Introduction
Colorectal metastases from gastric cancer rarely
occur without dissemination or direct infiltration and may be found
several years after gastrectomy (1–4).
Metastasis patterns, diagnosis, and treatment are being
investigated from reported cases. In colon metastasis not
originating from infiltration of gastric cancer, endoscopic
findings often reveal multiple polypoid or linitis plastica-like
lesions due to submucosal development (5). There are case reports (2,4,6) of
long-term survival following surgical treatment and chemotherapy
for recurrent lesions, and it is highly possible that treatment
will contribute to improving the prognosis. We aimed to examine the
characteristics of solitary colorectal metastasis from gastric
cancer by evaluating our case and other reported cases to improve
the medical treatment of colorectal metastasis from gastric
cancer.
Case report
Patient background, onset and
course
A 64-year-old man underwent distal gastrectomy for
gastric cancer in December 2010. He had a history of hypertension
(which was well controlled with antihypertensive medication) and
hyperlipidemia (total cholesterol was 150 mg/dl on
antihyperlipidemic medication). The pathological diagnosis was
poorly differentiated adenocarcinoma (Fig. S1), type 2, Se, ly +, v +, n +, T4a
N1 M0-stage IIIa (according to the 7th edition UICC) (Fig. 1). Postoperative adjuvant
chemotherapy with tegafur/gimeracil/oteracil potassium (S-1) was
administered in two courses. Computed tomography (CT) in May 2011
revealed peritoneal carcinomatosis; therefore, chemotherapy with
docetaxel was administered, given the recurrence. Subsequently,
chemotherapy was changed to bi-weekly paclitaxel therapy, and
complete remission (CR) was achieved. Contrast-enhanced CT scan
taken in December 2014 showed no recurrent lesions, and
chemotherapy was discontinued at the patient's request. Thereafter,
he remained under outpatient observation for stage IV gastric
cancer follow-up. After completion of chemotherapy in December
2014, tumor markers were measured once every 3 months, CT and
ultrasonography were performed once every 6 months, and
gastrofiberscopy was performed once a year. In February 2019, the
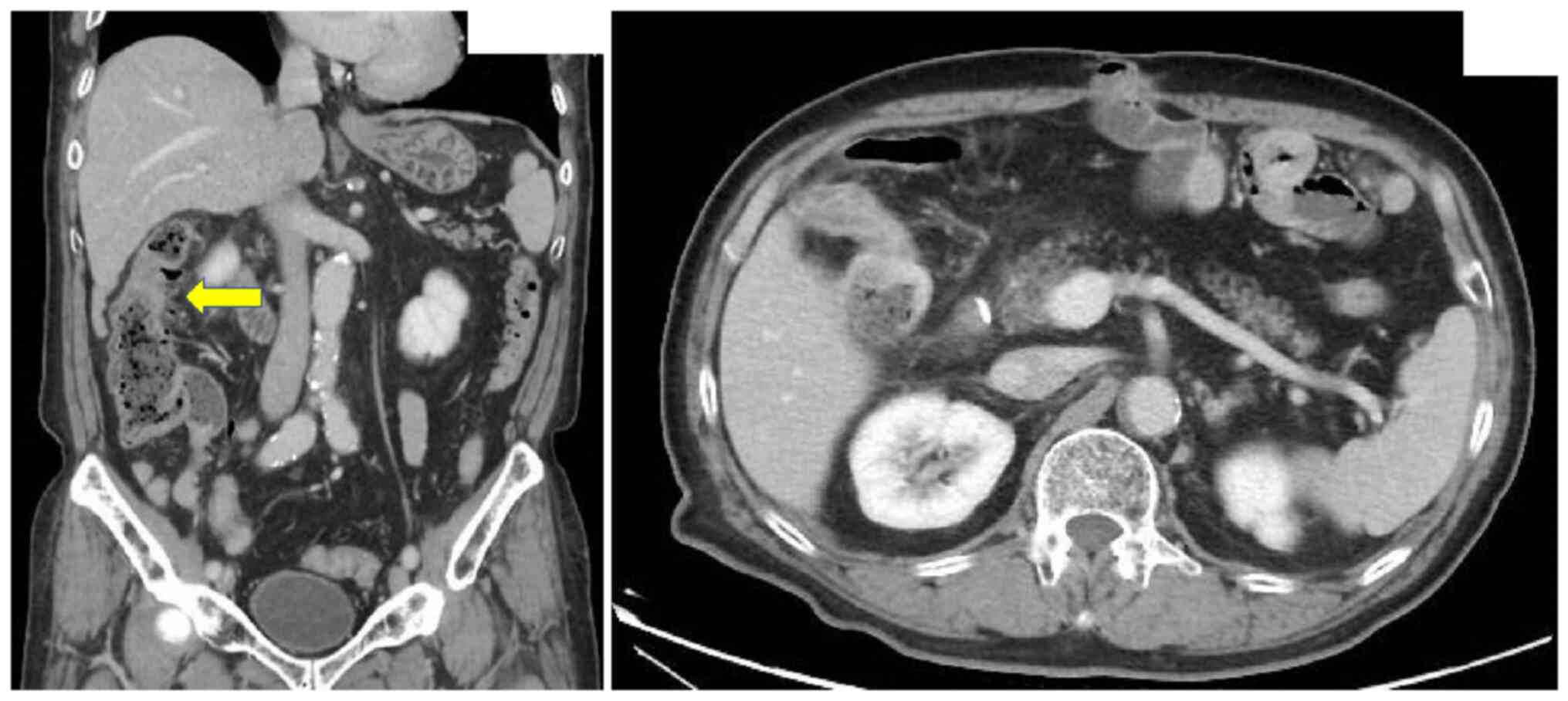
patient complained of discomfort in the right abdomen, and a CT
scan showed wall thickening from the ascending colon to the
transverse colon (Fig. 2). A
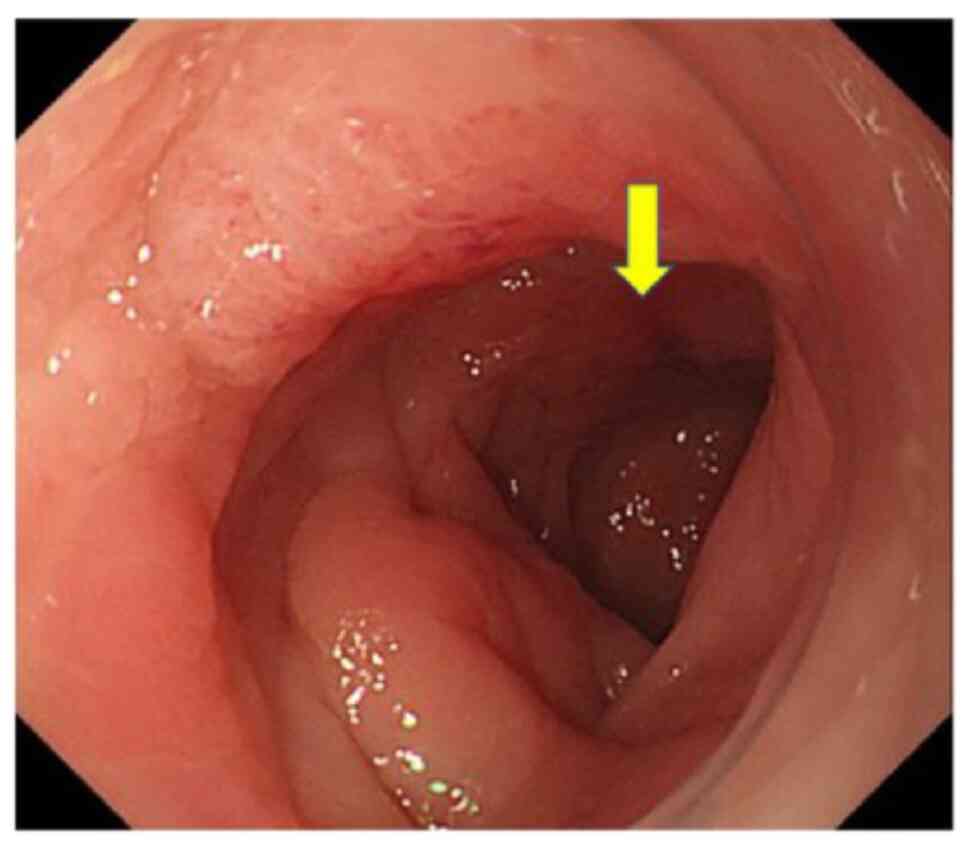
colonoscopy revealed stenosis and mucosal edema from the hepatic
flexure of the transverse colon to the ascending colon; diverticula
in the ascending colon was also detected. Biopsy pathology showed
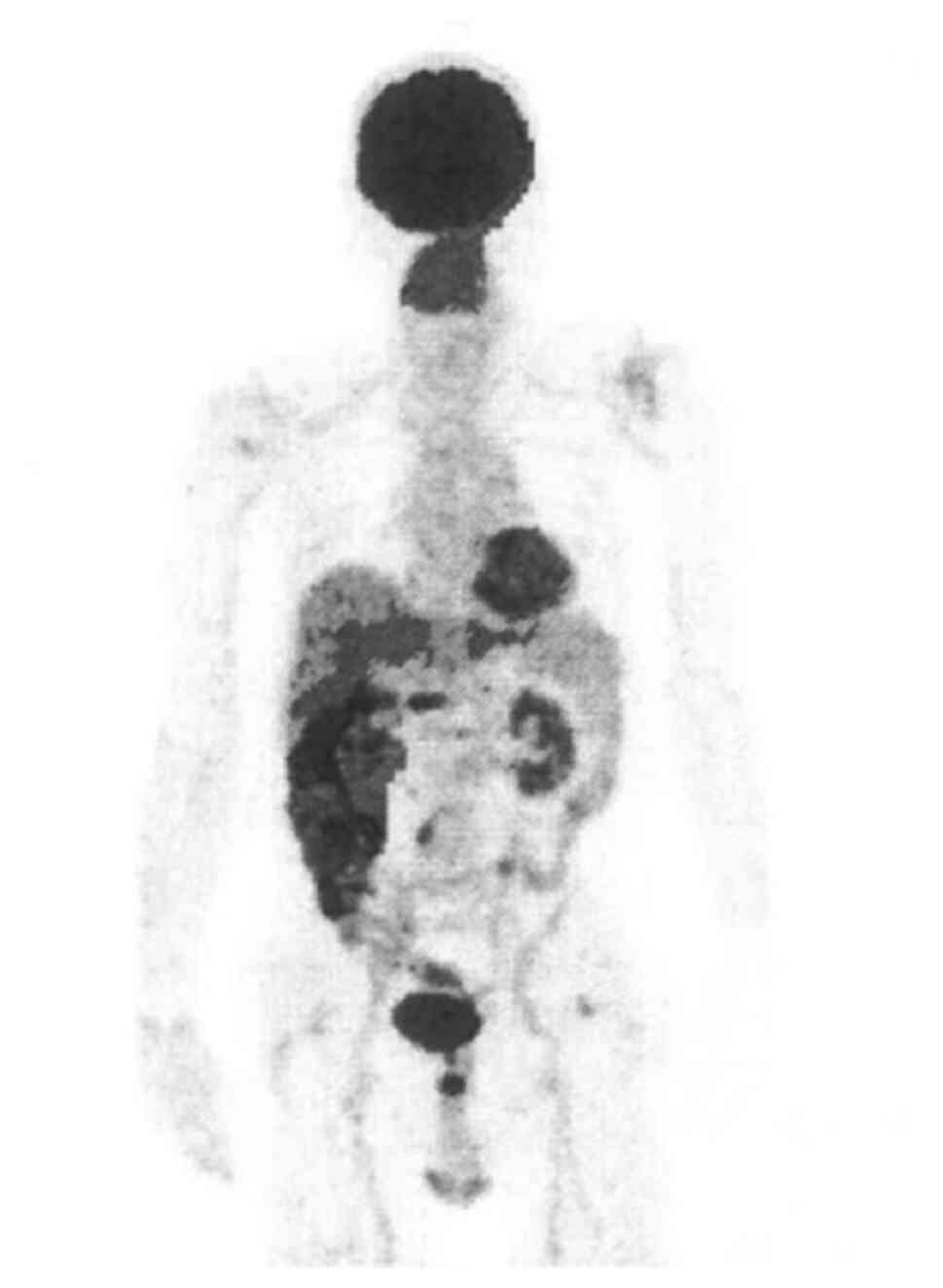
no malignant findings. Positron emission tomography-CT examination
(Fig. 3) showed accumulation of
fluorodeoxyglucose, and colorectal diverticulitis, potential
dissemination, and peritoneal carcinomatosis were further
diagnosed. The laparoscopic examination showed no ascites,
recurrence, or disseminated lesions. No colectomies were performed
since there was no obstruction, and no diagnosis of colon cancer
was made. Appendectomy was performed, and a white induration in the
peritoneum was biopsied; both specimens were submitted for
pathological diagnosis, but no malignant findings were observed. A
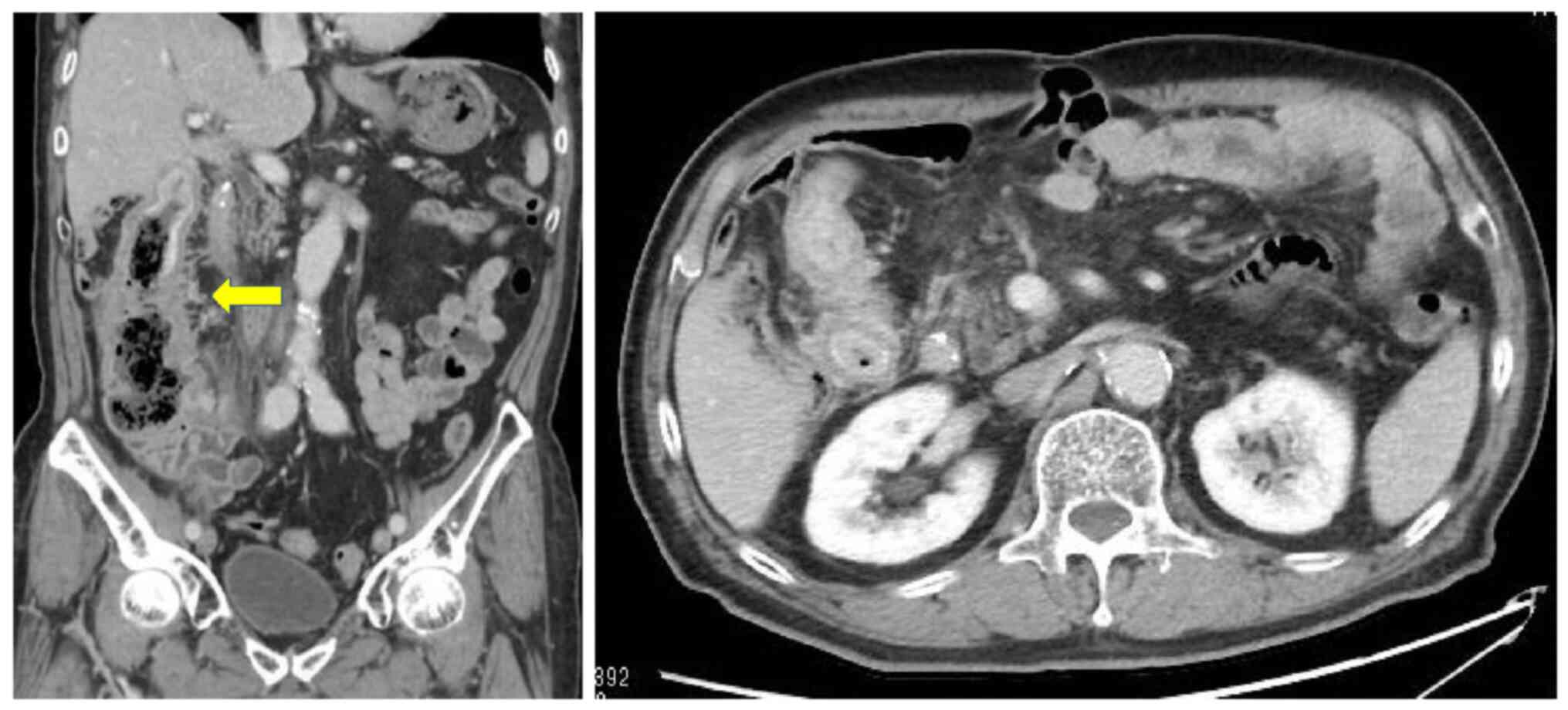
CT scan conducted in October 2019 (Fig.
4) showed worsening of the wall thickening of the ascending
colon. Furthermore, carcinoembryonic antigen levels increased to
23.7 ng/ml. Colonoscopy revealed extensive stenosis in the
transverse colon, and the biopsy showed no findings indicating
malignancy (Fig. 5). Despite these
results, right hemicolectomy was performed in November 2019 based
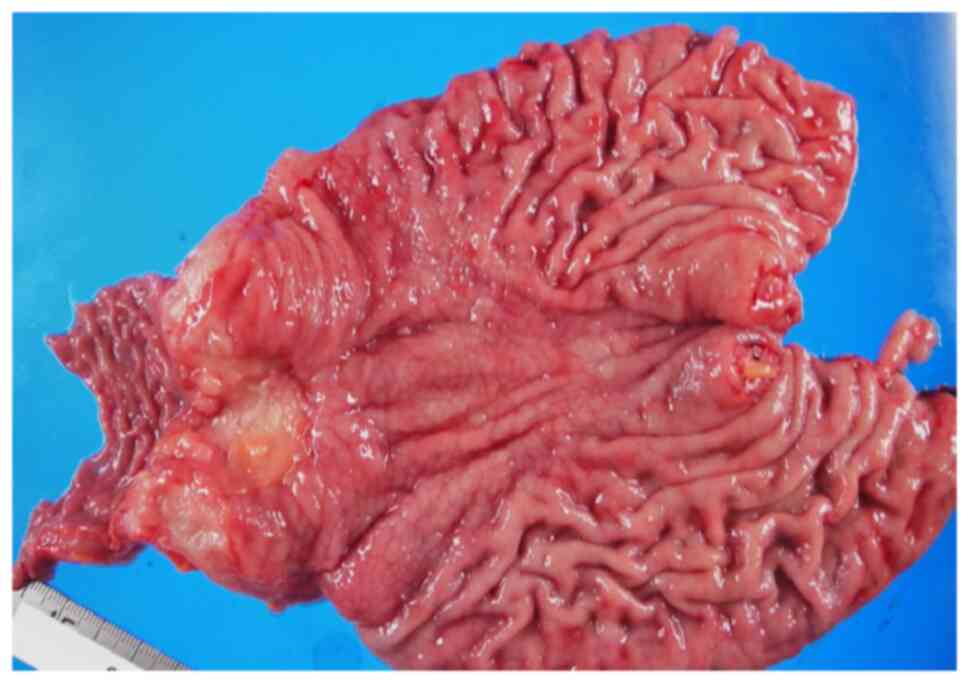
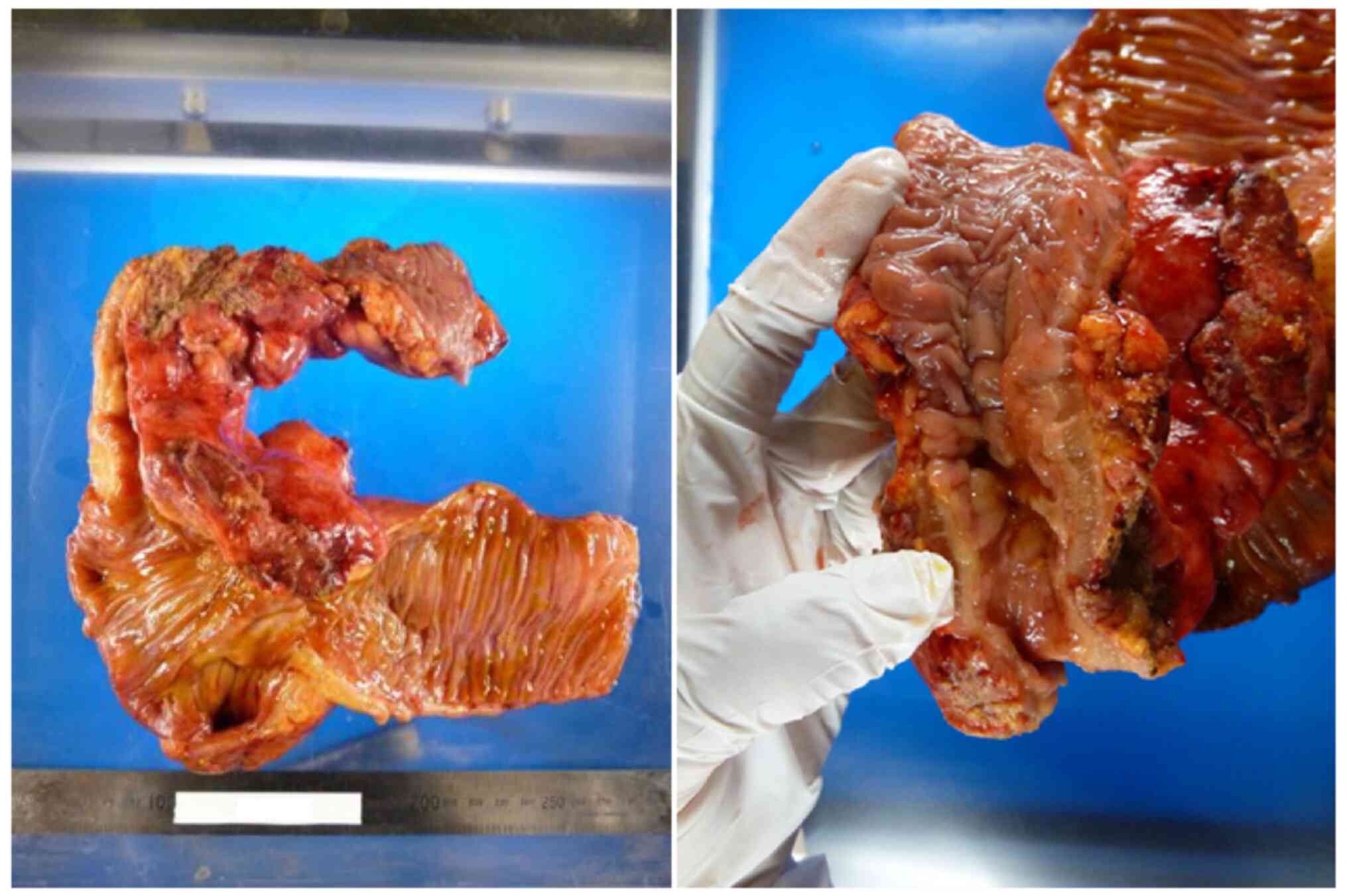
on a diagnosis of transverse colon cancer. Gross findings of the
resected specimen (Fig. 6) showed a
linitis plastica-like tumor that was 20×60 mm in size, extending
from the ascending to the transverse colon; the extent of mucosal
infiltration was unclear. The pathological diagnosis was poorly
differentiated adenocarcinoma, type 4, Se, ly0, v0, Pn1b, BD3, n0,
and Cy0, T4a N0 M0-stage IIb (according to the 8th edition UICC).
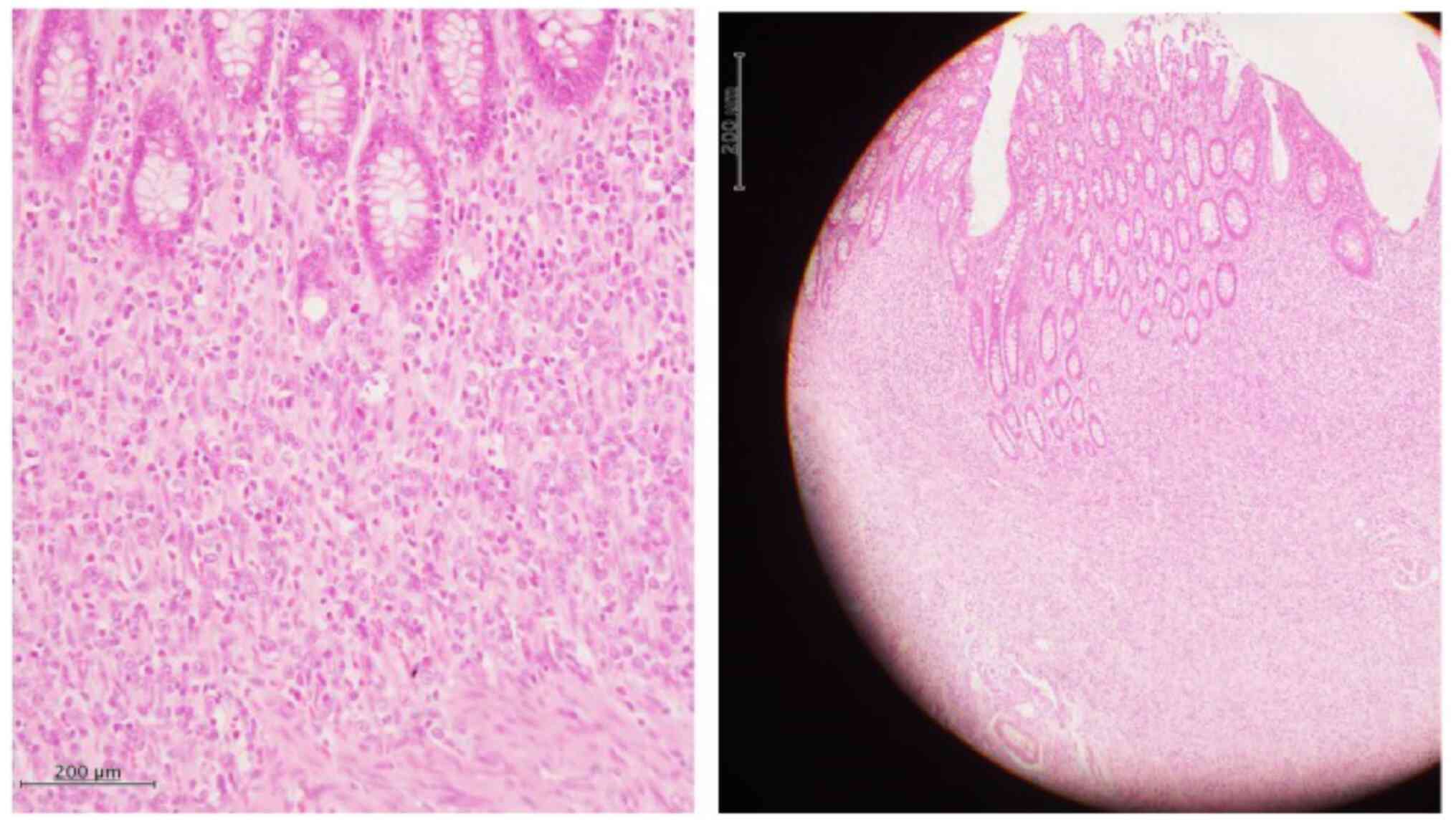
Microscopic findings (Fig. 7)
showed atypical cells that infiltrated and proliferated in a
cord-like or individual-cell manner with a fibrous stroma and that
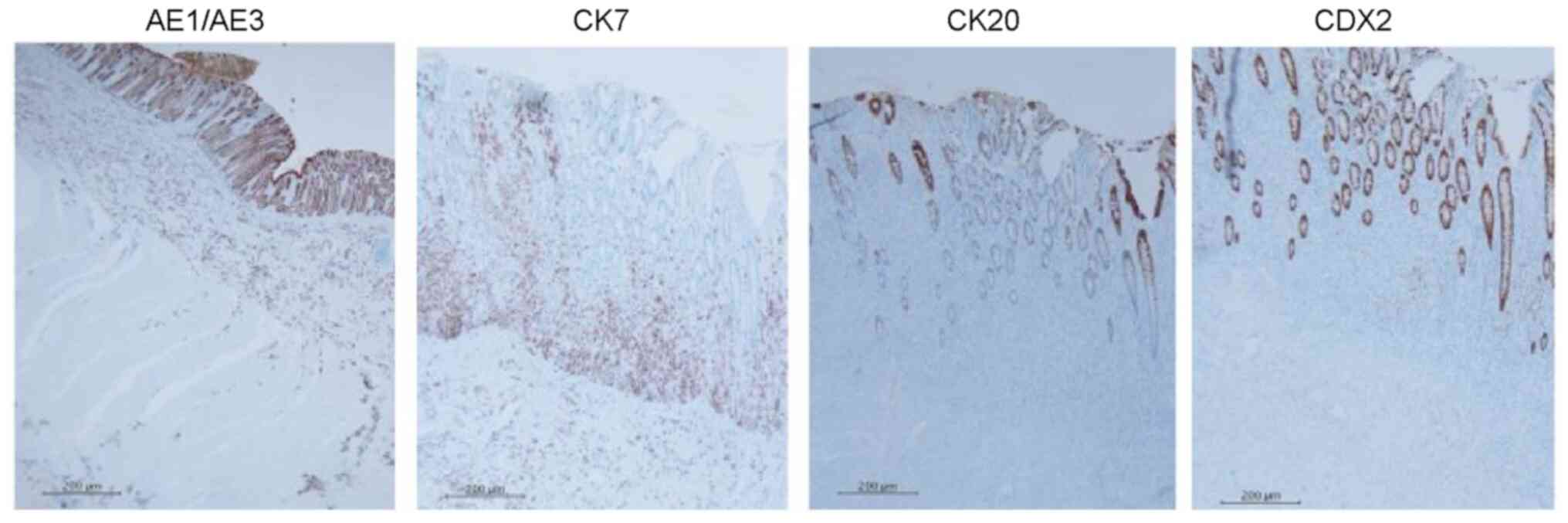
the spread extended to all layers. Tumor cells were AE1/AE3
positive, cytokeratin (CK)7 positive, CK20 negative, and caudal
type homeobox 2 (CDX2) negative on immunostaining (Fig. 8), and gastric cancer recurrence was
diagnosed. Thereafter, S-1, oxaliplatin chemotherapy → ramucirumab,
paclitaxel chemotherapy → nivolumab chemotherapy → irinotecan
chemotherapy → docetaxel chemotherapy were administered; however,
the patient died of peritoneal carcinomatosis in July 2021.
Analysis method from case reports
To investigate the characteristics of colon
metastasis from gastric cancer, we performed a literature search of
English abstracts published on colon metastasis and gastric cancer
in the Cochrane Library and PubMed databases. The data were
obtained directly from case reports searched in the literature and
the present case. We did not acquire any patient cohort data during
this study. From the previous case reports, we extracted data on
the following factors: age, sex, gastric cancer pathology,
macroscopic feature of gastric cancer, other metastatic sites
besides the colorectum, metastatic site in the colorectum,
chemotherapy after the diagnosis of colorectal metastasis, and
prognosis. We tested for significant differences in each factor
between the synchronous (n=13) and metachronous (n=24) gastric
cancer colonic metastasis case groups. The prognosis after
diagnosis of colon metastasis between the two groups was
determined.
Statistical analysis
Categorical data were represented as n (%).
Continuous data were described as mean ± standard deviation (SD).
To compare 13 syncronous and 24 metacronous colon metastasis case
groups, Fisher's exact test was used to determine significant
differences between covariates for gastric cancer pathology,
macroscopic features, recurrent colon tumor macroscopic features,
other metastatic sites besides the colorectum, metastatic site in
the colorectum, and chemotherapy after the diagnosis of colorectal
metastasis. Continuous data for age were analyzed by unpaired
t-test. Prognosis was analyzed using Kaplan-Meier survival curve
and log-rank test. Estimated mean survival, estimated median
survival, and their respective standard errors (SE) and 95%
confidence intervals (CI) were calculated. All statistical analyses
were performed using IBM SPSS Statistics for Windows, version 22.0
(IBM Japan, Ltd., Tokyo, Japan). A two-sided P-<0.05 was
considered significant.
Discussion
We described the case of a patient with stage IV
gastric cancer who underwent chemotherapy and achieved CR. He was
found to have developed colorectal metastases 8 years and 10 months
after gastrectomy and died 1 year and 8 months after undergoing a
right hemicolectomy.
Regarding cancer metastasis from other organs to the
gastrointestinal tract, the most reported metastatic site is the
colorectum, followed by the stomach and duodenum (7). The proportion of metastatic lesions
from other organs in colorectal cancer is considered to be
approximately 1%; however, it may be slightly higher at postmortem
(7). In addition, the detection of
colorectal metastases is thought to increase a greater awareness of
the need to follow up on primary tumors (7). The most common primary organs of
colorectal metastases are the lung, ovary, mammary gland, prostate,
kidney, and skin (7). The incidence
of multiple primary cancer after gastric cancer surgery is 2.0-7.6%
(8), and colorectal cancer often
occurs. In a single institutional report of secondary primary
colorectal carcinoma in Asia (9),
17 of 1,031 resected colorectal cancers had metastasized from other
sites. Of the 17 cases, six and five were breast and gastric
cancers, respectively. The incidence of gastric cancer is high in
East Asia (9). Genes are involved
in the carcinogenic mechanism, but in cancers that occur in
multiple organs, it is important to know whether they are
metastases or multiple individual cancer occurrences.
Classification of a cancer as metastatic or second primary cancer
is essential. The criteria for multiple secondary tumors (MPCs) are
reported by Warren and Gates in 1932 (10). According to them, ‘each of the
tumors must present a definite picture of malignancy, each must be
distinct, and the probability of one being a metastasis of the
other must be excluded’. Malignant lesions with characteristics
other than these are treated as metastases. Evidence of such
metastasis to distant organs is confirmed from pathological
features; however, recent determinations using immunostaining are
believed to have increased accuracy. Immunohistochemical staining
is effective in determining whether the lesions are of the same
origin; this is reportedly effective in demonstrating metastasis
(11,12). Immunohistochemical markers are
useful in diagnosing metastatic adenocarcinoma with increased
sensitivity and specificity to determine the primary site (12,13).
The staining pattern of CDX2, CK7, and CK20 reveals whether the
metastasis is gastric or colorectal (12). CDX2 is a homeobox gene that encodes
a gut-specific transcription factor and is expressed in the
epithelial cell nuclei of the intestine from the duodenum to the
rectum. AE1/AE3 stains almost all epithelial cells. Gastric cancer
is generally CK7-positive and CK20-negative, while colon cancer is
generally CK7-negative and CK20-positive. Our findings revealed a
similar pattern (CK7-positive and CK20-negative) of
immunohistochemical staining. However, immunohistochemical staining
does not aid in determining whether rare cancers in multiple organs
occur due to the same gene.
In our case, the patient achieved CR from stage IV
gastric cancer after chemotherapy, and CT scan revealed colon
metastasis 8 years and 10 months postoperatively. To investigate
the characteristics of colon metastasis from gastric cancer, we
summarized the reported cases of gastric cancer colon metastasis.
Synchronous colorectal metastases differ from metachronous; we
found 24 metachronous (1–4,6,14–29)
(Table I) and 13 synchronous cases
(5,25,30–40),
as classified and listed in Table
II. Several reports have set the boundary between synchronous
and metachronous metastases as diagnosed after 2 months, 6 months,
and 1 year from the primary diagnosis. Our report is based on the
Surveillance Epidemiology and End Results approach, which has the
largest number of cases of metachronous cancer. According to this
classification (41), metachronous
cancer is defined as cancer of other organs diagnosed ≥2 months
after the first cancer.
 | Table I.Cases of metachronous gastric cancer
colorectal metastasis. |
Table I.
Cases of metachronous gastric cancer
colorectal metastasis.
|
|
|
|
|
|
|
|
|
|
|
| RCT |
|
|
|---|
|
|
|
|
|
|
| PGT |
|
|
|
|---|
| First author/s,
year | No. | Age, years | Sex | N | Et |
| SCM: OMS | BP | Pathology | MF | DFI, months | CACO | Outcome | (Refs.) |
|---|
| MF | Stage | LNM | Pathology | CTX |
|---|
| Ogiwara et
al, 1994 | 1 | 53 | F | JPN | UN | IIc+III
(advanced) | UN | Yes | Por | UN | D, S: none | Por | UN | Pol | 132 | UN | UN | (1) |
| Tanakaya et
al, 2004 | 2 | 68 | M | JPN | UN | Type IV | IIIA | Yes | Sig, Por | UN | IC: none | AC | Sig, Por | Pol | 96 | UN | Death 16 Mo after
SS | (14) |
| Tseng et al,
2004 | 3 | 74 | M | UN | UN | UN | UN | Yes | UN | UN | S, R: UN | AC | AC | Pol | 108 | Yes | Death 6 Mo after
SS | (15) |
| Pace et al,
2009 | 4 | 77 | M | ITA | UN | Type II or III | IIIA | Yes | Sig, Mod | Yes | A:none | Por | Por, Sig | UN | 15 | UN | UN | (16) |
| Lim et al,
2011 | 5 | 43 | F | KOR | UN | Type III | II | Yes | Por | UN | R:none | NDM | Por | LPL | 34 | FOL-FOX | Alive 14 Mo after
SS | (17) |
| Mayumi et
al, 2013 | 6 | 74 | M | JPN | UN | Type III | IV | No | Por | 1st, S-1; 2nd, S-1,
CDDP; 3rd, CPT | T: ovary | UN | Por | Type II | 38 | 1st,
CPT; 2nd, PTX | Death 28 Mo after
SS | (18) |
|
|
|
|
|
|
|
|
|
|
|
| T:intes-tine | UN | Por | UN | 49 | Capecia tbine,
CDDP | Death 17 Mo after
3rd Sur |
|
| Watanabe et
al, 2013 | 7 | 63 | M | JPN | UN | Type II | IIB | No | Mod | TS-1 | T:Per | NDM | Mod-Por | Type II | 34 | Yes | Alive 15 Mo after
SS | (19) |
| Noji et al,
2014 | 8 | 61 | M | JPN | UN | UN | II | No | Por, Sig | UN | T:LN | UN | Por, Sig | UN | 108 | Refused | Alive 17 Mo after
SS | (2) |
| Noji et al,
2014 | 9 | 46 | F | JPN | UN | UN | IIIA | Yes | Sig | Yes | R: ovary, LN | UN | Mod, Por | Pol | 108 | Yes | Alive 24 Mo after
SS | (2) |
| Oh et al,
2014 | 10 | 69 | F | USA | UN | Type II or III | II | Yes | Por | 1st, FOL FOX; 2nd,
ECX | S:UN | Por | No resec tions | Type II or III | 47 | 1st, FOL
FOX; 2nd, FOL FIRI | UN | (20) |
| Tomita et
al, 2015 | 11 | 70 | F | JPN | UN | Type IV | IIIA | Yes | Sig | S-1 | T:UN | NDM | Sig | UN | 33 | S-1 | Death 37 Mo after
SS | (21) |
|
|
|
|
|
|
|
|
|
|
|
| A:Per | UN | No resec tions | UN | 47 | S-1, DOC | Death 23 Mo after
3rd Sur |
|
| Fujimoto et
al, 2016 | 12 | 58 | F | JPN | UN | 0-IIc+IIa | Ia | No | Por, Sig | 1st, S-1; 2nd, S-1,
CDDP, RTX | S: ovary | UN | Por, Sig | 0-IIc | 28 | S-1, DOC | Alive 30 Mo after
SS | (6) |
| Ikeda et al,
2016 | 13 | 69 | F | JPN | UN | 0-IIc Sig | Ib | Yes | Por, Sig | No | T:LN | None | Por, | SMTL | 47 | Refused | Death 7 Mo after
SS | (22) |
| Uemura et
al, 2016 | 14 | 64 | M | JPN | UN | 0-I | Ia | No | W | No | R:LN | W | W | Pol | 24 | UN | Alive 6 Mo after
SS | (23) |
| Higuchi et
al, 2018 | 15 | 55 | M | JPN | UN | Type III | IV | Yes | Mod Por | S-1 | C:none | NDM | Mod- | SMTL | 10 | FP | Death 20 Mo after
SS | (24) |
| Higuchi et
al, 2018 | 16 | 59 | M | JPN | UN | Type IV | IIIB | Yes | Tub, Por | S-1 | T:UN | Por. Sig | Por. Sig | LPL | 23 | S-1 | Death 25 Mo after
SS | (24) |
| Su et al,
2018 | 17 | 78 | M | TW | UN | Advan ced GC | IIIB | UN | Por | FOL FOX | R, T:UN | UN | Por | Type II | 18 | UN | Death 6 Mo after
SS | (25) |
| Mizuguchi et
al, 2018 | 18 | 60′ | M | JPN | UN | Type III | IIB | Yes | Tub, Por | S-1 | A: LN | Por | Tub, Por | LPL | 26 | None | Death 6 Mo after
SS | (26) |
| Elghali et
al, 2019 | 19 | 62 | M | TUN | UN | UN | IB | No | Sig | No | T: LN | UN | Sig | UN | 96 | Yes | UN | (3) |
| Yang et al,
2020 | 20 | 57 | M | CN | UN | UN | IIIA | Yes | Por, Sig | SOX | A: skin, LN | Por | Por. Sig | LPL | 30 | Yes | Death 9 Mo after
SS | (27) |
| Okazaki et
al, 2020 | 21 | 70 | F | JPN | UN | UN | IV | UN | Por | SOX | C: none | NDM | Por | Pol | 48 | SOX | Alive 12 Mo after
SS | (28) |
| Then et al,
2021 | 22 | 66 | M | USA | UN | UN | UN | UN | UN | UN | T:UN | NDM | AC | UN | 24 | None | UN | (29) |
| Wang et al,
2021 | 23 | 42 | M | CN | UN | UN | IIIA | Yes | Por | L-OHP, CF,
5-FU | RS: Per | UN | Por | Mass | 70 | PTX, CF, 5-FU | Death 76 Mo after
SS | (4) |
|
|
|
|
|
|
|
|
|
|
|
| S: LN | Por | Por | Pol | 110 | Epiru bicin, L-OHP,
Capecita bine | Death 44 Mo after
3rd Sur |
|
|
|
|
|
|
|
|
|
|
|
|
| S LN | Por | Por | Mass | 138 | Suniti nib +
S-1 | Death 16 Mo after
4th Sur |
|
|
|
|
|
|
|
|
|
|
|
|
| S: LN | Por | Por | Mass | 151 | UN | Death 3 Mo after
5th Sur |
|
| Present study | 24 | 73 | M | JPN | Asian | Type II | IIIA | Yes | Por | 1st, S-1; 2nd, DOC;
3rd, PTX. | T: none | NDM | Por | LPL | 118 | 1st, SOX; 2nd, RAM,
PTX; 3rd Niv; 4th, CPT; 5th, DOC | Death 20 Mo after
SS | - |
 | Table II.Cases of synchronous gastric cancer
colorectal metastasis. |
Table II.
Cases of synchronous gastric cancer
colorectal metastasis.
|
|
|
|
|
|
|
| PGT | RCT |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | No. | Age, years | Sex | N | Et | Macroscopic
features | Stage | Pathology | SCM: OMS | BP | MF | CAC | Outcome | (Refs.) |
|---|
| Metayer et
al, 1991 | 1 | 65 | M | FR | UN | Type II or III | IV | Sig | C, T, S:UN | Sig | Pol | None | Death within a
month after DX | (30) |
| Tomikashi et
al, 2002 | 2 | 57 | M | JPN | UN | Type III | IV | Por | All colon:
bones | Por | Pol | FP | Alive 7 months
after DX | (31) |
| Lee et al,
2004 | 3 | 41 | M | TW | UN | Type IV | IV | Sig | T, D, S:WB, LN | Por, Sig | Pol | HDFL | Death 1 month after
DX | (32) |
| Nakamura et
al, 2008 | 4 | 86 | F | JPN | UN | Type III | IV | Por, Sig | T:Per | Por, Sig | 0-IIa+IIc | None | Death 10 months
after DX | (33) |
| Tural et al,
2012 | 5 | 74 | F | TUR | UN | UN | IV | Well | R:none | Well | Pol | TCF | Alive several
months after CTX | (34) |
| Reyes-Diaz et
al, 2012 | 6 | 58 | M | ES | UN | UN | IV | Sig | C, A, S, R:LN | Sig | UN | CDDP | Death 12 months
after DX | (35) |
| Gao et al,
2014 | 7 | 80 | M | CN | UN | Type II or III | IV | Por, Sig | A, T, D, S:UN | Sig | Pol | Xeloda | Death 3 months
after DX | (36) |
| Lee et al,
2015 | 8 | 55 | F | KOR | UN | 0-IIc | IV | Sig | S, R: bone | Sig | Pol | FOL FOX | Alive >16 months
after CTX | (37) |
| Patel et al,
2016 | 9 | 59 | F | USA | CAU | UN | IV | Sig | A, T, S:LN | Sig | LPL | FOL FOX | Alive 1 months
after surgery | (38) |
| Makker et
al, 2016 | 10 | 60 | F | UN | UN | Type I or II | IV | Sig | R:B, L, Pl | Por, Sig | Pol+LPL | DCFL | Death 12 months
after DX | (39) |
| Sonoda et
al, 2017 | 11 | 81 | M | JPN | Asian | Type III | IV | Por, Sig | T:Per | Por, Sig | 0-IIa | UN | UN | (5) |
| Su et al,
2018 | 12 | 77 | M | TW | UN | UN | IV | Por | R, T:UN | NDM | Pol | FOL FOX | Death 6 months
after DX | (25) |
| Meheershahi et
al, 2020 | 13 | 55 | M | USA | UN | Type IV | IV | Por | S:UN | Por, Sig | Type II | UN | UN | (40) |
CT is reportedly effective in diagnosing metastasis
to the colorectum after gastric cancer surgery. It has a very high
rate of detection, especially when a helical CT outcome is
interpreted by a radiologist (42).
Most of the reported cases of gastric cancer and colorectal
metastasis were diagnosed by CT and endoscopy. Diagnostic
colonoscopy was performed in almost all cases for both metachronous
and synchronous colon metastases, and the findings were polypoid or
linitis plastica-like. In many of the reviewed cases, the biopsy
results obtained using colonoscopy were not described, and no
malignant findings were reported (17,19,24,25,28,29)
(Tables I and II). In our case, more than 8 years had
passed since the gastric cancer surgery, and no malignant findings
were obtained upon examination. Therefore, the large intestine was
not resected during the second laparoscopic surgery, and the
diagnosis of colon metastasis was slightly delayed. Endoscopic
biopsy diagnosis may be ineffective in colorectal metastasis due to
the presence of metastatic lesions under the mucosa (42). Even if no malignant findings are
revealed by biopsy, resection is a better option when it is
feasible based on the diagnostic imaging.
Similar to a previous report (42), most of the colon metastases from
gastric cancer (Tables I and
II) are poorly differentiated and
signet ring cell adenocarcinomas. In general, when the tissue is
intestinal, the metastases are often hematogenous, whereas when it
is poorly differentiated (diffuse type), the metastases are often
lymphatic/disseminated (43). It is
assumed that there is a mechanism responsible for the many
hematogenous metastases of poorly differentiated adenocarcinoma to
the colorectum from gastric cancer. Poorly differentiated and
signet ring cell adenocarcinomas are rare primary colorectal
cancers, and metastasis from gastric cancer should be considered
when biopsy results by colonoscopy show such findings (1).
Sonoda et al (5) divided and investigated the metastatic
pathways of colorectal metastasis from gastric cancer without
peritoneal carcinomatosis, as follows: i) metastatic pathway from
the gastrointestinal lumen; ii) lymphatic metastasis; and iii)
hematogenous metastasis. According to them, there are many
metastases from the gastrointestinal lumen, but the route is not
considered in each report. Lymphatic metastasis to the colorectum,
other than the transverse colon, is unlikely, and as reported by
Noji et al (2), metastasis
to the colorectum from the ovary or disseminated lesion is also
possible. In this report (Tables I
and II), many cases show
macroscopic findings that cause submucosal infiltration, and many
do not show malignant findings on the mucosal surface. Since
metastasis from the gastrointestinal tract often presents with
mucosal lesions and is often transplanted to the anastomotic
stapler site, it is considered that the frequency of gastric cancer
metastasis to the colon via the gastrointestinal tract is extremely
low. Lymphatic metastasis is unlikely in colonic metastasis from a
site distant from the stomach without dissemination, and therefore,
hematogenous metastasis is mostly considered. Cancer stem cells are
involved in carcinogenesis and metastasis, and their markers are
attracting attention in research. It is known that CD44 and
G-protein coupled receptor 5 (LGR5) are closely related to gastric
cancer (44,45). Although these were not investigated
in the case reported in our study, we believe that evaluating these
markers will be useful in future research on colorectal metastasis
from gastric cancer.
There are cases in which signet ring cell
adenocarcinoma of the mammary gland (46,47)
and small-cell carcinoma of the lung present with metastases to the
colon (48) with submucosal
infiltration. In addition, cases in which signet ring cell
adenocarcinoma of the sigmoid colon and rectum metastasized to the
stomach (49,50), and poorly differentiated
adenocarcinoma of the mammary gland metastasized to the stomach
(51,52) with submucosal invasion have been
reported. In many reports, metastatic lesions from other organs
that grow under the mucosa of organs of the gastrointestinal tract
show polypoid or linitis plastica-like findings (46–52).
In general, metastases from other organs to the gastrointestinal
tract are often hematogenous, are thought to infiltrate submucosal
lymphatics, and develop as linitis plastica (16,53).
Regarding gastrointestinal metastasis from breast cancer, many are
estrogen and progesterone receptor-positive, suggesting a
relationship with chemocain (51).
In addition, the metastasis is related to cancer-related adipocytes
(54), and the relationship with
submucosal and subserosal adipocytes of the gastrointestinal tract
is also considered, although currently, it remains unclear. When
tumor cell metastases occur submucosally, the smooth muscle acts as
a barrier to tumor infiltration. Furthermore, it is thought that
the tumor growth suppression mechanism of the muscle is involved in
the growth and infiltration of cancer in the submucosa (55). In our case, colonic metastasis
occurred at the same site as the colonic diverticulum. It has been
reported (56) that the microbiome
is involved in cancer development. In our case, metastasis of the
microbiome is unlikely, considering that the metastasis developed
mainly in the submucosa. Furthermore, after cancer onset, the
patient had achieved CR for a long time. In addition, in the
referenced previous case report, the role of the microbiome was not
mentioned; therefore, the relationship between metastasis and
infection is unknown.
In cases of metastasis, it is possible that cancer
lesions that have already metastasized before chemotherapy
administration for gastric cancer may recur when chemotherapy is
discontinued. Although patient prognosis has been extended due to
advances in chemotherapy for advanced gastric cancer, there is no
standard protocol for discontinuing chemotherapy for stage IV
gastric cancer. Therefore, it is difficult to decide on the
discontinuation of chemotherapy, as in the reported case.
In summary, a comprehensive study of published cases
of gastric cancer colon metastasis, comparison between macroscopic
classifications of gastric cancer, and these macroscopic features
have not been reported. In our study, no differences were observed
between the synchronous and metachronous metastasis groups.
Similarly, there were no significant differences between the two
groups in terms of sex, gastric cancer pathology, macroscopic
feature of gastric cancer, other metastatic sites besides the
colorectum, metastatic site in the colorectum, and chemotherapy
after the diagnosis of colorectal metastasis. In cases of
metachronous colorectal metastases, the rate of early gastric
cancer was higher, and the stage was considered lower than in cases
of synchronous metastases. According to the characteristics of
recurrence (Tables I and II), the stage of gastric cancer differs
depending on the age at which gastric cancer develops; therefore, a
comparison was made based on the age at which the metastasis to the
colorectum was diagnosed (Table
III). For synchronous and metachronous metastases, the mean age
of patients was 65.2±13.2 years and 63.2±10.4 years, respectively,
and there was no significant difference between the two groups
(P=0.608). In metachronous cases, the period from the initial
surgery for gastric cancer to the discovery of colorectal
metastasis was 55.3±9.9 months, which is considered a long
interval. The average ± standard error of prognosis after diagnosis
of colorectal metastases was 22.90±3.33 months and 8.37±1.73 months
for metachronous and synchronous colorectal metastases,
respectively (Table IV). The
prognosis for metachronous metastasis was significantly longer than
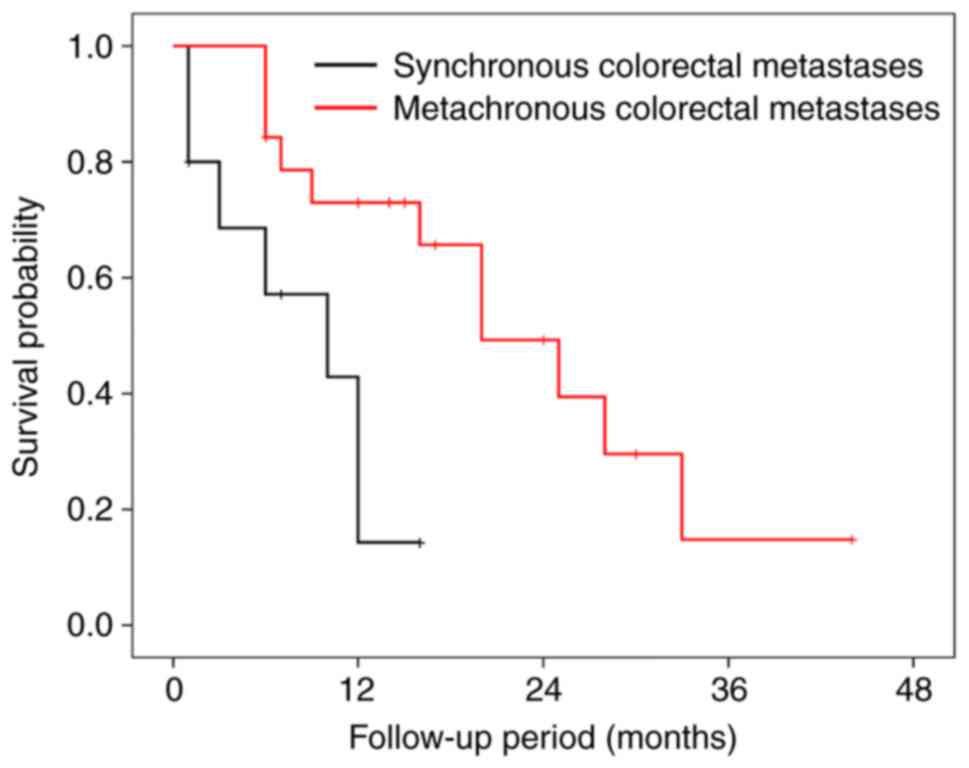
for synchronous metastasis (P=0.008) (Fig. 9). According to this case, both
synchronous and metachronous metastases were of stage IV, but the
prognosis of synchronous metastases was shorter than the overall
survival rate (57) of stage IV
gastric cancer.
 | Table III.Comparison between patients with
synchronous gastric cancer colorectal metastases and patients with
metachronous gastric cancer colorectal metastases. |
Table III.
Comparison between patients with
synchronous gastric cancer colorectal metastases and patients with
metachronous gastric cancer colorectal metastases.
|
| Synchronous
colorectal metastases | Metachronous
colorectal metastasis |
|
|---|
|
|
|
|
|
|---|
| Variables | No. | Value | No. | Value | P-value |
|---|
| Mean age ± SD,
years | 13 | 65.2±13.2 | 23 | 63.2±10.4 | 0.608a |
| Gastric cancer
pathology, n (%) | 13 |
| 22 |
|
>0.999b |
|
Poorly
differentiated |
| 12 (92.3) |
| 19 (86.4) |
|
|
Well-to-moderate
differentiated |
| 1 (7.7) |
| 3 (13.6) |
|
| Gastric cancer
macroscopic features, n (%) | 9 |
| 16 |
|
>0.999b |
|
Early |
| 1 (11.1) |
| 3 (18.8) |
|
|
Advanced |
| 8 (88.9) |
| 13 (81.3) |
|
| Recurrent colon
tumor macroscopic features, n (%) | 12 |
| 19 |
| 0.274b |
|
Polypoid or
early |
| 9 (75.0) |
| 10 (52.6) |
|
|
Advanced |
| 3 (25.0) |
| 9 (47.4) |
|
| Other metastatic
sites besides the colorectum, n (%) | 9 |
| 18 |
| 0.201b |
|
Yes |
| 8 (88.9) |
| 11 (61.1) |
|
|
No |
| 1 (11.1) |
| 7 (38.9) |
|
| Metastatic site in
the colorectum, n (%) | 13 |
| 24 |
| 0.667b |
|
Colon |
| 8 (61.5) |
| 18 (75.0) |
|
|
Rectum |
| 2 (15.4) |
| 3 (12.5) |
|
|
Colon and
rectum |
| 3 (23.1) |
| 3 (12.5) |
|
| Chemotherapy, n
(%) | 11 |
| 19 |
|
>0.999b |
|
Yes |
| 9 (81.8) |
| 15 (78.9) |
|
|
No |
| 2 (18.2) |
| 4 (21.1) |
|
 | Table IV.Prognostic comparison between
patients with synchronous and metachronous gastric cancer
colorectal metastases. |
Table IV.
Prognostic comparison between
patients with synchronous and metachronous gastric cancer
colorectal metastases.
|
| Mean survival,
months | Median survival,
months |
|---|
|
|
|
|
|---|
| Groups | Estimated mean
survival | SE | 95% CI | Estimated median
survival | SE | 95% CI |
|---|
| Synchronous | 8.37 | 1.73 | 4.98–11.77 | 10 | 4.88 | 0.43–19.57 |
| Metachronous | 22.90 | 3.33 | 16.36–29.43 | 20 | 4.55 | 11.07–28.93 |
| Whole | 19.11 | 2.84 | 13.53–24.69 | 20 | 4.88 | 10.44–29.56 |
We found that metachronous colorectal metastasis
from gastric cancer has a better prognosis than synchronous
metastasis. First, the prognosis after gastric cancer surgery
without MPC is better than with MPC. Synchronous MPC has a worse
prognosis than metachronous MPC; however, when MPC occurs, the
prognosis of metachronous colorectal metastasis deteriorates
rapidly. This phenomenon has been reported as a reason for the
treatment effect for gastric cancer in metachronous secondary
primary cancer (58). Therefore, in
gastric cancer metastasis, the therapy is expected to affect the
colorectal metastasis.
In terms of prognosis after the diagnosis of colonic
metastases, metachronous metastasis was associated with a favorable
outcome when considering the reported case. The reasons for this
are as follows: i) when metachronous gastric cancer has
metastasized to the colorectum, the cancer lesions often remain
localized or do not spread widely; ii) the effect of chemotherapy
and resection on colonic metastases improves prognosis.
As reported by Mayumi et al (18) and Wang et al (4), long-term survival has been achieved in
some cases by performing several resections and chemotherapy
treatments for repeated recurrence. This suggests that surgical
resection and chemotherapy may be effective for gastric metastasis
to the colorectum.
The use of chemotherapy is based on the treatment
guidelines of each country. In the reported cases, there were cases
in which the prognosis was considered to have improved by
administering chemotherapy using the same protocol or the same
anticancer drug after recurrence due to colorectal metastasis
(6,18,20,21,24).
In cases of metachronous recurrence, if the chemotherapy protocol
before recurrence was effective and the chemotherapy was
discontinued, the same treatment should be considered first.
However, it would be good to consider changing the protocol if
lesion progression is observed.
Future research on the mechanisms of cancer
metastasis and analysis of similar cases is expected to lead to
findings that will guide the development of novel and rational
treatment approaches. In particular, research advances involving
biomarkers, immunohistochemistry, and liquid biopsies should be
expected.
Limitation: Since some case reports did not describe
the stage and type of gastric cancer at the time of diagnosis or
the pathological results of the endoscopic biopsy performed to
diagnose colon metastasis, these items could not be statistically
processed and examined.
In conclusion, colon metastases from gastric cancer
may develop even after a prolonged period of treatment for gastric
cancer and often result in polypoid, linitis plastica-like
submucosal endoscopic findings. Such findings are similar to those
of hematogenous colonic metastases from other organs. The results
of biopsy pathology often show no malignant findings; however,
poorly differentiated adenocarcinoma and signet ring cell carcinoma
are common. When such findings are obtained, metastatic colorectal
cancer should be suspected. It has been suggested that treatment
for gastric cancer may have a therapeutic effect on gastric cancer
colorectal metastasis and that resection and/or chemotherapy for
gastric cancer metastases are effective.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Koji
Nomura (Department of Pathology, The Jikei University Katsushika
Medical Center, Tokyo, Japan) for his pathological assessment. This
abstract was presented at the 45th conference of Japanese College
of Surgeons (December 22, 2020-December 24, 2020; Kurume, Japan),
and was published as Abstract no. O4-04 (J-GLOBAL ID:
202102291766857226 21A0001180).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK, MI, SRY, HA, MO, FY and KE contributed to the
study conception and design. Material preparation, data collection
and analysis were performed by SK, SRY and HA. The first draft of
the manuscript was written by SK, and all authors commented on
previous versions of the manuscript. SK and MI confirm the
authenticity of all the raw data. KE gave final approval of the
version to be published. All authors have read and approved the
final manuscript.
Ethics approval and consent for
participate
Ethical review and approval were waived for this
case report because of the retrospective nature of the published
data.
Patient consent for publication
Written informed consent for publication was
obtained from the patient antemortem.
Authors' information
SK is a member of the Japan Surgical Society, the
Japanese Society of Gastroenterological Surgery, the Japanese
Society of Gastroenterology, the Japan Gastroenterological
Endoscopy Society, the Japan Society of Clinical Oncology, and the
Japanese Gastric Cancer Association.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogiwara H, Konno H, Kitayama Y, Kino I and
Baba S: Metastases from gastric adenocarcinoma presenting as
multiple colonic polyps: Report of a case. Surg Today. 24:473–475.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noji T, Yamamura Y, Muto J, Kuroda A,
Koinuma J, Yoshioka T, Murakawa K, Otake S, Hirano S and Ono K:
Surgical resection of colorectal recurrence of gastric cancer more
than 5 years after primary resection. Int J Surg Case Rep.
5:954–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elghali MA, Fadhl H, Hamila F, Mestiri S,
Jarrar MS and Letaief R: Colon metastasis, 8 years after
gastrectomy, for stage I gastric cancer. J Gastrointest Cancer.
50:127–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Ma P, Liu K, Xu D and Liu Q:
Gastric cancer with repeated metastasis in the colonic lumen: A
case report and multi-surgical experience. J Int Med Res.
49:30006052110184202021.PubMed/NCBI
|
|
5
|
Sonoda H, Kawai K, Yamaguchi H, Murono K,
Kaneko M, Nishikawa T, Otani K, Sasaki K, Yasuda K, Tanaka T, et
al: Lymphogenous metastasis to the transverse colon that originated
from signet-ring cell gastric cancer: A case report and review of
the literature. Clin Res Hepatol Gastroenterol. 41:e81–e86. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujimoto D, Hirono Y, Goi T and Yamaguchi
A: Sigmoid colonic metastasis by lymphatic spread occurring with
unilateral Krukenberg tumor considered to be caused by stage IA
early gastric cancer: A case report. Oncol Lett. 11:668–672. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galanopoulos M, Gkeros F, Liatsos C,
Pontas C, Papaefthymiou A, Viazis N, Mantzaris GJ and Tsoukalas N:
Secondary metastatic lesions to colon and rectum. Ann
Gastroenterol. 31:282–287. 2018.PubMed/NCBI
|
|
8
|
Kim C, Chon H, Kang B, Kim K, Jeung HC,
Chung H, Noh S and Rha S: Prediction of metachronous multiple
primary cancers following the curative resection of gastric cancer.
BMC Cancer. 13:3942013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kan JY, Hsieh JS, Pan YS, Wang WM, Chen
FM, Jan CM, Huang YS, Huang TJ and Wang JY: Clinical
characteristics of patients with sporadic colorectal cancer and
primary cancers of other organs. Kaohsiung J Med Sci. 22:547–553.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and statistical study.
Am J Cancer. 16:1358–1414. 1932.
|
|
11
|
Vidarsdottir H, Tran L, Nodin B, Jirström
K, Planck M, Jönsson P, Mattsson JSM, Botling J, Micke P and
Brunnström H: Immunohistochemical profiles in primary lung cancers
and epithelial pulmonary metastases. Hum Pathol. 84:221–230. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Kim BH, Kim JH, Lee S and Kang
GH: Panels of immunohistochemical markers help determine primary
sites of metastatic adenocarcinoma. Arch Pathol Lab Med.
131:1561–1567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong J, Chen Y and Wang LJ: Emerging
molecular basis of hematogenous metastasis in gastric cancer. World
J Gastroenterol. 22:2434–2440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanakaya K, Takeuchi H, Yasui Y, Takeda A,
Umeda Y and Murakami I: Metastatic carcinoma of the colon similar
to Crohn's disease: A case report. Acta Med Okayama. 58:217–220.
2004.PubMed/NCBI
|
|
15
|
Tseng LJ, Jao YT, Wu CJ, Lin RC, Kuo JY,
Chang KK and Mo LR: Metastatic gastric carcinoma presenting as
multiple submucosal colonic cysts. Gastrointest Endosc. 59:317–318.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pace U, Contino G, Chiappa A, Bertani E,
Bianchi PP, Fazio N, Renne G, Di Meglio G and Andreoni B:
Metachronous colon metastases from gastric adenocarcinoma: A case
report. Case Rep Oncol. 2:92–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim SW, Huh JW, Kim YJ and Kim HR:
Laparoscopic low anterior resection for hematogenous rectal
metastasis from gastric adenocarcinoma: A case report. World J Surg
Oncol. 9:1482011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayumi K, Terakura M, Hamano G, Ikebe T,
Takemura M and Hori T: A case of repeated postoperative recurrence
of gastric cancer treated via laparoscopic approach. Gan To Kagaku
Ryoho. 40:2176–2178. 2013.(In Japanese). PubMed/NCBI
|
|
19
|
Watanabe M, Narusaka T, Hayashi D,
Matsumura T, Nonaka Y, Kurose M, Tokuda N and Miyake T: A case of
metastatic carcinoma of the colon after curative resection for
gastric cancer. Gan To Kagaku Ryoho. 40:2182–2184. 2013.(In
Japanese). PubMed/NCBI
|
|
20
|
Oh SY, Cunningham J and Saif MW: Colonic
metastasis from gastric cancer. Clin Colorectal Cancer. 13:255–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita Y, Fujii Y, Miura S, Fujita J,
Morioka E, Kaida D, Ohonishi T, Ohono Y, Noguchi M, Funaki H, et
al: A successful case of treatment of colonic metastasis and
peritoneal recurrence of type 4 gastric cancer by using colectomy
and chemotherapy. Gan To Kagaku Ryoho. 42:1591–1593. 2015.(In
Japanese). PubMed/NCBI
|
|
22
|
Ikeda K, Sato T, Maezawa Y, Kano K,
Satoyoshi T, Segami K, Nakajima T, Ogata T, Cho H and Yoshikawa T:
Case of colon metastasis from early gastric cancer 4 years after
laparoscopic assisted distal gastrectomy. Gan To Kagaku Ryoho.
43:2208–2210. 2016.(In Japanese). PubMed/NCBI
|
|
23
|
Uemura N, Kurashige J, Kosumi K, Iwatsuki
M, Yamashita K, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida
N, et al: Early gastric cancer metastasizing to the rectum,
possibly via a hematogenous route: A case report and review of
literature. Surg Case Rep. 2:582016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higuchi I, Akiyama Y, Ishikawa A, Hosomi
S, Tanigawa T, Hasuike Y and Miyamoto M: Two cases of colon
metastasis of gastric cancer by different metastasis pathway. Gan
To Kagaku Ryoho. 45:127–129. 2018.(In Japanese). PubMed/NCBI
|
|
25
|
Su WC, Tsai HL, Wu CC, Tsai SY, Yeh YS, Ma
CJ and Wang JY: Two rare cases of synchronous and metachronous
colonic metastases in patients with advanced gastric cancer. World
J Surg Oncol. 16:212018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizuguchi N, Koyama M, Obana A, Kitamura
K, Matsumura T, Okada K, Karikomi K, Masamura S, Suzuki H and Suwa
T: A case of metachronous ascending colon metastasis from gastric
cancer that was difficult to diagnose. Gan To Kagaku Ryoho.
45:2075–2077. 2018.(In Japanese). PubMed/NCBI
|
|
27
|
Yang S, Liu XL, Guo XL, Song B, Li SZ, Sun
XF and Feng Y: Solitary metastasis to the skin and colon from
gastric cancer after curative gastrectomy and chemotherapy: A case
report. Medicine (Baltimore). 99:e215322020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okazaki Y, Shibutani M, Nagahara H,
Fukuoka T, Iseki Y, Wang E, Maeda K, Hirakawa K and Ohira M: A case
of submucosal-like tumor at cecum that was diagnosed as colon
metastasis from gastric cancer. Gan To Kagaku Ryoho. 47:2343–2345.
2020.(In Japanese). PubMed/NCBI
|
|
29
|
Then EO, Grantham T, Deda X, Ramachandran
R and Gaduputi V: Metastatic gastric cancer to the colon. World J
Oncol. 12:127–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metayer P, Antonietti M, Oumrani M, Hemet
J, Lemoine F and Basuyau J: Metastases of a gastric adenocarcinoma
presenting as colonic polyposis. Report of a case. Dis Colon
Rectum. 34:622–623. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tomikashi K, Mitsufuji S, Kanemasa H,
Sakai M, Wakabayashi N and Tsuchihashi Y: Gastric cancer metastatic
to the colon. Gastrointest Endosc. 55:5612002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HC, Yang MT, Lin KY, Tu HY, Zhang TA
and Chen PH: Metastases from gastric carcinoma to colon in the form
of multiple flat elevated lesions: A case report. Kaohsiung J Med
Sci. 20:552–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura H, Fu K, Fukui H, Hurlstone DP,
Kaji Y, Ishikawa T and Fujimori T: A solitary colonic metastasis
from gastric cancer detected at an early stage. Gastrointest
Endosc. 67:1000–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tural D, Selçukbiricik F, Erçalışkan A,
Inanç B, Günver F and Büyükünal E: Metachronous rectum metastases
from gastric adenocarcinoma: A case report. Case Rep Med.
2012:7268412012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reyes-Díaz ML, Torres-Arcos C,
Oliva-Mompeán F, Curado-Soriano A, Lizarralde-Gómez CJ and
Cuaresma-Soriano F: Colonic metastasis of diffuse signet ring cells
gastric carcinoma. Rev Esp Enferm Dig. 104:442–443. 2012.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao B, Xue X, Tai W, Zhang J, Chang H, Ma
X, Qi Y, Cui L, Yan F and Pan L: Polypoid colonic metastases from
gastric stump carcinoma: A case report. Oncol Lett. 8:1119–1122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee IH, Lee JE, Byeon SW, Lee HJ, Huo SM,
Yoon SB, Kim JS, Lee SH and Roh SY: A case of advanced gastric
cancer presenting as multiple colonic lymphoid hyperplasia. Korean
J Gastroenterol. 66:221–226. 2015.(In Korean). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patel YA, McCall SJ, Zhang X, Jaffe T and
Shimpi RA: Radiographic and endoscopic regression of metastatic
gastric cancer to the colon in the setting of 5-aminosalicylic acid
use. J Gastrointest Oncol. 7:E88–E92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Makker J, Karki N, Sapkota B, Niazi M and
Remy P: Rare presentation of gastroesophageal carcinoma with rectal
metastasis: A case report. Am J Case Rep. 17:611–615. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehershahi S, Mantri N, Sun H, Shaikh D
and Patel H: Metastasis from gastric signet ring cell
adenocarcinoma presenting as a rectosigmoid stricture: A rare case.
Cureus. 12:e85522020.PubMed/NCBI
|
|
41
|
Curtis RE, Freedman DM, Ron E, Ries LAG,
Hacker DG, Edward BK, Tucker MA and Fraumeni JF Jr: New
malignancies following cancer survivors: SEER cancer registries,
1973–2000. National Cancer Institute; Bethesda, MD, USA: pp. 9–14.
2006
|
|
42
|
Jang HJ, Lim HK, Kim HS, Cho EY, Lee SJ,
Kim KA and Choi D: Intestinal metastases from gastric
adenocarcinoma: Helical CT findings. J Comput Assist Tomogr.
25:61–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duarte I and Llanos OL: Patterns of
metastases in intestinal and diffuse types of carcinoma of the
stomach. Hum Pathol. 12:237–242. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Razmi M, Ghods R, Vafaei S, Sahlolbei M,
Saeednejad Zanjani L and Madjd Z: Clinical and prognostic
significances of cancer stem cell markers in gastric cancer
patients: A systematic review and meta-analysis. Cancer Cell Int.
21:1392021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi YJ, Kim N, Lee HS, Park SM, Park JH,
Yoon HY, Shin CM, Park YS, Kim JW and Lee DH: Expression of
leucine-rich repeat-containing G-protein coupled receptor 5 and
CD44: Potential implications for gastric cancer stem cell marker. J
Cancer Prev. 21:279–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fayemi AO, Ali M and Braun EV: Metastatic
carcinoma simulating linitis plastica of the colon. A case report.
Am J Gastroenterol. 71:311–314. 1979.PubMed/NCBI
|
|
47
|
Malhotra A, Guturu P, Basim MS and Raju
GS: A rare case of breast cancer metastasis presenting as linitis
plastica of the stomach and colon (with videos). Gastrointest
Endosc. 70:552–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hsiao SC, Chen YH, Lo CC and Lin CI: A
noteworthy treatment of metastatic small-cell lung cancer with
afatinib, followed by subsequent development of rare metastatic
lesions in the ascending and sigmoid colon. Cancer Rep (Hoboken).
3:e12432020.PubMed/NCBI
|
|
49
|
Li B, Li Q, Li X, Zhang G and Shen L:
Signet-ring cell cancer of the colon presenting as facial and
gastroduodenal metastasis 7 years after sigmoidectomy. Endoscopy.
46 (Suppl 1):E220–E221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Iwai N, Okuda T, Harada T, Oka K, Hara T,
Inada Y, Tsuji T, Komaki T, Dohi O, Yoshida N, et al: Gastric
metastasis from colorectal cancer mimicking a submucosal tumor.
Case Rep Gastroenterol. 14:338–345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fu JX, Zou YN, Long-Li L and Wang XJ:
Widespread metastasis to the stomach 10 years after primary breast
cancer: A case report and review of the literature. Medicine
(Baltimore). 99:e225272020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Woo J, Lee JH, Lee KE, Sung SH and Lim W:
Gastric metastasis as the first presentation one year before
diagnosis of primary breast cancer. Am J Case Rep. 19:354–359.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feczko PJ, Collins DD and Mezwa DG:
Metastatic disease involving the gastrointestinal tract. Radiol
Clin North Am. 31:1359–1373. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nieman KM, Romero IL, Van Houten BV and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Harryman WL, Marr KD, Hernandez-Cortes D,
Nagle RB, Garcia JGN and Cress AE: Cohesive cancer invasion of the
biophysical barrier of smooth muscle. Cancer Metastasis Rev.
40:205–219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khatun S, Appidi T and Rengan AK: The role
played by bacterial infections in the onset and metastasis of
cancer. Curr Res Microb Sci. 2:1000782021.PubMed/NCBI
|
|
57
|
Sano T, Coit DG, Kim HH, Roviello F,
Kassab P, Wittekind C, Yamamoto Y and Ohashi Y: Proposal of a new
stage grouping of gastric cancer for TNM classification:
International gastric cancer association staging project. Gastric
Cancer. 20:217–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ikeda Y, Saku M, Kawanaka H, Nonaka M and
Yoshida K: Features of second primary cancer in patients with
gastric cancer. Oncology. 65:113–117. 2003. View Article : Google Scholar : PubMed/NCBI
|