokines such as
interleukin-1 (IL-1), IL-6 and tumor necrosis factor-α (29,30).
ALB has been demonstrated to protect the host against tumorigenesis
by stabilizing cell growth and DNA replication, buffering changes
in various biochemical reactions, such as catalytic chemical
reactions, binding and dissolving various compounds, and
maintaining sex hormone homeostasis (31). Therefore, in a sense, ALB can
reflect the immune and inflammatory status of the host. A small
sample study (32) showed that ALB
level was an independent predictor of early mortality in patients
with esophageal cancer, and could be combined with other biomarkers
to provide prognostic information and guide treatment. The present
study demonstrated that ALB was associated with the prognosis of
immunotherapy in patients with metastatic ESCC. In a retrospective
study, Qi et al (33) found
that preoperative ALB is significantly associated with OS upon
univariate analysis, but not upon multivariate analysis, implying
that ALB is associated with prognosis, but is not an independent
prognostic factor. In the present study, ALB was an independent
risk factor for survival outcome in patients with metastatic ESCC
treated with camrelizumab. The differences between these findings
may be related to the differences in cut-off values, treatment
methods and baseline characteristics of the patients.
Lymphocytes, one of the basic components of cellular
immunity, inhibit the proliferation and invasion of tumor cells by
cytokine-mediated cytotoxicity (34). The decrease in lymphocytes before
treatment is a factor indicating a poor prognosis for patients with
cancer, which may be associated with the immunosuppressive
microenvironment of the host (35).
A previous study (34) showed that
lymphopenia is an independent prognostic factor for survival
outcomes in metastatic breast cancer, advanced soft-tissue sarcoma
and non-Hodgkin's lymphoma. PNI, which is calculated from serum ALB
and peripheral blood lymphocyte levels, is a comprehensive index
reflecting the nutritional and immune status of the host (36). This suggests that it has a potential
predictive value for immunotherapy in patients with cancer. A
retrospective analysis of 123 patients with advanced non-small cell
lung cancer treated with PD-1 inhibitors found that PNI was an
independent predictor for early progression and survival outcomes
(37). The results of two other
studies (33,38) revealed that preoperative PNI was an
independent prognostic factor for OS in patients with ESCC after
surgery and that it could be used as a biomarker to predict
survival outcomes. This association was also observed in the
present study.
BMI is one of the common standards used to measure
the degree of obesity and health, which can reflect the nutritional
status of the body. Research in China has revealed that a decrease
in preoperative BMI is significantly associated with poor
postoperative survival outcomes in patients with gastric cancer or
adenocarcinoma of the gastroesophageal junction (39). In another cohort study of 615
patients with ESCC who underwent esophagectomy or
chemoradiotherapy, a high BMI before treatment was found to be an
independent prognostic factor for long-term survival (40), which was similar to the present
results.
To the best of our knowledge, the association
between nutritional indicators and the efficacy and prognosis of
patients metastatic ESCC treated with immunotherapy is still
largely unknown. To the best of our knowledge, this association was
first observed in the present study. A previous study demonstrated
that some specific nutrients may be related to the etiology or
severity of cervical cancer (41),
which implied that the type of nutrition may affect the progression
of disease or treatment efficacy. In addition, ALB, BMI and PNI can
reflect the nutritional and immune status of the body, and it is
easy to conduct quality control for them in studies. Furthermore,
compared with the common biomarkers for predicting immunotherapy,
such as PDL-1, MMR and TMB, the nutritional indicators used in this
study were cost-effective and are easily available in clinical
practice. Since the specific nutrients were not taken into account
in this study, it may be an aim of future research. However, there
are also some limitations in the present study. Firstly, selection
bias is inevitable in the present study as it is a single-center,
small-sample, retrospective analysis. Therefore, further validation
of the findings is required in large-sample, multicenter,
prospective studies. Secondly, although the results are similar to
those of previous studies, due to the lack of a unified cut-off
value in various studies, it may be difficult to repeat the
conclusions in other experiments. Consequently, consistent cut-off
values need to be explored in subsequent studies.
In summary, the present study revealed that PNI, ALB
and BMI are effective predictors to evaluate the survival outcomes
in patients with metastatic ESCC treated with camrelizumab.
Furthermore, PNI, ALB and BMI may be independent prognostic factors
in these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and DL were responsible for study conception and
design. Administrative support and the χ2 test and Cox
proportional hazard models were conducted by GH and CZ. WX, JX, WZ
and PJ enrolled the study subjects and performed survival analyses
and the follow-up for all patients. JW collected and assembled the
data, and performed ROC curve analysis. Data analysis and
interpretation was performed by JL. The manuscript was written by
JL. JL, GH, CZ, JW, WX, JX, WZ, PJ and DL confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study involving human participants was reviewed
and approved by the Ethics Committee of the Medical School of
Yangzhou University (Xinghua, China). As it is a non-interventional
retrospective study, the requirement for informed consent was
waived. Privacy was maintained and identifiable information of all
patients was kept confidential and used in compliance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Y, Qin S, Luo S, Li Z, Cheng Y, Fan Y,
Sun Y, Yin X, Yuan X, Li W, et al: Pembrolizumab versus
chemotherapy for patients with esophageal squamous cell carcinoma
enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open.
7:1003412022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato K, Cho BC, Takahashi M, Okada M, Lin
CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al:
Nivolumab versus chemotherapy in patients with advanced oesophageal
squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:1506–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y,
Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al: Camrelizumab versus
investigator's choice of chemotherapy as second-line therapy for
advanced or metastatic oesophageal squamous cell carcinoma
(ESCORT): A multicentre, randomised, open-label, phase 3 study.
Lancet Oncol. 21:832–842. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal squamous-cell carcinoma.
N Engl J Med. 386:449–462. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reck M, Schenker M, Lee KH, Provencio M,
Nishio M, Lesniewski-Kmak K, Sangha R, Ahmed S, Raimbourg J, Feeney
K, et al: Nivolumab plus ipilimumab versus chemotherapy as
first-line treatment in advanced non-small-cell lung cancer with
high tumour mutational burden: Patient-reported outcomes results
from the randomised, open-label, phase III CheckMate 227 trial. Eur
J Cancer. 116:137–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayne ST, Playdon MC and Rock CL: Diet,
nutrition, and cancer: Past, present and future. Nat Rev Clin
Oncol. 13:504–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barreira JV: The role of nutrition in
cancer patients. Nutr Cancer. 73:2849–2850. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buzby GP, Mullen JL, Matthews DC, Hobbs CL
and Rosato EF: Prognostic nutritional index in gastrointestinal
surgery. Am J Surg. 139:160–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakatani M, Migita K, Matsumoto S,
Wakatsuki K, Ito M, Nakade H, Kunishige T, Kitano M and Kanehiro H:
Prognostic significance of the prognostic nutritional index in
esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis
Esophagus. 30:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bozkaya Y, Köstek O, Sakin A, Özyükseler
DT, Şakalar T and Çil İ: Is the prognostic nutritional index a
prognostic and predictive factor in metastatic non-small cell lung
cancer patients treated with first-line chemotherapy? Support Care
Cancer. 28:2273–2282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida
K and Kusunoki M: Prognostic nutritional index predicts
postoperative outcome in colorectal cancer. World J Surg.
37:2688–2692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (Eastern Cooperative Oncology Group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rice TW, Gress DM, Patil DT, Hofstetter
WL, Kelsen DP and Blackstone EH: Cancer of the esophagus and
esophagogastric junction-Major changes in the American Joint
Committee on Cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:304–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chalian H, Töre HG, Horowitz JM, Salem R,
Miller FH and Yaghmai V: Radiologic assessment of response to
therapy: Comparison of RECIST Versions 1.1 and 1.0. Radiographics.
31:2093–2105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brahmer JR, Abu-Sbeih H, Ascierto PA,
Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E,
Johnson DB, et al: Society for Immunotherapy of Cancer (SITC)
clinical practice guideline on immune checkpoint inhibitor-related
adverse events. J Immunother Cancer. 9:e0024352021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C and Lu FC: The guidelines for
prevention and control of overweight and obesity in Chinese adults.
Biomed Environ Sci. 17 Suppl:1–36. 2004.PubMed/NCBI
|
|
25
|
Wei W, Zeng H, Zheng R, Zhang S, An L,
Chen R, Wang S, Sun K, Matsuda T, Bray F and He J: Cancer
registration in China and its role in cancer prevention and
control. Lancet Oncol. 21:e342–e349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan H, Cai S, Ji J, Jiang Z, Liang H, Lin
F and Liu X: The impact of nutritional status, nutritional risk,
and nutritional treatment on clinical outcome of 2248 hospitalized
cancer patients: A multi-center, prospective cohort study in
Chinese teaching hospitals. Nutr Cancer. 65:62–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rocha NP and Fortes RC: Total lymphocyte
count and serum albumin as predictors of nutritional risk in
surgical patients. Arq Bras Cir Dig. 28:193–196. 2015.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Dai Y, Zhou F, Long Z, Li Y, Liu B,
Xie D, Tang J, Tan J, Yao K, et al: The prognostic role of
preoperative serum albumin/globulin ratio in patients with bladder
urothelial carcinoma undergoing radical cystectomy. Urol Oncol.
34:484 e481–484 e8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brenner D, Blaser H and Mak TW: Regulation
of tumour necrosis factor signalling: Live or let die. Nat Rev
Immunol. 15:362–374. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seaton K: Albumin concentration controls
cancer. J Natl Med Assoc. 93:490–493. 2001.PubMed/NCBI
|
|
32
|
Nassri A, Zhu H, Wang DH and Ramzan Z:
Serum albumin at diagnosis is an independent predictor of early
mortality in veteran patients with esophageal cancer. Nutr Cancer.
70:1246–1253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qi Q, Song Q, Cheng Y and Wang N:
Prognostic significance of preoperative prognostic nutritional
index for overall survival and postoperative complications in
esophageal cancer patients. Cancer Manag Res. 13:8585–8597. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ray-Coquard I, Cropet C, Van Glabbeke M,
Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P,
Labidi I, et al: Lymphopenia as a prognostic factor for overall
survival in advanced carcinomas, sarcomas, and lymphomas. Cancer
Res. 69:5383–5391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Giorgi U, Mego M, Scarpi E, Giuliano M,
Giordano A, Reuben JM, Valero V, Ueno NT, Hortobagyi GN and
Cristofanilli M: Relationship between lymphocytopenia and
circulating tumor cells as prognostic factors for overall survival
in metastatic breast cancer. Clin Breast Cancer. 12:264–269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
37
|
Liu N, Jiang A, Zheng X, Fu X, Zheng H,
Gao H, Wang J, Liang X, Tian T, Ruan Z and Yao Y: Prognostic
Nutritional Index identifies risk of early progression and survival
outcomes in advanced non-small cell lung cancer patients treated
with PD-1 inhibitors. J Cancer. 12:2960–2967. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirahara N, Tajima Y, Fujii Y, Kaji S,
Yamamoto T, Hyakudomi R, Taniura T, Miyazaki Y, Kishi T and
Kawabata Y: Preoperative prognostic nutritional index predicts
long-term surgical outcomes in patients with esophageal squamous
cell carcinoma. World J Surg. 42:2199–2208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao X, Pan Y, Han W, Hu C, Wang C, Chen L,
Guo Y, Shi Y, Pan Y, Xie H, et al: Association of systemic
inflammation and body mass index with survival in patients with
resectable gastric or gastroesophageal junction adenocarcinomas.
Cancer Biol Med. 18:283–297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu WS, Fang WZ, Liu CY, Pan KY, Ding R, Li
XH and Duan CH: Prognostic significance of combined pretreatment
body mass index (BMI) and BMI loss in patients with esophageal
cancer. Cancer Manag Res. 11:3029–3041. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Potischman N, Hoover RN, Brinton LA,
Swanson CA, Herrero R, Tenorio F, de Britton RC, Gaitan E and
Reeves WC: The relations between cervical cancer and serological
markers of nutritional status. Nutr Cancer. 21:193–201. 1994.
View Article : Google Scholar : PubMed/NCBI
|