Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
6th most common human cancer and develops from the mucosal
epithelium in the oral cavity, pharynx, and larynx (1). Various cellular and molecular
parameters influence individual immunological behaviors as well as
different responses to therapeutic treatments in patients with head
and neck cancer (2,3). In this context extracellular vesicles
(EVs), especially exosomes, have gained increasing attention
(4–6). Exosomes are small-sized (30–150 nm)
EVs that are released by almost all types of cells and can be found
in all body fluids. Tumor-derived exosomes play an important role
for the intercellular communication and promote tumor progression
and immune suppression (6,7).
We have recently shown that HNSCC patients with
advanced tumor stages reveal significantly increased levels of
plasma derived CD16+ total exosomes, suggesting CD16
positive exosomes as potential liquid biomarkers to characterize
the individual situation of HNSCC patients (8). CD16 (FcRIIIA) is part of the Fc
receptor (FcR) family and is known to be expressed on different
immune cells such as natural killer (NK) cells, neutrophils and
certain monocyte subsets (9,10). It
has also been shown that cells of the human monocyte leukaemia cell
line THP-1 (Tohoku Hospital Pediatrics-1) as well as THP-1 derived
exosomes are strongly positive for CD16 (8).
Furthermore, our recent data revealed individual
heterogeneous distributions of CD14/CD16 monocyte subset abundances
in the peripheral blood of HNSCC patients compared to healthy
donors, with elevated monocytic expression of checkpoint molecule
PD-L1 in CD16+ non-classical monocytes in certain
individuals (11). Briefly,
peripheral blood monocytes can be subdivided based on their CD14 (a
lipopolysaccharide co-receptor) and CD16 cell surface expression
(10,12,13).
CD14++CD16− ‘classical’ monocytes have their
origin in the bone marrow from where they enter the periphery. The
so called ‘intermediate’ (CD14+CD16+) and
‘non-classical’ (CD14dim+CD16+) monocytes are
considered as pro-inflammatory and more differentiated subtypes
with specialized immune functions such as viral defense (14,15).
Under normal conditions in healthy individuals, these subsets
comprise each an amount of 5–10% of the entirety of peripheral
blood monocytes. Increased abundances of these pro-inflammatory
subsets have been identified in different acute and chronic
inflammatory diseases (16–18). In cholangiocarcinoma as well as in
colorectal cancer increased percentages of CD16+ blood
monocytes were associated with higher densities of tumor-associated
macrophages (TAM) (19,20). Similarly, it has recently been
observed that exosomes have an important impact on the
communication between TAMs and cancer cells and therefore might be
a promising target for innovative immunotherapeutic approaches
(21).
However, the origin of plasma derived
CD16+ exosomes in HNSCC patients and their role within
the immune-regulatory network of circulating monocytes has not been
investigated so far. This study aimed to investigate the context of
CD16+ plasma-derived exosomes in terms of the
differentiation patterns of peripheral blood CD14/CD16 monocyte
subsets and the clinicopathological parameters in HNSCC patients.
Furthermore, monocyte subsets were analyzed with regard to the
expression patterns of adhesion molecules CD29 and CX3CR1 as well
as immune checkpoint molecule programmed death ligand 1 (PD-L1) to
broaden our understanding of the interplay of circulating monocyte
subsets and exosomes as potential bioliquid mediators and
indicators of tumor infiltration and progression.
Materials and methods
Ethics statement
All patients were subjected to standard clinical and
surgical treatment between April and September 2021 at the
Department of Otorhinolaryngology, University Hospital
Schleswig-Holstein, Campus Luebeck, and have given their written
informed consent for the following investigations. The study was
approved by the local ethics committee of the University of Luebeck
(approval number 16–278) and conducted in accordance with the
ethical principles for medical research formulated in the WMA
Declaration of Helsinki.
Exosome isolation
Exosomes were isolated from plasma samples of
healthy volunteers and head and neck cancer patients by size
exclusion chromatography as described previously (22). In short, freshly thawed plasma was
sequentially centrifuged at 2,000 × g for 10 min and 10,000 × g for
30 min at 4°C. Following, the supernatant was filtered through 0.22
µm syringe-driven filters (Millipore, Burlington, MA, USA). 1 ml
aliquots were loaded on pre-packed sepharose columns and eluted
with PBS. Sequential 1 ml fraction #4 was collected and used for
downstream analyses. Total exosome protein concentration was
measured using Pierce BCA Protein Assay (Thermofisher Scientific,
Waltham, MA, USA) according to manufacturer's instructions.
Exosomes were concentrated using 100 kDa cutoff centrifugal filters
(Millipore).
Characterization of exosomes
Exosomes were characterized by western blot,
nanoparticle tracking analysis and transmission electron
microscopy. These methods are in line with the minimal information
for studies of extracellular vesicle (MISEV) 2018 guidelines
(23) and are routinely performed
as described in our previous publication (8) (EV-TRACK ID: EV200068).
Bead-based flow cytometry of
exosomes
Immune capture and bead-based flow cytometry were
carried out as described previously (8,24,25).
In short, 10 µg exosomes in 100 µl PBS were incubated with 1 µg
biotin-labeled anti-CD63 (Cat: 353018, Biolegend, San Diego, CA,
USA) for 2 h at room temperature on a shaker. Next, 10 µl ExoCap
Streptavidin magnetic beads (MBL Life Science, Woburn, MA, USA)
were added and incubated for another 2 h at room temperature on a
shaker. Samples were washed using a magnetic rack and subsequently
stained with the following antibodies/isotype controls for 1 h at
room temperature on a shaker: CD14-PE (0.5 µg, Cat: 12-0149-42) and
IgG1-PE (0.5 µg, Cat: 12-4714-42) (both from
eBioscience/Thermofisher Scientific), CD16-APC (0.8 µg, Cat: 36076)
and IgG1-APC (0.8 µg, Cat: 400122) (both from Biolegend),
CD44v3-APC (10 µl, FAB5088A) and IgG2b-APC (10 µl, IC0041A) (both
from R&D, Minneapolis, MN, USA). The stained complexes were
washed twice using a magnetic rack and finally resuspended in 300
µl PBS for flow cytometry. Detection was performed using a Gallios
flow cytometer with Kaluza 1.0 software (Beckman Coulter, Brea, CA,
USA) and 10000 events were acquired. Data are presented as relative
fluorescent intensity (RFI) which is the mean fluorescence
intensity of the stained sample divided by the mean fluorescence
intensity of the corresponding isotype control.
Blood collection and patient data
All blood donors have signed an informed written
consent, and were clarified about the content of the proposed
study. Blood (8 ml per patient) was drawn by venipuncture into a
sodium citrate containing S-Monovette (Sarstedt; Nümbrecht,
Germany). Blood samples were collected from healthy donors (n=10; 6
female/4 male; mean age of 59, range from 22 to 84) and head and
neck cancer patients (n=25; 7 female/18 male; mean age of 64, range
from 50 to 86). All analyzed HNSCC patients were treatment naïve.
Blood samples were centrifuged at 1,000 × g for 10 min. Plasma
specimens were stored in aliquots at −80°C. The clinicopathological
characteristics of patients are listed in Table I.
 | Table I.Clinicopathological parameters. |
Table I.
Clinicopathological parameters.
|
| Patients
(n=25) |
|---|
|
|
|
|---|
|
Characteristics | n | % |
|---|
| Sex |
|
|
|
Male | 18 | 72 |
|
Female | 7 | 28 |
| Age (years) |
|
|
|
≤65 | 12 | 48 |
|
>65 | 13 | 52 |
| Tumor site |
|
|
|
Pharynx | 13 | 52 |
|
Larynx | 5 | 20 |
| Oral
cavity | 7 | 28 |
| Tumor stage |
|
|
|
T1-t2 | 16 | 64 |
|
T3-t4 | 9 | 36 |
| HPV status |
|
|
|
Positive | 11 | 44 |
|
Negative | 14 | 56 |
| Alcohol abuse |
|
|
|
Yes | 5 | 20 |
| No | 20 | 80 |
| Tobacco
consumption |
|
|
|
Yes | 16 | 64 |
| No | 9 | 36 |
FACS analysis of monocyte subsets
For FACS analyisis, 20 µl of citrate blood was
diluted in 80 µl PBS within 4 h after blood collection. Staining of
blood cells was performed with the following antibodies (diluted
1:50) for 25 min staining in the dark: CD45-PE (Cat: 368510),
CD14-FITC (Cat: 367116), CD16-BV-510 (Cat: 302048), HLA-DR-APC-Cy7
(Cat: 307618), PD-L1-APC (Cat: 329708), CD29-PE-Cy7 (Cat: 303026)
and CX3CR1-BV421 (Cat: 341620) (all from Biolegend, San Diego,
USA). Afterward, 650 µl RBC Lysis Buffer (Biolegend) were added to
the samples for another 20 min before the samples were centrifuged
(400 × g for 5 min) and supernatants were discarded. Cell pellets
were resuspended in 100 µl fresh PBS and flow cytometry
measurements were performed using a MACSQuant 10 flow cytometer
(Miltenyi Biotec, Bergisch-Gladbach, Germany). Measured data were
analyzed using the FlowJo software version 10.0 (FlowJo, LLC,
Ashland, USA). All antibody titrations and compensations were
performed beforehand. For whole blood measurements, at least 100
000 CD45+ leukocytes were analyzed. Gating of monocyte
subsets was performed as described before (26).
Immunohistochemistry and
Evaluation
IHC staining was performed according to the
manufacturer's instructions, using the Ventana Discovery (Ventana
Medical System, Roche, Basel, Switzerland) automated staining
system. In brief, slides were stained with the anti-PD-L1 antibody
(rabbit monoclonal antibody, clone E1L3N, RTU; Cell Signaling,
Danvers, MA, USA) and samples were evaluated by two independent
pathologists. The tumor positivity score (TPS) was calculated as
the percentage of tumor cells with positive PD-L1 membrane staining
(range 0–100%).
Statistical analysis
GraphPad Prism Version 7.0f (GraphPad Software,
Inc., San Diego, CA, USA) was used for unpaired student's t-Tests
for statistical analysis of all data presented here. Analyses of
patients samples were performed once per sample. The mean and
standard errors (SEM) are presented. P<0.05 was considered to
indicate a statistically significant difference. Correlation
analysis between different parameters was calculated using
multivariate regression with the Pearson correlation coefficient.
P<0.05 (*), P<0.01 (**), and P<0.001 (***).
Results
Characterization of exosomes from
plasma of healthy donors and HNSCC patients
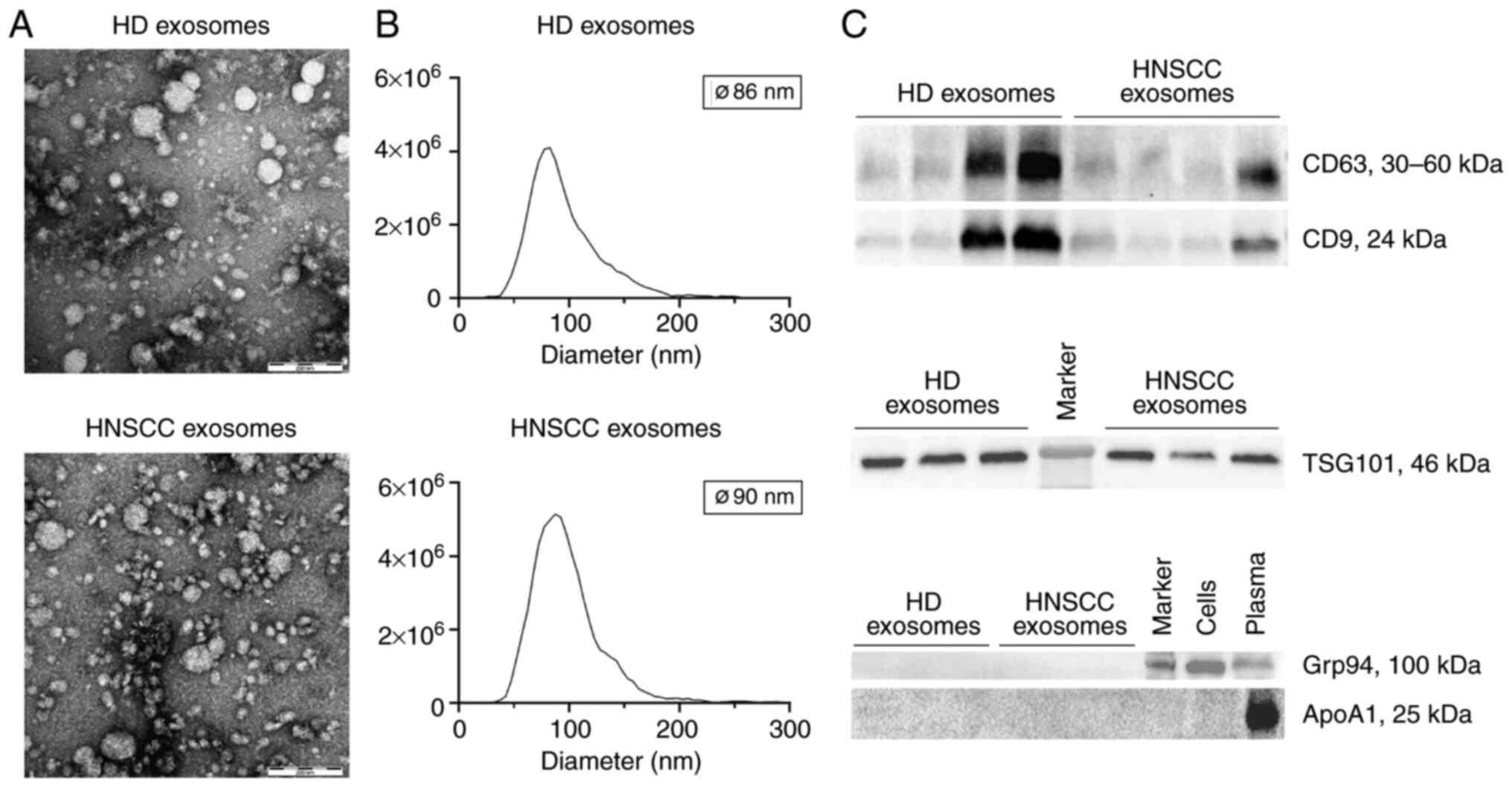
Exosomes isolated from plasma of healthy donors and
HNSCC patients were evaluated for morphology, size and protein
composition by transmission electron microscopy, nanoparticle
tracking and western blot analysis. Isolated vesicles had a
circular shape (Fig. 1A) and ranged
from 30 to 200 nm with median diameters around 90 nm (Fig. 1B). Exosome preparations were
positive for the tetraspanins CD63 and CD9 as well as the endosomal
marker TSG101 but negative for the non-exosomal marker Grp94 and
apolipoprotein ApoA1 (Fig. 1C).
According to the MISEV 2018 guidelines (23), the described results allow for
exosome nomenclature. Exosomal surface levels of CD44v3, used as
tumor marker, were higher in HNSCC patients compared to healhty
donors (HD) (Fig. 1D), indicating
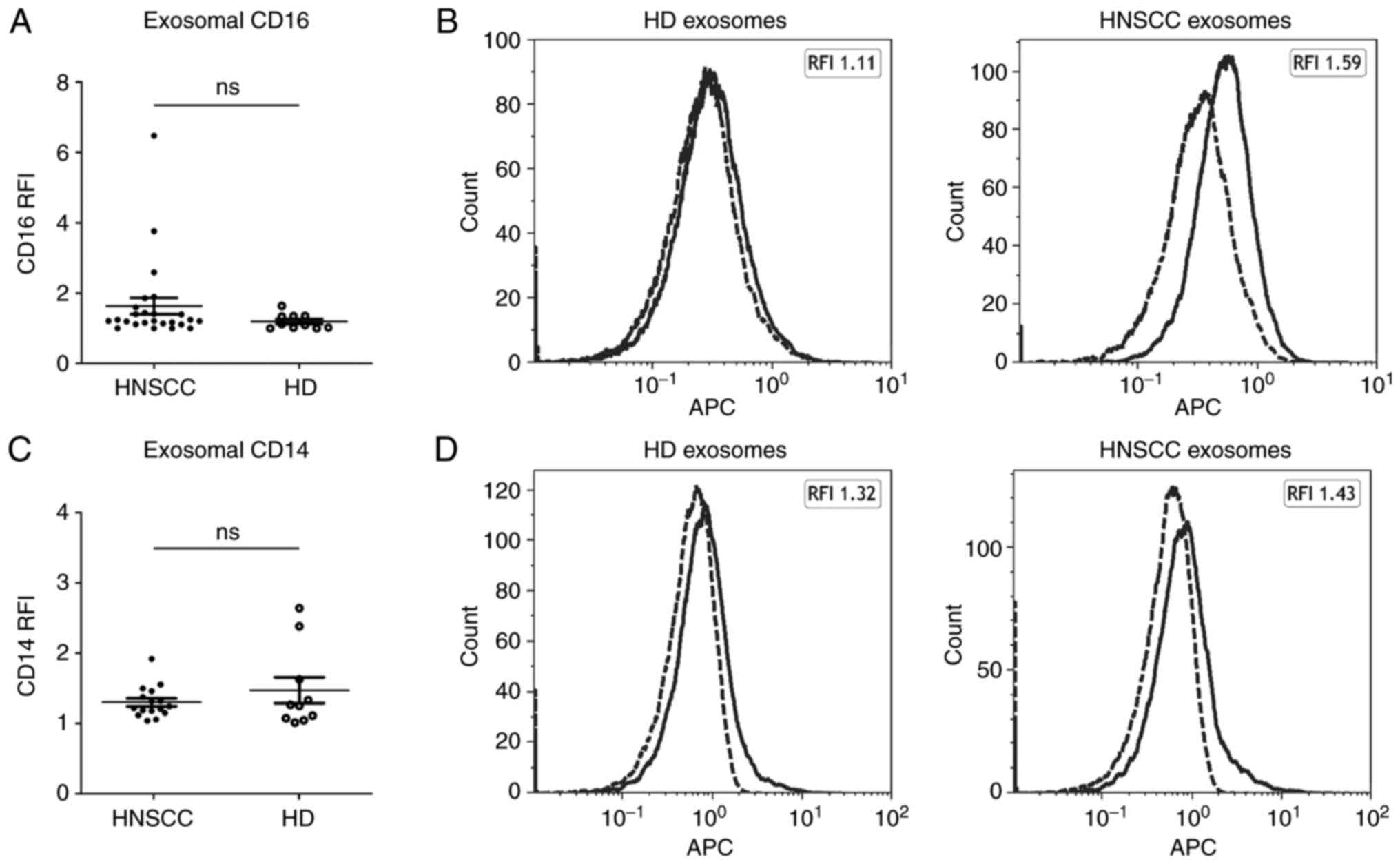
the presence of tumor-derived exosomes (TEX). Analysis of exosomal
CD16 and CD14 surface values revealed no significant overall
differences in HNSCC patients compared to healthy volunteers, but
individual patients with increased levels of plasma derived
CD16+ exosomes (Fig.
2).
CD14/CD16 monocyte subsets of the
HNSCC cohort
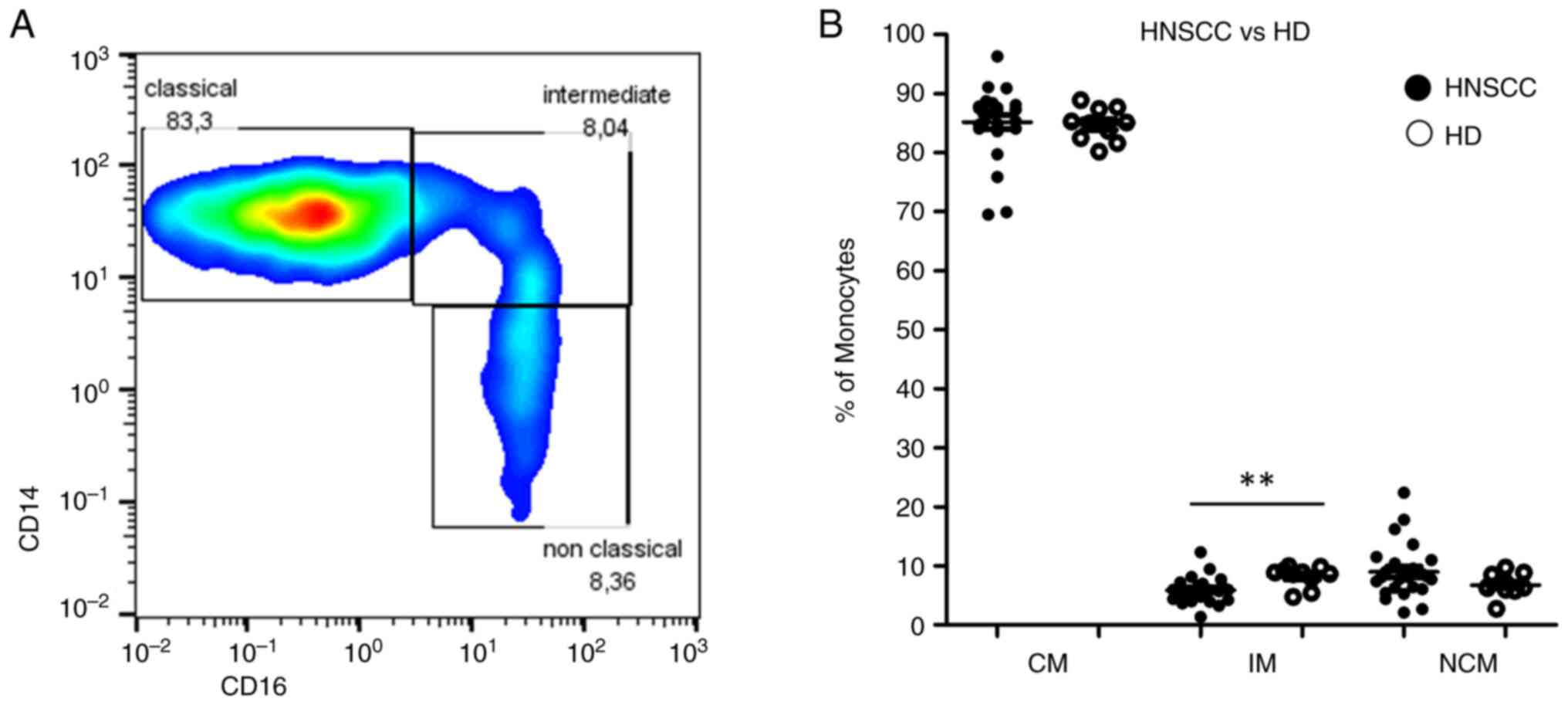
Circulating monocytes were divided into the three
subsets CD14++CD16− (classical),
CD14++CD16+ (intermediate) and
CD14dim+CD16+ (non-classical) using flow
cytometry (27). Briefly, CD45 was
used as a pan leukocyte marker for whole blood measurement and
monocytes were first roughly gated by their FSC/SSC characteristics
and the positivity for CD14 and CD16. Neutrophil granulocytes and
NK-cells were excluded by their missing HLA-DR expression.
Remaining B cells were excluded by the help of their lack of CD14
expression. Remaining monocytes were subgated into
CD14++CD16− (classical),
CD14++CD16+ (intermediate) and
CD14dim+CD16+ (non-classical) monocytes.
HNSCC patients revealed similar median percentages of both
classical and non-classical monocyte subsets compared to healthy
donors, but stronger dispersions of individual distributions. Our
measurements identified significantly increased percentages of
intermediate monocytes in healthy donors and a drop of classical
monocytes accompanied by an increase of non-classical monocytes in
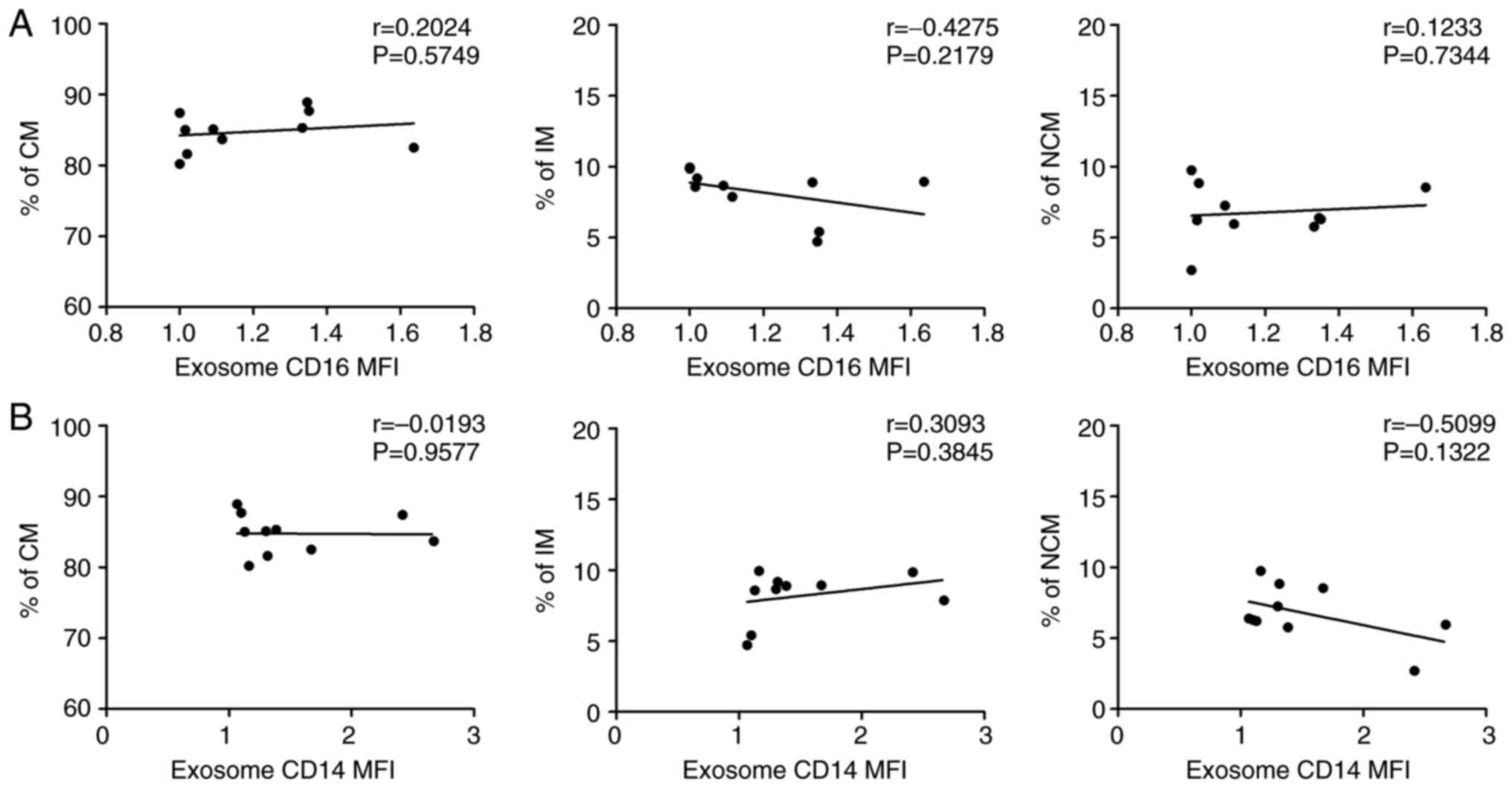
four HNSCC patients (Fig. 3).
Further Pearson's correlation analyses between the pathological
records of the intra-tumoral PD-L1 evaluation (tumor positivity
score, TPS) and CD16 positive exosomes and monocytes revealed no
significant correlations (Fig.
S1).
Correlation of CD16+
exosomes and monocyte subset alterations
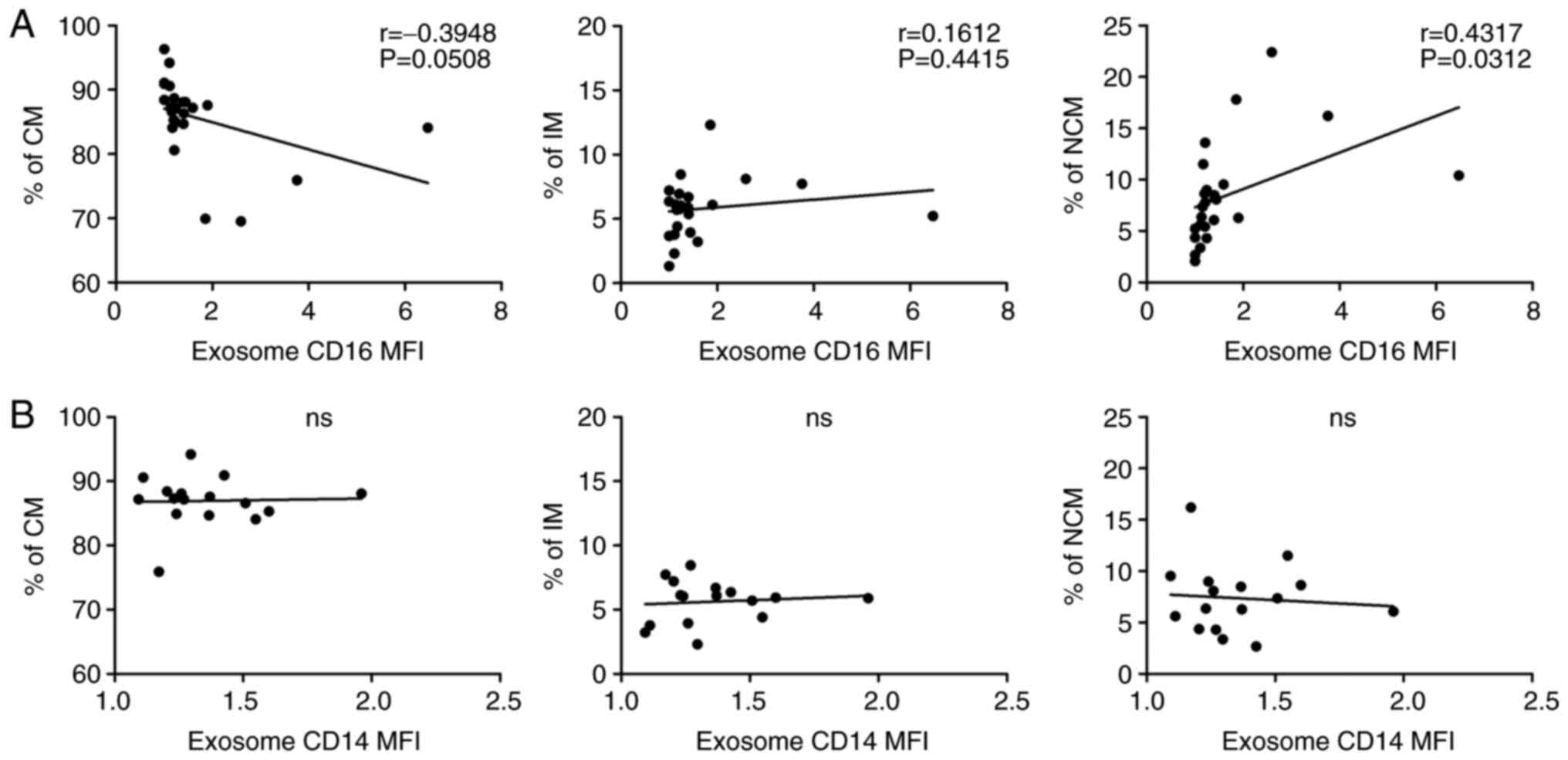
Our data revealed heterogeneous abundances of both
plasma derived CD16+ exosomes and circulating monocyte
subsets in the analysed HNSCC patients. Correlation analysis
between these parameters revealed a significant positive
correlation (P=0.0312) between CD16+ exosomes and
CD16+ non-classical monocytes but not CD16+
intermediate monocytes in head and neck cancer patients (Fig. 4A). Additional correlation analysis
between exosomal CD14, revealed no significant correlation
(Fig. 4B). In contrast, correlation
analyisis of both plasma derived CD14+ and
CD16+ exosomes and circulating monocyte subsets in the
analyzed healthy donors revealed no significant correlations
(Fig. 5). Analysis of
CD16+ exosomes from HPV positive and HPV negative
patients revealed no significant differences (Fig. S2).
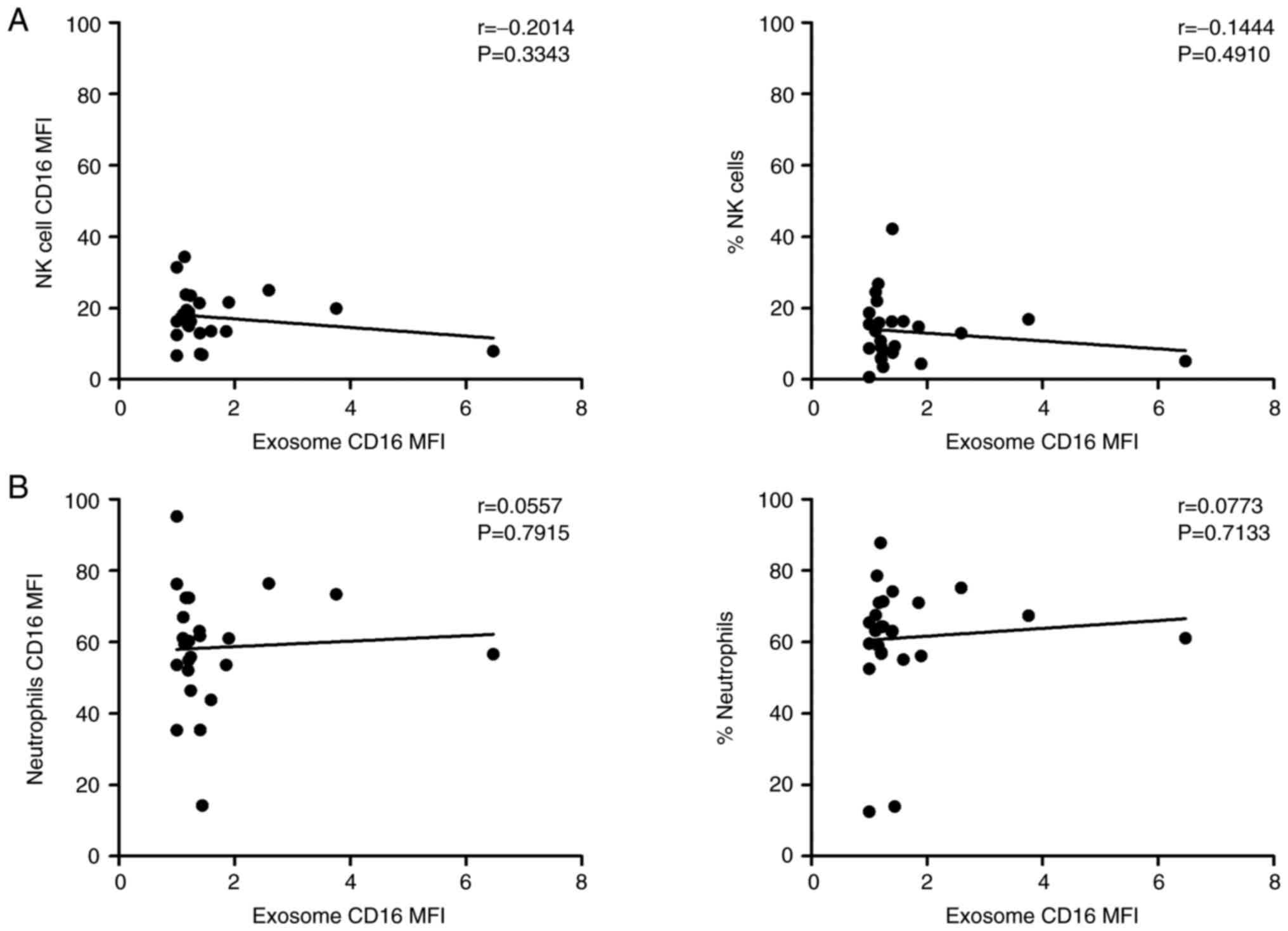
In addition, correlation analyses between exosomal
CD16 percentages and CD16 expression of natural killer (NK) cells
and neutrophils were performed. Our data revelaed no significant
correlation, which corroborates the conection between circulating
CD16+ monocytes and plasma CD16 exosomes in HNSCC
patients (Fig. 6). Furthermore,
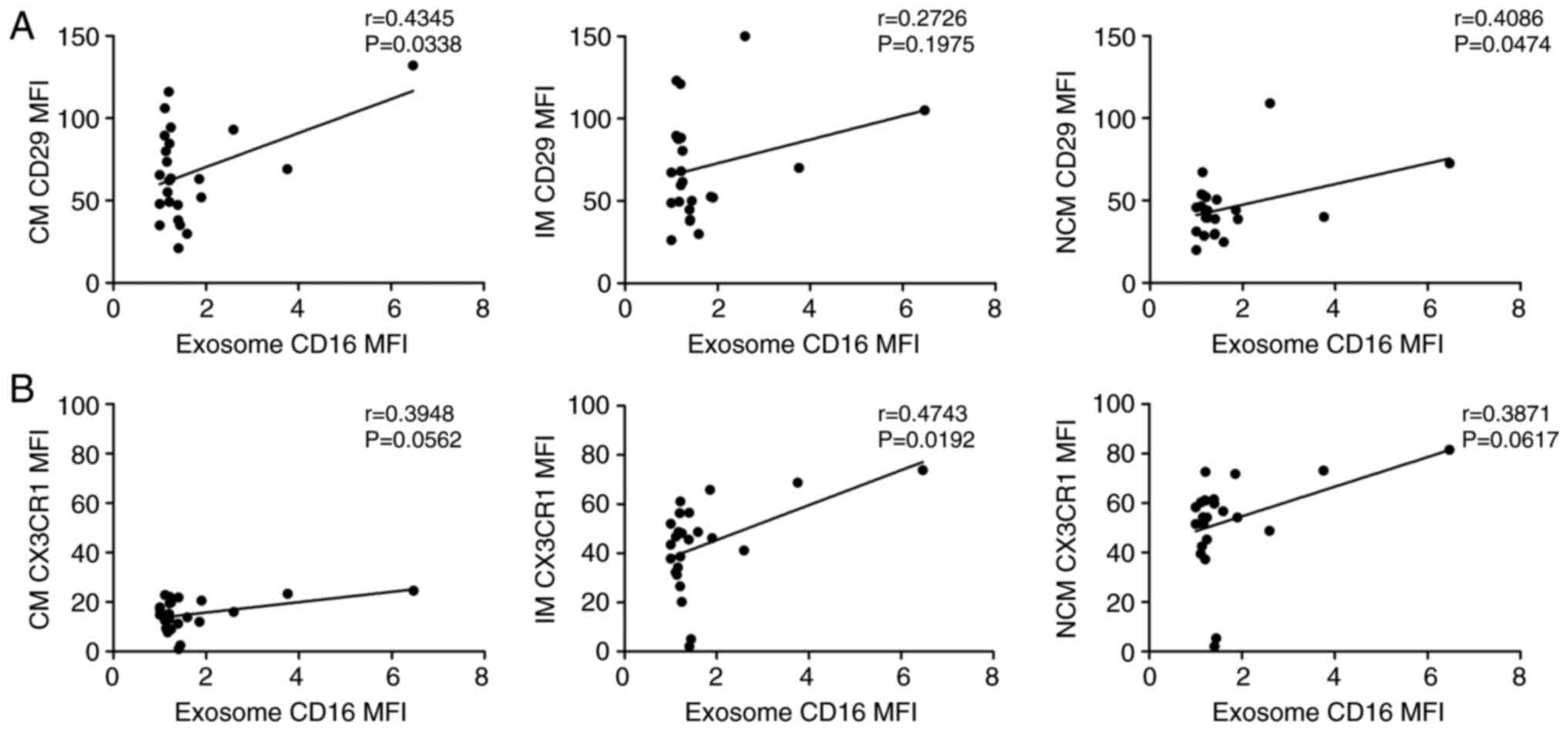
expression of adhesion molecules CD29 (integrin β1) and CX3CR1 was
measured in all three monocyte subsets and correlated with the
abundances of plasma derived CD16+ exosomes. Our data
revealed significant positive correlations between CD16 plasma
derived exosomes and CD29 expression on classical (P=0.0338) and
non-classical monocytes (P=0.0474) monocytes as well as a
significant correlation between CX3CR1 and CD16 plasma derived
exosomes expression on intermediate monocytes (P=0.0192) (Fig. 7).
Of note, the four patients with the strongest
monocyte subset alterations all suffer from oropharyngeal cancer,
thus located in a lymphoid-tissue-rich anatomical region that may
have a systemic influence on the immune regulation in the
peripheral blood.
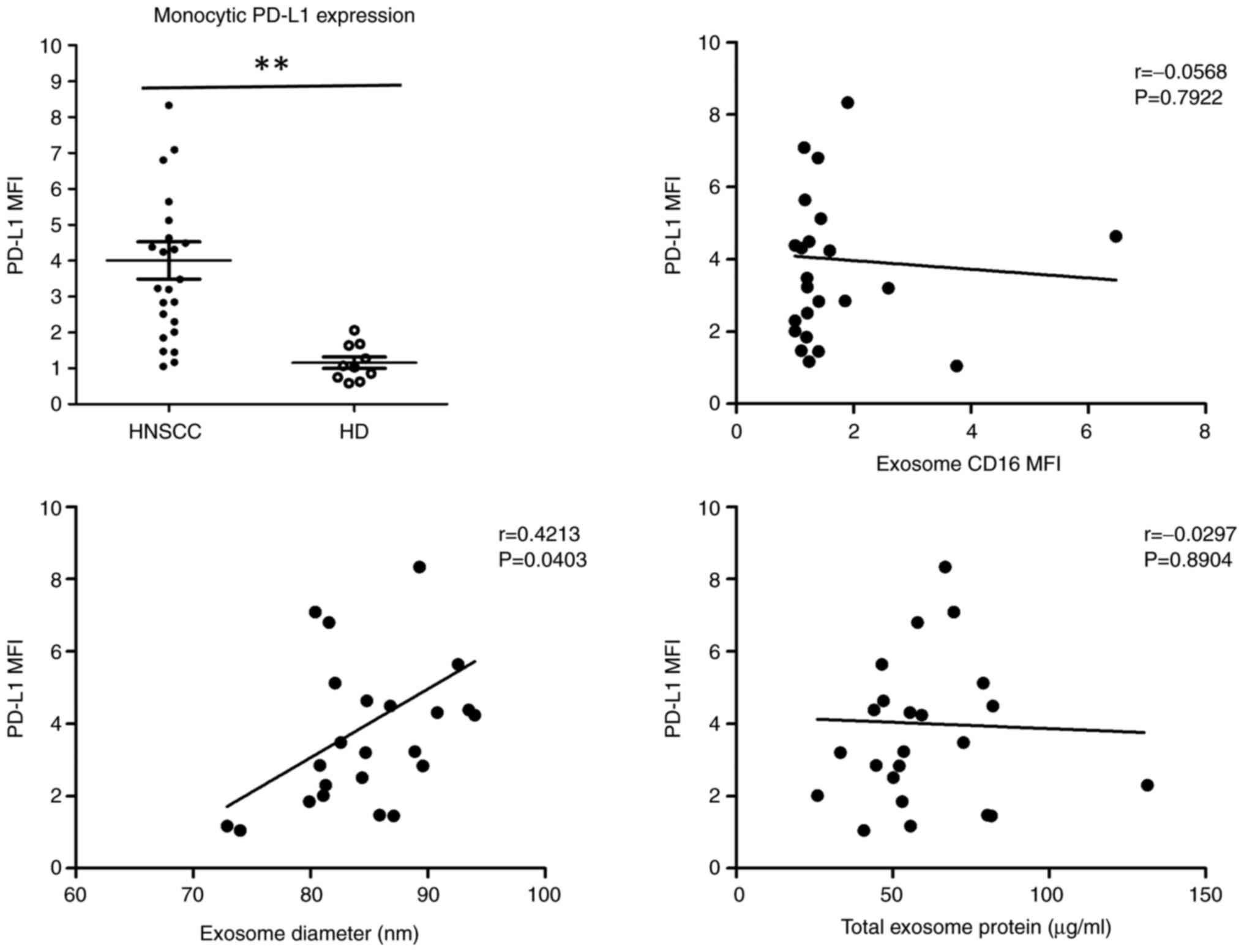
Next, the PD-L1 expression of monocytes was analyzed
and correlated with exosome CD16 expression, exosome diameter
values (nm) and total exome protein (µg/ml). Our data indicated
significantly increased monocytic PD-L1 expression in HNSCC
patients compared to healthy donors (P=0.0014) (Fig. 8A). Correlation analysis revealed no
significant correlations between monocytic PD-L1 expression and
exosomal CD16 and total exosomal protein levels, but a significant
correlation between monocytic PD-L1 and exosome diameter values
(Fig. 8). Further correlation
analysis revealed a positive significant correlation between
exosome diameter (nm) and percentages of classical monocytes as
well as a significant negative correlation between exosome diameter
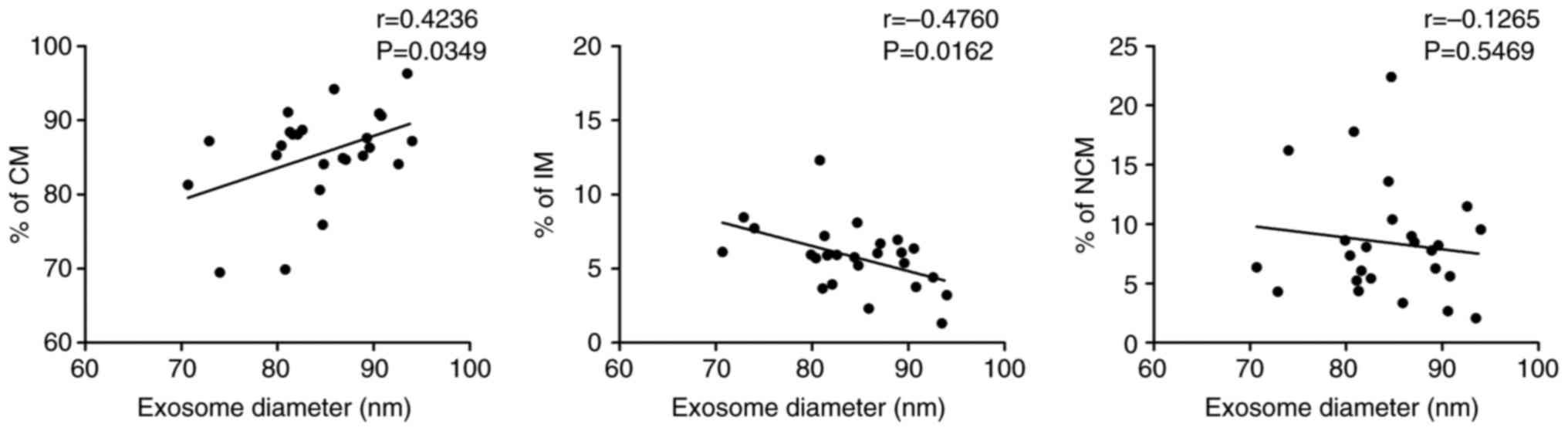
(nm) and percentages of intermediate monocytes (Fig. 9).
Discussion
The present study was undertaken to investigate the
possible connection between plasma derived CD16 exosomes and
abundances and characteristics of circulating CD14/CD16 monocyte
subsets in head and neck cancer. In general, the investigations
revealed no significant overall differences of exosomal CD16 and
CD14 values between HNSCC patients and healthy donors, but certain
individuals with increased abundances of CD16+ exosomes.
Monocyte subsets were distinguished using flow cytometry and
revealed overall heterogeneous distributions in HNSCC patients and
significantly increased percentages of intermediate monocytes in
healthy donors. However, there are conflicting observations
concerning the shift of certain monocyte subsets in head and neck
cancer in the literature so far. In a recent study, investigations
on a cohort of different HNSCC subtypes found lower abundances of
intermediate and higher percentages of classical monocytes in
comparison to healthy donors (28).
Another cohort of 22 oropharyngeal cancer patients revealed
increased percentages of classical monocytes and no differences of
non-classical monocytes compared to healthy donors (29). These different observations are most
likely due to the different sizes of the analyzed patient cohorts
and the manifold individual parameters in each cohort that
influence the monocyte subset distribution. This is as well an
acknowledged limitation of the present study. We found a
significant positive correlation between plasma derived CD16
exosomes and circulating CD16+ non-classical monocytes,
suggesting these cells as potential origin of CD16 plasma derived
exosomes in head and neck cancer. It is most likely that the
induction of CD16+ monocytes in certain individuals is
tumor driven, since a reconstitution of monocyte subset
distribution was observed after tumor resection in patients with
cholangiocarcinoma (30). Besides
their correlation with clinical parameters, plasma exosomes have as
well been identified as a promising bioliquid indicator for
treatment response and tumor progression in head and neck cancer
(31).
In this study, total exosomes from plasma of HNSCC
patients and HD were analyzed and they represent a mixture of
exosomes derived from normal, tumor and immune cells. We confirmed
the presence of CD44v3+ TEX in HNSCC patients. Previous studies
showed that HNSCC patients have higher amounts of total exosomes
compared to HD and that these are mainly tumor- and immune
cell-derived (22,24,25,32).
Remarkably, CD63 and CD9 showed interindivudal variability while
TSG101 was the most stable exosomal marker. This variance of
exosomal markers is commonly observed between different tumor cell
lines (33) and patients (32,34).
For exosome nomenclature, however, the presence of both
transmembrane proteins (CD9, CD63) and cytosolic proteins (TSG101)
recovered in exosomes need to be analyzed, irrespective of the
exact composition.
Fc receptor CD16 (FcRIIIA) is known to be expressed
as well on natural killer (NK) cells and neutrophils. We can't
distinguish from which cell populations CD16 exosomes derive but
studies suggest reduced NK cell numbers expressing CD16 (35,36)
and downregulated CD16 on NK cells (37,38) in
HNSCC, rendering it less likely that CD16 NK cells contribute to
CD16 exosomes to a large extent. Correspondingly, our correlation
analyses between exosomal CD16 percentages and CD16 expression of
natural killer (NK) cells and neutrophils revealed no significant
correlation, which corroborates the connection between circulating
CD16+ monocytes and plasma-derived CD16 exosomes in
HNSCC patients. Our data revealed significant correlations between
CD16 plasma derived exosomes and CD29 (integrin β1) expression on
classical and non-classical monocytes. β1- and β2-integrins play an
important role in the regulation of cell attachment and migration
of immune cells in inflammatory processes (39). These data may indicate an impact of
plasma derived exosomes on the adhesion characteristics and
intra-tumoral migration of circulating monocytes. It has recently
been shown in colorectal cancer, that tumor cell-derived spondin 2
triggers the transendothelial migration of monocytes via the
integrin β1/PYK2 axis. Furthermore, inhibition of the integrin
β1/PYK2 axis was found to impair the transendothelial migration of
monocytes as well as the cancer-promoting functions of
tumor-associated macrophages (40).
Furthermore, we identified a significant correlation between
CD16+ plasma derived exosomes and CX3CR1 expression on
intermediate monocytes. Adhesion molecule CX3CR1 is required for
monocyte crawling along the blood vessels by mediating the
interaction with the endothelium (41). Correspondingly, CX3CR1 is as well
associated with atherosclerosis and vascular inflammatory processes
(42,43). In patients with skin cancer, CX3CR1
has been shown to contribute to the accumulation of
tumor-associated macrophages (44).
Measurements revealed significantly increased
monocytic PD-L1 expression in HNSCC patients compared to healthy
patients, which corroborates earlier studies (11). Checkpoint molecule PD-L1 plays an
important role in tumor immune escape mechanisms by inhibiting
T-cell responses via its counterpart programmed death 1 (PD1)
(45). Further correlation analysis
revealed a significant correlation between monocytic PD-L1
expression and measured exosome diameter values, maybe due to
increased protein cargo of certain exosome subsets.
Surprisingly, further correlation analysis revealed
a significant positive correlation between exosome diameter (nm)
and percentages of classical monocytes as well as a significant
negative correlation between exosome diameter (nm) and percentages
of intermediate monocytes. This means that increased abundances of
intermediate monocytes accompany with a reduced size of plasma
exosomes in HNSCC patients.
It has recently been shown in prostate cancer (PC)
patients that plasmatic exosomes were smaller in size compared to
healthy individuals (46). It has
been proposed that the altered characteristics of plasmatic
exosomes in PC patients may be driven by tumor microenvironmental
conditions, such as acidity (47)
as well as the tumor mass (48,49).
However, our data suggest that characteristics of
plasma exosomes and immune alterations of circulating monocytes in
HNSCC patients are directly related or at least accompany upon
tumor driven alterations of the individual microenvironment. The
establishment of individual bioliquid markers for diagnosis and
therapy of cancer patients requires the long term comprehensive
analysis of countless molecular and cellular parameters in
correlation with the clinical situation. There will not be the ‘one
biomarker’ with clinical relevance for all patients but rather a
complex interaction of various potential biomarkers for each
individual cancer patient. Thus, the present work is just a small
but novel and necessary contribution to the big picture. This is
the first study to show that plasma-derived exosomes can inform us
about the state of monocyte subsets in corresponding blood samples
in HNSCC patients. Not only a correlation with non-canonical
monocytes was visible, but also with adhesion molecules and
checkpoint molecule PD-L1, which enable immune cells to migrate and
to regulate immune functions.
An acknowledged limitation of the present study is
the relatively small size of the analyzed patient cohort. Further
investigations on larger patient cohorts are required to understand
the biological induction as well as the clinical consequences of
the exosome mediated inter-monocytic communication in HNSCC
patients. Furthermore, patient outcome over a longer period of time
should be observed to assess the potential usability of the
analyzed parameters as bioliquid markers for patient prognosis and
therapy response prediction.
We do not propose the direct use of exosome CD16 for
the treatment of HNSCC, but we focus on its use as marker for the
level of immune suppression. By understanding the immune status of
patients, appropriate therapies can be selected. Our data suggest
that not only tumor derived but also monocyte derived exosomes
participate in the communication and immune alteration within the
circulating monocyte subpopulations in head and neck cancer.
Plasma-derived exosomes have the potential to reflect the immune
status of the patient with the long-term aim of easily
characterizing the TME as high or low immune suppressive and
facilitating the selection of the most suitable therapy.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mrs Kristin Loyal
(Department of Otorhinolaryngology, University Hospital
Schleswig-Holstein, Campus Luebeck, Luebeck, Germany) for their
technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH, DH, CI, JF, MNT and RP carried out the molecular
studies, data curation and statistical analysis. LH, CB, TKH, KLB,
MNT and RP participated in the design and coordination of the study
and helped to draft the manuscript. MNT and RP confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients were treated surgically at the
Department of Otorhinolaryngology, University Hospital
Schleswig-Holstein, Campus Luebeck, and have given their written
informed consent for the following investigations. The study was
approved by the local ethics committee of the University of Luebeck
(approval no. 16-278) and conducted in accordance with the ethical
principles for medical research formulated in the WMA Declaration
of Helsinki. Informed consent was obtained from all subjects
involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang P, Li S, Zhang T, Cui F, Shi JH,
Zhao F and Sheng X: Characterization of molecular subtypes in head
and neck squamous cell carcinoma with distinct prognosis and
treatment responsiveness. Front Cell Dev Biol. 9:7113482021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10:5402019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Canning M, Guo G, Yu M, Myint C, Groves
MW, Byrd JK and Cui Y: Heterogeneity of the head and neck squamous
cell carcinoma immune landscape and its impact on immunotherapy.
Front Cell Dev Biol. 7:522019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whiteside TL: The effect of tumor-derived
exosomes on immune regulation and cancer immunotherapy. Future
Oncol. 13:2583–2592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whiteside TL: Exosomes carrying
immunoinhibitory proteins and their role in cancer. Clin Exp
Immunol. 189:259–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whiteside TL: Exosomes in cancer: Another
mechanism of tumor-induced immune suppression. Adv Exp Med Biol.
1036:81–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofmann L, Ludwig S, Schuler PJ, Hoffmann
TK, Brunner C and Theodoraki MN: The potential of CD16 on
plasma-derived exosomes as a liquid biomarker in head and neck
cancer. Int J Mol Sci. 21:37392020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barros-Martins J, Bruni E, Fichtner AS,
Cornberg M and Prinz I: OMIP-084: 28-Color full spectrum flow
cytometry panel for the comprehensive analysis of human γδ T cells.
Cytometry A. 101:856–861. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziegler-Heitbrock L: Blood monocytes and
their subsets: Established features and open questions. Front
Immunol. 6:4232015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Idel C, Loyal K, Rades D, Hakim SG,
Schumacher U, Bruchhage KL and Pries R: Smoking-, alcohol-, and
age-related alterations of blood monocyte subsets and circulating
CD4/CD8 T cells in head and neck cancer. Biology (Basel).
11:6582022.PubMed/NCBI
|
|
12
|
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM
and Wong SC: The three human monocyte subsets: Implications for
health and disease. Immunol Res. 53:41–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel AA, Zhang Y, Fullerton JN, Boelen L,
Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B,
et al: The fate and lifespan of human monocyte subsets in steady
state and systemic inflammation. J Exp Med. 214:1913–1923. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyette LB, Macedo C, Hadi K, Elinoff BD,
Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG and
Metes DM: Phenotype, function, and differentiation potential of
human monocyte subsets. PLoS One. 12:e01764602017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jakubzick CV, Randolph GJ and Henson PM:
Monocyte differentiation and antigen-presenting functions. Nat Rev
Immunol. 17:349–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossol M, Kraus S, Pierer M, Baerwald C
and Wagner U: The CD14(bright) CD16+ monocyte subset is expanded in
rheumatoid arthritis and promotes expansion of the Th17 cell
population. Arthritis Rheum. 64:671–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moniuszko M, Bodzenta-Lukaszyk A, Kowal K,
Lenczewska D and Dabrowska M: Enhanced frequencies of CD14++CD16+,
but not CD14+CD16+, peripheral blood monocytes in severe asthmatic
patients. Clin Immunol. 130:338–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azeredo EL, Neves-Souza PC, Alvarenga AR,
Reis SR, Torrentes-Carvalho A, Zagne SM, Nogueira RM,
Oliveira-Pinto LM and Kubelka CF: Differential regulation of
toll-like receptor-2, toll-like receptor-4, CD16 and human
leucocyte antigen-DR on peripheral blood monocytes during mild and
severe dengue fever. Immunology. 130:202–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subimerb C, Pinlaor S, Khuntikeo N,
Leelayuwat C, Morris A, McGrath MS and Wongkham S: Tissue invasive
macrophage density is correlated with prognosis in
cholangiocarcinoma. Mol Med Rep. 3:597–605. 2010.PubMed/NCBI
|
|
20
|
Schauer D, Starlinger P, Reiter C, Jahn N,
Zajc P, Buchberger E, Bachleitner-Hofmann T, Bergmann M, Stift A,
Gruenberger T and Brostjan C: Intermediate monocytes but not
TIE2-expressing monocytes are a sensitive diagnostic indicator for
colorectal cancer. PLoS One. 7:e444502012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang H, Zhou L, Shen N, Ning X, Wu D,
Jiang K and Huang X: M1 macrophage-derived exosomes and their key
molecule lncRNA HOTTIP suppress head and neck squamous cell
carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell
Death Dis. 13:1832022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong CS, Funk S, Muller L, Boyiadzis M and
Whiteside TL: Isolation of biologically active and morphologically
intact exosomes from plasma of patients with cancer. J Extracell
Vesicles. 5:292892016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Theodoraki MN, Matsumoto A, Beccard I,
Hoffmann TK and Whiteside TL: CD44v3 protein-carrying tumor-derived
exosomes in HNSCC patients' plasma as potential noninvasive
biomarkers of disease activity. Oncoimmunology. 9:17477322020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Theodoraki MN, Hoffmann TK and Whiteside
TL: Separation of plasma-derived exosomes into CD3(+) and CD3(−)
fractions allows for association of immune cell and tumour cell
markers with disease activity in HNSCC patients. Clin Exp Immunol.
192:271–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polasky C, Steffen A, Loyal K, Lange C,
Bruchhage KL and Pries R: Reconstitution of monocyte subsets and
PD-L1 expression but Not T cell PD-1 expression in obstructive
sleep apnea patients upon PAP therapy. Int J Mol Sci. 22:113752021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polasky C, Steffen A, Loyal K, Lange C,
Bruchhage KL and Pries R: Redistribution of monocyte subsets in
obstructive sleep apnea syndrome patients leads to an imbalanced
PD-1/PD-L1 cross-talk with CD4/CD8 T cells. J Immunol. 206:51–58.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakakura K, Takahashi H, Motegi SI,
Yokobori-Kuwabara Y, Oyama T and Chikamatsu K: Immunological
features of circulating monocyte subsets in patients with squamous
cell carcinoma of the head and neck. Clin Immunol. 225:1086772021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi H, Sakakura K, Tada H, Kaira K,
Oyama T and Chikamatsu K: Prognostic significance and population
dynamics of peripheral monocytes in patients with oropharyngeal
squamous cell carcinoma. Head Neck. 41:1880–1888. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subimerb C, Pinlaor S, Lulitanond V,
Khuntikeo N, Okada S, McGrath MS and Wongkham S: Circulating
CD14(+) CD16(+) monocyte levels predict tissue invasive character
of cholangiocarcinoma. Clin Exp Immunol. 161:471–479. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Theodoraki MN, Laban S, Jackson EK, Lotfi
R, Schuler PJ, Brunner C, Hoffmann TK, Whiteside TL and Hofmann L:
Changes in circulating exosome molecular profiles following
surgery/(chemo)radiotherapy: Early detection of response in head
and neck cancer patients. Br J Cancer. 125:1677–1686. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshioka Y, Konishi Y, Kosaka N, Katsuda
T, Kato T and Ochiya T: Comparative marker analysis of
extracellular vesicles in different human cancer types. J Extracell
Vesicles. 2:2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hofmann L, Abou Kors T, Ezić J, Niesler B,
Röth R, Ludwig S, Laban S, Schuler PJ, Hoffmann TK, Brunner C, et
al: Comparison of plasma- and saliva-derived exosomal miRNA
profiles reveals diagnostic potential in head and neck cancer.
Front Cell Dev Biol. 10:9715962022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bose A, Chakraborty T, Chakraborty K, Pal
S and Baral R: Dysregulation in immune functions is reflected in
tumor cell cytotoxicity by peripheral blood mononuclear cells from
head and neck squamous cell carcinoma patients. Cancer Immun.
8:102008.PubMed/NCBI
|
|
36
|
Türkseven MR and Oygür T: Evaluation of
natural killer cell defense in oral squamous cell carcinoma. Oral
Oncol. 46:e34–e37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watanabe M, Kono K, Kawaguchi Y, Mizukami
Y, Mimura K, Maruyama T, Izawa S and Fujii H: NK cell dysfunction
with down-regulated CD16 and up-regulated CD56 molecules in
patients with esophageal squamous cell carcinoma. Dis Esophagus.
23:675–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dasgupta S, Bhattacharya-Chatterjee M,
O'Malley BW Jr and Chatterjee SK: Inhibition of NK cell activity
through TGF-beta 1 by down-regulation of NKG2D in a murine model of
head and neck cancer. J Immunol. 175:5541–5550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ou Z, Dolmatova E, Lassègue B and
Griendling KK: β1- and β2-integrins: Central players in regulating
vascular permeability and leukocyte recruitment during acute
inflammation. Am J Physiol Heart Circ Physiol. 320:H734–H739. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang C, Ou R, Chen X, Zhang Y, Li J,
Liang Y, Zhu X, Liu L, Li M, Lin D, et al: Tumor cell-derived SPON2
promotes M2-polarized tumor-associated macrophage infiltration and
cancer progression by activating PYK2 in CRC. J Exp Clin Cancer
Res. 40:3042021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Auffray C, Fogg D, Garfa M, Elain G,
Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G and
Geissmann F: Monitoring of blood vessels and tissues by a
population of monocytes with patrolling behavior. Science.
317:666–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McDermott DH, Halcox JP, Schenke WH,
Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA and Murphy PM:
Association between polymorphism in the chemokine receptor CX3CR1
and coronary vascular endothelial dysfunction and atherosclerosis.
Circ Res. 89:401–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tacke F, Alvarez D, Kaplan TJ, Jakubzick
C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et
al: Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1
to accumulate within atherosclerotic plaques. J Clin Invest.
117:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ishida Y, Kuninaka Y, Yamamoto Y, Nosaka
M, Kimura A, Furukawa F, Mukaida N and Kondo T: Pivotal involvement
of the CX3CL1-CX3CR1 axis for the recruitment of M2
tumor-associated macrophages in skin carcinogenesis. J Invest
Dermatol. 140:1951–1961.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Logozzi M, Mizzoni D, Di Raimo R, Giuliani
A, Maggi M, Sciarra A and Fais S: Plasmatic exosome number and size
distinguish prostate cancer patients from healthy individuals: A
prospective clinical study. Front Oncol. 11:7273172021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Logozzi M, Mizzoni D, Angelini DF, Di
Raimo R, Falchi M, Battistini L and Fais S: Microenvironmental pH
and exosome levels interplay in human cancer cell lines of
different histotypes. Cancers (Basel). 10:3702018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Logozzi M, De Milito A, Lugini L, Borghi
M, Calabrò L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi
E, et al: High levels of exosomes expressing CD63 and caveolin-1 in
plasma of melanoma patients. PLoS One. 4:e52192009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rodríguez Zorrilla S, Pérez-Sayans M, Fais
S, Logozzi M, Gallas Torreira M and García García A: A pilot
clinical study on the prognostic relevance of plasmatic exosomes
levels in oral squamous cell carcinoma patients. Cancers (Basel).
11:4292019. View Article : Google Scholar : PubMed/NCBI
|