Introduction
In recent years, the incidence of colon cancer has
increased year by year in China, and currently ranks third (with an
incidence rate of ~28/100,000), only after lung and gastric cancers
(1). At present, the clinical
treatment of patients with colon cancer is still based on the
comprehensive treatment mode of surgical resection plus adjuvant
chemoradiotherapy. The FOLFOX (oxaliplatin + calcium leucovorin +
5-fluorouracil, i.e., L-OHP+CF+5-Fu) regimen is currently a common
chemotherapy regimen used to treat colon cancer. Colon cancer has a
relatively low survival rate after chemotherapy (the five-year
survival rate is ~31% in China) (2), which is detrimental to human health
(2,3). In 2011, there were >12 million
newly diagnosed cases of colon cancer worldwide, ranking it third
for all malignant tumors in men, and the incidence in females was
second only to breast cancer (4).
At an early stage, colon cancer lacks typical signs and symptoms,
and most patients are at the advanced stages (often with
metastases) at the point of diagnosis, missing the opportunity for
optimum treatment efficacy (5). The
treatment of colon cancer is mainly based on surgical resection,
combined with radiotherapy, chemotherapy and molecular-targeted
therapy (6,7). Hence, it is of great importance to
develop strategies for the prevention and treatment of colon cancer
(8).
Tumor necrosis factor-α (TNF-α) is an important
pleiotropic cellular signaling protein (cytokine), which has been
associated with systemic inflammatory responses in the development
of autoimmune diseases, including diabetes, and various cancers,
such as colon, bladder, liver, stomach and breast cancer (9,10).
TNF-α also exerts functions in angiogenesis by promoting
endothelial cell proliferation and increasing the expression of
pro-angiogenic factors (11,12).
In addition, TNF-molecules (such as E-cadherin and β-catenin)
(13,14). In numerous malignant tumors (such as
colon and kidney cancer), elevated TNF-α levels have been detected,
which predicted poor prognosis of patients (15,16).
In addition, TNF-α binds directly to its receptor, which leads to
abnormal activation of the NF-κB, JNK and MAPK signaling pathways,
further resulting in abnormal expression of numerous chronic
inflammatory genes and the release of inflammatory factors and
chemokines (such as semaphoring 3D and MMP3) (17–20).
Tetrandrine (Tet) is a bisbenzylisoquinoline
alkaloid calcium antagonist, which can reduce the total peripheral
vascular resistance and lower the blood pressure (21) (no reflex heart rate increases when
blood pressure is reduced), increase cardiac output and muscle
relaxation, is antipyretic, has analgesic and anti-inflammatory
effects and exerts certain effects on various tumor cells (such as
pituitary adenoma and nasopharyngeal carcinoma cells) (22,23).
In addition, tetrandrine has been used for the treatment of lung
cancer, in combination with low-dose radiation (24,25).
Furthermore, tetrandrine can also be used in the treatment of
patients with simple silicosis and coal sputum lung, early mild
hypertension, rheumatic pain, joint pain and neuralgia (26–28).
Studies have demonstrated that as a calcium antagonist, tetrandrine
can directly and effectively inhibit intracellular
calcium-dependent TNF-α production and can also indirectly inhibit
the expression and production of TNF-α by cells (such as glial
cells-neurons and monocytes) (29,30).
However, whether the application of tetrandrine in the adjuvant
chemotherapy of colon cancer is effective and efficient has not
been fully elucidated.
In the present study, the efficacies and underlying
mechanisms of tetrandrine combined with neoadjuvant chemotherapy in
colon cancer were explored. Reverse transcription-quantitative
(RT-q) PCR, western blotting, MTT assays and ELISA were performed
to detect indicators in tumor tissue and blood samples from
patients with colon cancer, subjected to tetrandrine combined with
neoadjuvant chemotherapy.
Materials and methods
Patients and ethics
In total 46 patients with colon cancer with
neuropathic pain who were admitted to the First Hospital of Zibo
City (Shandong, China) between December 2015 and August 2018 were
enrolled in the present study. All patients underwent tumor
resection after neoadjuvant chemotherapy for colon cancer. Among
these patients, 26 patients who did not take tetrandrine during
chemotherapy were included in the control group, while 20 patients
who had tetrandrine during chemotherapy were assigned to the
experimental group. Tumors and blood samples (10 ml) were collected
from all the patients and the paraneoplastic negative tissues were
collected as control. In the control group, there were 16 males and
10 females, age range 35–68 years, median age of 52.2 years; while
in the experimental group, there were 12 males and 8 females, age
range 33–69 years and median age of 51.8 years. All of the patients
suffered from first-time disease onset and were diagnosed and
evaluated by pathologists and oncologists from the First Hospital
of Zibo City. All the patients were subjected to 5-Fu-based
adjuvant chemotherapy. Prior written and informed consent were
obtained from every patient and the study was approved by the
Ethics Review Board of the First Hospital of Zibo City.
Low-dose chemotherapy regimens and
inclusion/exclusion criteria
Patient inclusion criteria were as follows: i)
Patients who received a cycle of the L-OHP+CF+5-Fu regimen every 3
weeks [oxaliplatin (130 mg/m2, day 1) + calcium
leucovorin (200 mg/m2, days 1–5) and 5-Fu (300
mg/m2, days 1–5)]; ii) patients that had at least 1
measurable lesion before receiving L-OHP+CF+5-Fu adjuvant
chemotherapy; iii) patients who did not receive chemotherapy within
6 months before receiving adjuvant chemotherapy or radiation
therapy within 3 months of having received adjuvant chemotherapy;
iv) patients who received 4 cycles of chemotherapy; and v) patients
that after receiving adjuvant chemotherapy, based on a doctor's
assessment could have the tumor removed. Subjects that did not meet
the inclusion criteria were excluded from the present study.
Patients in the experimental and treatment groups were subjected to
the same basic treatments, mainly hydration diuretic treatment and
routine liver protection, antiemetic, nutritional support and
symptomatic treatment. General symptomatic treatment included a
high-quality protein, low-salt and low-sodium diet; adequate energy
and vitamins and appropriate exercise. During the chemotherapy
period, the patients from the experimental group took tetrandrine
tablets (specification: 20 mg/tablet; National Pharmaceutical
Standard H20063338; Beihai Sunshine Pharmaceutical Co., Ltd.),
according to the recommended dosage (60 mg 3 times per day, during
chemotherapy), while the patients in the control group did not take
the drug. The patients were followed-up after each
chemotherapy.
Specimen collection
Following completion of chemotherapy, the patients
underwent tumor resection and the specimens were collected. The
freshly resected tumor tissues were kept at 4°C. The tissue sample
was cut into 1 cm × 1 cm pieces with surgical scissors in a sterile
environment and stored in liquid nitrogen. The remaining tumor
tissue was cut into 1 cm × 1 cm pieces (weight and dimensions were
the same so that the tissue could fully release inflammatory
factors), which were added into 1 ml complete DMEM medium
containing 10% FBS (both Thermo Fisher Scientific, Inc.),
supplemented with 100 U/ml penicillin and 0.1 mg/ml streptomycin,
and incubated in a 37°C (5% CO2) incubator. After 24 h,
the supernatant was collected and subjected to centrifugation at
1,000 × g at 4°C for 15 min. The supernatant was collected in a 1.5
ml centrifugation tube and stored at −20°C.
Peripheral blood (10 ml) was collected from all
patients on the day of chemotherapy under fasting conditions.
Monocytes were obtained with the Human Monocyte Separation kit
(cat. no. P9260; Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's instructions. Some of the
blood sample was centrifuged at 1,000 × g for 10 min, which led to
separation of the sera and red blood cells and the serum was
collected and stored at −20°C.
Cell culture
The monocytes were cultured in a 37°C, 5%
CO2 incubator for 1–2 h. Then adherent cells which
represented the mononuclear cells were cultured with DMEM culture
medium containing 10% FBS (Thermo Fisher Scientific, Inc.) for 24 h
before experiments. The HCT116 cell line was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and
cultured in a 37°C, 5% CO2 incubator with DMEM culture
medium (Thermo Fisher Scientific Inc.) containing 10% FBS for 72 h
prior to the MTT assay. HCT116 cells were challenged with the
tissue culture supernatant a 37°C for 24 h.
RT-q PCR
Total RNA was extracted from the serum and tissue
samples with TRIzol® (cat. no. R0016; Beyotime Institute
of Biotechnology) according to the manufacturer's instructions.
cDNA was obtained by reverse transcription with the TIANScript II
cDNA First Strand Synthesis Kit (Tiangen Biotech Co., Ltd.),
according to the manufacturer's instructions. The RT-q PCR was
performed with the SuperReal PreMix (SYBR Green) (cat. no. FP204;
Tiangen Biotech Co., Ltd.) on the PCR-iQ5 RT-qPCR instrument
(Bio-Rad Laboratories Inc.). The primer sequences were as follows:
TNF-α forward, 5′-AGACCCTCACACTCAGATCATCTTC-3′ and reverse
5′-CTCCGCTTGGTGGTTTGCTA-3′; and β-actin forward
5′-CACCAGGGCGTGATGGT-3′ and reverse, 5′-CTCAAACATGATCTGGGTCAT-3′.
The 20-µl PCR system consisted of 10 µl RT-qPCR-Mix, 0.5 µl primer
each, 2 µl cDNA and 7 µl ddH2O. The thermocycling
conditions used were as follows: 95°C for 3 min; 30 cycles of 94°C
for 15 sec, 58°C for 30 sec and 72°C for 1 min; followed by 72°C
for 5 min. The expression levels of the target gene were calculated
using the 2−ΔΔCq method (31). β-actin was used as the internal
control.
Western blotting
Monocytes (1×106 cells; after being
cultured for 4 h) and tissues were lysed with lysis buffer
(Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. Protein concentration was determined
using the bicinchoninic acid (BCA) method. Then, 20 µg protein per
lane was separated with 10% SDS-PAGE and then electronically
transferred onto PVDF membranes. Following blocking with 5% nonfat
milk at room temperature for 1 h, the membrane was incubated with
rabbit anti-human anti-TNF-α (1:1,000; cat. no. ab6671; Abcam,) or
rabbit anti-human anti-β-actin (1:5,000; cat. no. ab129348; Abcam)
primary antibody at 4°C overnight. The membrane was then incubated
with the goat anti-rabbit secondary antibody (1:3,000; cat. no.
ab6721; Abcam) at room temperature for 1 h. Color development was
performed with the ECL method (cat. no. ab65623; Abcam) and the
protein bands were acquired and analyzed with the Image Lab v.3.0
software (Bio-Rad Laboratories, Inc.). β-actin was used as the
loading control.
ELISA
Blood samples were centrifuged at 1,000 × g for 10
min for separating sera and red blood cells. The serum and tissue
culture supernatants (after culturing with 1 ml complete medium
supplemented with double antibodies, at 37°C for 12 h) were used as
specimens. ELISA was performed with the following kits, according
to the manufacturer's instructions: Human TNF-α ELISA kit (cat. no.
ab181421; Abcam), human IL-1β ELISA kit (cat. no. ab100562; Abcam),
human IL-6 ELISA kit (cat. no. ab46027; Abcam), human IL-15 ELISA
kit (cat. no. ab218266; Abcam), human chemokine ligand (CCL)2 ELISA
kit (cat. no. ab179886; Abcam), human CCL20 ELISA kit (cat. no.
ab178015; Abcam), human CCL5 ELISA kit (ab174446; Abcam), human
chemokine (C-X-C) motif ligand (CXCL) 1 ELISA kit (cat. no.
ab190805; Abcam), human CXCL2 ELISA kit (cat. no. ab184862; Abcam),
human CXCL3 ELISA kit (cat. no. ab234574; Abcam), human CXCL5 ELISA
kit (cat. no. ab212163; Abcam) and human CXCL10 ELISA kit (cat. no.
ab83700; Abcam). Standard and sample wells were set separately. The
standard wells were loaded with 50 µl standards at indicated
concentrations. The sample wells were added with 10 µl sample,
followed by the addition of 40 µl dilution. Nothing was added into
the blank well. Except for the blank wells, 100 ml horseradish
peroxidase (HRP)-labeled detection antibody was added to the
standard and sample wells, which were then sealed with a sealing
membrane and incubated for 1 h. After washing, 50 µl substrate A
and B each was added into each well, followed by incubation at 37°C
for 15 min. Then 50 µl stop solution was added into each well. The
optical density (OD) values at 450 nm were measured with the GloMax
20/20 luminometer (Promega Inc.) within 15 min.
MTT assay
HCT116 colon cancer cells (commonly used for their
highly invasive and proliferative nature) in the logarithmic growth
phase were collected and seeded onto the 96-well plates at a
density of 2×103 cells/well. Cell viability was assessed
with the MTT assay (cat. no. JRDC000003; Kilton Biotechnology
(Shanghai) Co., Ltd.). After 24, 48 and 72 h, respectively, all
media were replaced with serum-free medium and 20 µl MTT (5 mg/ml)
was added into each well and incubated in the dark at 37°C for 4 h.
After the medium was discarded, 150 µl DMSO was added into each
well and the plate was shaken in the dark for 5 min. The absorbance
(A) at 490 nm was measured to reflect the cell proliferation or
number. The experiment was performed in triplicate.
Statistical analysis
Data were expressed as mean±SD. Statistical analysis
was performed with the SPSS 18.0 (SPSS Inc.) software package.
Unpaired t-tests were used for the pairwise group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
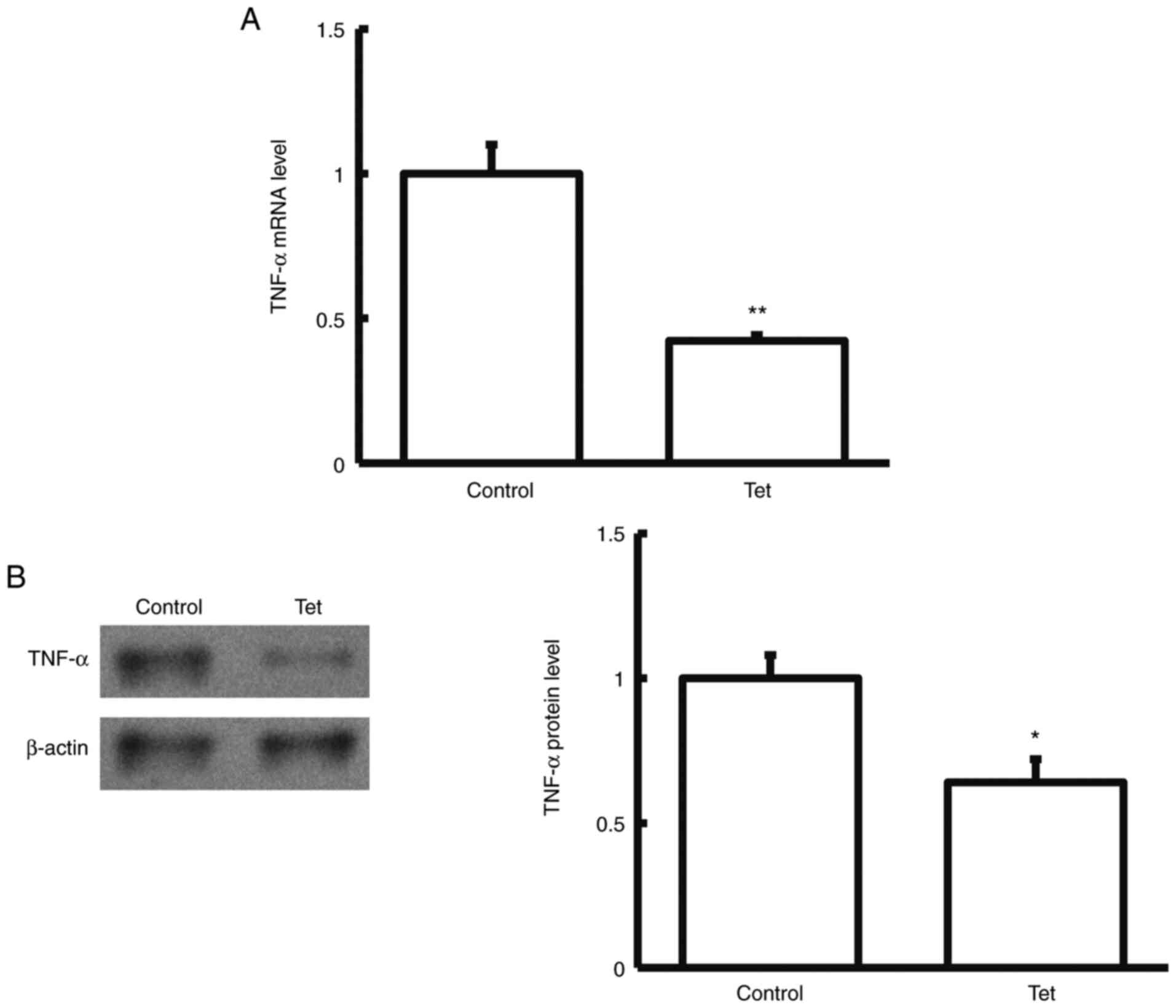
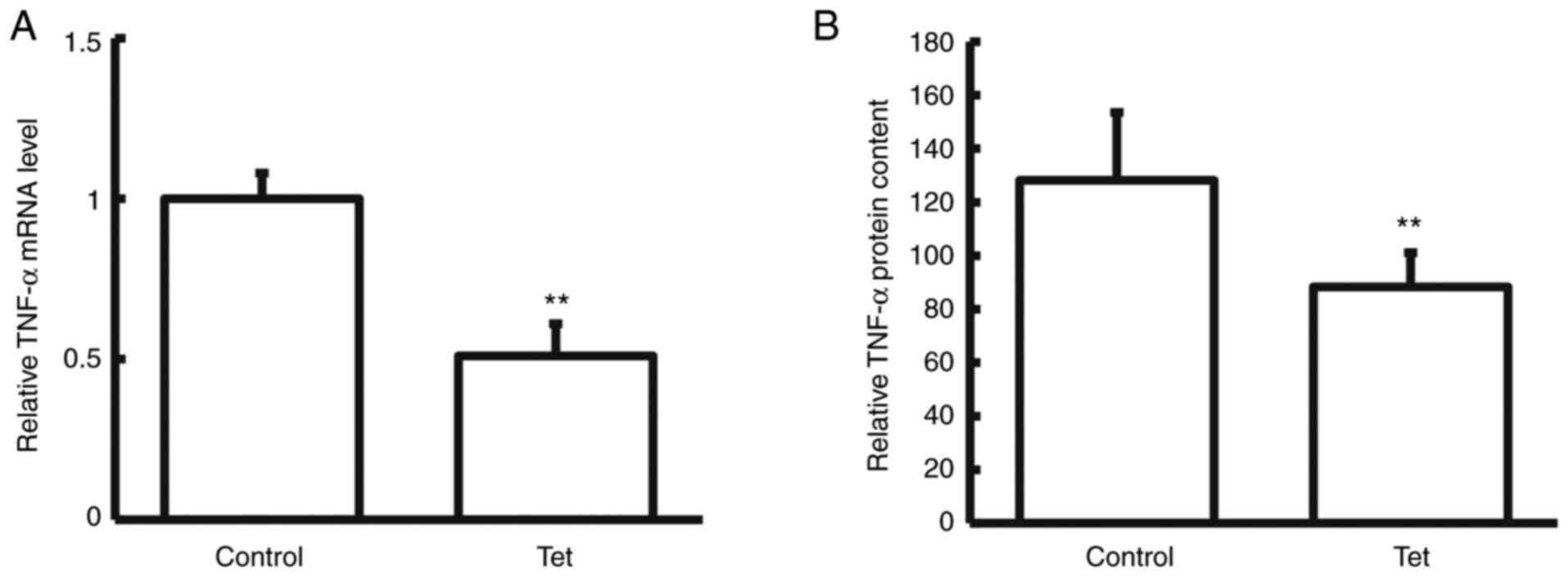
Tetrandrine decreases TNF-α expression
in tumor tissues and blood samples
To detect the expression levels of TNF-α expression
in tumor tissues and blood samples, RT-q PCR, western blotting and
ELISA were performed. Compared with the control group, TNF-α mRNA
and protein levels in the tumor tissues and blood samples
significantly declined in patients with colon cancer treated with
tetrandrine tablets during neoadjuvant chemotherapy (P<0.05;
Figs. 1 and 2). These results suggested that the
combination of tetrandrine tablets reduced TNF-α expression in
neoadjuvant chemotherapy for colon cancer.
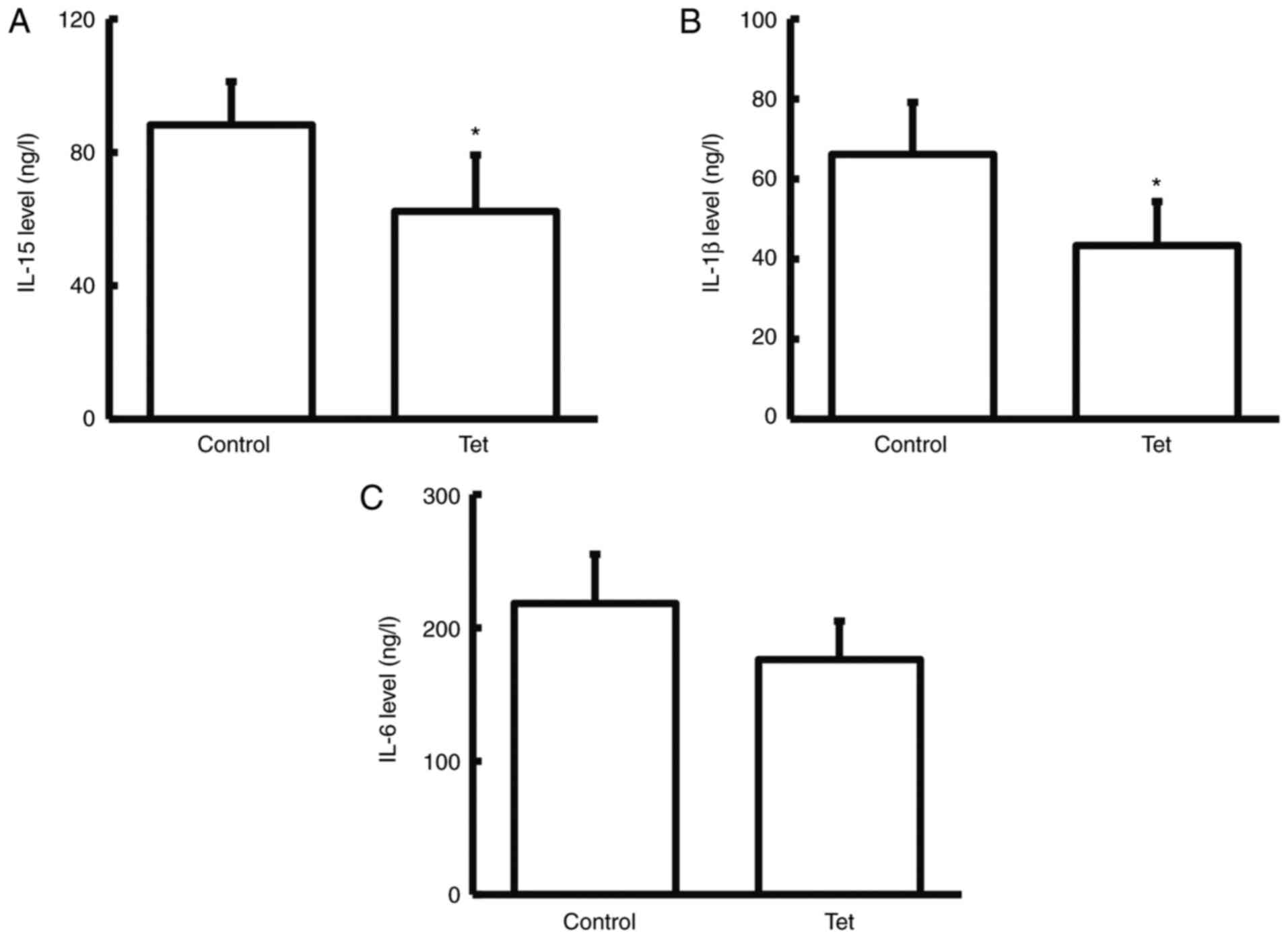
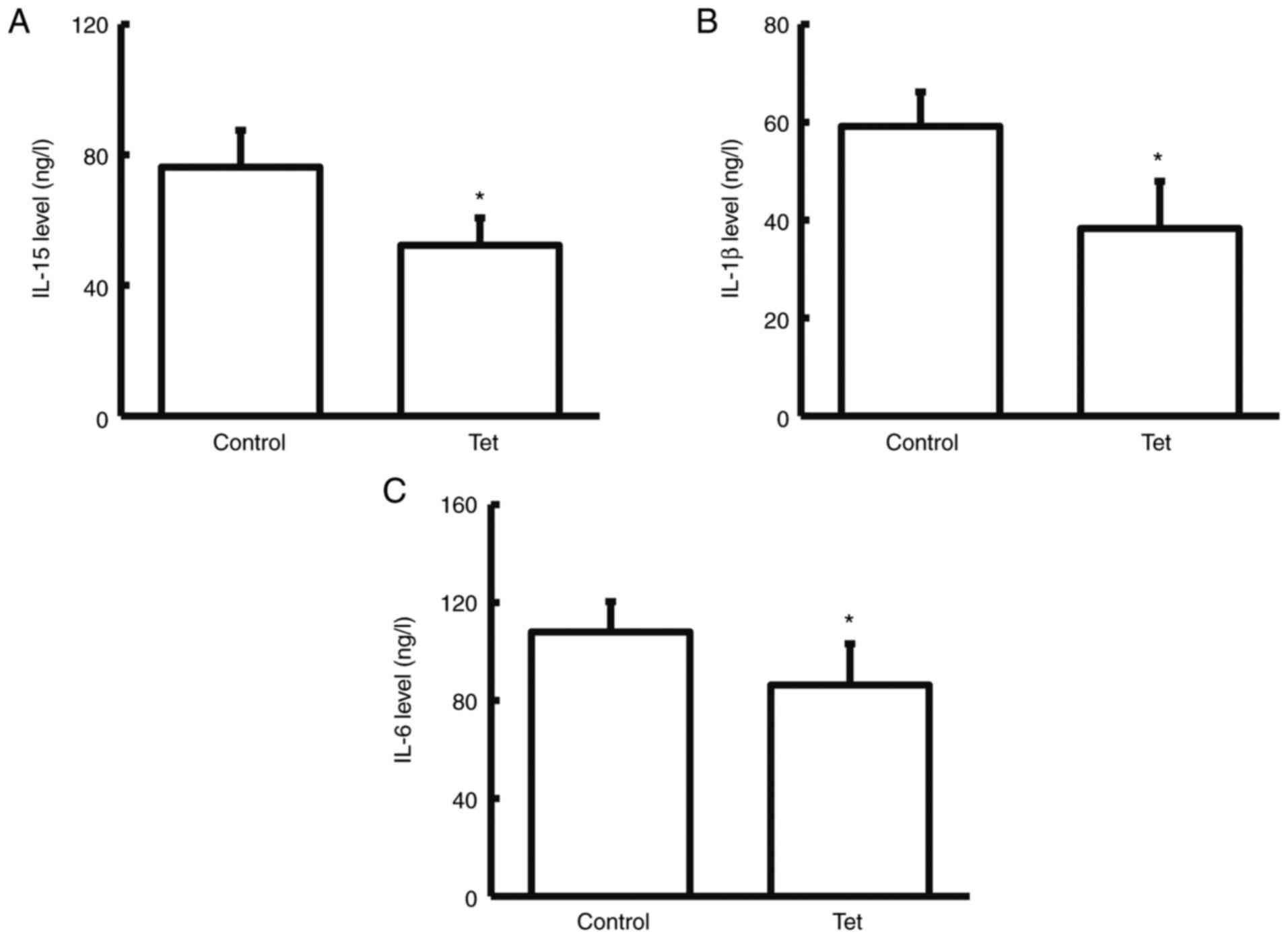
Tetrandrine reduces serum levels of
IL-15, IL-1β and IL-6
To detect the expression levels of
inflammation-related factors (IL-15, IL-1β and IL-6) in serum
samples (32), ELISA was performed.
Compared with the control group, the serum levels of IL-15, and
IL-1β significantly declined (while IL-6 did not significantly
decline) in the patients taking tetrandrine tablets during the
neoadjuvant chemotherapy (P<0.05; Fig. 3). These results indicated that
tetrandrine can reduce the release of inflammatory factors in the
blood of patients with colon cancer.
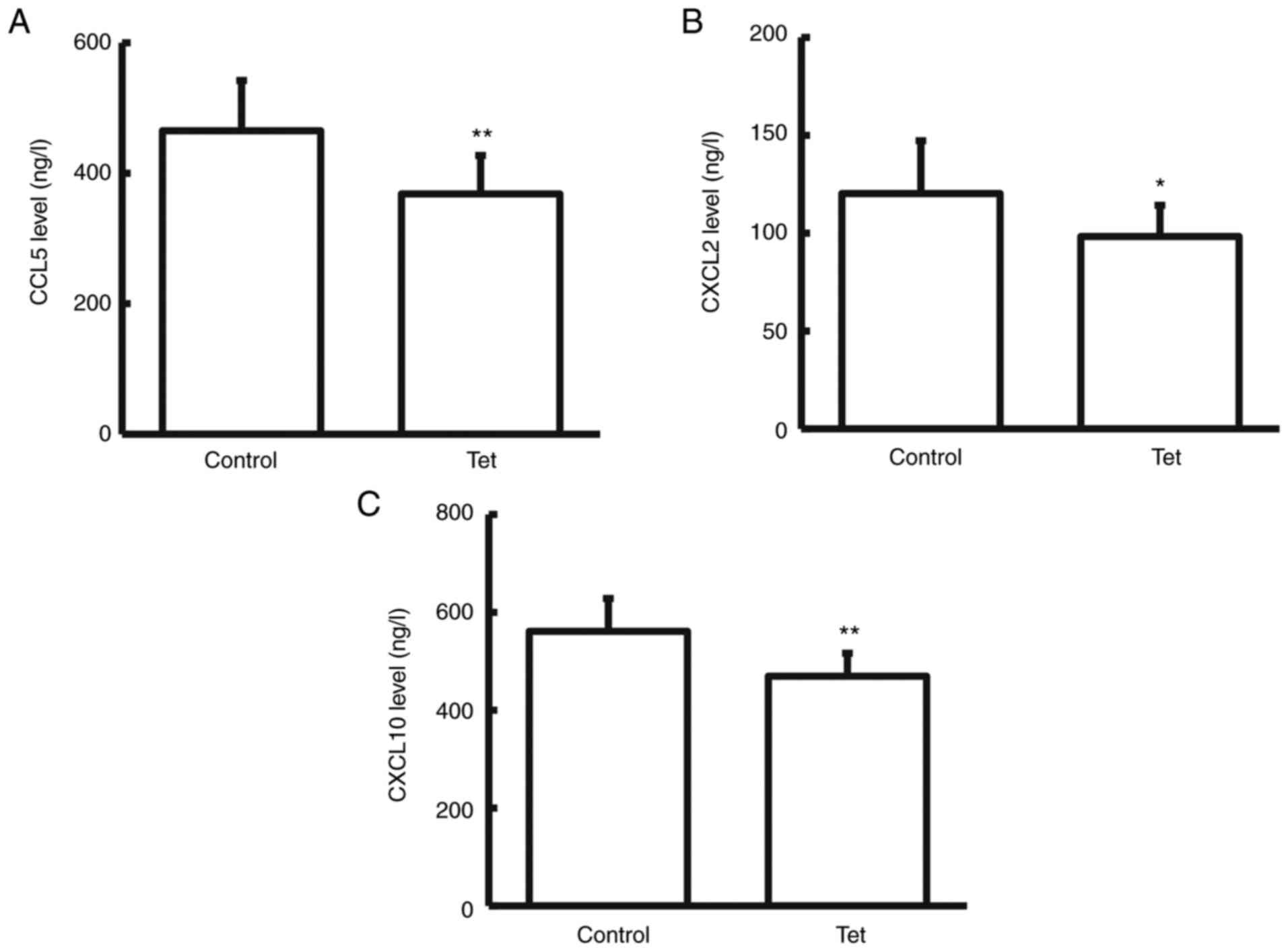
Tetrandrine reduces CCL5, CXCL2 and
CXCL10 content in colon cancer tissue culture supernatant
Release of inflammatory cytokines and chemokines
represents one of the common features of colon cancers (33). The chemotactic factors in the
surgically resected fresh colon cancer tissue culture medium were
detected with ELISA. It was revealed that compared with the control
group, CCL5, CXCL2 and CXCL10 expression levels in the tissue
culture supernatant significantly declined in the patients taking
tetrandrine tablets during chemotherapy (P<0.05 for CXCL2;
P<0.01 for CCL5 and CXCL10; Fig.
4). No significant differences were observed in the levels of
the other chemokines investigated, such as CCL20, CCL2, CXCL1,
CXCL3 and CXCL5 (data not shown). These results suggested that
tetrandrine can reduce the release of chemokines from colon cancer
cells.
Tetrandrine reduces release of IL-15,
IL-1β and IL-6 in monocytes cultured with colon cancer tissue
culture supernatant
Human blood mononuclear cells were cultured in the
conditioned medium prepared from the surgically resected fresh
colon cancer tissue and the release of IL-15, IL-1β and IL-6 in
monocytes was detected. Compared with the control group, the
release of IL-15, IL-1β and IL-6 in monocytes cultured with colon
cancer tissue culture supernatant was significantly reduced
(P<0.05; Fig. 5). Taken
together, these results suggested that tetrandrine can reduce the
release of colon cancer inflammatory cytokines in the colonic
infiltrating monocytes.
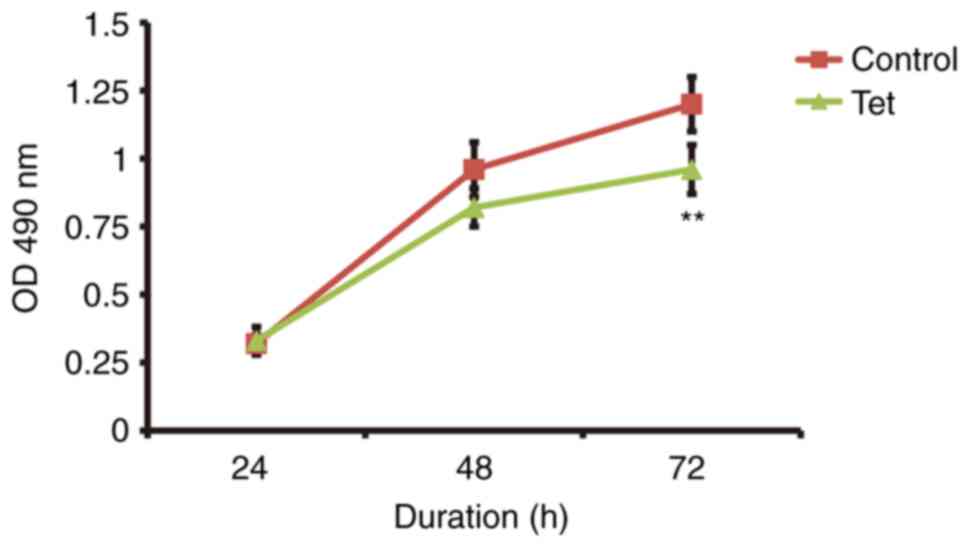
Tetrandrine decreases colon cancer
cell proliferation
HCT 116 cells (colon cancer cells) were cultured in
a conditioned medium prepared from the surgically resected fresh
colon cancer tissue and the cell proliferation was assessed with
the MTT assay. The proliferation of the colon cancer cells cultured
with the conditioned medium prepared from the surgically resected
fresh colon cancer tissues from the patients taking the tetrandrine
tablet during the chemotherapy was significant decreased compared
with those taking no tetrandrine (P<0.05; Fig. 6).
Discussion
In the present study, the clinical efficacy of the
combination application of tetrandrine in the neoadjuvant
chemotherapy for patients with colon cancer was assessed. The
changes of TNF-α expression in tumor tissues and blood samples, the
changes of the expression levels of IL-15, IL-1β, and IL-6 in the
blood samples and the release of chemokines in the tumor tissue
culture supernatants were detected. In addition, after culturing
with the tumor tissue supernatant, the release of human monocyte
inflammatory factors from the monocytes of patients with colon
cancer and the proliferation of colon cancer cell lines were
investigated. The results of the present study preliminarily
verified the anti-inflammatory effects of tetrandrine in the
neoadjuvant chemotherapy for colon cancers.
The massive secretion of TNF-α cytokines in the
tumor microenvironment can accelerate the growth and spread of
cancer cells (34). At the same
time, cancer cells can bypass the immune system, promote the
process of epithelial-mesenchymal transition and cause distant
metastasis (35). Silencing of
TNF-α expression in triple-negative breast cancer can inhibit the
proliferation of TNBC cells and promote its apoptosis through the
NF-κB pathway (36). In the TNBC
mouse model, TNF-related apoptosis-inducing ligand receptor-2 can
inhibit the proliferation and metastasis of cancer cells (37). A previous study has demonstrated the
expression of TNF-α in the cytoplasm of solid tumors (such as such
as lung cancer and colon cancer) (38). There is an important relationship
between the expression of TNF-α in tumor tissues and tumor
development (39). Tumor cells
produce TNF-α and autocrine TNF-α promotes tumor cell growth and
directly promotes tumor invasion (40,41).
The drug tetrandrine used in the present study is a
natural non-selective Ca2+ channel blocker, which is
very similar to the slow channel blocker verapamil (42). By inhibiting Ca2+,
tetrandrine can inhibit the effect and release of allergic media,
reduce myocardial contractility, dilate peripheral blood vessels
and relax muscles (42). Studies
have reported that tetrandrine can eliminate oxygen free radicals,
protect islet β-cell membranes, reduce Ca2+ overload,
prevent excessive aggregation of superoxide in cells and finally
reduce islet cell apoptosis (43,44).
It is known that tetrandrine may serve a certain role in fighting
against inflammation (45). At
present, chronic inflammation has been recognized as one of the
most common physiological causes for tumors and it is even believed
that most tumors are caused by chronic inflammation (46). The control of inflammatory factors
in tumors is also a new direction for preventing and treating
cancers (47). The results of the
present study demonstrated that administration of tetrandrine
during neoadjuvant chemotherapy in patients with colon cancer
reduced the expression of TNF-α in tumor tissues, suggesting that
tetrandrine may inhibit colon cancer by reducing TNF-α expression
to a certain extent. When colon cancer HCT116 cells were cultured
in the present study with the supernatant of tumor tissue culture
from patients treated with tetrandrine, cell proliferation
dramatically declined, further confirming that the administration
of tetrandrine during neoadjuvant chemotherapy can slow the growth
of colon cancer.
It has been demonstrated that IL-15, IL-1β, and IL-6
are also very important factors in inflammatory processes (48). IL-15, IL-1β, and IL-6 in the tumor
microenvironment can promote tumor proliferation and metastasis,
strongly affecting disease prognosis (49–51).
In addition, chemokines released by colon cancer cells can recruit
immune cells to local infiltration within tumor tissues, amplifying
the inflammatory response in tumor tissues, accelerating tumor
progression and reducing the therapeutic effect of chemotherapy
drugs (such as mitochondrial pyruvate carrier 1 and CCL7) (52,53).
The relevant chemokines (CCL2, CCL5, CCL20, CXCL1, CXCL2, CXCL3,
CXCL5 and CXCL10) in colon cancer tissue culture supernatants were
assessed in the present study. The results of the present study
demonstrated that the release of CCL5, CXCL2 and CXCL10 in colon
cancer tissue culture supernatants from patients taking tetrandrine
were relatively low, compared with those not taking tetrandrine,
suggesting that administration of tetrandrine during the
neoadjuvant chemotherapy process significantly reduced release of
chemokines in colon cancer cells and decreased the recruitment of
immune cells. In addition, the release of IL-15, IL-1β, and IL-6 in
human monocytes cultured with colon cancer tissue culture
supernatants was detected in the present study. The results
demonstrated that the release amount of IL-15, IL-1β and IL-6 in
these human monocytes cultured from colon cancer tissue supernatant
from patients taking tetrandrine was significantly reduced compared
with patients not taking tetrandrine, which further confirmed that
the administration of tetrandrine during the adjuvant chemotherapy
may reduce the activity and recruitment of human monocytes.
The present study had several limitations. For
example, only some indicators in the blood samples and the effects
on cells have been studied. A follow-up study on the patients'
condition in the tested groups (i.e., overall survival, progression
free survival and relapse rate) needs to be performed to determine
whether the administration of tetrandrine upon neoadjuvant therapy
in colon cancer is indeed beneficial.
In conclusion, the results of the present study
demonstrated that the administration of tetrandrine during
neoadjuvant chemotherapy for colon cancer reduced the inflammatory
response and release of chemokines in cancer tissues, which may
delay tumor growth and the recruitment of immune cells. Hence,
tetrandrine may have a positive effect on patients with colon
cancers, reducing inflammatory indicators in the patient's
blood.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL, FY, BW, DL and BL contributed to the study
design, experimental performance, data collection and analysis and
manuscript preparation. All authors have read and approved the
final manuscript. JL, FY, BW, DL and BL confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of the First Hospital of Zibo City. Prior written and
informed consent were obtained from every patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chesney TR, Metz JJ, Nadler A, Quereshy
FA, Ashamalla S, Acuna SA and Swallow CJ: Long-term outcomes of
resection for locoregional recurrence of colon cancer: A
retrospective descriptive cohort study. Eur J Surg Oncol.
47:2390–2397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haber PK, Puigvehí M, Castet F, Lourdusamy
V, Montal R, Tabrizian P, Buckstein M, Kim E, Villanueva A,
Schwartz M and Llovet JM: Evidence-based management of
Hepatocellular Carcinoma: Systematic review and meta-analysis of
randomized controlled trials (2002–2020). Gastroenterology.
161:879–898. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thosani N, Guha S and Singh H: Colonoscopy
and colorectal cancer incidence and mortality. Gastroenterol Clin
North Am. 42:619–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nasseri Y and Langenfeld SJ: Imaging for
colorectal cancer. Surg Clin North Am. 97:503–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simon K: Colorectal cancer development and
advances in screening. Clin Interv Aging. 11:967–976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hon KW, Abu N, Ab Mutalib NS and Jamal R:
miRNAs and lncRNAs as predictive biomarkers of response to FOLFOX
therapy in colorectal cancer. Front Pharmacol. 9:8462018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang K and Karin M: Tumor-elicited
inflammation and colorectal cancer. Adv Cancer Res. 128:173–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33 (Suppl 1):S79–S84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong H, Jiang L, Lin Y, He C, Zhu G, Du Q,
Wang X, She F and Chen Y: TNF-alpha promotes lymphangiogenesis and
lymphatic metastasis of gallbladder cancer through the
ERK1/2/AP-1/VEGF-D pathway. BMC Cancer. 16:2402016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao Z, Liu Q, Mao F, Wu J and Lei T:
TNF-α-induced VEGF and MMP-9 expression promotes hemorrhagic
transformation in pituitary adenomas. Int J Mol Sci. 12:4165–4179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka T, Imamura T, Yoneda M, Irie A, Ogi
H, Nagata M, Yoshida R, Fukuma D, Kawahara K, Shinohara M and
Nakayama H: Enhancement of active MMP release and invasive activity
of lymph node metastatic tongue cancer cells by elevated signaling
via the TNF-α-TNFR1-NF-κB pathway and a possible involvement of
angiopoietin-like 4 in lung metastasis. Int J Oncol. 49:1377–1384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thanos S and Vanselow J: The effect of
central and peripheral neuroglia on the regeneration of the optic
nerve. Fortschr Ophthalmol. 86:172–175. 1989.(In German).
PubMed/NCBI
|
|
15
|
Olsen RS, Nijm J, Andersson RE, Dimberg J
and Wagsater D: Circulating inflammatory factors associated with
worse long-term prognosis in colorectal cancer. World J
Gastroenterol. 23:6212–6219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sang C, Zhang J, Zhang Y, Chen F, Cao X
and Guo L: TNF-α promotes osteoclastogenesis through JNK
signaling-dependent induction of Semaphorin3D expression in
estrogen-deficiency induced osteoporosis. J Cell Physiol.
232:3396–3408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aye IL, Jansson T and Powell TL: TNF-α
stimulates System A amino acid transport in primary human
trophoblast cells mediated by p38 MAPK signaling. Physiol Rep.
3:e125942015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchavanakit N, Saengtong W,
Manokawinchoke J and Pavasant P: TNF-α stimulates MMP-3 production
via PGE2 signalling through the NF-kB and p38 MAPK pathway in a
murine cementoblast cell line. Arch Oral Biol. 60:1066–1074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sedger LM and McDermott MF: TNF and
TNF-receptors: From mediators of cell death and inflammation to
therapeutic giants-past, present and future. Cytokine Growth Factor
Rev. 25:453–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu XH, Gan YC, Xu GB, Chen T, Zhou H, Tang
JF, Gu Y, Xu F, Xie YY, Zhao XY and Xu RZ: Tetrandrine citrate
eliminates imatinib-resistant chronic myeloid leukemia cells in
vitro and in vivo by inhibiting Bcr-Abl/β-catenin axis. J Zhejiang
Univ Sci B. 13:867–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyu L, Hu Y, Yin S, Wang L, Ye F, Wang M,
Zhou Y, Ma W, Chen C, Jiang Y, et al: Autophagy inhibition enhances
anti-pituitary adenoma effect of tetrandrine. Phytother Res.
35:4007–4021. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Yao Z, Lai X, Bao H, Li Y, Li S,
Chang L and Zhang G: Tetrandrine sensitizes nasopharyngeal
carcinoma cells to irradiation by inducing autophagy and inhibiting
MEK/ERK pathway. Cancer Med. 9:7268–7278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho HS, Chang SH, Chung YS, Shin JY, Park
SJ, Lee ES, Hwang SK, Kwon JT, Tehrani AM, Woo M, et al:
Synergistic effect of ERK inhibition on tetrandrine-induced
apoptosis in A549 human lung carcinoma cells. J Vet Sci. 10:23–28.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye LY, Hu S, Xu HE, Xu RR, Kong H, Zeng
XN, Xie WP and Wang H: The effect of tetrandrine combined with
cisplatin on proliferation and apoptosis of A549/DDP cells and A549
cells. Cancer Cell Int. 17:402017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Tsai YH and Tseng SH: The
potential of tetrandrine as a protective agent for ischemic stroke.
Molecules. 16:8020–8032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional Chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Idec-Sadkowska I, Andrzejak R,
Antonowicz-Juchniewicz J and Kaczmarek-Wdowiak B: Trials of casual
treatment of silicosis. Med Pr. 57:271–280. 2006.(In Polish).
PubMed/NCBI
|
|
29
|
Wang B, Yang L, Yan HL, Wang M and Xiao
JG: Effect of tetrandrine on calcium-dependent tumour necrosis
factor-alpha production in glia-neurone mixed cultures. Basic Clin
Pharmacol Toxicol. 97:244–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrante A, Seow WK, Rowan-Kelly B and
Thong YH: Tetrandrine, a plant alkaloid, inhibits the production of
tumour necrosis factor-alpha (cachectin) hy human monocytes. Clin
Exp Immunol. 80:232–235. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lunjani N, Tan G, Dreher A, Sokolowska M,
Groeger D, Warwyzniak M, Altunbulakli C, Westermann P, Basera W,
Hobane L, et al: Environment-dependent alterations of immune
mediators in urban and rural south African children with atopic
dermatitis. Allergy. 77:569–581. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wierdak M, Surmiak M, Milian-Ciesielska K,
Rubinkiewicz M, Rzepa A, Wysocki M, Major P, Kłęk S and Pędziwiatr
M: Immunonutrition changes inflammatory response in colorectal
cancer: Results from a pilot randomized clinical trial. Cancers
(Basel). 13:14442021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weitzenfeld P, Kossover O, Körner C,
Meshel T, Wiemann S, Seliktar D, Legler DF and Ben-Baruch A:
Chemokine axes in breast cancer: Factors of the tumor
microenvironment reshape the CCR7-driven metastatic spread of
luminal-A breast tumors. J Leukoc Biol. 99:1009–1025. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Tang N, Wang C, Xiao L, Yu M, Zhao
L, Cai H, Han L, Xie C and Zhang Y: TNF-α-inducing protein of
Helicobacter pylori induces epithelial-mesenchymal transition (EMT)
in gastric cancer cells through activation of IL-6/STAT3 signaling
pathway. Biochem Biophys Res Commun. 484:311–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiao Y, He H, Jonsson P, Sinha I, Zhao C
and Dahlman-Wright K: AP-1 is a key regulator of proinflammatory
cytokine TNFα-mediated Triple-negative breast cancer progression. J
Biol Chem. 291:5068–5079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strekalova E, Malin D, Good DM and Cryns
VL: Methionine deprivation induces a targetable vulnerability in
triple-negative breast cancer cells by enhancing TRAIL receptor-2
expression. Clin Cancer Res. 21:2780–2791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lebrec H, Ponce R, Preston BD, Iles J,
Born TL and Hooper M: Tumor necrosis factor, tumor necrosis factor
inhibition, and cancer risk. Curr Med Res Opin. 31:557–574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stamova S, Ott-Rötzer B, Smetak H,
Schäffler K, Eder R, Fink I, Hoffmann P, Reichert TE, Beckhove P
and Spanier G: Characterization and ex vivo expansion of rare in
situ cytokine secreting T cell populations from tumor tissue and
blood of oral squamous cell carcinoma patients. J Immunol Methods.
496:1130862021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pilling AB, Hwang O, Boudreault A, Laurent
A and Hwang C: IAP Antagonists enhance apoptotic response to
enzalutamide in castration-resistant prostate cancer cells via
autocrine TNF-α Signaling. Prostate. 77:866–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee J, Tian Y, Chan ST, Kim JY, Cho C and
Ou JH: TNF-α induced by hepatitis C Virus via TLR7 and TLR8 in
hepatocytes supports interferon signaling via an autocrine
mechanism. PLoS Pathog. 11:e10049372015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li P, Zou J, Dong Y, Jiang J, Liang W and
Li D: Tetrandrine, a potent antifungal agent: Inhibits mycelial
growth and virulence of Botrytis cinerea. Phytopathology.
111:1152–1157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Serag El-Dien MM, Abdou AG, Asaad NY, Abd
El-Wahed MM and Kora MAEM: Intratumoral FOXP3+ regulatory T cells
in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol.
25:534–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu JY, Feng CP, Li X, Chang MC, Meng JL
and Xu LJ: Immunomodulatory and antioxidative activity of Cordyceps
militaris polysaccharides in mice. Int J Biol Macromol. 86:594–598.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao H, Kong L, Shen J, Ma Y, Wu Z, Li H
and He Y: Tetrandrine inhibits the occurrence and development of
frozen shoulder by inhibiting inflammation, angiogenesis, and
fibrosis. Biomed Pharmacother. 140:1117002021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eapen MS, Hansbro PM, Larsson-Callerfelt
AK, Jolly MK, Myers S, Sharma P, Jones B, Rahman MA, Markos J, Chia
C, et al: Chronic obstructive pulmonary disease and lung cancer:
Underlying pathophysiology and new therapeutic modalities. Drugs.
78:1717–1740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dai P, Li J, Ma XP, Huang J, Meng JJ and
Gong P: Efficacy and safety of COX-2 inhibitors for advanced
non-small-cell lung cancer with chemotherapy: A meta-analysis. Onco
Targets Ther. 11:721–730. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Minciullo PL, Catalano A, Mandraffino G,
Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S
and Basile G: Inflammaging and anti-inflammaging: The role of
cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz).
64:111–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng
Z and Liu L: Serum levels of IL-6, IL-8, and IL-10 are indicators
of prognosis in pancreatic cancer. J Int Med Res. 46:5228–5236.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Manohar M, Kandikattu HK, Verma AK and
Mishra A: IL-15 regulates fibrosis and inflammation in a mouse
model of chronic pancreatitis. Am J Physiol Gastrointest Liver
Physiol. 315:G954–G965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee CH, Chang JS, Syu SH, Wong TS, Chan
JY, Tang YC, Yang ZP, Yang WC, Chen CT, Lu SC, et al: IL-1β
promotes malignant transformation and tumor aggressiveness in oral
cancer. J Cell Physiol. 230:875–884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cabrero-de Las Heras S and
Martinez-Balibrea E: CXC family of chemokines as prognostic or
predictive biomarkers and possible drug targets in colorectal
cancer. World J Gastroenterol. 24:4738–4749. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Itatani Y, Kawada K, Inamoto S, Yamamoto
T, Ogawa R, Taketo MM and Sakai Y: The role of chemokines in
promoting colorectal cancer invasion/metastasis. Int J Mol Sci.
17:6432016. View Article : Google Scholar : PubMed/NCBI
|