Introduction
Stage III non-small cell lung cancer (NSCLC)
accounts for one third of NSCLC at the time of initial diagnosis,
and stage IIIA includes small tumors (T1a-T2b) with N2 involvement,
large tumors (T3-T4) with N1 involvement and T4N0 [International
Association for the Study of Lung Cancer (IASLC)/Union for
International Cancer Control (UICC)/TNM staging system, 8th
edition] (1). The survival of
patients with stage IIIA NSCLC is poor: 55% of patients are alive
at 24 months and the 5-year survival rate is 36% (2). Radiotherapy is an important treatment
for which there are indications in all stages of lung cancer
(3). For unresectable stage III
NSCLC, thoracic radiotherapy with concurrent chemotherapy is the
standard of care for the majority of patients (4). However, in patients with resected
III-N+ NSCLC, postoperative radiotherapy (PORT) is not recommended
in all patients. On one hand, PORT had a negative effect on the
survival rate of patients with pN0 and pN1 disease (5). On the other hand, the effect of PORT
on stage IIIA-N2 NSCLC has been controversial. There have been two
large randomized clinical trial (RCT) studies (6,7)
reporting the value of PORT for completely resected stage IIIA-N2
NSCLC. One is the LungART (6) study
from Europe, which enrolled 501 patients (252 in the PORT group and
249 in the control group). The results confirmed that PORT reduced
the mediastinal recurrence rate but did not significantly improve
disease-free survival (DFS) rate (47 vs. 44%) or overall survival
(OS) rate (67 vs. 69%). Another is the PORT-C study from China
(7), which used modified
intent-to-treat analysis and included 364 patients (184 in the PORT
group and 180 in the observation group). There was no significant
difference in 3-year OS (78.3 vs. 82.8%; P=0.93), but PORT
significantly improved the 3-year local recurrence-free survival
(LRFS) rate (66.5 vs. 59.7%; P=0.03). In addition, there was no
significant difference in 3-year DFS (40.5 vs. 32.7%; P=0.20).
However, in a pre-planned yet exploratory analysis of the PORT-C
study (7), DFS significantly
differed after stratification according to the number of detected
lymph nodes (DLNs) and positive lymph nodes (PLNs) [hazard ratio
(HR), 0.75; P=0.04].
As stage N2 NSCLC is a group of heterogeneous
diseases, the efficacy of PORT may differ among subgroups with
different clinicopathological features, such as the number of N2
stations (8,9), the number of N2 PLNs (10), histological type (11,12),
smoking status (13), radiotherapy
technology (14) and sex (15). Stage N2 NSCLC has a high risk of
local recurrence (35–60%); therefore, some patients may still
benefit from PORT (16,17). However, we hypothesised that not all
patients may benefit from PORT. The present study aimed to screen
the potential benefitting population of PORT through
clinicopathological subgroup analysis.
Materials and methods
Patient selection
Between October 2010 and September 2016, 288
consecutive patients with pathologically confirmed stage IIIA-N2
NSCLC (according to the 7th edition of IASLC/UICC/TNM) (2) were included in the study. Patients who
survived >4 months after radical resection at the Beijing Chest
Hospital (Beijing, China) were included. The main eligibility
criteria were as follows: i) Eastern Cooperative Oncology Group
performance status (PS) (18) of 0
or 1; ii) not having received neo-adjuvant chemotherapy or
chemoradiotherapy; and iii) information about tumor
characteristics, pathology and follow-up data being available. The
mean age was 58 years (range, 31–80 years) and 63.2% of the
included patients were male. The medical records and follow-up data
of the patients were retrospectively analysed.
Treatment
The surgical methods for the 288 patients were
divided into thoracic (261 cases) and thoracoscopic surgery (27
cases). Surgery included single lobectomy (212 cases), compound
lobectomy (18 cases), sleeve resection (12 cases) and total lung
resection (pneumonectomy; 46 cases). Surgical patients met the
following criteria: i) PS of 0 or 1; ii) not having received
neoadjuvant chemotherapy or chemoradiotherapy; iii) R0 radical
surgical resection; and iv) complete mediastinal lymph node
dissection or systematic mediastinal lymph node sampling performed
during surgery.
Postoperative adjuvant chemotherapy (POCT) was
administered with a cisplatin or carboplatin-based regimen
(cisplatin, 75 mg/m2; carboplatin, area under the plasma
drug concentration-time curve, 5), with a median of 4 cycles. A
minority of patients did not receive POCT due to asthenia, refusal
or the physician's decision (38 cases). PORT was performed on 61
patients. The administration of PORT was based on the radiation
oncologist's decision or the surgeon's referral. Radiotherapy
techniques included 3D conformal radiotherapy (21 cases) and
intensity-modulated radiotherapy (40 cases). The clinical target
volume (CTV) included subcarinal, ipsilateral paratracheal and
ipsilateral hilar nodes, as well as involved nodes. The surgical
margin of the stump was also included in the CTV. The planning
target volume was defined as the CTV plus 0.5-0.8-cm margins. The
therapies were administered with a linear accelerator using a 6–8
MV X-ray at 180–200 cGy per fraction, 5 days per week, for a mean
total radiation dose of 5,198 cGy. PORT was initiated at a mean of
4.38 months after surgery.
Follow-up, evaluation of toxicity and
survival
Patients were regularly followed up every 3 months
after surgery for the first 2 years and every 6–12 months
thereafter. The last follow-up time was December 2019. Radiation
pneumonitis and esophagitis were graded according to The Radiation
Therapy Oncology Group criteria (19) and Common Terminology Criteria for
Adverse Event version 4.0 (20),
respectively. LRFS was defined from the day of surgery to the day
of local recurrence (including surgical margin, ipsilateral hilar
and/or mediastinum) or the last follow-up. OS was measured from the
day of surgery to the date of death from any cause or to the last
follow-up.
Statistical analysis
SPSS statistical software (version 26.0; IBM Corp.)
was used for the statistical analyses. Data are presented as n (%).
Due to the small number of positive cases (PORT group) and the
large research time span in this retrospective case-controlled
study, more data deviation and confounding variables could lead to
unreliable results. Therefore, the regression data were analysed by
the propensity score-matching (PSM) method, and patients with
similar baseline data were matched to obtain the effect of the
approximate RCT. Multiple logistic regression was used to calculate
the propensity score of the PORT group (1:1 matching). Covariates
included sex, age, smoking index [the pack-year index, which is
calculated by multiplying the smoking period (years) by the number
of packs of cigarettes smoked per day] (21), type of surgery, pathological tissue
type, pathological T stage, number of N2 stations, number of N2
PLNs and POCT. Categorical variables were compared using the
χ2 test. The Kaplan-Meier method and the log-rank test
were used for univariate analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 288 analysable patients were included in
the present study, of which 61 underwent PORT. Before PSM, there
were 61 patients in the PORT group and 227 patients in the non-PORT
group. There were statistically significant differences between the
two groups for the type of surgery and POCT (performed vs. not
performed), while there were no statistically significant
differences for the other clinical factors. The clinical data of
these patients were matched according to their PORT status with the
PSM method (1:1 matching). A total of 60 patients were included in
the PORT group and 60 patients were included in the non-PORT group
after PSM. The general clinical data of the patients are shown in
Table I. All factors were
comparable after PSM matching (P>0.05).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Before PSM | After PSM |
|---|
|
|
|
|
|---|
| Patient
characteristic | Non-PORT, n (%)
(n=227) | PORT, n (%)
(n=61) | P-value | Non-PORT, n (%)
(n=60) | PORT, n (%)
(n=60) | P-value |
|---|
| Sex |
|
| 0.446 |
|
| 0.709 |
|
Female | 81 (35.7) | 25 (41.0) |
| 23 (38.3) | 25 (41.7) |
|
|
Male | 146 (64.3) | 36 (59.0) |
| 37 (61.7) | 35 (58.3) |
|
| Age, years |
|
| 0.128 |
|
| 0.827 |
|
<65 | 156 (68.7) | 48 (78.7) |
| 46 (76.7) | 47 (78.3) |
|
|
≥65 | 71 (31.3) | 13 (21.3) |
| 14 (23.3) | 13 (21.7) |
|
| Smoking index,
pack-year |
|
| 0.072 |
|
| 0.705 |
|
<400 | 112 (49.3) | 38 (62.3) |
| 39 (65.0) | 37 (61.7) |
|
|
≥400 | 115 (50.7) | 23 (37.7) |
| 21 (35.0) | 23 (38.3) |
|
| Type of
surgery |
|
| 0.024 |
|
| >0.999 |
|
Lobectomy | 185 (81.5) | 57 (93.4) |
| 56 (93.3) | 56 (93.3) |
|
|
Pneumonectomy | 42 (18.5) | 4 (6.6) |
| 4 (6.7) | 4 (6.7) |
|
| Histology |
|
| 0.753 |
|
| 0.540 |
|
SCC | 66 (29.1) | 19 (31.1) |
| 15 (25.0) | 18 (30.0) |
|
|
Non-SCC | 161 (70.9) | 42 (68.9) |
| 45 (75.0) | 42 (70.0) |
|
| Pathological T
stage |
|
| 0.544 |
|
| 0.274 |
|
T1-2 | 178 (78.4) | 50 (82.0) |
| 44 (73.3) | 49 (81.7) |
|
|
T3 | 49 (21.6) | 11 (18.0) |
| 16 (26.7) | 11 (18.3) |
|
| No. of N2
stations |
|
| 0.622 |
|
| 0.261 |
|
Single | 63 (27.8) | 15 (24.6) |
| 10 (16.7) | 15 (25.0) |
|
|
Multiple | 164 (72.2) | 46 (75.4) |
| 50 (83.3) | 45 (75.0) |
|
| No. of N2 positive
nodes |
|
| 0.135 |
|
| 0.850 |
|
1-3 | 110 (48.5) | 23 (37.7) |
| 22 (36.7) | 23 (38.3) |
|
|
≥4 | 117 (51.5) | 38 (62.3) |
| 38 (63.3) | 37 (61.7) |
|
| POCT |
|
| 0.031 |
|
| 0.309 |
|
No | 35 (15.4) | 3 (4.9) |
| 1 (1.7) | 3 (5.0) |
|
|
Yes | 192 (84.6) | 58 (95.1) |
| 59 (98.3) | 57 (95.0) |
|
Survival
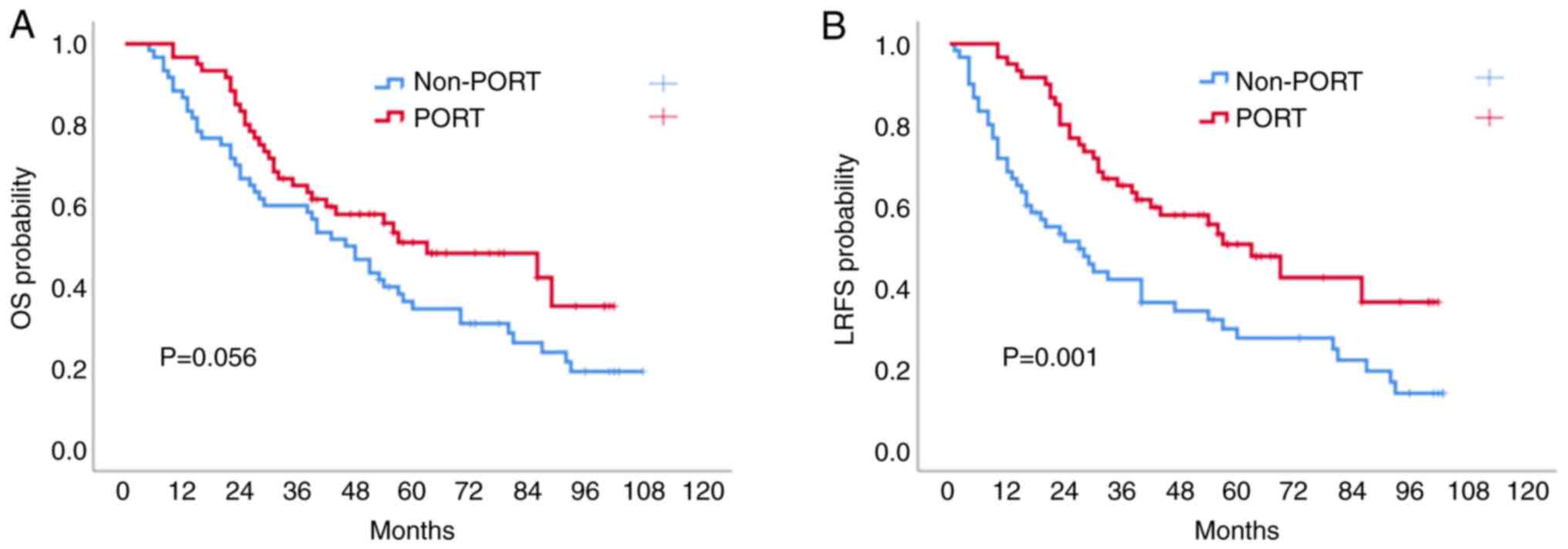
The median survival time of the 120 patients was 53
months. In Kaplan-Meier univariate analysis, the 1-, 3- and 5-year
OS rates in the PORT group were 95.0, 63.2 and 48.2%, respectively,
while the rates of the non-PORT group were 86.7, 58.3 and 34.5%,
respectively (P=0.056; Fig. 1A);
however, there was no significant difference between the two
groups. The 1-, 3- and 5-year LRFS rates in the PORT group were
95.0, 65.0 and 47.5%, respectively, while the rates of the non-PORT
group were 68.3, 41.7 and 27.3%, respectively (P=0.001; Fig. 1B).
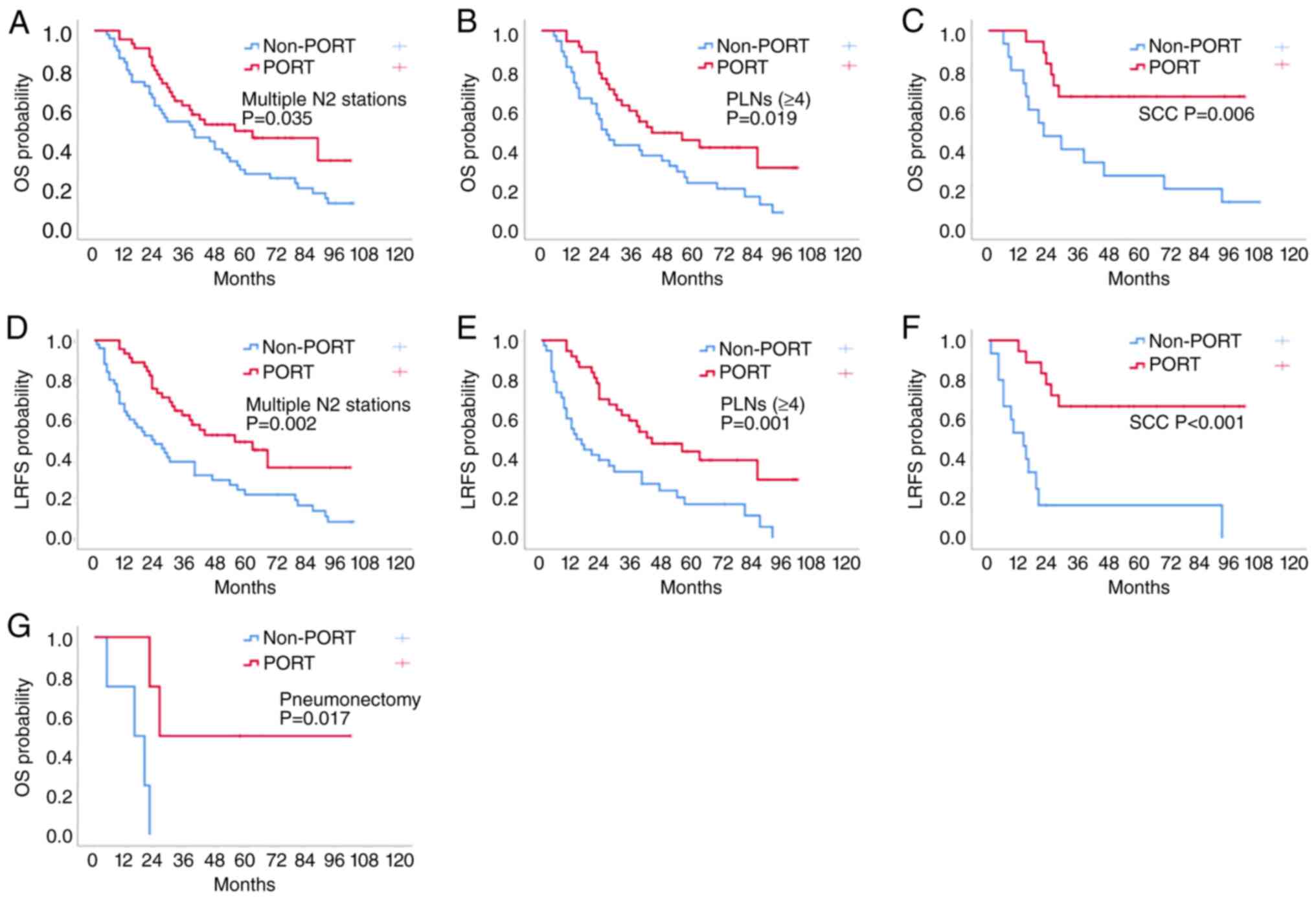
The Kaplan-Meier method was also used to analyse the
effect of PORT on OS and LRFS in patients in different subgroups
based on clinicopathological features. In the subgroups of number
of N2 stations (multiple stations; P=0.035; Fig. 2A), number of N2 PLNs (≥4; P=0.019;
Fig. 2B), histology (squamous cell
carcinoma; P=0.006; Fig. 2C) and
type of surgery (pneumonectomy, P=0.017; Fig. 2G), the 5-year OS time of the PORT
group was significantly prolonged compared with that of the
non-PORT group. In the additional clinicopathological subgroups,
there was no significant difference between the two groups in terms
of sex, age, smoking index, type of surgery (lobectomy), histology
(non-squamous cell carcinoma), T stage, number of N2 stations
(single station), number of N2 PLNs (1–3) or
POCT in terms of the 5-year OS rate (Table II).
 | Table II.OS and LRFS rates of patients with
different clinicopathological features according to the use of PORT
after propensity score-matching. |
Table II.
OS and LRFS rates of patients with
different clinicopathological features according to the use of PORT
after propensity score-matching.
|
| 5-year OS rate | 5-year LRFS
rate |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | Non-PORT, % | PORT, % | P-value | Non-PORT, % | PORT, % | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 33.5 | 59.5 | 0.203 | 27.2 | 58.7 | 0.037 |
|
Male | 34.9 | 45.9 | 0.137 | 27.2 | 45.9 | 0.008 |
| Age, years |
|
|
|
|
|
|
|
<65 | 34.5 | 48.5 | 0.198 | 25.0 | 47.8 | 0.008 |
|
≥65 | 33.3 | 59.3 | 0.131 | 34.3 | 59.3 | 0.064 |
| Smoking index,
pack-year |
|
|
|
|
|
|
|
<400 | 40.3 | 57.1 | 0.166 | 33.3 | 56.4 | 0.028 |
|
≥400 | 23.8 | 41.2 | 0.121 | 15.4 | 41.2 | 0.005 |
| Type of
surgery |
|
|
|
|
|
|
|
Lobectomy | 36.9 | 50.7 | 0.149 | 29.3 | 50.2 | 0.006 |
|
Pneumonectomy | 0.0 | 50.0 | 0.017 | 0.0 | 50.0 | 0.051 |
| Histology |
|
|
|
|
|
|
|
SCC | 26.7 | 66.7 | 0.006 | 16.7 | 66.7 | <0.001 |
|
Non-SCC | 36.9 | 45.6 | 0.560 | 31.9 | 44.8 | 0.170 |
| Pathological T
stage |
|
|
|
|
|
|
|
T1-2 | 35.5 | 51.5 | 0.173 | 29.2 | 50.8 | 0.012 |
|
T3 | 31.3 | 45.5 | 0.179 | 21.9 | 45.5 | 0.050 |
| No. of N2
stations |
|
|
|
|
|
|
|
Single | 70.0 | 55.9 | 0.613 | 58.3 | 55.9 | 0.823 |
|
Multiple | 27.5 | 49.3 | 0.035 | 22.0 | 48.7 | 0.002 |
| No. of N2 positive
nodes |
|
|
|
|
|
|
|
1-3 | 54.2 | 61.0 | 0.778 | 44.7 | 61.0 | 0.213 |
|
≥4 | 23.0 | 44.6 | 0.019 | 17.2 | 43.8 | 0.001 |
| POCT |
|
|
|
|
|
|
|
No | 0.0 | 66.7 | 0.083 | 0.0 | 66.7 | 0.083 |
|
Yes | 35.1 | 50.2 | 0.077 | 27.7 | 49.7 | 0.002 |
Compared with that in the non-PORT group, the 5-year
LRFS rate in the PORT group was significantly different in several
clinicopathological subgroups. Notably, in the PORT group, the
5-year LRFS rate was improved in three subgroups: Number of N2
stations (multiple stations; P=0.002; Fig. 2D), number of N2 PLNs (≥4; P=0.001;
Fig. 2E) and histology (squamous
cell carcinoma; P<0.001; Fig.
2F). There were also statistically significant differences
observed in terms of sex, age (<65 years), smoking index, type
of surgery (lobectomy), pT stage (T1-2) and POCT (yes) (Table II). Therefore, for subgroups of
patients with multiple N2 stations, N2 positive lymph nodes ≥4 and
squamous cell carcinoma, PORT significantly increased the OS and
LRFS rates (Table II).
Discussion
A meta-analysis published in 1998 (5) demonstrated that PORT had an adverse
effect on patients with NSCLC after complete resection (HR, 1.21;
95% CI, 1.08-1.34). Subgroup analysis demonstrated that for
patients with N0-N1 NSCLC, there was no significant improvement in
survival rate, which was related to the then available 2D
radiotherapy technology (22).
However, for patients with N2 NSCLC, the value of PORT was not
clear. With the improvements of radiotherapy technology, the damage
to normal tissues caused by radiotherapy has decreased. Therefore,
the role of PORT in patients with resected N2 NSCLC should be
re-evaluated. In 2006, a retrospective analysis of 7,465
postoperative patients with stage II–III NSCLC based on the
Surveillance, Epidemiology and End Results (SEER) database
demonstrated that although PORT reduced the survival rate of
patients with N0-1 NSCLC, it also improved the survival rate of
patients with N2 NSCLC (HR, 0.855; P=0.008) (23). Similarly, in 2015, a retrospective
analysis of patients with stage pN2 NSCLC after radical operation
(stratified based on the use of PORT) based on the National Cancer
Database demonstrated that the 5-year median OS time in the PORT
group was significantly increased compared with that in the
non-PORT group (45.2 and 40.7 months, respectively), and the 5-year
OS rate was 34.7% in the non-PORT group, while that in the PORT
group was 39.8% in (P=0.014) (24).
In 2018, an updated meta-analysis also demonstrated that PORT
increased the 5-year OS rate by 8% (P=0.008) in patients with
resectable stage IIIA-N2 NSCLC, with significantly increased DFS
rate (HR, 0.70; P<0.0001) and LRFS rate (HR, 0.37; P<0.0001)
(25). Based on the aforementioned
conclusions, the value of PORT after radical resection of stage
IIIA-N2 NSCLC remains controversial and there is still a lack of
high-level evidence.
Recently, two large RCT studies (6,7)
evaluated the efficacy of PORT in stage IIIA-N2 NSCLC with complete
resection. The European LungART (6)
study was the first randomised phase 3 study evaluating the role of
3D-conformal PORT after a considered complete resection of stage
IIIA-N2 NSCLC, which demonstrated no decrease in the risk of death
or disease progression for PORT. The 3-year OS rate was 69% in the
control group and 67% in the PORT group. In the present study, the
5-year OS rates of the PORT group and non-PORT group after PSM were
48.2 and 34.5%, respectively. PORT tended to increase the OS rate,
but it did not achieve statistical significance (P=0.056). In the
present study, lymph node status subgroups, such as multiple lymph
node stations and ≥4 nodes subgroups, were important subgroups of
patients who benefited from PORT, which was not explored in the
LungART study. One interesting aspect is that, compared with the
LungART study, the ratio of multiple N2 stations in the present
study was higher. The lymph node metastasis subgroups (multiple N2
stations and N2 positive lymph nodes ≥4) may be important subgroups
of patients who may benefit from PORT. The present study also
demonstrated a significant improvement in LRFS rate by PORT (5-year
LRFS, 47.5 vs. 27.3%; P=0.001). The same conclusion was reached in
another large RCT study (7), with
the number of DLNs and PLNs supporting the hypothesis that the PORT
group had an improved prognosis (P=0.04) after stratified analysis.
It reached a conclusion that the cohort with a higher number of
PLNs may benefit from PORT. Since N2 stage NSCLC is a group of
heterogeneous diseases, and not all patients may benefit from PORT,
further research is required to precisely identify the population
that may benefit from PORT based on more detailed clinical
characteristics.
The efficacy of PORT after radical surgery in
patients with stage IIIA-N2 NSCLC is affected by numerous clinical
factors. Among these, mediastinal PLN status is the most studied.
Riquet et al (26) compared
patients with multiple station N2 metastasis and single station N2
metastasis in N2 stage NSCLC after complete resection who had
5-year OS rates of 28.5 and 17.2%, respectively (P=0.0002). The
purpose of PORT is to reduce the recurrence rate in the
mediastinum, and it may reduce distant metastasis. Matsuguma et
al (8) retrospectively analysed
stage IIIA-N2 NSCLC after complete resection. The results
demonstrated that in patients with multiple station metastasis N2,
the DFS rate in the PORT group (41.7%) was significantly higher
than that of the non-PORT group (5.9%; P=0.02). Another
retrospective study of patients with multiple station pN2 NSCLC
also concluded that the local control rate (66.0 vs. 29.4%;
P=0.011) and DFS rate (43.2 vs. 16.6%; P=0.037) were significantly
improved in the PORT group in contrast to the non-PORT group
(9). Wang et al (10) analysed 3,377 patients with stage
IIIA-N2 NSCLC in the SEER database and demonstrated that the use of
PORT significantly improved OS rate (HR, 0.803; 95% CI,
0.687-0.938; P=0.006) and lung cancer-specific survival rate (HR,
0.794; 95% CI, 0.671-0.94; P=0.007) in the positive lymph nodes
(n>3) subgroup, while the use of PORT was not associated with an
advantage in the positive lymph nodes (n≤3) subgroup. The efficacy
of PORT in patients with different pathological types after radical
resection of stage IIIA-N2 NSCLC is also different. In patients
with resectable N2 NSCLC, squamous cell carcinoma has a higher
local failure rate (21 vs. 14%) and a lower distant failure rate (7
vs. 11%) compared with adenocarcinoma (25). Therefore, the use of PORT to
eradicate minimal residual disease may reduce the risk of tumour
metastasis in the mediastinum (27). Hui et al (11) retrospectively analysed 221 patients
with stage IIIA-N2 NSCLC after resection and demonstrated that PORT
increased the 5-year OS rate, and the subgroup analysis
demonstrated that PORT significantly improved the OS of groups with
squamous cell carcinoma (P=0.013) and N2 PLNs ≥4 (P=0.025).
Therefore, the contribution of PORT was different in different
histological types of stage IIIA-N2 NSCLC after resection, and PORT
may be more beneficial in patients with squamous cell carcinoma.
The present study found that PORT was beneficial in the multiple
station N2, N2 PLNs ≥4 and squamous cell carcinoma subgroups.
However, there were some limitations of the present
study. Firstly, this was a retrospective study with small subgroup
analyses and the findings are exploratory rather than definite, and
the number of patients who received PORT was small. Resected
pIIIA-N2 NSCLC is a heterogeneous group of diseases, and not all
patients benefited from PORT in the present study. However, it is
interesting to note that the LungART trial did not explore the
clinical subgroups benefiting from PORT in detail (6). Secondly, after PSM, there was a
smaller proportion of patients treated with pneumonectomy or POCT
in the subgroup, which may have reduced the statistical efficiency
of the conclusions made. Thirdly, the present study was
retrospective and with the shortcoming that pathologists did not
report the extranodal extension (ENE) of nodal metastases. ENE is
an important prognostic factor in NSCLC (28). It is suggested that ENE should be
added as an important subgroup factor for future RCT studies to
evaluate the role of PORT in IIIA-N2 NSCLC after radical
resection.
In conclusion, PORT is not a novel clinical process
after NSCLC resection; however, the role of PORT in patients with
stage III-N2 NSCLC has remained controversial for a long time. Some
results from the LungART trial are unpublished and explorative
analysis from this trial will be reported in detail in the future
(6). The present study indicated
that there may be some patients who may benefit from PORT. In
total, 82% of European radiation oncology experts still use PORT
for patients with stage III pN2 NSCLC with risk factors, such as
multi-station/-level lymph nodes (29). The present study demonstrated that
there may be some subgroups of patients after resection of pIIIA-N2
NSCLC, such as multiple station N2, N2 PLNs ≥4 and squamous cell
carcinoma subgroups, which may benefit from PORT.
Acknowledgements
The authors acknowledge helpful revisions by Dr Ji
Ming Wang (Laboratory of Cancer ImmunoMetabolism, Center for Cancer
Research, National Cancer Institute at Frederick, Frederick, MD,
USA).
Funding
This project was supported by funding from The Academic Research
Project from the Beijing Tuberculosis and Thoracic Tumor Research
Institute/Beijing Chest Hospital (Beijing, China; grant no.
2019-2-14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT conceived the idea for the study. BL took part in
all planning. CT, GL, YX, GX, TZ, JH, FL and BL designed the study,
collected the data, analyzed the data, and drafted the article. CT
and FL assisted in the application of statistical methods. CT and
BL confirm the authenticity of all the raw data. All authors
critically revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Academic Research
Project at the Beijing Tuberculosis and Thoracic Tumor Research
Institute/Beijing Chest Hospital (Beijing, China; ethical approval
no. 2019-47) approved the present study. Written informed consent
was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PORT
|
postoperative radiotherapy
|
|
NSCLC
|
non-small cell lung cancer
|
|
RCT
|
randomized clinical trial
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
LRFS
|
local recurrence-free survival
|
|
DLNs
|
detected lymph nodes
|
|
PLNs
|
positive lymph nodes
|
|
POCT
|
postoperative adjuvant
chemotherapy
|
|
CTV
|
clinical target volume
|
|
PSM
|
propensity score-matching
|
References
|
1
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) Edition of the TNM Classification for Lung
Cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Groome PA, Bolejack V, Crowley JJ, Kennedy
C, Krasnik M, Sobin LH and Goldstraw P; IASLC International Staging
Committee; Cancer Research and Biostatistics; Observers to the
Committee; Participating Institutions, : The IASLC lung cancer
staging project: Validation of the proposals for revision of the T,
N, and M descriptors and consequent stage groupings in the
forthcoming (seventh) edition of the TNM classification of
malignant tumours. J Thorac Oncol. 2:694–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinod SK and Hau E: Radiotherapy treatment
for lung cancer: Current status and future directions. Respirology.
25 (Suppl 2):S61–S71. 2020. View Article : Google Scholar
|
|
4
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-Year survival outcomes from the PACIFIC
Trial: Durvalumab after chemoradiotherapy in stage III
Non-Small-Cell lung cancer. J Clin Oncol. 40:1301–1311. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postoperative radiotherapy in
non-small-cell lung cancer, . Systematic review and meta-analysis
of individual patient data from nine randomised controlled trials.
PORT Meta-analysis Trialists Group. Lancet. 352:257–263. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Pechoux C, Pourel N, Barlesi F, Lerouge
D, Antoni D, Lamezec B, Nestle U, Boisselier P, Dansin E, Paumier
A, et al: Postoperative radiotherapy versus no postoperative
radiotherapy in patients with completely resected non-small-cell
lung cancer and proven mediastinal N2 involvement (Lung ART): An
open-label, randomised, phase 3 trial. Lancet Oncol. 23:104–114.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N,
Zhou Z, Liang J, Lv J, Feng Q, et al: Effect of postoperative
radiotherapy for patients with pIIIA-N2 Non-Small cell lung cancer
after complete resection and adjuvant chemotherapy: The phase 3
PORT-C randomized clinical trial. JAMA Oncol. 7:1178–1185. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuguma H, Nakahara R, Ishikawa Y,
Suzuki H, Inoue K, Katano S and Yokoi K: Postoperative radiotherapy
for patients with completely resected pathological stage IIIA-N2
non-small cell lung cancer: Focusing on an effect of the number of
mediastinal lymph node stations involved. Interact Cardiovasc
Thorac Surg. 7:573–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim BH, Kim HJ, Wu HG, Kang CH, Kim YT,
Lee SH and Kim DW: Role of postoperative radiotherapy after
curative resection and adjuvant chemotherapy for patients with
pathological stage N2 non-small-cell lung cancer: A propensity
score matching analysis. Clin Lung Cancer. 15:356–364. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Ma Z, Yang X, Wang Y, Xu Y, Xia W,
Chen R, Qiu M, Jiang F, Yin R, et al: Choice of postoperative
radiation for stage IIIA pathologic N2 non-small cell lung cancer:
Impact of metastatic lymph node number. Radiat Oncol. 12:2072017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hui Z, Dai H, Liang J, Lv J, Zhou Z, Feng
Q, Xiao Z, Chen D, Zhang H, Yin W and Wang L: Selection of proper
candidates with resected pathological stage IIIA-N2 non-small cell
lung cancer for postoperative radiotherapy. Thorac Cancer.
6:346–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan C, Tao X, Zheng D, Pan Y, Ye T, Hu H,
Xiang J, Zhang Y, Chen H and Sun Y: The lymph node status and
histologic subtypes influenced the effect of postoperative
radiotherapy on patients with N2 positive IIIA non-small cell lung
cancer. J Surg Oncol. 119:379–387. 2019.PubMed/NCBI
|
|
13
|
Nguyen SK, Masson-Cote L, Fortin A and
Dagnault A: Influence of smoking status on treatment outcomes after
post-operative radiation therapy for non-small-cell lung cancer.
Radiother Oncol. 96:89–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Billiet C, Decaluwe H, Peeters S,
Vansteenkiste J, Dooms C, Haustermans K, De Leyn P and De Ruysscher
D: Modern post-operative radiotherapy for stage III non-small cell
lung cancer may improve local control and survival: A
meta-analysis. Radiother Oncol. 110:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kou P, Wang H, Lin J, Zhang Y and Yu J:
Male patients with resected IIIA-N2 non-small-cell lung cancer may
benefit from postoperative radiotherapy: A population-based
survival analysis. Future Oncol. 14:2371–2381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Betticher DC, Hsu Schmitz SF, Totsch M,
Hansen E, Joss C, von Briel C, Schmid RA, Pless M, Habicht J, Roth
AD, et al: Prognostic factors affecting long-term outcomes in
patients with resected stage IIIA pN2 non-small-cell lung cancer:
5-year follow-up of a phase II study. Br J Cancer. 94:1099–1106.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Douillard JY, Rosell R, De Lena M, Riggi
M, Hurteloup P and Mahe MA; Adjuvant Navelbine International
Trialist Association, : Impact of postoperative radiation therapy
on survival in patients with complete resection and stage I, II, or
IIIA non-small-cell lung cancer treated with adjuvant chemotherapy:
The adjuvant Navelbine International Trialist Association (ANITA)
Randomized Trial. Int J Radiat Oncol Biol Phys. 72:695–701. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, Mcfadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Cancer Institute, Cancer Therapy
Evaluation Program, . Common Terminology Criteria for Adverse
Events v.3.0 and v.4.0 (CTCAE). 2006.
|
|
21
|
Alberg AJ, Ford JG and Samet JM; American
College of Chest Physicians, : Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (3 Suppl):29S–55S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miles EF, Kelsey CR, Kirkpatrick JP and
Marks LB: Estimating the magnitude and field-size dependence of
radiotherapy-induced mortality and tumor control after
postoperative radiotherapy for non-small-cell lung cancer:
Calculations from clinical trials. Int J Radiat Oncol Biol Phys.
68:1047–1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lally BE, Zelterman D, Colasanto JM,
Haffty BG, Detterbeck FC and Wilson LD: Postoperative radiotherapy
for stage II or III non-small-cell lung cancer using the
surveillance, epidemiology, and end results database. J Clin Oncol.
24:2998–3006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson CG, Patel AP, Bradley JD, Dewees
T, Waqar SN, Morgensztern D, Baggstrom MQ, Govindan R, Bell JM,
Guthrie TJ, et al: Postoperative radiotherapy for pathologic N2
non-small-cell lung cancer treated with adjuvant chemotherapy: A
review of the National Cancer Data Base. J Clin Oncol. 33:870–876.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakib N, Li N, Zhu X, Li D, Li Y and Wang
H: Effect of postoperative radiotherapy on outcome in resectable
stage IIIA-N2 non-small-cell lung cancer: An updated meta-analysis.
Nucl Med Commun. 39:51–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riquet M, Bagan P, Le Pimpec Barthes F,
Banu E, Scotte F, Foucault C, Dujon A and Danel C: Completely
resected non-small cell lung cancer: Reconsidering prognostic value
and significance of N2 metastases. Ann Thorac Surg. 84:1818–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai H, Hui Z, Ji W, Liang J, Lu J, Ou G,
Zhou Z, Feng Q, Xiao Z, Chen D, et al: Postoperative radiotherapy
for resected pathological stage IIIA-N2 non-small cell lung cancer:
A retrospective study of 221 cases from a single institution.
Oncologist. 16:641–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luchini C, Veronese N, Nottegar A, Cheng
M, Kaneko T, Pilati C, Tabbo F, Stubbs B, Pea A, Bagante F, et al:
Extranodal extension of nodal metastases is a poor prognostic
moderator in non-small cell lung cancer: A meta-analysis. Virchows
Arch. 472:939–947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suveg K, Le Pechoux C, Faivre-Finn C,
Putora PM, De Ruysscher D, Widder J, Van Houtte P, Troost EGC,
Slotman BJ, Ramella S, et al: Role of postoperative radiotherapy in
the management for resected NSCLC-Decision criteria in clinical
routine Pre- and Post-LungART. Clin Lung Cancer. 22:579–586. 2021.
View Article : Google Scholar : PubMed/NCBI
|