Introduction
According to global cancer statistics from GLOBOCAN
2020, the number of cases of breast cancer in women exceeded that
of lung cancer to make it the most frequently diagnosed cancer,
with an estimated 2.3 million new cases (11.7%) compared with 2.24
million cases of lung cancer (11.4%) (1). In China, researchers performed a
descriptive secondary analysis of the GLOBOCAN 2020 data and when
the incidence was stratified by sex, breast cancer was also the
most common type of cancer among women (2). Signet ring cell breast carcinoma
(SRCBC), a rare variation on invasive lobular carcinoma, was first
described in 1976 (3). The
carcinoma is histologically defined by the presence of
intracytoplasmic, mucin-rich vacuoles that cause nuclear
displacement toward one cell pole, forming a crescent or signet
ring shape, in >20% of its tumor cells (4,5). In
previous years, carcinoma with signet ring cell differentiation has
been categorized as invasive breast carcinoma, and to date, only a
few cases have been reported (6–8). The
prevalence of SRCBC is postulated to be as high as 2–4.5% of all
breast carcinoma cases (9,10). The prognosis is mainly associated
with the stage. Patients with SRCBC exhibit a more advanced disease
stage, with higher mortality rates, compared with other
histological subtypes of breast cancer. In a previous study,
stage-based survival analyses found 5- and 10-year survival rates
of 26.9 and 8.8%, respectively, for stage IV SRCBC. Patients in a
lower stage had higher rates of locoregional therapy using surgery
and radiation, while patients in a higher stage were more likely to
receive systemic chemotherapy (5).
Cases of brain metastasis of carcinoma with signet ring cell
differentiation in invasive breast cancer are rare, as previously
noted (9,10). In the present study, the case of a
patient diagnosed with carcinoma with signet ring cell
differentiation for advanced invasive breast cancer is
reported.
Case report
A 40 years-old female patient was referred to the
Third Affiliated Hospital of Shenzhen University in January 2018.
In January 2015, she had presented with a nodule 1×1 cm in size in
the upper quadrant of the right breast. In June 2017, the breast
nodule raised the skin surface and was ~3×3 cm in size. In that
year, the patient went to the hospital for treatment, and the
doctor recommended surgery; however, the patient refused the
proposed treatment. In October 2017, the right breast mass had
rapidly increased to ~110×110 mm in size and occupied the right
breast. At the same time, due to metastasis to the right axillary
lymph node, the right upper limb blood circulation of the patient
was restricted, right upper limb swelling was observed and pain
appeared. In December 2017, the patient suffered from slight facial
puffiness and chest tightness, as well as difficulty swallowing
when eating dry food. Therefore, soft and liquid diets were
advised. The patient had no past history or family history of
breast disease.
Physical examination revealed the patient's face and
right upper limb were significantly swollen, and restriction in
lifting the upper right limb was observed. Multiple enlarged lymph
nodes were observed in the right neck, and the right
supraclavicular and axillary lymph nodes, measuring 50×30 mm, were
fused. Irregularly shaped multiple fused lumps of ~110×110 mm in
size occupied the entire right breast and were mainly solid and
partially cystic with tenderness. Varicose veins were observed on
the chest wall, the skin on the right breast was red and swollen,
and redness and swelling had invaded the left chest wall. The skin
of the right breast, sternum and upper quadrant of the left breast
was hard (Fig. 1A). Satellite
nodules could be palpated subcutaneously in the right upper
quadrant and left upper quadrant of the left breast. The patient's
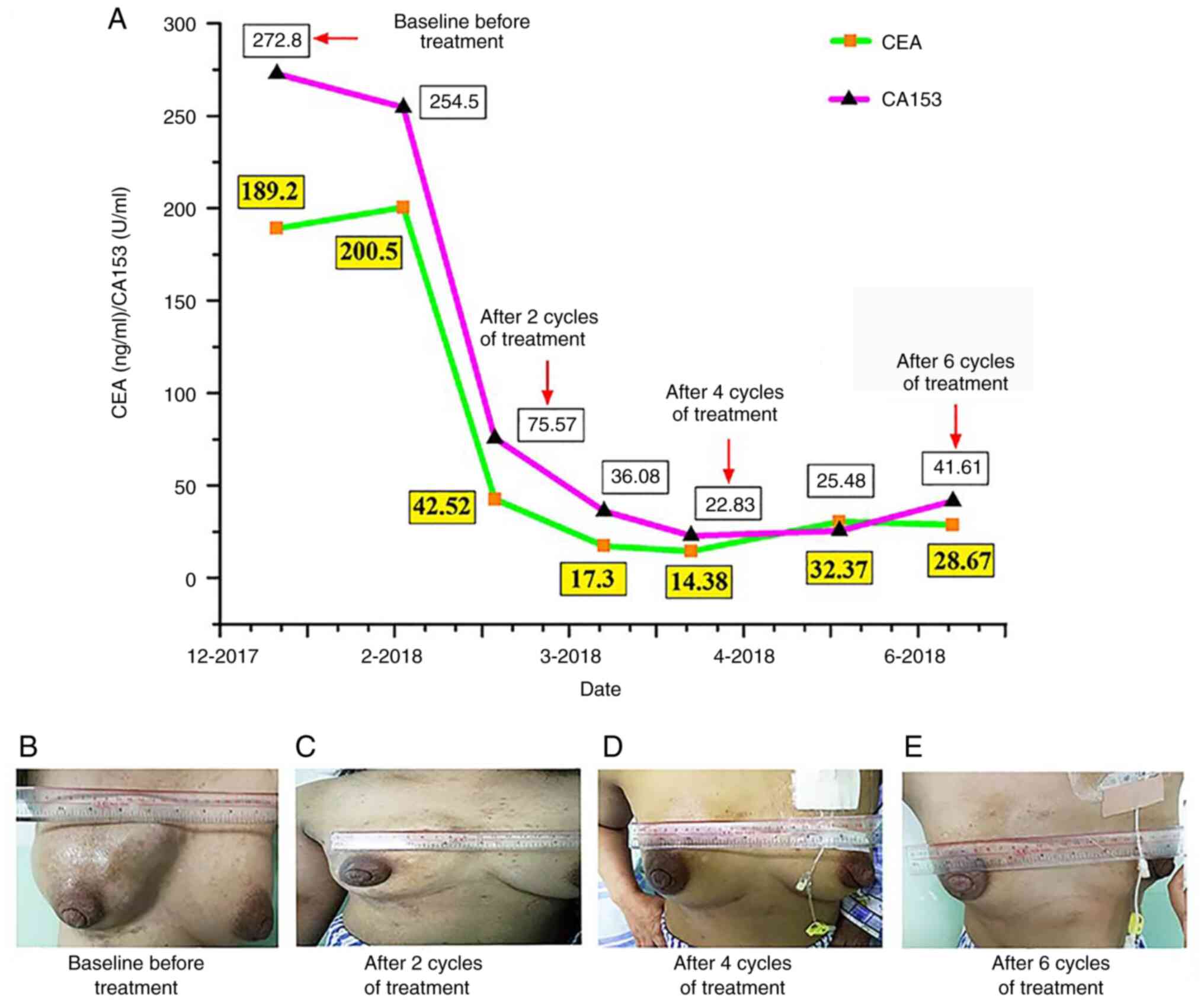
laboratory study results revealed elevated carcinoembryonic antigen
(CEA) and carbohydrate antigen 153 (CA153) levels of 189.2 (0–5)
ng/ml and 272.8 (0–25) U/ml, respectively (Fig. 1).
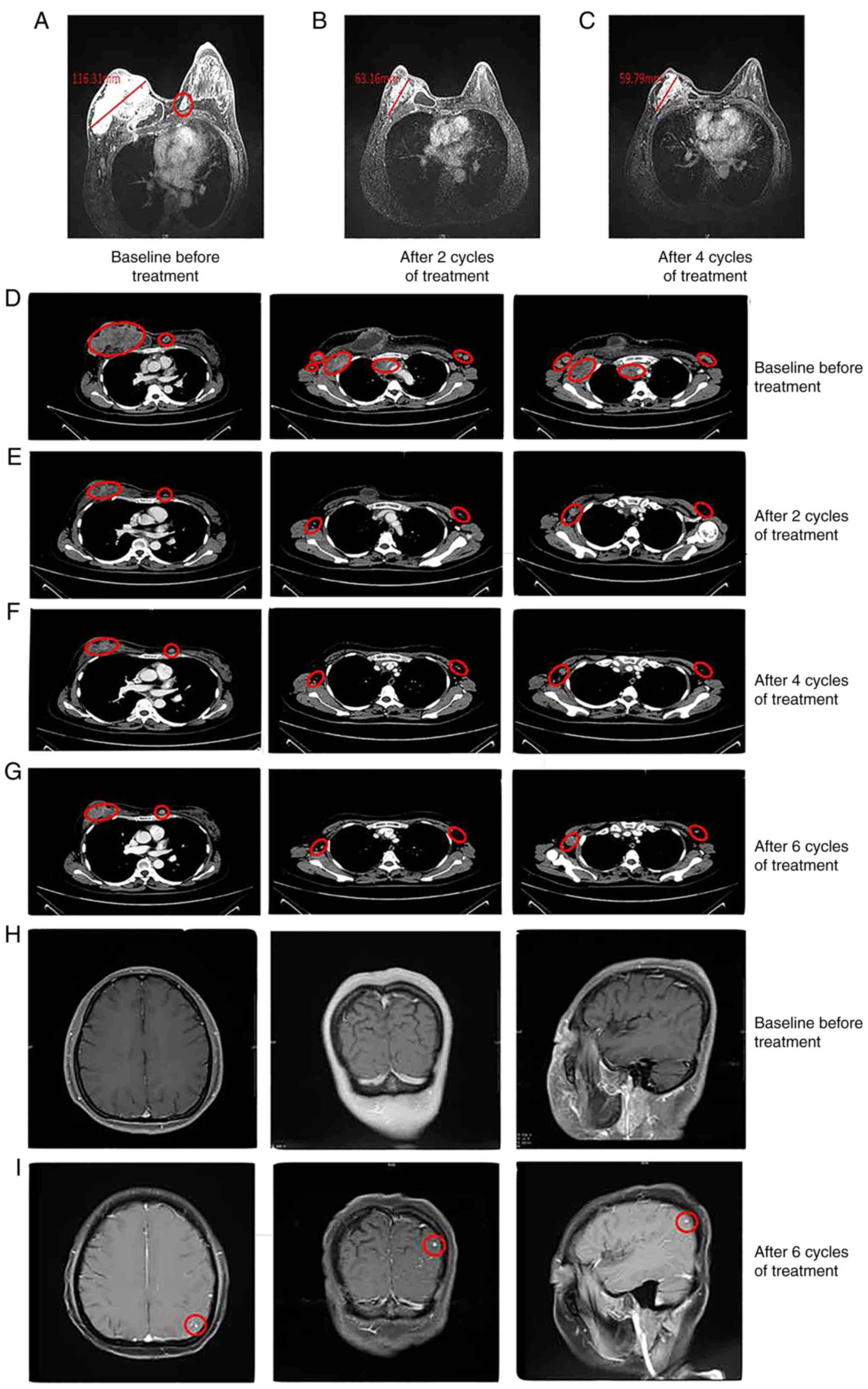
MRI of the breast showed a large mass in the right
breast with an irregular shape and a size of ~116×113×69 mm. The
skin of the right breast was significantly thickened, with
involvement of the right pectoralis major. Irregular signals could
be observed past the left areola and the upper left quadrant of the
left breast, which were attributed to nodules (Fig. 2A). A contrast-enhanced CT scan of
the chest showed multiple soft-tissue shadows on the right breast
area, and some lesions had fused and become unevenly strengthened.
The third anterior right rib was invaded, and the bone was broken.
Numerous swollen lymph node metastases were observed around the
lesions in the breast area, on both sides of the armpit, on the
right subclavian lymph node, on both sides of the neck and on the
mediastinum (Fig. 2D). At this
time, MRI of the head (Fig. 2H) and
contrast-enhanced CT of the lung did not show evidence of
metastatic disease (Fig. 2D).
Contrast-enhanced CT scanning of the abdomen and gastroscopy were
performed, and no organ-derived lesions other than the
breast-occupying lesions were identified. Based on the patient's
symptoms, signs and imaging examination, it was hypothesized that
the primary disease originated from the breast. According to the
8th American Joint Committee on Cancer staging system for breast
cancer, the Tumor-Node-Metastasis classification was T4bN3cM1 stage
IV (11).
Pathological examination by ultrasound-guided
core-needle biopsy with three biopsies revealed carcinoma with
signet ring cell differentiation. The biopsy tissue was fixed using
10% neutral formaldehyde solution at room temperature for 1–2 h.
The tissue was dehydrated and embedded in paraffin and sectioned at
4 µm. Hematoxylin and eosin staining was at room temperature for
5–10 min and 30 sec respectively. Then 3%
H2O2 was used for blocking at room
temperature for 4 min. An Olympus optical microscope (Olympus
Corporation) was used to observe, and magnification or scale bar
was shown in the figures (Figs. 3
and 4). Microscopically, tumor
cells were arranged in a solid nest-like or pawn-like arrangement,
in which ~80% of the tumor cell cytoplasm was translucent and
contained mucin that was signet ring cell-like, and some tumor cell
cytoplasm was eosinophilic (Fig.
3B). Immunohistochemical (IHC) staining employed ready-to-use
primary antibodies including ER (cat. no. 790–4325; Roche
Diagnostics), PR (cat. no. 790-4296; Roche Diagnostics), Her-2
(cat. no. 790-4493; Roche Diagnostics), Ki67 (cat. no. 790-4286;
Roche Diagnostics), p63 (cat. no. 790-4509; Roche Diagnostics),
E-cad (cat. no. MAB-0738; Fuzhou Maixin Biotechnology Development
Co., Ltd.), p120 (cat. no. MAB-0621; Fuzhou Maixin Biotechnology
Development Co., Ltd.), GATA3 (cat. no. MAB-0695; Fuzhou Maixin
Biotechnology Development Co., Ltd.), SYN (cat. no. MAB-0742;
Fuzhou Maixin Biotechnology Development Co., Ltd.), CgA (cat. no.
MAB-0707; Fuzhou Maixin Biotechnology Development Co., Ltd.), SMMHC
(cat. no. MAB-0121; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), (cat. no. CK)7 (cat. no. Kit-0021; Fuzhou Maixin
Biotechnology Development Co., Ltd.), CK20 (cat. no. Kit-0025;
Fuzhou Maixin Biotechnology Development Co., Ltd.), 34βE12 (cat.
no. Kit-0020; Fuzhou Maixin Biotechnology Development Co., Ltd.),
CDX-2 (cat. no. RMA-0631; Fuzhou Maixin Biotechnology Development
Co., Ltd.), CK5/6 (cat. no. ZM-0313; OriGene Technologies, Inc.)
were used at 37°C for 32 min. Ready-to-use secondary antibodies
(cat. no. 760-500; Roche Diagnostics) and conjugated peroxidase
were used at 37°C for 8 min. HRP/DAB was also used and chromogen
detection performed with an Ultra View Universal DAB Detection kit
(cat. no. 760-500; Roche Diagnostics GmbH). IHC staining results
were positive for cytokeratin (CK)7, CK20 (section), tumor protein
p120 (cytoplasm), 34βE12 and Ki67 (70%) (Fig. 3) and negative for E-cadherin
(E-cad), p63, GATA-binding factor 3 (GATA3), CK5/6, homeobox
protein CDX-2 (CDX-2), estrogen receptor (ER), progesterone
receptor (PR), receptor tyrosine-protein kinase erbB-2 (Her-2),
smooth muscle myosin heavy chain (SMMHC), synaptophysin (SYN) and
chromogranin A (CgA). Approximately 20% of the tumor cell area had
a ductal carcinoma immunophenotype with neuroendocrine expression
(Fig. 3A). The IHC staining results
of this area were positive for E-cad, p120 (membrane), GATA3, CK7,
ER, PR, SYN, CgA and Ki67 (Fig. 4),
and negative for CK20, CK5/6, CDX-2, p63, Her-2, 34βE12 and
SMMHC.
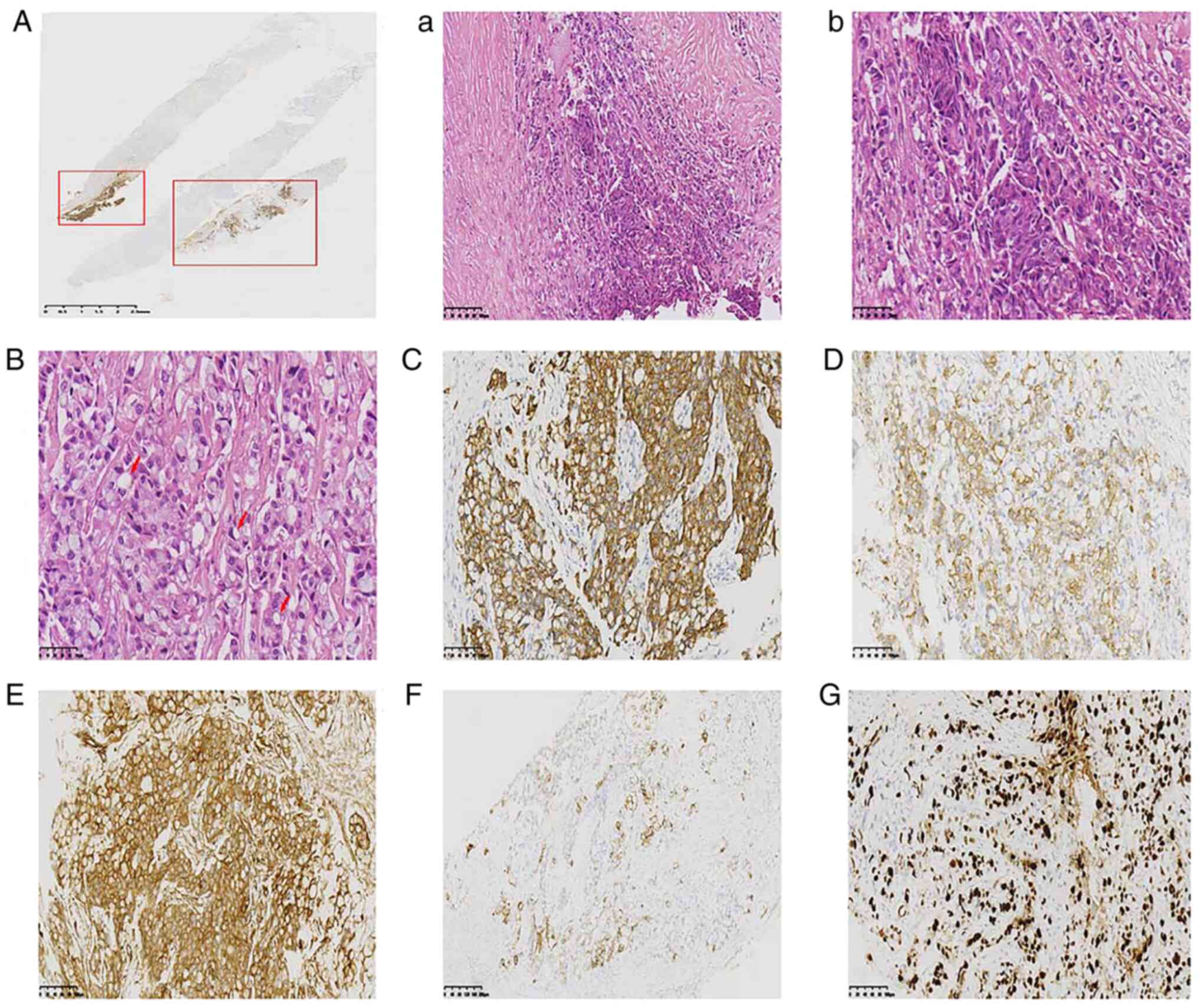 | Figure 3.(A) Microscopically, ~20% of the tumor
cell area had a ductal carcinoma immunophenotype that was positive
for E-cad. Tumor cells were arranged in (A-a) solid nest-like
(H&E staining; magnification, ×200; scale bar is 100 µm) or
(A-b) pawn-like (H&E staining; magnification, ×400; scale bar,
50 µm) arrangements, in which (B) ~80% of the tumor cell cytoplasm
was translucent and contained mucin, which was signet ring
cell-like (H&E staining; magnification, ×400; scale bar, 50
µm). Immunohistochemistry findings of the tumor showed that the
signet ring cell carcinoma component was positive for (C) CK7, (D)
CK20 (section), (E) tumor protein p120 (cytoplasm) (magnification,
×200), (F) 34βE12 and (G) Ki67 (70%) (magnification, ×100; scale
bar, 200 µm). CK, cytokeratin. |
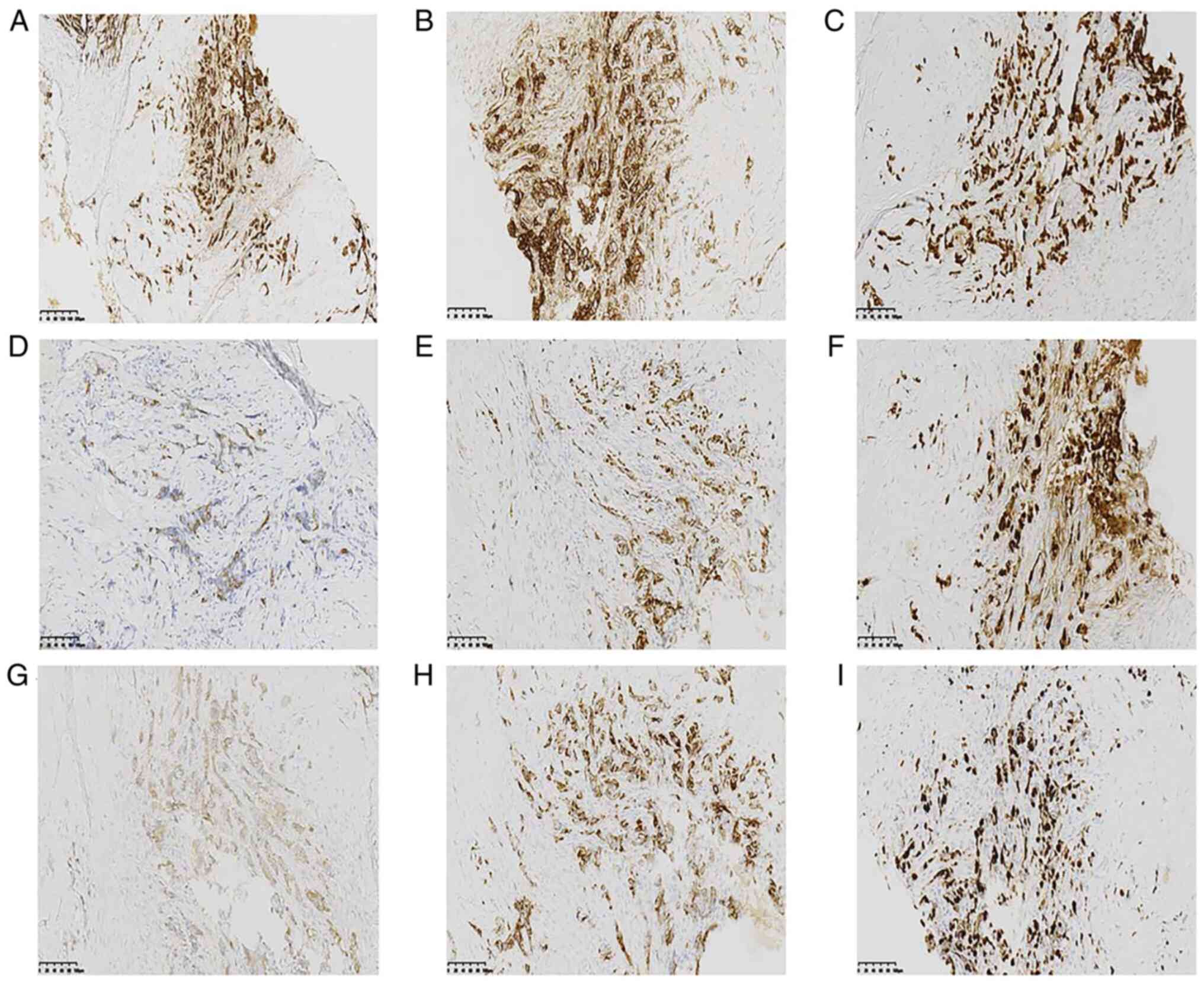 | Figure 4.Immunohistochemical staining results
for the ductal carcinoma immunophenotype with neuroendocrine
expression, which was positive for (A) E-cadherin (magnification,
×100), (B) tumor protein p120 (cytoplasm), (C) GATA-binding factor
3, (D) cytokeratin 7, (E) estrogen receptor, (F) progesterone
receptor (70%), (G) synptophysin, (H) chromogranin A, and (I) Ki67
(magnification, ×200; scale bar, 100 µm). |
When referred to the Third Affiliated Hospital of
Shenzhen University in January 2018, the patient had a Karnofsky
performance status (KPS) score of 90 (12). Considering the status of advanced
breast cancer, the tumor stage and the pathology, and in accordance
with current guidelines and specifications, the clinician first
sought to administer rescue chemotherapy with the patient's
consent. The doxorubicin hydrochloride and paclitaxel liposomes
(AT) regimen was selected for the reason that the tumor should be
shrunk rapidly, to relieve the patient's symptoms quickly. On the
other hand, paclitaxel is usually combined with salvage
chemotherapy for breast cancer and is considered if the patient has
not received anthracycline therapy. Therapy with paclitaxel
liposomes and doxorubicin hydrochloride liposomes (135 and 35
mg/m2, respectively, on day 1 of a 21-day cycle) was
initiated in January 2018. The patient tolerated chemotherapy well,
and after two cycles of treatment, the symptoms of facial and right
upper limb swelling were significantly reduced, and the patient
achieved a partial response (PR) with an obvious decrease in the
size of the target lesion (Figs.
1B, 2B and E). CEA and CA153
levels were also decreased (Fig.
1). After four cycles of treatment, the swelling of the right
breast was further reduced (Fig.
1C), and repeated MRI and CT scans showed a PR with a slight
decrease in the target lesion compared with that after 2 cycles of
treatment (Fig. 2C and F). Tumor
marker levels were also further decreased; however, a slight
increase was noted after the 5th cycle of treatment (Fig. 1). Considering that the patient's
previous clinical symptoms had improved significantly (Fig. 1D), and the imaging evaluation
revealed a PR, the original treatment was considered for the 6th
cycle, and the patient was prepared for radiotherapy five times a
week with a dose of 2 Gy for ~5-6 min, 25 times (5 weeks).
During preparation for radiotherapy, the patient
suddenly developed symptoms of headache and dizziness that were
paroxysmal, accompanied by nausea and vomiting. Later, the patient
developed a walking disorder, gradually became unresponsive and had
one seizure (KPS score of 20). On physical examination, the
patient's vital signs were stable, and consciousness was lethargic,
arousable and responsive to painful stimuli. The patient was
sensitive to light reflexes, had cognitive impairment, could not
answer simple questions and did not cooperate with the physical
examination. Although neck stiffness was suspected in this patient,
pathological signs were negative. Routine blood, liver and kidney
function, ion tests and other laboratory tests showed no apparent
abnormalities, while the CA153 level was increased. Enhanced MRI
examination showed new lesions under the left parietal cortex
(Fig. 2I) compared with the
baseline examination before chemotherapy (Fig. 2H). Although the single brain
metastasis detected by imaging was small, the patient exhibited
obvious symptoms of central nervous system (CNS) pathology. The
patient's family did not consent to breast MRI examination which
was attributed to the patient's inability to cooperate with the
long-term MRI examination under the current conditions, so only a
chest enhanced CT examination was performed (Fig. 2G). Since the patient could not be
placed in the lateral lying position, the family considered the
patient's condition to be significantly more severe, and so did not
consider further vertebral puncture to detect cerebrospinal fluid
pathology and refused radiotherapy. The patient returned to Huizhou
Central People's Hospital for supportive treatment and died in July
2018.
Discussion
Primary signet ring cell carcinoma (SRCC) of the
breast is a rare disease that originates from both invasive lobular
carcinoma and ductal carcinoma (13). Only a few cases of SRCC of the
breast have been reported, and its prevalence ranges from 2 to 4.5%
of total breast cancer cases (9,10). In
the 2012 classification of breast carcinomas by the World Health
Organization, carcinoma with signet ring cell differentiation was
classified as invasive breast carcinoma (14). Breast metastases of gastric SRCC may
be difficult to distinguish from primary breast carcinoma. New
antibodies for immunohistochemistry have been revealed to be useful
for the differential diagnosis of such cases. For example, research
has shown that primary breast SRCC is usually CK7-positive but
CK20-negative, whereas gastrointestinal SRCC is typically
CK20-positive but CK7-negative. Together with ER staining results,
the expression patterns for CK7 and CK20 can be used for
distinguishing between gastrointestinal SRCC and breast SRCC
(15).
In the present case report, the IHC staining results
were positive for CK7 and CK20 (section) and negative for ER.
Considering that the specimen was obtained from a puncture biopsy
and that there were tissue limitations such as size and location,
these findings do not fully reflect the actual histological type.
The microscopic appearance of the patient's puncture specimen
largely conformed to the main components of SRCC. Notably, ~80% of
the tumor cell cytoplasm in the pathological tissue was translucent
and contained mucin, which appeared signet ring cell-like, and ~20%
of the tumor cell area exhibited a ductal carcinoma immunophenotype
with neuroendocrine expression. Research has shown that patients
with multifocal breast cancer who have heterogeneous tumors in
terms of the molecular phenotype have significantly shorter
disease-free survival times (16).
Carcinoma with signet ring cell differentiation associated with
invasive breast cancer often presents with lymphatic metastasis and
is associated with a high Ki67 index (3). In addition, the mortality rate is
higher for SRCC than that for other forms of mammary carcinoma
without signet ring cells (9). The
aforementioned tumor biological behavior and molecular pathology
are in line with the findings of the present case.
Recent studies have shown that a large tumor burden
(17,18), axillary lymph involvement, high
tumor number (18,19), high histological grade (17), triple-negative breast cancer
(18,20), high Ki67 index (21), BRCA1 mutation (22) and breast cancer metastasis to the
CNS (20) are associated with
reduced survival time in breast cancer. The present case was
consistent with these findings, although the BRCA gene was not
tested for in this case.
With regard to the treatment process of this
disease, after the fifth cycle of treatment, although the patient's
imaging evaluation resulted in a PR, the CEA and CA153 tumor
markers were slightly elevated, indicating that the tumor might
have begun to exhibit drug resistance. The elevated levels also
suggested a poor prognosis. The onset of neurological symptoms in
this case was sudden. Although the brain metastasis was small, the
patient exhibited clinical symptoms such as unresponsiveness,
headache, dizziness, neck pain, walking disorder and epilepsy,
which were accompanied by nausea and vomiting. The symptoms led to
a significant decrease in the patient's physical strength and
quality of life, which seriously affected the patient's willingness
to receive further treatment.
In conclusion, carcinoma with signet ring cell
differentiation associated with invasive breast cancer is a rare
malignant tumor that should be distinguished from metastases of
signet ring cells to the breast. Pathological and clinical
characteristics should be used for the diagnosis of this type of
carcinoma. The prognosis of this tumor type is usually poor
(3). Therefore, early detection and
timely and standardized treatment are the keys to successfully
treating this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WY and WBG designed the case report. SD, YYL, LTW,
FPR and HFW made substantial contributions to acquisition of data,
and analysis and interpretation of data. HW and GDH advised on
patient treatment or analyzed patient data. WY drafted the
manuscript. FPR and WBG revised it critically for important
intellectual content and gave final approval of the version to be
published. WY and SD confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The husband of the patient provided written informed
consent for the use of the patient information for research
purposes, including the publication of case details and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Zhang Z, Li X, Lin Q, Chen G, Lu J,
Zeng Y, Hu D, Huang K, Lin Z and Yan J: Poorer prognosis of primary
signet-ring cell carcinoma of the breast compared with mucinous
carcinoma. PLoS One. 11:e01620882016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frost AR, Terahata S, Yeh IT, Siegel RS,
Overmoyer B and Silverberg SG: The significance of signet ring
cells in infiltrating lobular carcinoma of the breast. Arch Pathol
Lab Med. 119:64–68. 1995.PubMed/NCBI
|
|
5
|
Mehdi M, Kong AL, Frebault J, Huang S,
Huang CC and Cortina CS: Prognostic outcomes of signet ring cell
carcinoma of the breast. J Surg Res. 264:138–148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Feng YF, Wei WD, Liu P, Xie ZM, Wang
J and Xie XM: Signet-ring cell carcinoma of the breast: A case
report. World J Surg Oncol. 11:1832013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda N, Fujishima N, Ohara M, Hirouchi
T, Mizuno K and Lee GH: Invasive ductal carcinoma of the breast
with signet-ring cell and mucinous carcinoma components: Diagnostic
utility of immunocytochemistry of signet-ring cells in aspiration
cytology materials. Diagn Cytopathol. 35:171–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chatterjee D, Bal A, Das A, Kohli PS,
Singh G and Mittal BR: Invasive duct carcinoma of the breast with
dominant signet-ring cell differentiation: A microsatellite stable
tumor with aggressive behavior. Appl Immunohistochem Mol Morphol.
25:720–724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hull MT, Seo IS, Battersby JS and Csicsko
JF: Signet-ring cell carcinoma of the breast: A clinicopathologic
study of 24 cases. Am J Clin Pathol. 73:31–35. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merino MJ and Livolsi VA: Signet ring
carcinoma of the female breast: A clinicopathologic analysis of 24
cases. Cancer. 48:1830–1837. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American joint
committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mor V, Laliberte L, Morris JN and Wiemann
M: The karnofsky performance status scale. An examination of its
reliability and validity in a research setting. Cancer.
53:2002–2007. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karabagli P and Kilic H: Primary pure
signet cell carcinoma of the breast: A case report and review of
the literature. Breast Cancer. 20:363–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sinn HP and Kreipe H: A brief overview of
the WHO classification of breast tumors, 4th edition, focusing on
issues and updates from the 3rd edition. Breast Care (Basel).
8:149–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tot T: The role of cytokeratins 20 and 7
and estrogen receptor analysis in separation of metastatic lobular
carcinoma of the breast and metastatic signet ring cell carcinoma
of the gastrointestinal tract. APMIS. 108:467–472. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan YS, Mao QX, Li LF, Sun YD, Wang L and
Cui SD: Intertumoral heterogeneity of molecular phenotype and
analysis of prognosis in multifocal and multicentric breast cancer.
Zhonghua Zhong Liu Za Zhi. 38:833–838. 2016.(In Chinese).
PubMed/NCBI
|
|
17
|
Nguyen D, Yu J, Reinhold WC and Yang SX:
Association of independent prognostic factors and treatment
modality with survival and recurrence outcomes in breast cancer.
JAMA Netw Open. 3:e2072132020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deniz M, DeGregorio A, DeGregorio N, Bekes
I, Widschwendter P, Schochter F, Ernst K, Scholz C, Bauer EC,
Aivazova-Fuchs V, et al: Differential prognostic relevance of
patho-anatomical factors among different tumor-biological subsets
of breast cancer: Results from the adjuvant SUCCESS A study.
Breast. 44:81–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evcimik T, Degerli MS and Bilgic T: The
effect of prognostic factors on long-term protection in breast
cancer. Cureus. 12:e88782020.PubMed/NCBI
|
|
20
|
Mohar-Betancourt A, Alvarado-Miranda A,
Torres-Dominguez JA, Cabrera P, Lara-Medina F, Villarreal-Gómez YS
and Reynoso-Noverón N: Prognostic factors in patients with breast
cancer and brain metastasis as the first site of recurrence. Salud
Publica Mex. 60:141–150. 2018.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanyilmaz G, Yavuz BB, Aktan M, Karaağaç
M, Uyar M and Findik S: Prognostic importance of Ki-67 in breast
cancer and its relationship with other prognostic factors. Eur J
Breast Health. 15:256–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huszno J, Kolosza Z and Grzybowska E:
BRCA1 mutation in breast cancer patients: Analysis of prognostic
factors and survival. Oncol Lett. 17:1986–1995. 2019.PubMed/NCBI
|