|
1
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
Society of Clinical Oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poh SS, Soong YL, Sommat K, Lim CM, Fong
KW, Tan TW, Chua ML, Wang FQ, Hu J and Wee JT: Retreatment in
locally recurrent nasopharyngeal carcinoma: Current status and
perspectives. Cancer Commun (Lond). 41:361–370. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie
A, Mendenhall WM, Rinaldo A, Rodrigo JP, Saba NF, Strojan P, et al:
Management of locally recurrent nasopharyngeal carcinoma. Cancer
Treat Rev. 79:1018902019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chidharla A, Parsi M and Kasi A:
Cetuximab, in StatPearls. 2022, StatPearls Publishing,
Copyright© 2022. StatPearls Publishing LLC; Treasure
Island, FL: 2022
|
|
6
|
Tu C, Zeng Z, Qi P, Li X, Guo C, Xiong F,
Xiang B, Zhou M, Liao Q, Yu J, et al: Identification of genomic
alterations in nasopharyngeal carcinoma and nasopharyngeal
carcinoma-derived Epstein-Barr virus by whole-genome sequencing.
Carcinogenesis. 39:1517–1528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang P, Wu SK, Wang Y, Fan ZX, Li CR,
Feng M, Xu P, Wang WD and Lang JY: p53, MDM2, eIF4E and EGFR
expression in nasopharyngeal carcinoma and their correlation with
clinicopathological characteristics and prognosis: A retrospective
study. Oncol Lett. 9:113–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng X, Zhou Y, Tao Y and Liu S:
Nasopharyngeal carcinoma: The role of the EGFR in epstein-barr
virus infection. Pathogens. 10:11132021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quatrale AE, Petriella D, Porcelli L,
Tommasi S, Silvestris N, Colucci G, Angelo A and Azzariti A:
Anti-EGFR monoclonal antibody in cancer treatment: In vitro and in
vivo evidence. Front Biosci (Landmark Ed). 16:1973–1985. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Depenni R, Cossu Rocca M, Ferrari D,
Azzarello G, Baldessari C, Alù M, Nolé F, Codecà C, Boscolo G,
Piccininni M, et al: Clinical outcomes and prognostic factors in
recurrent and/or metastatic head and neck cancer patients treated
with chemotherapy plus cetuximab as first-line therapy in a
real-world setting. Eur J Cancer. 115:4–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang R, Yang L and Zhu X: Nimotuzumab, an
Anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal
carcinoma. Cancer Control. 28:10732748219893012021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Zhou Y, Zhang X, Fu S, Lin Z, Fang
W, Yang Y, Huang Y, Zhao H, Hong S and Zhang L: Anti-epidermal
growth factor receptor monoclonal antibody plus palliative
chemotherapy as a first-line treatment for recurrent or metastatic
nasopharyngeal carcinoma. Cancer Med. 9:1721–1732. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun XS, Liang YJ, Li XY, Liu SL, Chen QY,
Tang LQ and Mai HQ: Palliative chemotherapy with or without
anti-EGFR therapy for de novo metastatic nasopharyngeal carcinoma:
A propensity score-matching study. Drug Des Devel Ther.
13:3207–3216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Liang R and Zhu X: Anti-EGFR
therapies in nasopharyngeal carcinoma. Biomed Pharmacother.
131:1106492020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analysis: The PRISMA statement. Int J Surg. 8:336–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.aspApril
3–2023
|
|
17
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1538. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cochran WG: The Combination of Estimates
from Different Experiments. Int Biometric Soc. 10:101–129. 1954.
View Article : Google Scholar
|
|
19
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duval S and Tweedie R: Tweedie: Trim and
fill: A simple funnel-plot-based method of testing and adjusting
for publication bias in meta-analysis. Biometrics. 56:455–463.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
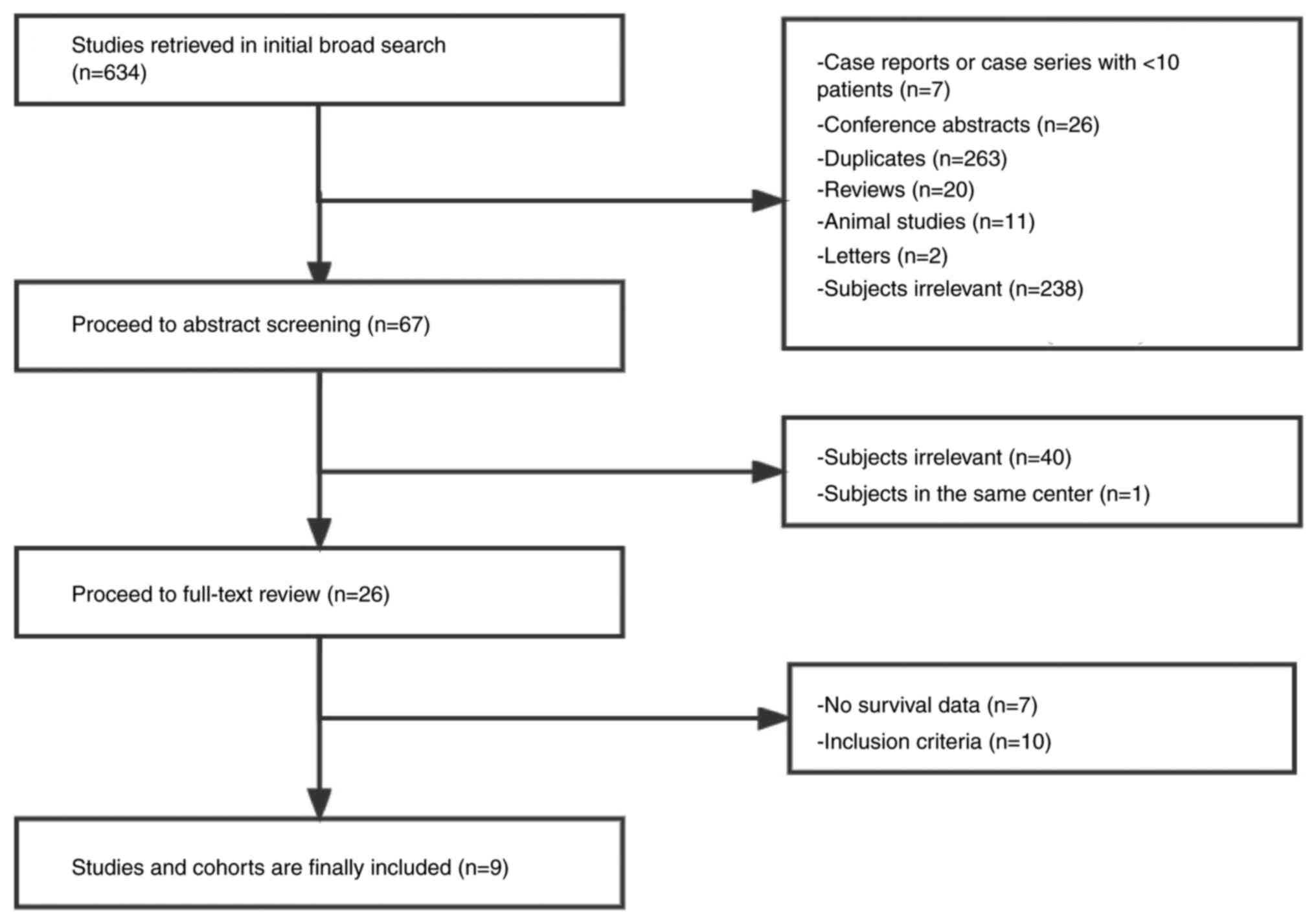
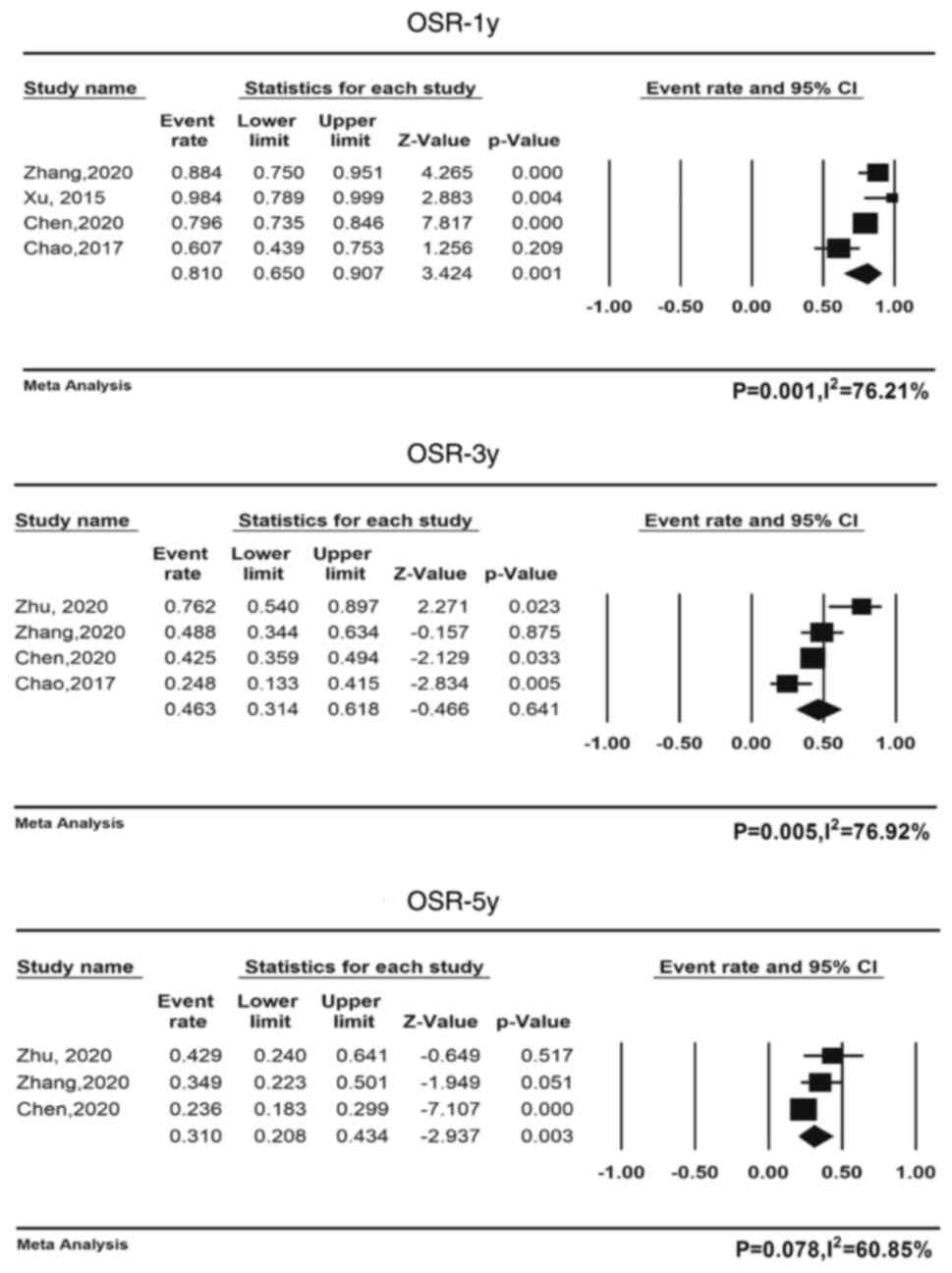
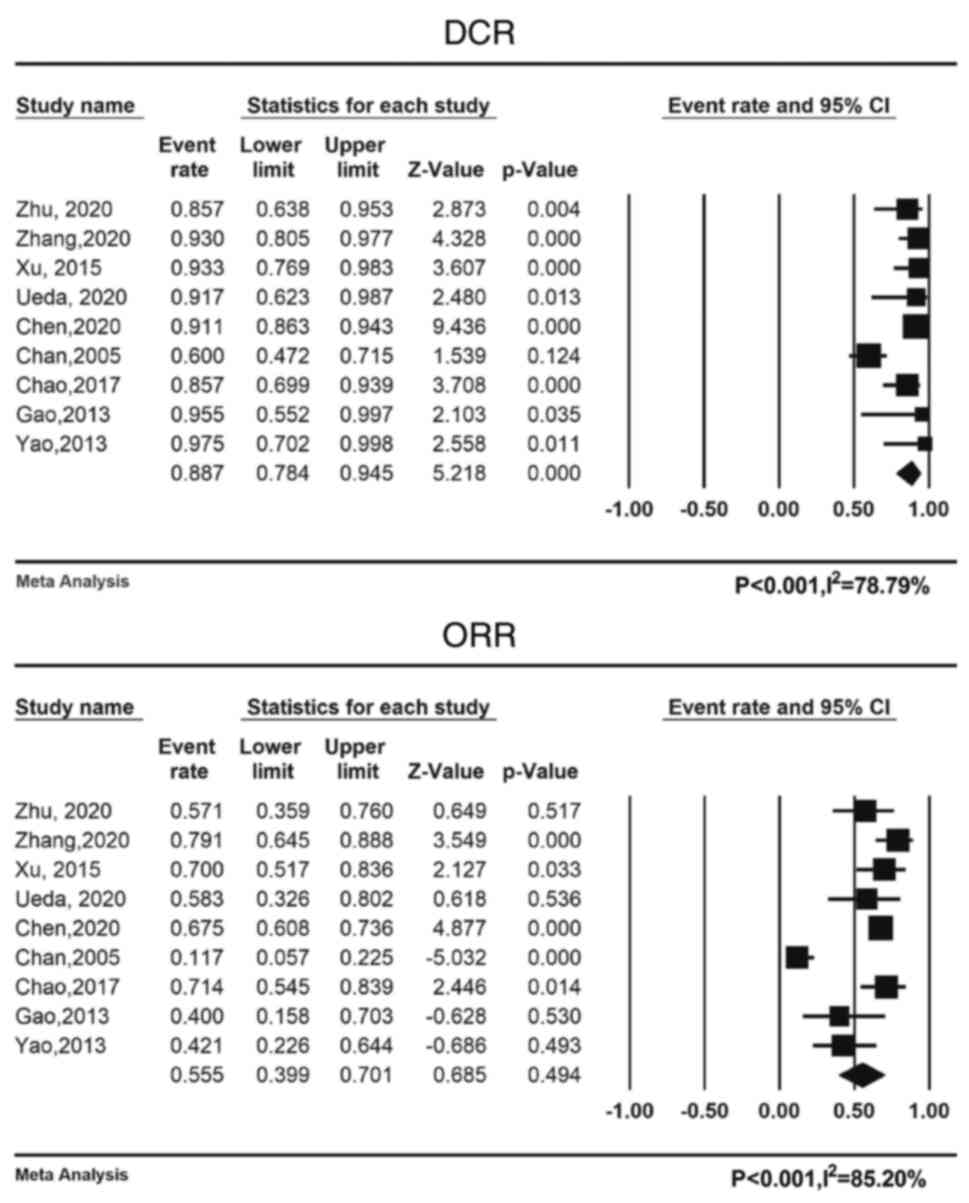
Zhu Y, Yang S, Zhou S, Yang J, Qin Y, Gui
L, Shi Y and He X: Nimotuzumab plus platinum-based chemotherapy
versus platinum-based chemotherapy alone in patients with recurrent
or metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol.
12:17588359209537382020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al:
Multicenter, phase II study of cetuximab in combination with
carboplatin in patients with recurrent or metastatic nasopharyngeal
carcinoma. J Clin Oncol. 23:3568–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ueda Y, Enokida T, Okano S, Fujisawa T,
Ito K and Tahara M: Combination treatment with paclitaxel,
carboplatin, and cetuximab (PCE) as first-line treatment in
patients with recurrent and/or metastatic Nasopharyngeal carcinoma.
Front Oncol. 10:5713042020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Huang H, Li X, Huang Y, Chen C,
Fang X, Wang Z, Guo C, Lam S, Fu X, et al: Long-term survival of
patients with chemotherapy-naïve metastatic nasopharyngeal
carcinoma receiving cetuximab plus docetaxel and cisplatin regimen.
Front Oncol. 10:10112020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao C, Miao J, Shen G, Li J, Shi M, Zhang
N, Hu G, Chen X, Hu X, Wu S, et al: Anti-epidermal growth factor
receptor (EGFR) monoclonal antibody combined with cisplatin and
5-fluorouracil in patients with metastatic nasopharyngeal carcinoma
after radical radiotherapy: A multicentre, open-label, phase II
clinical trial. Ann Oncol. 30:637–643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao HB and Zheng DY: The application of
cetuximab in patients with local advanced nasopharyngeal carcinoma.
Guangdong Med J. 34:2244–2246. 2013.
|
|
27
|
Yao HQ, Yang CL, Yang G and Yang YB: The
effect of cetuximab combined with gemcitabine in the treatment of
the advanced nasopharyngeal carcinoma followed by Paclitaxel. Anhui
Med Pharm J. 19:1391–1392. 2015.
|
|
28
|
Xu T, Ou X, Shen C and Hu C: Cetuximab in
combination with chemoradiotherapy in the treatment of recurrent
and/or metastatic nasopharyngeal carcinoma. Anticancer Drugs.
27:66–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong S, Zhang Y, Yu G, Peng P, Peng J, Jia
J, Wu X, Huang Y, Yang Y, Lin Q, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin as First-line therapy for
recurrent or metastatic nasopharyngeal carcinoma: Final overall
survival analysis of GEM20110714 phase III study. J Clin Oncol.
39:3273–3282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Xuan J, Yang Z, Han A, Xing L, Yue
J, Hu M and Yu J: The expression of epidermal growth factor
receptor and Ki67 in primary and relapse nasopharyngeal cancer: A
micro-evidence for anti-EGFR targeted maintenance therapy. Med
Oncol. 29:1448–1455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XS, Liu SL, Liang YJ, Chen QY, Li XY,
Tang LQ and Mai HQ: The role of capecitabine as maintenance therapy
in de novo metastatic nasopharyngeal carcinoma: A propensity score
matching study. Cancer Commun (Lond). 40:32–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
You R, Liu YP, Huang PY, Zou X, Sun R, He
YX, Wu YS, Shen GP, Zhang HD, Duan CY, et al: Efficacy and safety
of locoregional radiotherapy with chemotherapy vs chemotherapy
alone in de novo metastatic nasopharyngeal carcinoma: A multicenter
phase 3 randomized clinical trial. JAMA Oncol. 6:1345–1352. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
27:1536–1543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee V, Kwong D, Leung TW, Lam KO, Tong CC
and Lee A: Palliative systemic therapy for recurrent or metastatic
nasopharyngeal carcinoma-How far have we achieved? Crit Rev Oncol
Hematol. 114:13–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan C, Xu XH, Xu L, Liu Y, Sun M, Ni LH,
Wang XL, Chen Z, Zhang K, Wan HL and Zeng G: Cetuximab versus
nimotuzumab for the treatment of advanced nasopharyngeal carcinoma:
A network meta-analysis. J buon. 22:1004–1010. 2017.PubMed/NCBI
|
|
37
|
Blettner M, Sauerbrei W, Schlehofer B,
Scheuchenpflug T and Friedenreich C: Traditional reviews,
meta-analyses and pooled analyses in epidemiology. Int J Epidemiol.
28:1–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poonacha TK and Go RS: Level of scientific
evidence underlying recommendations arising from the National
Comprehensive Cancer Network clinical practice guidelines. J Clin
Oncol. 29:186–1891. 2011. View Article : Google Scholar : PubMed/NCBI
|