Introduction
Nasopharyngeal carcinoma (NPC) is a malignant
epithelial tumor of the nasopharynx that is common in Southeast
Asia and North Africa (1). Distant
metastases and local recurrence after primary curative treatment
are the most common causes of treatment failure (2,3). For
recurrent NPC and NPC with distant metastasis, the mainstream
treatment option still remains palliative systemic chemotherapy.
Platinum-containing two-drug or three-drug regimens were
recommended as first-line chemotherapy for RM-NPC by The Chinese
Society of Clinical Oncology (CSCO) in 2021 (1). Immunotherapy combined with
chemotherapy has also proved to be a promising treatment approach,
although it has always been controversial when combined with
anti-epidermal growth factor receptor (EGFR) monoclonal antibodies
(4).
The erb-b2 receptor tyrosine kinase 2 family of
receptors includes epidermal growth factor receptor (EGFR). The
EGFR signaling pathway is a critical regulator of cell
differentiation, proliferation, migration, angiogenesis and
apoptosis of cancer cells (5).
Overexpression of EGFR is common in NPC (6) and certain studies have indicated that
patients with high EGFR mRNA expression levels have worse prognoses
than those with low expression levels (7). Furthermore, a study analyzing clinical
samples from a cohort revealed a association between EGFR
overexpression and the clinical stage, distant metastasis state and
advanced tumor-node-metastasis stage of patients with NPC (8).
Anti-EGFR monoclonal antibodies including cetuximab
(CTX) and nimotuzumab (NTZ) were discussed in the present study. A
recombinant chimeric human/mouse IgG1 monoclonal antibody called
CTX binds to EGFR and blocks the binding of EGF and other ligands
through competitive binding. In contrast to NTZ, CTX binds to EGFR
with greater specificity and affinity, competing for ligand binding
and thereby blocking ligand-induced EGFR tyrosine kinase activation
(5). Although NTZ is an IgG1
monoclonal antibody against human EGFR, this humanization lessens
the immunogenicity of the substance (9). Thus, combined use of CTX/NTZ with
palliative chemotherapy (PCT) may be a therapeutic option for
patients with recurrent and/or metastatic NPC (RM-NPC).
In comparison to chemotherapy alone, combination
treatment with CTX was reported to improve response rates,
progression-free survival (PFS) and overall survival (OS) of
patients with recurrent and/or metastatic squamous cell head and
neck cancer (10); however, primary
NPC was an exclusion criterion in that study. In terms of biology,
epidemiology, histology, natural history and therapeutic response,
NPC is distinct from other head and neck malignancies (6). According to previous reports, the
majority of NPC cases have high EGFR expression, which is
independently associated with poor prognosis (11). To assess the effectiveness of
EGFR-targeted therapies (CTX/NTZ) in combination with chemotherapy
in RM-NPC, various retrospective studies have been conducted
(12,13). Since 2004, a combination of CTX/NTZ
and PCT has been trialed for treating RM-NPC and the results were
documented in multiple case series. The median PFS (mPFS) was 8.9
months (95% CI: 7.7-10.0 months) and the median OS (mOS) was 29.1
months (95% CI: 23.5-34.6 months) in the study by Chen et al
(12) including 203 patients with
RM-NPC who underwent first-line chemotherapy with an anti-EGFR
antibody. The PFS and OS rates at 1, 3 and 5 years were 35.5 and
79.6%, 15.2 and 42.5%, and 11.6 and 23.6%, respectively (12). Thus, this treatment appears to
achieve promising antitumor activity with tolerable toxicity.
However, the effectiveness of CTX/NTZ and PCT was comparable to
that of single PCT treatment among de novo metastatic patients with
NPC as per the propensity score reported by Sun et al
(13). The addition of CTX to
concurrent radiochemotherapy (CCRT) may worsen the acute mucositis
and skin reactions, and the addition of anti-EGFR drugs to CCRT for
patients with de novo metastatic NPC may not be beneficial.
In light of the clinical effect of CTX added to
RM-NPC treatment, only a Phase III randomized, controlled,
multi-center trial (NCT02633176) comparing cisplatin, docetaxel
plus CTX with cisplatin and docetaxel has been reported (14). Thus, there appears to be a lack of
credible evidence supporting the use of EGFR-targeted treatments
for RM-NPC. Accordingly, the present study aimed to examine the
available literature on the combined use of CTX/NTZ with PCT for
RM-NPC.
Materials and methods
Study protocol
Preferred Reporting Items for Systematic Reviews and
Meta-Analysis standards were followed in the present study
(15). Systematic searches in the
Pubmed, EMBASE, the Cochrane library, WanFang Data and China
National Knowledge Infrastructure databases were conducted up to
February 15, 2022. All terms that may be used to refer to
chemotherapy and RM-NPC were included in the search terms.
Accordingly, searches were conducted in these databases using the
following terms: (‘recurrent/metastatic nasopharyngeal carcinoma’
OR ‘mNPC’ OR ‘recurrent/metastatic nasopharynx cancer’ OR
‘recurrent/metastatic nasopharyngeal tumor’ OR
‘recurrent/metastatic nasopharyngeal neoplasms’ OR ‘advanced
nasopharyngeal carcinoma’) AND (‘cetuximab’ OR ‘CTX’ OR ‘targeted
therapy’ OR ‘anti-EGFR’ OR ‘nimotuzumab’ OR ‘NTZ’) AND
‘chemotherapy’. In addition, the reference lists of relevant
articles were searched to identify further studies. English- and
Chinese-language articles were included. Unpublished research was
excluded from the search. XLN and HCY evaluated the included
studies independently and sequent disagreements were resolved by
discussion with a third investigator (DZ).
Selection criteria
All of the following criteria were required to be
met by the studies to be eligible for inclusion in the present
meta-analysis: i) Studies with at least 10 patients with RM-NPC;
ii) clinical trials, prospective studies or retrospective research;
and iii) containing information on at least one topic on survival
(OS, PFS, 1-, 2-, 3- and 5-year OS rates), short-term effects
[objective response rate (ORR), disease control rate (DCR)] and
safety. The following were applied as the exclusion criteria: i)
Letters, case reports, animal or in vitro research, reviews,
conference articles and abstracts; ii) studies for which full-text
articles could not be retrieved or those with insufficient data;
and iii) duplicate reports.
After checking the titles of the studies that were
searched, reviews, duplicates, animal or in vitro research,
and case reports were removed. The studies were filtered to
determine whether they met the inclusion criteria and to check for
relevance to the study subject by reviewing the abstracts. When
multiple studies had been published by the same center, the study
with the greatest number of RM-NPC cases was included, as long as
it met the inclusion criteria. Finally, a full-text review was
performed on the filtered articles to determine whether they were
relevant to the study subject and met all inclusion criteria. Two
independent investigators performed the entire study selection
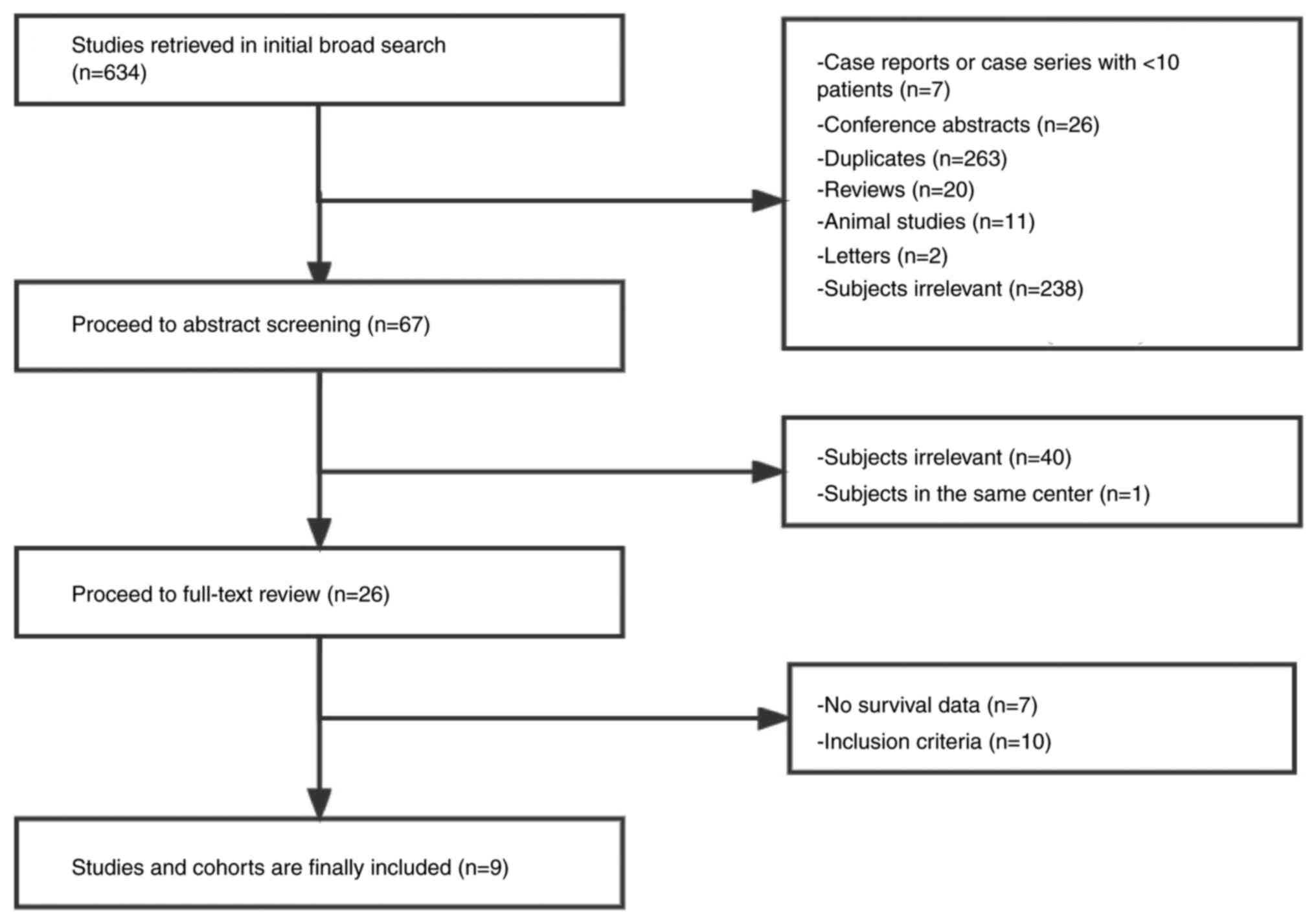
process. Fig. 1 depicts the process
of study selection.
Data extraction
The following data were independently extracted by
two reviewers: i) First author, year of publication, country,
design type, number of participants, inclusion period, age, sex,
stage, treatment, NTZ/CTX, chemotherapy regimens and radiotherapy.
The total relevant data and subgroup characteristics were extracted
from noncomparative studies. ii) Antitumor efficacy indices [drug
responses including complete response, partial response, stable
disease, ORR and DCR; survival outcomes including mOS, mFPS, 1-,
2-, 3- and 5-year OS rate]. iii) All grade 3–4 adverse events (AEs)
were also extracted. All original data were entered into related
tables by XLN and HCY and a third reviewer (DZ) rectified any
discrepancies.
Quality assessment
As the majority of the included studies were
retrospective in nature, two authors (DZ and JY) evaluated the
quality of the included studies using the Nottingham Ottawa Scale
(NOS) (16). By analyzing three
domains-selection, comparability and outcome for cohort studies, or
exposure for case-control studies-the NOS rates the quality of
clinical trials. A report with a NOS score of 7 to 9 was considered
to be of high quality, whereas one with a score of 4 to 6 was
considered medium quality.
Statistical analysis
Primary endpoints were mOS, mFPS, and 1-, 2-, 3- and
5-year OS rates. Secondary endpoints included ORR, DCR and
toxicities at grade 3 or higher. Further, mOS, mFPS and toxicities
were described in detail. To display the results of each analysis,
a forest plot was drawn. Heterogeneity was defined as a P-value of
the Cochran Q test being <0.1 and the I2 statistic
being >50% (17,18). If the data were significantly
heterogeneous (P<0.1, I2>50%), a random-effects
model was used; otherwise, a fixed-effects model was used for
analysis.
Publication bias for primary and secondary endpoints
was assessed visually using the asymmetry of the funnel plot and
quantitatively using Egger's test of intercept (19) and Duval and Tweedie's trim and fill
test (20). If the two-tailed
P-value in Egger's test was <0.1, Duval and Tweedie's trim test
was performed. Comprehensive Meta-Analysis software version 3 was
used for all statistical analyses (Biostat, Inc.).
Results
Study selection and features
Following the initial broad search using the search
terms, 634 studies were screened. The first screening eliminated
567 studies due to being duplicate studies, reviews, letters,
animal studies, conference abstracts and case reports or case
studies with 10 or fewer patients, or had an irrelevant topic. The
titles and abstracts of the remaining 67 studies were then
carefully reviewed and 40 studies with irrelevant topics were
further excluded. One study was omitted because it was published by
the same institute. The full-text contents of the remaining 26
studies were examined to determine whether they met all of the
inclusion criteria. A total of 7 studies were eliminated because
they only provided short-term efficacy with no survival data, and
10 studies were eliminated because they did not meet the inclusion
criteria. Finally, 9 studies (12,21–28)
comprising 435 patients (346 males and 89 females) were included in
the present analysis.
A total of 8 studies were retrospective in nature
and only 1 study was prospective. Of these 9 studies, 7 studies
were published in English journals and 2 studies were from Chinese
journals. The period of analysis of these studies was between 2004
and 2019. Overall, 207 patients were treated with NTZ, whereas 228
patients were treated with CTX. A total of 3 studies reported
outcomes of combined treatment with NTZ and PCT, 5 studies reported
outcomes of combined treatment with CTX and PCT, and only 1 of the
9 studies reported outcomes of combined treatment with NTZ/CTX and
PCT. The treatment with CTX/NTZ ranged from 2 to 31 cycles,
consistent with PCT. PCT regimens included Gemcitabine + platinum
(GP), Fluorouracil + platinum (PF), Paclitaxel + fluorouracil +
platinum (TPF) and Paclitaxel + carboplatin (PC). Furthermore, 2
studies also reported on combined radiotherapy with PCT. Table I, Table
II, Table III provide
summaries of the baseline characteristics, clinical outcomes and
grade 3–4 AEs of these included investigations, respectively. The
quality levels of all nine studies fell into the medium quality
range on the NOS scale (Table
IV).
 | Table I.Characteristics of included
trials. |
Table I.
Characteristics of included
trials.
| First author,
year | Country | Design type | Inclusion
period | n | Age, years | Males, % | Stage | CTX/NTZ | Treatment (dose and
cycles) | Chemotherapy | Radiotherapy | (Refs.) |
|---|
| Zhu, 2020 | China | Cohort | 2004-2018 | 49 | 49.08±10.72 | 81.6 | RM-NPC | NA | CT (5 cycles,
range=2-8) | TP, GP, PF | NA | (21) |
|
|
|
|
| 21 | 48.81±13.32 | 100 | RM-NPC | NTZ (12 cycles,
range=3-31) | CT (4 cycles,
range=2-8) | TP, GP, PF | NA |
|
| Zhang, 2020 | China | Prospective | 2006-2014 | 43 | 43 (23–63) | 83.7 | RM-NPC | CTX | CT (≤6 cycles) | TP | IMRT | (24) |
| Xu, 2015 | China | Retrospective | 2007-2011 | 30 | 44 (26–62) | 73.3 | RM-NPC | CTX (7 cycles,
range=3-18) | NA | GP, TP, TPF,
PC | IMRT | (28) |
| Ueda, 2020 | Japan | Retrospective | 2013-2019 | 14 | 59.6 (43–74) | 71.4 | RM-NPC | CTX continued until
disease progression or unacceptable toxicities | CT (6 cycles) | PC | NA | (23) |
| Chen, 2020 | China | Retrospective
cohort | 2007-2017 | 203 | 43 (12–72) | 82.8 | RM-NPC | CTX/NTZ | NA | GP, TP, TPF,
PF | NA | (12) |
| Chan, 2005 | China | Phase II study | NA | 60 | 44.5 (23–64) | 77 | III, IV | CTX (10 cycles,
range=1-30) | CT (≤8 cycles) | PF | NA | (22) |
| Zhao, 2019 | China | Phase II clinical
trial | 2012-2015 | 35 | 44 (29–67) | 85.7 | RM-NPC | NTZ (12
cycles) | CT (6 cycles) | PF | NA | (25) |
| Gao, 2013 | China | Retrospective | 2009-2012 | 12 | 35 | 83.3 | IV | NA | CT (≥2 cycles) | GP | NA | (26) |
|
|
|
|
| 10 | 50 | 80 | IV | CTX | CT (≥2 cycles) | GP | NA |
|
| Yao, 2013 | China |
| 2009-2012 | 18 | 45.1 | 83.3 | NA | NA | CT (2 cycles) | GP | NA | (27) |
|
|
|
|
| 19 | 45.3 | 78.9 | NA | NTZ (2 cycles) | CT (2 cycles) | GP | NA |
|
 | Table II.Clinical results of included
trials. |
Table II.
Clinical results of included
trials.
| First author,
year | n | M follow
up(months) | mOS | Survival
outcomes | Drug response,
% |
|---|
|
|
|---|
| mPFS | OSR-1y | OSR-2y | OSR-3y | OSR-5y | CR | PR | SD | ORR | DCR | (Refs.) |
|---|
| Zhu, 2020 | 49 | 62 | 25.6
(18.9–32.4) | 7.5 (6.6–8.4) | NR | NR | 36.7 | 25.4 | 4.1 (2) | 55.1 (27) | 32.7 (16) | 59.2 (29) | 91.8 (45) | (21) |
|
| 21 | 59 | 48.69
(35.6–61.6) | 8.5 (6.1–11.0) | NR | NR | 76.2 | 42.9 | 0 (0) | 57.1 (12) | 28.6 (6) | 57.1 (12) | 85.7 (18) |
|
| Zhang, 2020 | 43 | NA | 32.9
(18.2–47.5) | 18.3
(10.6–26.0) | 88.4 | 60.5 | 48.8 | 34.9 | 34.9 (15) | 44.2 (19) | 14 (6) | 79.1
(66.9–91.2) | 93 (85.4–100) | (24) |
| Xu, 2015 | 30 | NA | 23.6 | NR | 100 | 53.3 | NR | NR | 10.0 | 60.0 | 23.3 | 70.0 | 93.3 | (28) |
| Ueda, 2020 | 14 | 23.8 | Not reached | 4.1 (2.6–5.6) | NR | NR | NR | NR | 16.7 (2) | 41.7 (5) | 33.3 (4) | 58.3 (7) | 91.7 (11) | (23) |
| Chen, 2020 | 203 | 34.3 | 29.1
(23.5–34.6) | 8.9 (7.7–10.0) | 79.6 | NR | 42.5 | 23.6 | 3.9 | 63.6 | 23.6 | 67.5 | 91.1 | (12) |
| Chan, 2005 | 60 | NA | 7.8 | 2.7 | NR | NR | NR | NR | 0 | 11.7 | 48.3 | 11.7 | 60.0 | (22) |
| Zhao, 2019 | 35 | 13.2 | 16.3
(11.4–21.3) | 7 (5.8–8.2) | 60.7 | 35.4 | 24.8 | NR | 3 | 68.6 | 14.3 | 71.4 | 85.7 | (25) |
| Gao, 2013 | 12 | NA | 46.43 | 7.07 | NR | NR | NR | NR | CR+PR, 16.7 |
| 66.7 | 16.7 | 83.3 | (26) |
|
| 10 | NA | 39.97 | 11 | NR | NR | NR | NR | CR+PR, 40.0 |
| 60.0 | 40.0 | 100 |
|
| Yao, 2013 | 18 | NA | 29.3 | 7 | NR | NR | NR | NR | NR | NR | NR | 16.7 | 77.8 | (27) |
|
| 19 | NA | 40.2 | 11.2 | NR | NR | NR | NR | NR | NR | NR | 42.1 | 100 |
|
 | Table III.Grade 3–4 treatment-related adverse
effects. |
Table III.
Grade 3–4 treatment-related adverse
effects.
| First author,
year | N | Neutropenia
(%) | Leucopenia (%) | Anemia (%) | Thrombocytopenia
(%) | Vomiting Nausea
(%) | Decreased (%)
(%) | Alopecia
appetite | Neuropathy (%) | Acne-like (%) | Dermatitis rash
(%) | (%) | (Ref.) |
|---|
| Zhu, 2020 | 49 | 42.9 | 44.9 | 6.1 | NR | 4.1 | 4.1 | 0 | 10.2 | 2 | NR | NR | (21) |
|
| 21 | 42.9 | 28.6 | 4.8 | NR | 4.8 | 0 | 4.8 | 19 | 4.8 | NR | NR |
|
| Zhang, 2020 | 43 | 14 | 39.5 | 2.3 | 9.3 | 0 | 0 | NR | 0 | 0 | 11.6 | 0 | (24) |
| Xu, 2015 | 30 | 86.7 | NR | 26.7 | 10 | NR | NR | NR | NR | NR | 20 | NR | (28) |
| Ueda, 2020 | 14 | 21.4 | 28.3 | 0 | 0 | 7.1 | 7.1 | 0 | NR | 0 | 14.3 | NR | (23) |
| Chen, 2020 | 203 | NR | 43.4 | NR | 11.3 | 1 | 1 | 0 | NR | 0 | 1.5 | NR | (12) |
| Chan, 2005 | 60 | NR | 5 | 18.3 | 10 | NR | 6.7 | NR | NR | 6.7 | 11.7 | NR | (22) |
| Zhao, 2019 | 35 | NR | 62.9 | NR | NR | 22.9 | 25.7 | 17.2 | NR | NR | NR | NR | (25) |
| Gao, 2013 | 12 | NR | 33.3 | 0 | 16.7 | 0 | 0 | 0 | NR | NR | 0 | NR | (26) |
|
| 10 | NR | 40 | 40 | 40 | 0 | 0 | 0 | NR | NR | 0 | NR |
|
| Yao, 2013 | 18 | NR | 33.3 | 0 | 11.1 | 0 | 0 | NR | NR | NR | 0 | NR | (27) |
|
| 19 | NR | 36.8 | 0 | 36.8 | 0 | 0 | NR | NR | NR | 0 | NR |
|
 | Table IV.Newcastle-Ottawa Scale assessment of
the quality of studies included in the meta-analysis. |
Table IV.
Newcastle-Ottawa Scale assessment of
the quality of studies included in the meta-analysis.
|
| Selection | Comparability
control for important factor | Exposure |
|
|
|---|
|
|
|
|
|
|
|---|
| First author,
year | Adequate
definitions of the cases | Representativeness
of the cases | Selection of
controls | Definition of
controls | Ascertainment of
exposure | Same method of
ascertainment for cases and controls | Non-response
rate | Scores | (Refs.) |
|---|
| Zhu, 2020 | ★ | ★ | - | - | ★ | ★ | ★ | ★ | 6 | (21) |
| Zhang, 2020 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (24) |
| Xu, 2015 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (28) |
| Ueda, 2020 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (23) |
| Chen, 2020 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (12) |
| Chan, 2005 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (22) |
| Zhao, 2019 | ★ | ★ | - | - | - | ★ | ★ | ★ | 5 | (25) |
| Gao, 2013 | ★ | - | ★ | ★ | ★ | ★ | - | - | 5 | (26) |
| Yao, 2013 | ★ | - | ★ | ★ | ★ | ★ | - | - | 5 | (27) |
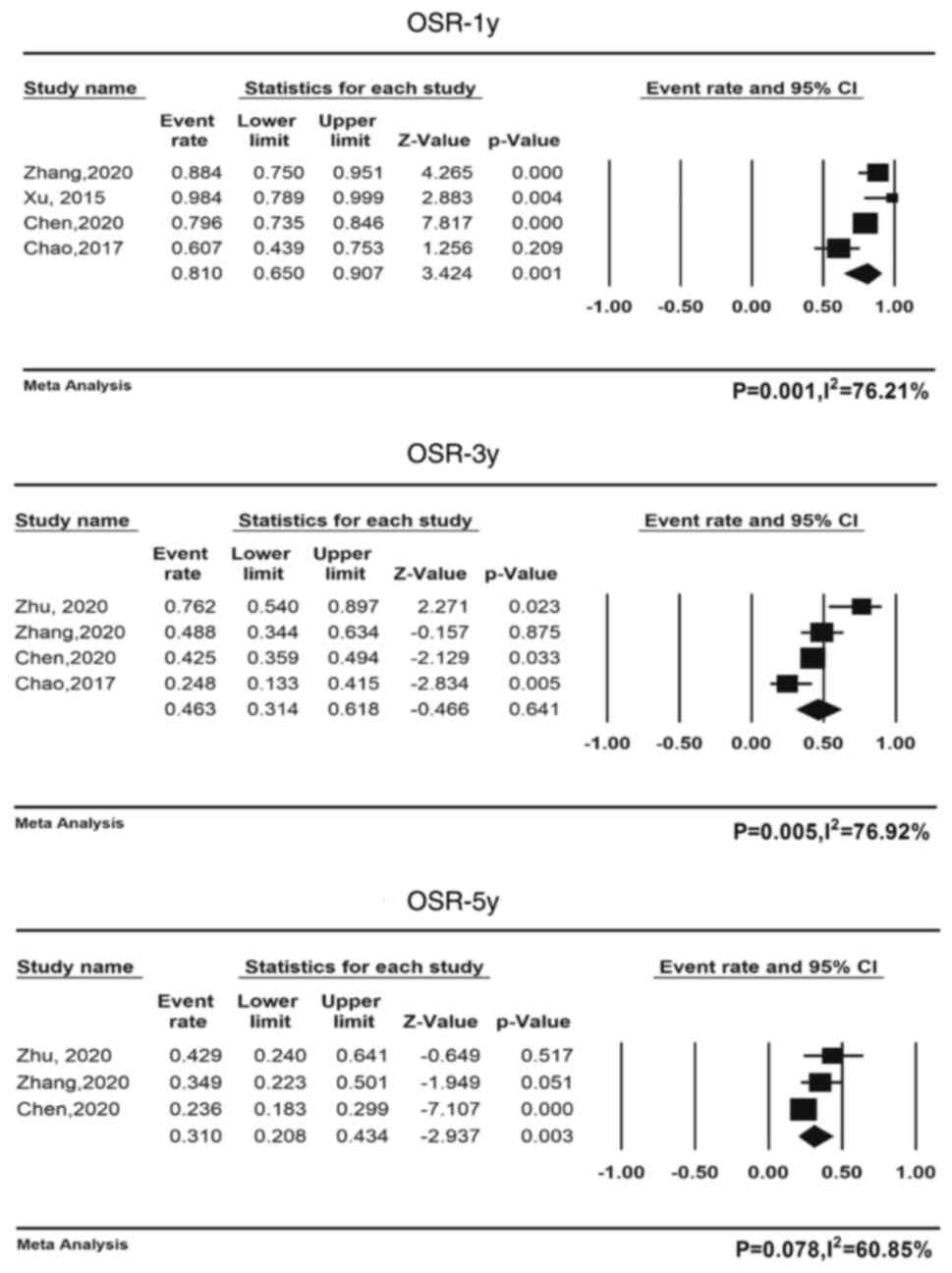
OS
Only 4 studies including 302 patients reported mOS
and its range (12,21,24,25);
the pooled mOS was 30.8 months (95% CI, 18.5-43.2,
I2=90.1%); 5 studies comprising 316 patients reported
mPFS and its range (12,21,23–25),
and the pooled mPFS was 7.9 months (95% CI, 5.4-10.2,
I2=89.5%). The pooled 1-year OS rate for all four
cohorts was 81.0% (95% CI: 65.0-90.7%). The pooled 2-year OS rates
were available for all three cohorts, with a pooled rate of 49.9%
(95% CI: 35.3-64.5%) (Table V), and
the pooled 3-year OS rates for all four cohorts were available,
with a pooled rate of 46.3% (95% CI: 31.4-61.8%), and the pooled
5-year OS rates for all three cohorts were also available, with a
pooled rate of 31.0% (95% CI: 20.8-43.4%). Fig. 2 displays a forest plot containing
the 1-, 3- and 5-year survival data. Table V provides an overview of the
combined survival rates.
 | Table V.Summary of pooled rates. |
Table V.
Summary of pooled rates.
|
| Rates, % (95%
CI) | P-value,
I2 | Effect model | Publication
bias | Trimmed result, %
(95% CI) |
|---|
| OSR-1y | 81 (65–90.7) | 0.001, 76.21 | Random | Yes | 77.6
(59.1–89.3) |
| OSR-2y | 49.9
(35.3–64.5) | 0.090, 58.47 | Random | No | / |
| OSR-3y | 46.3
(31.4–61.8) | 0.005, 76.92 | Random | No | / |
| OSR-5y | 31 (20.8–43.4) | 0.078, 60.85 | Random | Yes | 23.6
(15.5–34.1) |
| DCR | 88.7
(78.4–94.5) | <0.001,
78.79 | Random | Yes | 85.9
(75.6–92.3) |
| ORR | 55.6
(39.9–70.1) | <0.001,
85.20 | Random | No | / |
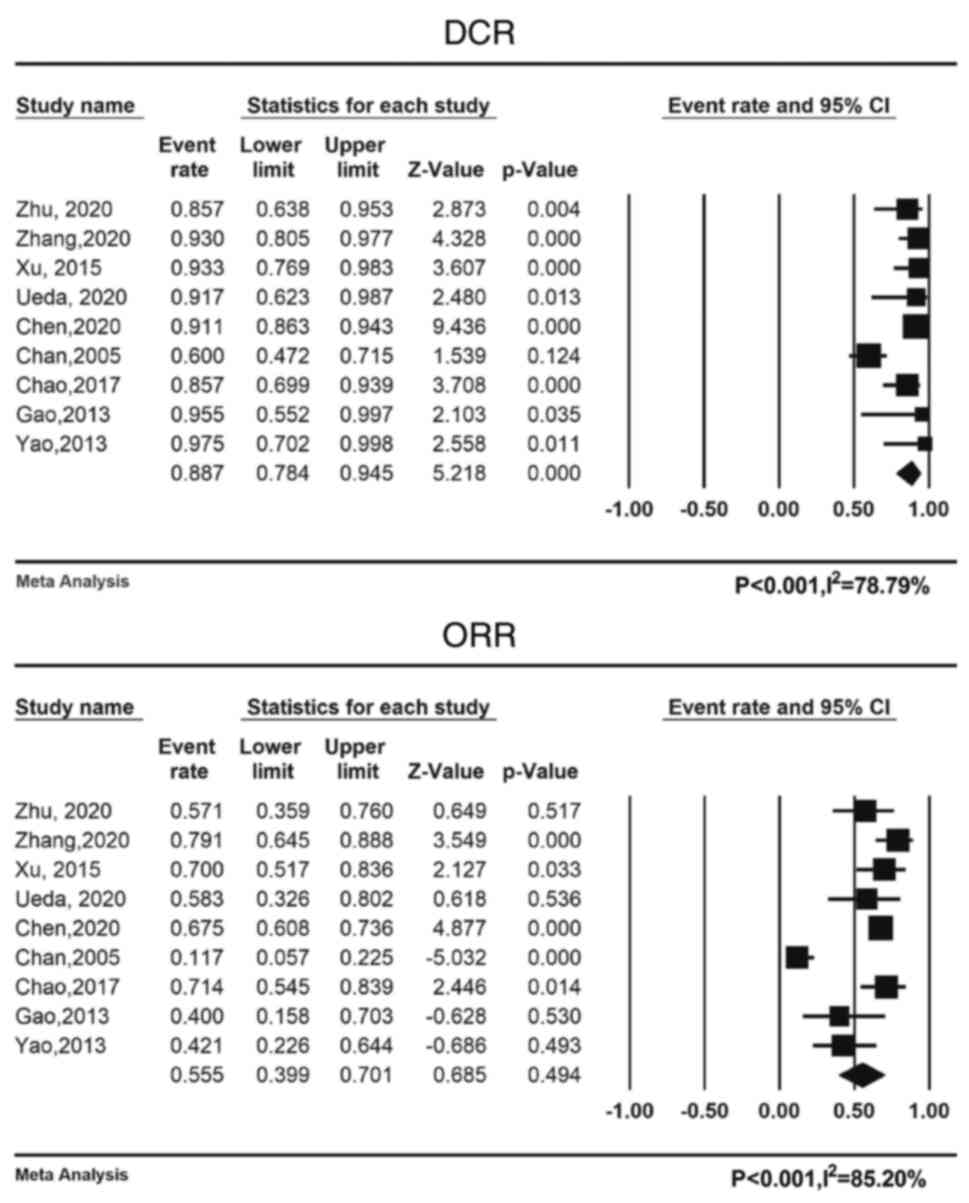
Pooled DCR and ORR rates were 88.7% (95% CI:
78.4-94.5%) and 55.6% (95% CI: 39.9-70.1%), respectively. Fig. 3 displays a forest plot of the ORR
and DCR. Table V provides an
overview of the pooled DCR and ORR. Due to the heterogeneity of the
included trials, a random-effects model was used to calculate the
ORR and DCR of the disease (I2=78.79 and 85.20%,
respectively; P<0.001 for both).
Treatment toxicities
A list of all 3–4 AEs from each included study was
compiled (Table VI). Neutropenia
(40.7%), leucopenia (32.6%), platelet count decrease (12.4%) and
anemia (12.7%) were the most common AEs. Other AEs included nausea
(3.5%), vomiting (4.0%), decreased appetite (2.5%), alopecia
(6.3%), neuropathy (1.5%) and acne-like rash (6.0%).
 | Table VI.Grade 3–4 AEs in included
studies. |
Table VI.
Grade 3–4 AEs in included
studies.
| AEs | Studies with
reported AEs | Events/total | % |
|---|
| Hematological
system AEs |
|
|
|
|
Neutropenia | 4 | 44/108 | 40.7 |
|
Leucopenia | 8 | 132/405 | 32.6 |
|
Anemia | 7 | 25/197 | 12.7 |
|
Thrombocytopenia | 7 | 47/379 | 12.4 |
| Digestive system
AEs |
|
|
|
|
Nausea | 7 | 12/345 | 3.5 |
|
Vomiting | 8 | 16/405 | 4.0 |
|
Decreased
appetite | 5 | 7/283 | 2.5 |
| Others |
|
|
|
|
Alopecia | 2 | 4/64 | 6.3 |
|
Neuropathy | 5 | 5/341 | 1.5 |
|
Acne-like
rash | 7 | 23/379 | 6.0 |
|
Dermatitis | 1 | 0/43 | 0.0 |
Publication bias
Publication bias was found for 1-year OS (P=0.608),
5-year OS (P=0.036) and DCR (P=0.247) using Egger's regression test
and on visual inspection of funnel plots (data not shown). Using
Duval and Tweedie's method, trimmed data for these three rates were
obtained (Table V). One study for
1-year overall survival, two for 5-year overall survival and four
for response rate were trimmed.
Discussion
To the best of our knowledge, the present study was
the first single-group rate meta-analysis to pool the efficacy of
the combination of CTX/NTZ and PCT in treating RM-NPC. Finally, 9
studies comprising 435 patients were included in the present study,
wherein 207 patients were treated with NTZ and 228 patients were
treated with CTX. These results mostly represent the efficacy and
toxicities in the Asian population, notably in China, as the vast
majority of the patients included in the present analysis had been
treated in China. In addition, the male:female ratio of the pooled
cohort of the present study was in line with that reported in the
literature. The incidence of NPC is much higher in males than in
females, with a ratio of ~2.5:1 in China in 2015 (1). RM-NPC is a set of heterogeneous
disorders that are typically broken down into three categories: De
novo metastasis, locoregional recurrence and locoregional
recurrence with distant metastasis (1).
The pooled mOS and mPFS were 30.8 and 7.9 months in
the present study, respectively, which appear higher than those
observed with standard PCT (29,30).
In 2021, a final OS analysis of the GEM20110714 phase III study: GP
vs. FP as first-line therapy for RM-NPC, reported a median OS of
22.1 months with GP vs. 18.6 months with FP. The OS rate with GP
vs. FP at 1, 3 and 5 years was 79.9, 31.0 and 19.2% compared with
71.8, 20.4 and 7.8%, respectively (29). By contrast, in the present study,
the pooled 1-, 2-, 3- and 5-year OS rates were 81.0, 49.9, 43.6 and
31.0%, respectively. The rate observed in the present study was
also higher than that observed with standard PCT. There are several
reasons for this, which may include the following: First, PCT was
administered for 2–8 cycles or until unacceptable toxicities
developed in included studies; CTX or NTZ was continued until
disease progression or unacceptable toxicities developed in certain
studies, wherein maintenance therapy may contribute to longer PFS
or OS (31,32); however, more evidence is still
required to confirm this in the future. Second, two out of nine
studies (24,28) concluded that adding local radiation
to chemotherapy significantly increased OS in patients with mNPC
who were responsive to treatment (33). Other palliative first-line systemic
treatment options included immunotherapy combined with gemcitabine
plus cisplatin and other chemotherapeutic regimens in the 2021 CSCO
guidelines. Yang et al (4)
compared camrelizumab plus GP with placebo plus GP in a randomised
phase 3 trial, and independent review committee-assessed PFS was
significantly longer in the camrelizumab group (median, 9.7 months)
than that in the placebo group (median, 6.9 months). Toripalimab
was added to GP chemotherapy as a first-line treatment for patients
with RM-NPC in a multicenter randomized phase 3 trial, which
demonstrated better PFS compared with GP alone and a tolerable
safety profile (34). Other
chemotherapeutic regimens, targeted therapy and most recently
immunotherapy have steadily developed as palliative systemic
treatment options in RM-NPC (35).
In the present study, a thorough analysis of various conventional
chemotherapy regimens was performed with an emphasis on
contemporary chemotherapeutic strategies, as well as the most
recent advancements in targeted medicine (4,34).
All grade 3–4 AEs reported in the included studies
were also gathered in the present study. The most common grade 3–4
AEs were neutropenia (40.7%), leucopenia (32.6%), platelet count
decrease (12.4%) and anemia (12.7%). Acne-like rash was another AE,
which was observed at a frequency of 6%. Unlike CTX and
small-molecule EGFR tyrosine kinase inhibitors, NTZ did not cause
any acne-like rash. NTZ is able to preserve the equilibrium between
the tethered and stretched EGFR conformations and does not obstruct
EGFR signaling at the basal level, which is essential for the
survival of healthy epithelial cells. These processes, along with
the intermediate affinity of NTZ for other anti-EGFR antibodies,
may account for the low level of side effects and low toxicity
observed in clinical settings (9).
Thus, NTZ may have a greater complete remission rate or overall
remission rate of primary tumors in NPC compared with cetuximab,
according to the findings of a network meta-analysis. However,
there was no difference in the 1- and 2-year OS rates between NTZ
and CTX (36).
The present study has a number of limitations.
First, meta-analysis of observational studies is debatable
(37) and heterogeneity among
studies in terms of varying patient characteristics and study
methods may have had an impact on pooled rates (38). Oncology does not always have solid
evidence; thus, therapeutic decisions may be based on observational
studies, numerous small trials or even just clinical experience
alone. Although randomized controlled trials provide the strongest
evidence, this is not always the case (39). A meta-analysis may be one of the few
ways available to evaluate therapeutic efficacy and safety, as
there are minimal observational study data regarding the
combination of CTX/NTZ and PCT to date, despite the fact that
RM-NPC is not an extremely rare disease. Second, most of the study
participants were Chinese, which may have biased the results.
Third, various therapy techniques had been used on the study
participants. Fourth, the number of included articles and patients
was small.
In conclusion, the current meta-analysis
demonstrated that the combination of CTX/NTZ with PCT may be a
feasible palliative treatment option for patients with RM-NPC.
However, high-quality evidence with large sample sizes is needed to
further validate the efficacy of EGFR-targeted therapies for
RM-NPC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
Conceptualization: XN, JZ, DZ, JY, HY; data
curation: XN, HY, DZ; formal analysis: XN, HY, DZ, JY;
investigation: XN, HY, DZ, JY, QD; methodology: XN, HY; project
administration: XN, JZ, DZ, JY, HY; software: XN, QD; supervision:
XN, DZ, QD; writing-original draft: XN, JZ; writing-review and
editing: XN, JZ, DZ, JY, HY, QD. XN and JZ confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RM-NPC
|
recurrent and/or metastatic
nasopharyngeal carcinoma
|
|
CTX
|
cetuximab
|
|
NTZ
|
Nimotuzumab
|
|
PCT
|
palliative chemotherapy
|
|
EGFR
|
epidermal growth factor receptor
|
|
mOS
|
median overall survival
|
|
PFS
|
progression-free survival
|
|
DFS
|
disease-free survival
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR (CR + PR)
|
objective response rate
|
|
DCR (CR + PR + SD)
|
disease control rate
|
|
SR
|
survival rate
|
|
AE
|
adverse event
|
|
CI
|
confidence interval
|
|
CSCO
|
Chinese Society of Clinical
Oncology
|
|
CCRT
|
concurrent radiochemotherapy
|
|
NOS
|
Nottingham Ottawa Scale
|
|
GP
|
Gemcitabine + Platinum
|
|
PF
|
Fluorouracil + Platinum
|
|
TPF
|
Paclitaxel + Fluorouracil +
Platinum
|
|
PC
|
Paclitaxel + Carboplatin
|
|
NA
|
not available
|
|
NR
|
no relevant statistical data
|
References
|
1
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
Society of Clinical Oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poh SS, Soong YL, Sommat K, Lim CM, Fong
KW, Tan TW, Chua ML, Wang FQ, Hu J and Wee JT: Retreatment in
locally recurrent nasopharyngeal carcinoma: Current status and
perspectives. Cancer Commun (Lond). 41:361–370. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie
A, Mendenhall WM, Rinaldo A, Rodrigo JP, Saba NF, Strojan P, et al:
Management of locally recurrent nasopharyngeal carcinoma. Cancer
Treat Rev. 79:1018902019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chidharla A, Parsi M and Kasi A:
Cetuximab, in StatPearls. 2022, StatPearls Publishing,
Copyright© 2022. StatPearls Publishing LLC; Treasure
Island, FL: 2022
|
|
6
|
Tu C, Zeng Z, Qi P, Li X, Guo C, Xiong F,
Xiang B, Zhou M, Liao Q, Yu J, et al: Identification of genomic
alterations in nasopharyngeal carcinoma and nasopharyngeal
carcinoma-derived Epstein-Barr virus by whole-genome sequencing.
Carcinogenesis. 39:1517–1528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang P, Wu SK, Wang Y, Fan ZX, Li CR,
Feng M, Xu P, Wang WD and Lang JY: p53, MDM2, eIF4E and EGFR
expression in nasopharyngeal carcinoma and their correlation with
clinicopathological characteristics and prognosis: A retrospective
study. Oncol Lett. 9:113–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng X, Zhou Y, Tao Y and Liu S:
Nasopharyngeal carcinoma: The role of the EGFR in epstein-barr
virus infection. Pathogens. 10:11132021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quatrale AE, Petriella D, Porcelli L,
Tommasi S, Silvestris N, Colucci G, Angelo A and Azzariti A:
Anti-EGFR monoclonal antibody in cancer treatment: In vitro and in
vivo evidence. Front Biosci (Landmark Ed). 16:1973–1985. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Depenni R, Cossu Rocca M, Ferrari D,
Azzarello G, Baldessari C, Alù M, Nolé F, Codecà C, Boscolo G,
Piccininni M, et al: Clinical outcomes and prognostic factors in
recurrent and/or metastatic head and neck cancer patients treated
with chemotherapy plus cetuximab as first-line therapy in a
real-world setting. Eur J Cancer. 115:4–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang R, Yang L and Zhu X: Nimotuzumab, an
Anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal
carcinoma. Cancer Control. 28:10732748219893012021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Zhou Y, Zhang X, Fu S, Lin Z, Fang
W, Yang Y, Huang Y, Zhao H, Hong S and Zhang L: Anti-epidermal
growth factor receptor monoclonal antibody plus palliative
chemotherapy as a first-line treatment for recurrent or metastatic
nasopharyngeal carcinoma. Cancer Med. 9:1721–1732. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun XS, Liang YJ, Li XY, Liu SL, Chen QY,
Tang LQ and Mai HQ: Palliative chemotherapy with or without
anti-EGFR therapy for de novo metastatic nasopharyngeal carcinoma:
A propensity score-matching study. Drug Des Devel Ther.
13:3207–3216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Liang R and Zhu X: Anti-EGFR
therapies in nasopharyngeal carcinoma. Biomed Pharmacother.
131:1106492020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analysis: The PRISMA statement. Int J Surg. 8:336–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.aspApril
3–2023
|
|
17
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1538. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cochran WG: The Combination of Estimates
from Different Experiments. Int Biometric Soc. 10:101–129. 1954.
View Article : Google Scholar
|
|
19
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duval S and Tweedie R: Tweedie: Trim and
fill: A simple funnel-plot-based method of testing and adjusting
for publication bias in meta-analysis. Biometrics. 56:455–463.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Yang S, Zhou S, Yang J, Qin Y, Gui
L, Shi Y and He X: Nimotuzumab plus platinum-based chemotherapy
versus platinum-based chemotherapy alone in patients with recurrent
or metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol.
12:17588359209537382020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al:
Multicenter, phase II study of cetuximab in combination with
carboplatin in patients with recurrent or metastatic nasopharyngeal
carcinoma. J Clin Oncol. 23:3568–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ueda Y, Enokida T, Okano S, Fujisawa T,
Ito K and Tahara M: Combination treatment with paclitaxel,
carboplatin, and cetuximab (PCE) as first-line treatment in
patients with recurrent and/or metastatic Nasopharyngeal carcinoma.
Front Oncol. 10:5713042020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Huang H, Li X, Huang Y, Chen C,
Fang X, Wang Z, Guo C, Lam S, Fu X, et al: Long-term survival of
patients with chemotherapy-naïve metastatic nasopharyngeal
carcinoma receiving cetuximab plus docetaxel and cisplatin regimen.
Front Oncol. 10:10112020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao C, Miao J, Shen G, Li J, Shi M, Zhang
N, Hu G, Chen X, Hu X, Wu S, et al: Anti-epidermal growth factor
receptor (EGFR) monoclonal antibody combined with cisplatin and
5-fluorouracil in patients with metastatic nasopharyngeal carcinoma
after radical radiotherapy: A multicentre, open-label, phase II
clinical trial. Ann Oncol. 30:637–643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao HB and Zheng DY: The application of
cetuximab in patients with local advanced nasopharyngeal carcinoma.
Guangdong Med J. 34:2244–2246. 2013.
|
|
27
|
Yao HQ, Yang CL, Yang G and Yang YB: The
effect of cetuximab combined with gemcitabine in the treatment of
the advanced nasopharyngeal carcinoma followed by Paclitaxel. Anhui
Med Pharm J. 19:1391–1392. 2015.
|
|
28
|
Xu T, Ou X, Shen C and Hu C: Cetuximab in
combination with chemoradiotherapy in the treatment of recurrent
and/or metastatic nasopharyngeal carcinoma. Anticancer Drugs.
27:66–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong S, Zhang Y, Yu G, Peng P, Peng J, Jia
J, Wu X, Huang Y, Yang Y, Lin Q, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin as First-line therapy for
recurrent or metastatic nasopharyngeal carcinoma: Final overall
survival analysis of GEM20110714 phase III study. J Clin Oncol.
39:3273–3282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Xuan J, Yang Z, Han A, Xing L, Yue
J, Hu M and Yu J: The expression of epidermal growth factor
receptor and Ki67 in primary and relapse nasopharyngeal cancer: A
micro-evidence for anti-EGFR targeted maintenance therapy. Med
Oncol. 29:1448–1455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XS, Liu SL, Liang YJ, Chen QY, Li XY,
Tang LQ and Mai HQ: The role of capecitabine as maintenance therapy
in de novo metastatic nasopharyngeal carcinoma: A propensity score
matching study. Cancer Commun (Lond). 40:32–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
You R, Liu YP, Huang PY, Zou X, Sun R, He
YX, Wu YS, Shen GP, Zhang HD, Duan CY, et al: Efficacy and safety
of locoregional radiotherapy with chemotherapy vs chemotherapy
alone in de novo metastatic nasopharyngeal carcinoma: A multicenter
phase 3 randomized clinical trial. JAMA Oncol. 6:1345–1352. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
27:1536–1543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee V, Kwong D, Leung TW, Lam KO, Tong CC
and Lee A: Palliative systemic therapy for recurrent or metastatic
nasopharyngeal carcinoma-How far have we achieved? Crit Rev Oncol
Hematol. 114:13–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan C, Xu XH, Xu L, Liu Y, Sun M, Ni LH,
Wang XL, Chen Z, Zhang K, Wan HL and Zeng G: Cetuximab versus
nimotuzumab for the treatment of advanced nasopharyngeal carcinoma:
A network meta-analysis. J buon. 22:1004–1010. 2017.PubMed/NCBI
|
|
37
|
Blettner M, Sauerbrei W, Schlehofer B,
Scheuchenpflug T and Friedenreich C: Traditional reviews,
meta-analyses and pooled analyses in epidemiology. Int J Epidemiol.
28:1–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poonacha TK and Go RS: Level of scientific
evidence underlying recommendations arising from the National
Comprehensive Cancer Network clinical practice guidelines. J Clin
Oncol. 29:186–1891. 2011. View Article : Google Scholar : PubMed/NCBI
|