Introduction
Epithelioid haemangioendothelioma (EHE) is an
extremely rare tumor of vascular origin that may occur in lung
(30%), liver (21%), liver plus lung (18%), single lung (12%) and
single bone (14%). The incidence of primary liver hepatic EHE
(HEHE) is one in a million (1). In
most cases, HEHE presents as an inert tumor, with clinical and
morphological features intermediate between hemangioma and
angiosarcoma. The rarity of HEHE and the non-specific nature of its
clinical manifestations mean that rates of misdiagnosis are high,
at about 60 to 80% (2). HEHE
diagnosis usually relies on histological, immunohistochemical and
molecular features but some imaging features are highly suggestive
of this lesion. Most existing case reports describe CT and MRI
imaging of HEHE, and rarely refer to two-dimensional ultrasound or
contrast-enhanced ultrasound (CEUS) (3). The possibility of HEHE should be
considered to avoid misdiagnosis, such as when a sign of
arterial-phase peripheral nodular hyperenhancement filling
internally with washout in the portal and late venous phases,
arterial phase marginal ring enhancement, entailing hypoenhancement
in the portal and late venous phases, or ‘reverse target sign’ are
presented. The current case report describes the ultrasonographic
findings of HEHE with the aim of improving the availability of
diagnostic instruments.
Case report
Patient profile, imaging and
laboratory results
A 38-year-old man presented for a health examination
and reported no physical discomfort. He had no positive signs on
physical examination and no significant disease history.
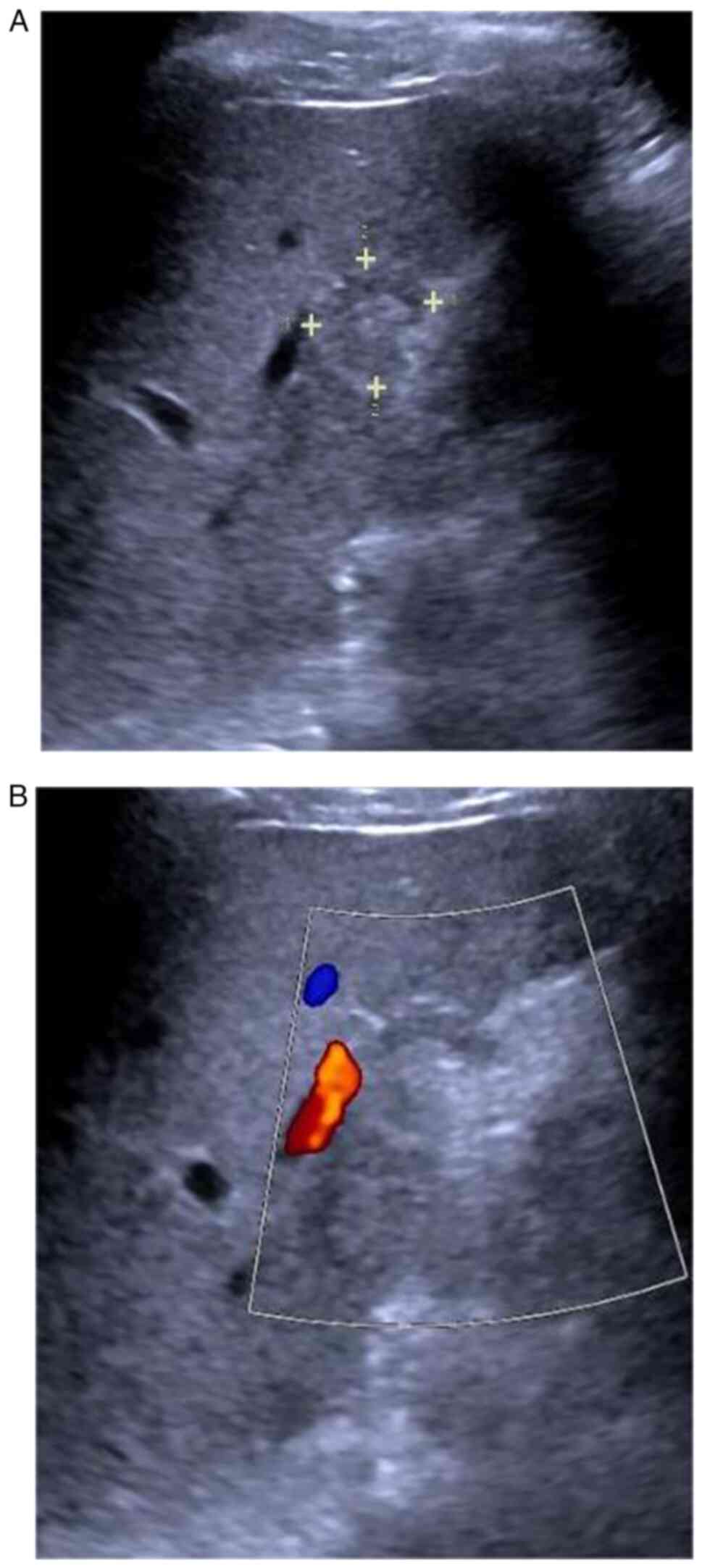
Two-dimensional ultrasound revealed a 2.0×1.8 cm hypoechoic nodule
in the lower segment of the right anterior lobe of the liver (S5
segment) with a clear boundary and regular shape (Fig. 1A). No blood flow signal was detected
in the nodule (Fig. 1B). The
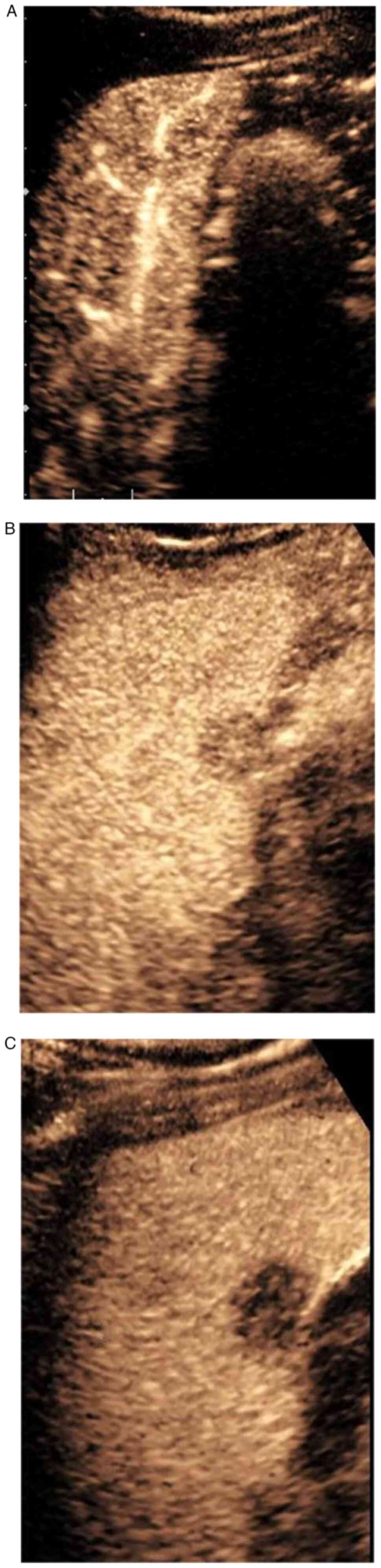
patient underwent CEUS (SIEMENS Sequoia) and the S5 segment
hypoechoic nodule was found to be synchronously enhanced with the
liver parenchyma in the arterial phase, exhibited slight
hyperenhancement in the periphery and slight hypoenhancement in the
interior, peaking at 19 s (Fig.
2A). The portal phase showed hypoenhancement, slightly higher
in the interior than at the periphery (Fig. 2B), no enhancement in the late venous
phase and showed ‘fast-forward and fast-out’ (Fig. 2C). Two possibilities emerged from
the CEUS results, cholangiocarcinoma or inflammatory pseudotumor.
Abdominal contrast-enhanced computed tomography (CT) revealed a
slightly hypodense nodule in the lower segment of the right
anterior lobe of the liver with patchy marginal enhancement.
Magnetic resonance imaging (MRI) showed an abnormal signal shadow
in the lower segment of the right anterior lobe of the liver,
indicating a chronic infectious or neoplastic lesion. Laboratory
test results were as follows: alpha-fetoprotein: 6.8 ng/ml;
carcinoembryonic antigen: 0.47 ng/ml; CA199: 8.09 U/ml; CA125: 12.5
U/ml; hepatitis B surface antigen negative and antibody positive.
Remaining laboratory parameters were unremarkable.
Surgical condition
The patient immediately went to West China Hospital
of Sichuan University for treatment. Right hepatic lesion
resection, cholecystectomy and intestinal adhesiolysis were
performed under general anesthesia. Intraoperative findings
revealed the following: ascites was not seen in the abdominal
cavity; the greater omentum and the transverse colon adhered to the
right hepatic margin, enlarged lymph nodes were not observed and
liver color and texture showed no significant abnormality. The S5
segment enclosed mass had the following features: approximately
2.0×2.0×2.1 cm in size, slightly hard consistency, well-demarcated
edges, an intact capsule and yellowish color in the cut
section.
Fluorescence in situ hybridization
detection (FISH)
The postoperative pathology confirmed that it was
HEHE, which was performed at West China Hospital of Sichuan
University including HE staining, immunohistochemistry, and FISH.
For accurate pathological diagnosis, fluorescence in situ
hybridization was performed. The reagents used include the first
antibody (application: IHC-P=1:400–800 IHC-F=1:400-800), 3%
hydrogen peroxide, normal goat serum working solution for blocking,
biotin labeled sheep anti rabbit IgG, horseradish enzyme labeled
chain enzyme ovalbumin working solution, diaminobenzidine staining
solution. Immunohistochemistry was performed on 4-µm
formalin-fixed, paraffin-embedded tissue sections after pressure
cooker antigen retrieval in citrate buffer using a polyclonal
anti-CAMTA1 antibody (15 min incubation; 1:1,000 dilution; Abcam).
Immunoreaction was carried out using a universal secondary antibody
(OriGene Technologies, Inc.).
Immunohistochemical results
The following immunohistochemical results were
obtained: CD34(+), CD31(+), CK7(+), ERG(+), Ki-67(5%+), PCK(−) and
EMA(−). CAMTA1 translocation including WWTR1-CAMTA1 gene fusion was
detected by molecular biological test. Pathological findings
revealed EHE (Fig. 3).
Follow-up
The patient's tumor did not recur during the
two-year follow-up.
Discussion
HEHE is very rare and accounts for less than 1% of
all vascular tumors (1). HEHE is
classified as malignant by the World Health Organization but is
clinically and histologically intermediate between benign
haemangioma and angiosarcoma and appears indolent in terms of
malignancy. The prognosis is usually good with 5-year survival
estimates between 40 and 60% (2).
No sign of tumor recurrence was found during two year post-surgical
follow-up of the current patient. HEHE has a non-specific clinical
presentation and the most common symptom is right upper quadrant
pain. Other symptoms may include ascites, asthenia, fatigue,
anorexia, nausea, vomiting and weight loss. Multiple manifestations
are common and 87.3% of HEHE cases in the largest published case
series were characterized by multiple lesions (4). Single-lesion types account for only 13
to 18% of all cases (3). The
present case involved a rare solitary nodule arising in the right
lobe of the liver.
Typical laboratory findings of HEHE patients include
increased levels of alkaline phosphatase and γ-glutamyl
transpeptidase and normal serum levels of alpha-fetoprotein,
carcinoembryonic antigen and CA199. Indeed, 15% of HEHE patients do
not have abnormal laboratory results (2). Alpha-fetoprotein, carcinoembryonic
antigen, CA199 and CA125 were all normal in the current case.
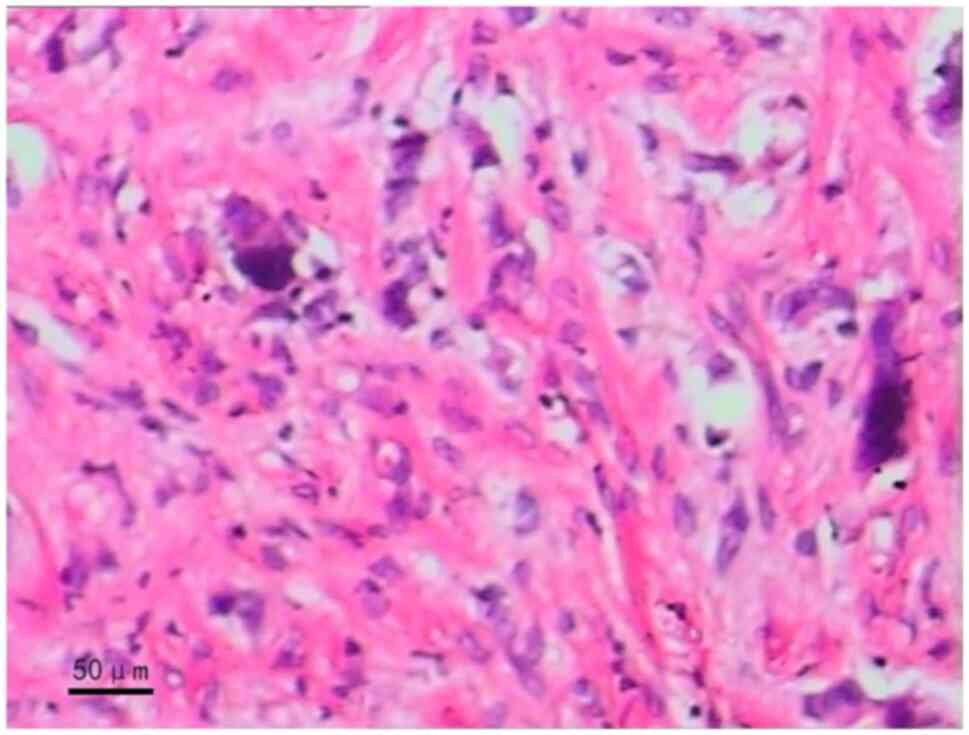
As shown in the figure, typical HEHE staining shows
that tumor cells have abundant cytoplasm, light eosinophilic and
glassy appearance, small nuclei, and fine nucleoli. Tumor cells can
see intracellular vacuoles, similar to the formation of primitive
vascular lumens, in which red blood cells can be seen, with fewer
mitotic images, suggesting diagnostic clues for EHE (Fig. 3). The definitive diagnosis of HEHE
is based on immunohistochemical findings. Around 90% of tumors
showed WWTR1-CAMTA1 gene fusion, 94% were positive for CD34 and 86%
positive for CD31 (5). These
characteristic features can be used to distinguish HEHE from other
lesions, such as epithelioid haemangioma and epithelioid
angiosarcoma. The current case showed positive immunohistochemical
staining for CAMTA1, CD31 and CD34.
Conventional CT, MRI and ultrasonography produce no
obvious specific findings for HEHE. Contrast-enhanced CT/MRI
involves an intermittent tomographic scan which is irradiating,
costly and has low reproducibility. The great advantages of CEUS
are the dynamic sweep in real time, its non-irradiating nature and
its high reproducibility. Exploration of HEHE by CEUS gives
additional imaging information with utility for diagnosis.
HEHE may be classified into three types depending on
imaging findings: uni-nodular, multifocal nodular and diffuse.
Early stage EHE may show nodular changes and present as uni-nodular
or multifocal nodular types. Nodules grow and merge over time,
forming a diffuse lesion (6). EHE
nodules often appear hypoechoic with indistinct margins on
two-dimensional ultrasound and a few nodules may have hypoechoic
halos. The internal echoes of the nodules are usually homogeneous
and anechoic areas can be observed inside a few which may indicate
hemorrhage and necrosis. HEHE is a vascular tumor but color Doppler
often reveals no blood flow-related information in the nodule,
perhaps due to the low capillary flow velocity and insufficient
color flow sensitivity of ultrasound instruments. Klinger et
al (7) described 3 types of
HEHE from CEUS examination: Type a: arterial-phase peripheral
nodular hyperenhancement filling internally with washout in the
portal and late venous phases; Type b: arterial phase marginal ring
enhancement, entailing hypoenhancement in the portal and late
venous phases; Type c: arterial-phase peripheral hypoenhancement
and internal isoenhancement (‘reverse target sign’) with or without
washout in the portal and late venous phases. The current case had
a contrast-enhanced ultrasound pattern consistent with a type b
nodule with rapid internal hypoenhancement of the nodule in the
arterial phase, slight hyperenhancement of the rim ring and
hypoenhancement in the portal and late venous phases. The cases
studied by Klinger et al (7)
were predominantly type c. Eight of 10 cases showed peripheral
hypoenhancement and internal isoenhancement in the arterial phase,
peripheral washout preceding the internal washout, peripheral
hypoenhancement and internal isoenhancement or no enhancement in
the portal and late venous phases, visualized as a ‘reverse target
sign’. Dong et al (8)
reported 72% of cases to be type b with remaining cases having no
obvious characteristics on angiography (Table I).
 | Table I.Different imaging findings of
HEHE. |
Table I.
Different imaging findings of
HEHE.
| Imaging
technique | Image
presentation | Formation
mechanism |
|---|
| Conventional CT | Slightly hypodense
nodule |
|
| Conventional MRI | Halo sign/target ring
sign | T1: Low signal core
with high signal edge (black target-likesign); T2: Heterogeneous
high signal intensity in the centre and low signal intensity at the
edge; (whitetarget-like sign) (15) |
| Enhanced CT/MRI | ‘Lollipop sign’ | The hepatic vein or
portal vein and its branches extend and terminate at the edge of
the nodule (13) |
| Two-dimensional
ultrasound | Hypoechoic nodules;
no blood flow information in the nodules; can be divided into
monodular nodular type, multifocal nodular type, and diffuse type
(6) |
|
| Contrast-enhanced
ultrasound | Type a: Peripheral
hyperenhancement in the arterial phase fills internally, with
clearance in the portal venous phase and delayed phase | i) Although the
growth is irregular, the structure of glandular vesicle and the
system remain intact |
|
| Type b: Marginal ring
enhancement in the arterial phase, hypoenhancement in the portal
venous phase and delayed phase | ii) HE staining is
regionally characteristic |
|
| Type c: Peripheral
hypoenhancement in the arterial phase and internal isoenhancement
(‘countertarget sign’) (7,8,10) | iii) Arteriovenous
fistulas in some areas (9) |
Pathologically, HEHE is characterized by marginal
invasive growth of tumor cells. The growth is irregular and
glandular vesicle structure and portal system remain intact. EHE
staining has regional characteristics, showing that: i) Tumor
peripheral epithelial cells grow along the hepatic terminal veins
and sinusoids and arteriovenous fistulas are present in some areas
(9), explaining the phenomenon of
rapid washout in the portal and delayed phases of type a and b
nodules; ii) conversely, the tumor center is less vascularized and
shows a significant stromal response and dense sclerosis (9), explaining lower internal enhancement
in the arterial phase than in the peripheral phase in type a and b
nodules; iii) the difference between a and b types largely stems
from peripheral vascular distribution. CEUS results of the current
case conformed to the classical EHE staining characteristics in the
pathological report. Klinger et al and Schweitzer et
al (7,10) reported low interstitial reaction and
dense sclerosis at the center of type c nodules from HE staining
and nodules had more internal vascularity than did those at the
periphery, resulting in significantly higher internal than external
enhancement in the arterial phase of c-type nodules. Moreover, the
degree of HEHE fibrosis has been reported to depend on the size of
the tumor lesion and blood vessel distribution is affected by the
degree of fibrosis (11).
Therefore, differences in the enhanced image are related to tumor
size and fibrosis degree, explaining the complexity and diversity
of HEHE CEUS patterns.
According to previous literature, the blood flow
perfusion of the liver and spleen can be affected by liver
diseases, such as cirrhosis. However, in this case, the blood flow
perfusion of the liver and spleen has not been studied, nor has it
been mentioned in the relevant literature, which needs further
study (12).
HEHE CT and MRI characteristics include the
‘lollipop sign’, a well-defined low-density mass on the enhanced
image resembling a lollipop. Moreover, venous obstruction in the
mass is manifested as a hypointense hepatic vein or portal vein or
branches perpendicular to the lesion and terminating at its
margins, forming a lollipop stem (13). Other imaging features include local
calcification in 20% of cases, capsular retraction in 10–25% of
cases, central hypodensity and peripheral enhancement (14,15)
(Table I).
The rarity of HEHE means that most case reports are
of individual cases, usually found incidentally by imaging
examination and an understanding of the imaging features is,
therefore, very important. Most existing case reports describe CT
and MRI images and rarely involve two-dimensional ultrasound or
CEUS. The current HEHE findings refer to two-dimensional ultrasound
and CEUS images with reference to the pathological basis. The
identification of slowly progressing nodules in lung, liver and
bone which show the characteristic imaging findings should allow
the possibility of HEHE to be entertained to avoid
misdiagnosis.
Acknowledgements
Not applicable.
Funding
This article was supported by Chengdu University of Traditional
Chinese Medicine (grant no. YYZX2021128).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, WT, YN and XM contributed to the study
conception and design. Data collection was performed by YL. Data
analysis was performed by WT and YN. The first draft of the
manuscript was written by WT and YN. XM guaranteed the completion
of the study. YL and WT confirm the authenticity of all the raw
data. WT and YN contributed equally to this manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sardaro A, Bardoscia L, Petruzzelli MF and
Portaluri M: Epithelioid Hemangioendothelioma: An overview and
update on a rare vascular tumor. Oncol Rev. 8:2592014.PubMed/NCBI
|
|
2
|
Ajay PS, Tsagkalidis V, Casabianca A,
Burchard PR, Melucci AD, Chacon A, Goyal S, Switchenko JM, Kooby
DA, Carpizo DR and Shah MM: A review of hepatic epithelioid
hemangioendothelioma-Analyzing patient characteristics and
treatment strategies. J Surg Oncol. 126:1423–1429. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong A, Dong H, Wang Y, Gong J, Lu J and
Zuo C: MRI and FDG PET/CT findings of hepatic epithelioid
hemangioendothelioma. Clin Nucl Med. 38:e66–e73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song W, Zhen Z, Li L, Ye J, Zhou S, Wu Q,
Xu L, Li H and Lin F: Epithelioid hemangioendothelioma of the
sternum. Thorac Cancer. 11:1741–1745. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jebastin Thangaiah J, Hanley K, Nomani L
and Policarpio Nicolas ML: Cytologic features and
immunohistochemical findings of epithelioid hemangioendothelioma
(EHE) in effusion: A case series. Diagn Cytopathol. 49:E24–E30.
2021. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Yu RS, Qiu LL, Jiang DY, Tan YB
and Fu YB: Contrast-enhanced multiple-phase imaging features in
hepatic epithelioid hemangioendothelioma. World J Gastroenterol.
17:3544–3553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klinger C, Stuckmann G, Dietrich CF,
Berzigotti A, Horger MS, Debove I, Gilot BJ, Pauluschke-Fröhlich J,
Hoffmann T, Sipos B and Fröhlich E: Contrast-Enhanced imaging in
hepatic epithelioid hemangioendothelioma: Retrospective study of 10
patients. Z Gastroenterol. 57:753–766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Y, Wang WP, Cantisani V, D'Onofrio M,
Ignee A, Mulazzani L, Saftoiu A, Sparchez Z, Sporea I and Dietrich
CF: Contrast-Enhanced ultrasound of histologically proven hepatic
epithelioid hemangioendothelioma. World J Gastroenterol.
22:4741–4749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi KH and Moon WS: Epithelioid
hemangioendothelioma of the liver. Clin Mol Hepatol. 19:315–319.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schweitzer N, Soudah B, Gebel M, Manns MP
and Boozari B: Gray scale and contrast-enhanced ultrasound imaging
of malignant liver tumors of vascular origin. United European
Gastroenterol J. 3:63–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Din NU, Rahim S, Asghari T, Abdul-Ghafar J
and Ahmad Z: Hepatic epithelioid hemangioendothelioma: Case series
of a rare vascular tumor mimicking metastases. Diagn Pathol.
15:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glišić TM, Perišić MD, Dimitrijevic S and
Jurišić V: Doppler assessment of splanchnic arterial flow in
patients with liver cirrhosis: Correlation with ammonia plasma
levels and MELD score. J Clin Ultrasound. 42:264–269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo L, Cai Z, Zeng S, Wang L, Kang Z, Yang
N and Zhang Y: CT and MRI features of hepatic epithelioid
haemangioendothelioma: A multi-institutional retrospective analysis
of 15 cases and a literature review. Insights Imaging. 14:22023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Hou P, Wang F, Li B and Gao J:
Primary hepatic malignant vascular tumors: A follow-up study of
imaging characteristics and clinicopathological features. Cancer
Imaging. 20:592020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou L, Cui MY, Xiong J, Dong Z, Luo Y,
Xiao H, Xu L, Huang K, Li ZP and Feng S: Spectrum of Appearances On
CT and MRI of hepatic epithelioid hemangioendothelioma. BMC
Gastroenterol. 15:692015. View Article : Google Scholar : PubMed/NCBI
|