Introduction
Lung cancer (LC), as the most frequently diagnosed
cancer, is the leading cause of cancer-associated death, with an
estimated 1.8 million deaths (accounting for 18% of all
cancer-associated deaths). The 5-year LC survival rate is 10 to 20%
in most countries based on patients diagnosed between 2010 and 2014
(1). LC is divided into small cell
LC (SCLC) and non-small cell LC (NSCLC). SCLC accounts for 15% of
all LC cases, whereas NSCLC accounts for 85% of all cases (2). NSCLC can be further subdivided into
adenocarcinoma (AC), squamous cell carcinoma (SC), and large cell
carcinoma (3). The major challenge
facing the management of LC is that the majority of patients are
diagnosed with advanced cancer in the first instance; when
diagnosed, >75% of patients are at stage III or IV (4,5).
Low-dose CT is a standard method for LC screening, although it has
a high false positive rate and carries the risk of potential
radiation hazards (6). Serum
neuron-specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1),
squamous cell carcinoma antigen (SCC), and progastrin-releasing
peptide (proGRP) are widely used biomarkers for LC (7,8).
However, the diagnostic efficacy of the aforementioned biomarkers
is insufficient for meeting the clinical diagnostic and therapeutic
requirements (7). To use biomarkers
for clinical conditions, biomarkers that improve the diagnostic
efficiency of LC are required.
Human epididymis secretory protein 4 (HE4),
glycosylated, acts as an extracellular protease inhibitor (9). While it was discovered in human
epididymal tissue cells, HE4 is typically expressed in a variety of
normal tissues, including the male reproductive system, respiratory
tract, and nasopharynx, amongst others (10). Conversely, HE4 expression is
increased in multiple tumor cell lines, such as ovarian, colon,
breast, lung, and renal cancer (11). As a result, HE4 is frequently
studied as a potential biomarker in various tumors.
HE4 was first used in the auxiliary diagnosis of
gynecological tumors. For the diagnosis of ovarian epithelial
carcinoma, the area under the curve (AUC) of HE4 was 0.92, while
the AUC of HE4 combined with CA125 improved to 0.94 (12). Researchers have found that not only
can HE4 be used as a screening tool for ovarian cancer, but also as
a marker of ovarian cancer recurrence (13,14).
As an independent prognostic factor in endometrial cancer, HE4 is
positively correlated with advanced lymph node metastasis of
endometrial cancer (15). Serum HE4
levels can be used as a marker for the diagnosis of early LC, in
which the AUC reached 0.82 (16).
The expression of HE4 was notably increased in both advanced LC and
node-positive LC groups. The overall survival time with high
expression of HE4 was considerably shorter, which indicated that
HE4 was an independent prognostic factor of LC (17). Recent research also found that HE4
autoantibodies may be a marker of early LC (18).
The results of the present study confirmed that HE4
had good diagnostic efficiency for LC, particularly for early-stage
LC. When HE4 was combined with NSE, CYFRA211, SCC, and ProGRP, the
diagnostic efficiency for LC was further improved. These results
further add to the body of evidence highlighting the value of HE4
as a marker of LC.
Materials and methods
Patients and healthy controls
The serum samples used in the present study were
collected during physical examinations on inpatients at the
Department of Thoracic Surgery of Tangshan People's Hospital
between January 2020 and May 2022. All volunteers signed an
informed consent form. The Ethics Committee at Tangshan People's
Hospital approved the collection and use of serum (approval no.
RMYY-LLKS-2019-0620-1). Serum was collected from 599 individuals,
including 201 healthy controls, 124 patients who were diagnosed
with benign lung diseases (BLD), and 274 with LC. The LC samples
included 259 NSCLC patients and 15 with SCLC (Table I). The inclusion and exclusion
criteria were: The LC patients had pathologically confirmed LC and
had no treatment before enrollment; The BLD patients had
pathologically confirmed benign diseases; Patients with a history
of any type of tumor or multiple tumors were excluded from the
study enrollment in the healthy control group, which was defined as
individuals who had no evidence of tumors at a recent health
checkup and comprehensive health assessment.
 | Table I.Characteristics of the study
participants. |
Table I.
Characteristics of the study
participants.
|
|
| Sex, n |
|
|---|
|
|
|
|
|
|---|
| Diagnosis | Total, n | Male | Female | Age median
(range) |
|---|
| Healthy control | 201 | 82 | 119 | 52.49 (29–82) |
| Benign lung
diseases | 124 | 66 | 58 | 56.47 (29–78) |
| Lung cancer | 274 | 152 | 122 | 59.32 (20–79) |
|
NSCLC | 259 | 141 | 118 | 58.64 (20–79) |
| Stage
I | 176 | 76 | 100 | 57.30 (20–77) |
| Stage
II | 7 | 7 | 0 | 63.28 (56–72) |
| AC | 223 | 107 | 116 | 57.97 (20–79) |
| SC | 36 | 34 | 2 | 62.83 (39–76) |
|
SCLC | 15 | 11 | 4 | 63.40 (47–76) |
Measurements
Approximately 3 ml whole blood was collected and
centrifuged for 10 min at 1,500 × g, 20°C. Serum was collected and
stored in a refrigerator at −80°C for later use. HE4, NSE,
CYFRA211, and ProGRP levels were detected using a Roche E601
electrochemiluminescence immunoassay analyzer (Roche Diagnostics),
and SCC was detected using an Abbott i2000 chemiluminescence
immunoassay analyzer (Abbott Pharmaceutical Co. Ltd.). The HE4,
NSE, CYFRA211 and ProGRP detection reagents purchased from Roche
Diagnostics, and the SCC detection reagent purchased from Abbott
Pharmaceutical Co. Ltd. were calibrated before detection.
Statistical analysis
For data processing, SPSS version 22.0 (IBM Corp.)
was used. The measurement data was skewed, thus, a Mann-Whitney U
test was used to compare the data between the two groups. A
Kruskal-Wallis H test followed by a Dunn's test was used to compare
the data between multiple groups. The receiver operating
characteristic (ROC) curve was used for the analysis of the
diagnostic efficiency. The data are presented as the median and
interquartile range (25,75).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum HE4 levels are increased in LC
patients
The serum HE4 levels in the LC group were
significantly higher than that of the healthy and benign lung
disease groups (both P<0.05). The serum HE4 levels were also
significantly higher in the AC, SC, and SCLC subgroups compared
with the healthy control group and BLD groups (all P<0.05;
(Fig. 1A). Furthermore, the
association between serum HE4 levels and the clinical
characteristics of LC cases was assessed. Serum HE4 levels in
patients with stage III and IV LC were significantly higher than
that in patients with stage I LC (P<0.05; Fig. 1B). Serum HE4 levels were found to be
associated with sex (P<0.05), age (P<0.05), tumor size
(P<0.05), T stage (P<0.05), N stage (P<0.05), M stage
(P<0.05), and AJCC stage III and IV (P<0.05) (Table II). Based on these results, serum
HE4 may serve as a potential marker of LC.
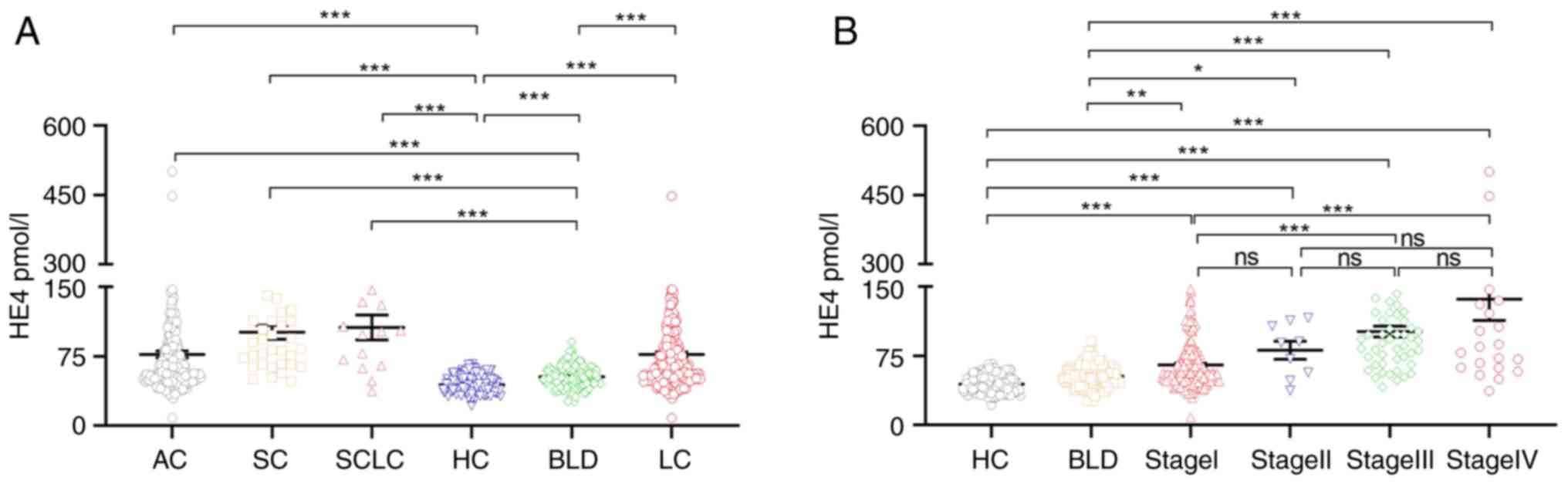 | Figure 1.HE4 serum levels in the samples from
patients with lung cancer, benign lung disease, and healthy
controls. (A) The serum HE4 levels in patients compared by disease
status. (B) The serum HE4 levels by disease stage and comparison
groups. LC n=274; LC included AC, SC, and SCLC. AC, n=223; SC,
n=36; and SCLC, n=15; BLD, n=124. HC, n=201. A Kruskal-Wallis H
test followed by a Dunn's test was used to compare the data between
HC, BLD, LC, AC, SC, SCLC, stage I, stage II, stage III, and stage
IV. *P<0.05, **P<0.01, ***P<0.001. ns, not significant;
HE4, human epididymis secretory protein 4; LC, lung cancer; AC,
adenocarcinoma; SC, squamous cell carcinoma; SCLC, small cell lung
cancer; BLD, benign lung disease. |
 | Table II.Association between serum HE4 levels
lung cancer characteristics. |
Table II.
Association between serum HE4 levels
lung cancer characteristics.
| Variable | N | HE4,
mol/la | P-value |
|---|
| Sex |
|
| 0.0001 |
|
Male | 152 | 81.33
(63.06-09.90) |
|
|
Female | 122 | 54.12
(46.55-65.55) |
|
| Age (years) |
|
| 0.0001 |
|
>60 | 140 | 81.53
(59.86-109.90) |
|
|
≤60 | 134 | 55.59
(46.94-71.73) |
|
| Tumor diameter
(cm) |
|
| 0.0001 |
| ≥3 | 29 | 90.16
(66.11-113.35) |
|
|
<3 | 175 | 55.97
(47.45-73.68) |
|
|
Unknown | 70 |
|
|
| T stage |
|
| 0.0001 |
| T1 | 163 | 56.18
(47.45-75.49) |
|
| T2 | 69 | 86.99
(67.95-113.35) | 0.0001 |
| T3 | 16 | 91.68
(68.35-144.23) | 0.0001 |
| T4 | 11 | 114.82
(55.42-156.90) | 0.0040 |
|
Unknown | 15 |
|
|
| N stage |
|
| 0.0001 |
|
Positive (N1-3) | 78 | 93.87
(67.01-133.98) |
|
|
Negative (N0) | 180 | 56.66
(47.54-76.26) |
|
|
Unknown | 16 |
|
|
| AJCC stage |
|
| 0.0001 |
| I | 176 | 56.67
(47.84-76.13) |
|
| II | 9 | 89.07
(52.49-110.25) | 0.3200 |
|
III | 52 | 93.20
(72.12-121.85) | 0.0001 |
| IV | 25 | 101.80
(64.69-160.26) | 0.0001 |
|
Unknown | 12 |
|
|
| Distant
metastasis |
|
| 0.0001 |
|
Absent | 239 | 63.20
(50.04-88.57) |
|
|
Present | 24 | 104.05
(68.29-168.70) |
|
|
Unknown | 11 |
|
|
HE4, NSE, CYFRA21-1, SCC, and proGRP
serum concentrations in LC patients and healthy controls
The serum concentrations of HE4, NSE, CYFRA21-1,
SCC, and proGRP in the LC, AC, SC, and SCLC patients as well as the
healthy controls are shown in Table
III. Compared with the healthy control group, the serum HE4,
NSE, CYFRA21-1, SCC, and proGRP were significantly increased in the
LC, AC, and SC subgroups (P<0.05), but only the serum
concentrations of HE4, NSE, CYFRA21-1, and proGRP were markedly
higher in SCLC patients (P<0.05), the serum concentrations of
SCC were not markedly higher in the SCLC subgroup (P>0.05).
Compared with the BLD group, the serum concentrations of HE4,
CYFRA21-1, and proGRP were markedly higher than those in the LC,
AC, SC, and SCLC groups (P<0.05); the serum concentrations of
NSE were markedly higher in the SC and SCLC patients (P<0.05),
but was not markedly higher in the LC and AC patients (P>0.05);
the serum concentrations of SCC were markedly higher in the LC and
SC patients (P<0.05), but was not markedly higher in the AC and
SCLC patients (P>0.05). Moreover, HE4 exhibited the most
substantial discriminative ability for LC (Figs. 1A and 2A-D).
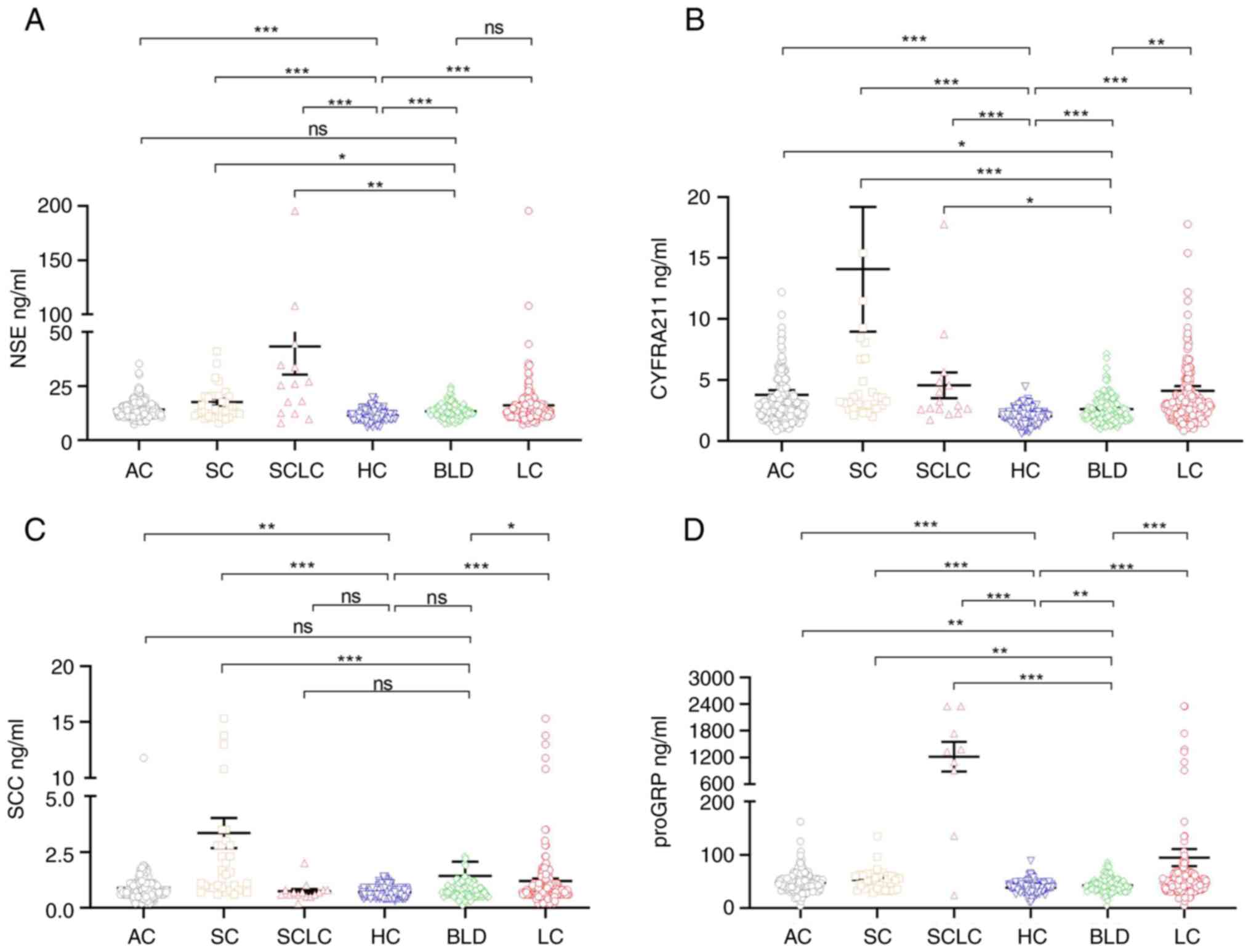 | Figure 2.NSE, CYFRA21-1, SCC, and proGRP serum
levels in patients with LC and in the HC group. The serum levels of
(A) NSE, (B) CYFRA21-1, (C) SCC, and (D) proGRP. A Kruskal-Wallis H
test was used to compare the data between HC, BLD, LC, AC, SC, and
SCLC. *P<0.05, **P<0.01, ***P<0.001. ns, not significant;
AC, adenocarcinoma; SC, squamous cell carcinoma; SCLC, small cell
lung cancer; BLD, benign lung diseases; HC, healthy control; NSC,
serum neuron-specific enolase; CYFRA21-1, cytokeratin 19 fragments;
SCC, squamous cell carcinoma antigen; proGRP, progastrin-releasing
peptide. |
 | Table III.Serum HE4, NSE, CYFRA21-1, SCC, and
proGRP levels in the different groups. |
Table III.
Serum HE4, NSE, CYFRA21-1, SCC, and
proGRP levels in the different groups.
|
|
| HE4, mol/l | NSE, ng/ml | CYFRA211,
ng/ml | SCC, ng/ml | proGRP, pg/ml |
|---|
|
|
|
|
|
|
|
|
|---|
| Diagnosis | n | Median | P25, P75 | Median | P25, P75 | Median | P25, P75 | Median | P25, P75 | Median | P25, P75 |
|---|
| Healthy
control | 201 | 41.73 | 36.99, 51.23 | 11.18 | 9.54, 13.12 | 2.01 | 1.55, 2.49 | 0.7 | 0.50, 0.90 | 38.65 | 33.87, 43.02 |
| Benign lung
diseasesa | 124 | 52.56 | 45.39, 61.19 | 13.17 | 11.54, 15.11 | 2.32 | 1.79, 3.23 | 0.8 | 0.60, 0.90 | 42.09 | 33.97, 48.72 |
| Lung
cancerb | 274 | 65.71 | 51.49, 97.94 | 13.71 | 11.51, 16.43 | 2.91 | 2.11, 3.85 | 0.8 | 0.60, 1.10 | 47.23 | 36.75, 58.31 |
|
NSCLCc | 259 | 65.23 | 51.22, 91.94 | 13.62 | 11.47, 16.24 | 2.91 | 2.09, 3.83 | 0.8 | 0.60, 1.10 | 46.59 | 36.08, 56.37 |
| Stage
Id | 176 | 56.6 | 47.80, 76.17 | 13.35 | 11.57, 15.54 | 2.62 | 1.89, 3.36 | 0.8 | 0.60, 1.00 | 45.96 | 36.86, 54.40 |
| Stage
IIe | 7 | 89.07 | 56.89, 113.30 | 16.24 | 13.37, 19.85 | 3.64 | 2.95, 11.49 | 0.8 | 0.60, 1.30 | 49.38 | 33.00, 54.29 |
|
ACf | 223 | 60.54 | 49.23, 86.70 | 13.58 | 11.45, 15.87 | 2.81 | 1.98, 3.58 | 0.8 | 0.60, 1.00 | 45.97 | 36.12, 55.04 |
|
SCg | 36 | 89.31 | 66.31, 120.25 | 14.78 | 11.62, 20.39 | 3.59 | 2.77, 9.09 | 1.55 | 0.90, 3.38 | 52.7 | 36.83, 61.09 |
|
SCLCh | 15 | 101.8 | 64.95, 133.70 | 25.98 | 12.54, 44.39 | 2.93 | 2.61, 4.88 | 0.7 | 0.60, 0.80 | 911.2 | 372.70,
1,738.00 |
Value of serum HE4, NSE, CYFRA21-1,
SCC, and proGRP for diagnosis of LC
ROC analysis was performed to better understand the
diagnostic value of serum HE4, NSE, CYFRA21-1, SCC, and proGRP for
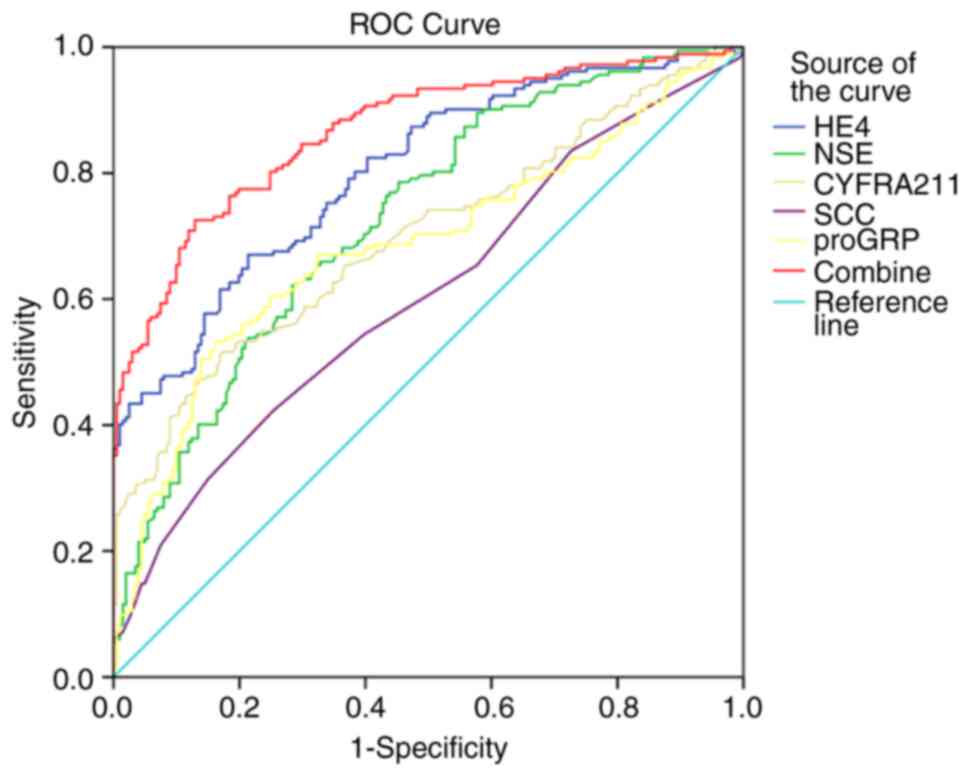
LC. The AUC of HE4 for discriminating LC from healthy controls was
0.851 (95% CI, 0.818-0.884) and 0.739 (95% CI, 0.695-0.783), 0.747
(95% CI, 0.704-0.790), 0.626 (95% CI, 0.577-0.676), and 0.700 (95%
CI, 0.653-0.747) for NSE, CYFRA21-1, SCC, and proGRP, respectively.
(Fig. 3A, Table IV). The cut-off values with a
specificity of 95% were 60.14 pmol/l for HE4, 16.01 µg/l for NSE,
3.14 µg/l for CYFRA21-1, 1.11 µg/l for SCC, and 54.20 ng/l for
proGRP. Furthermore, the AUC for serum HE4 combined with NSE,
CYFRA21-1, SCC, and proGRP for cancer diagnosis was 0.896 (95% CI,
0.868-0.923).
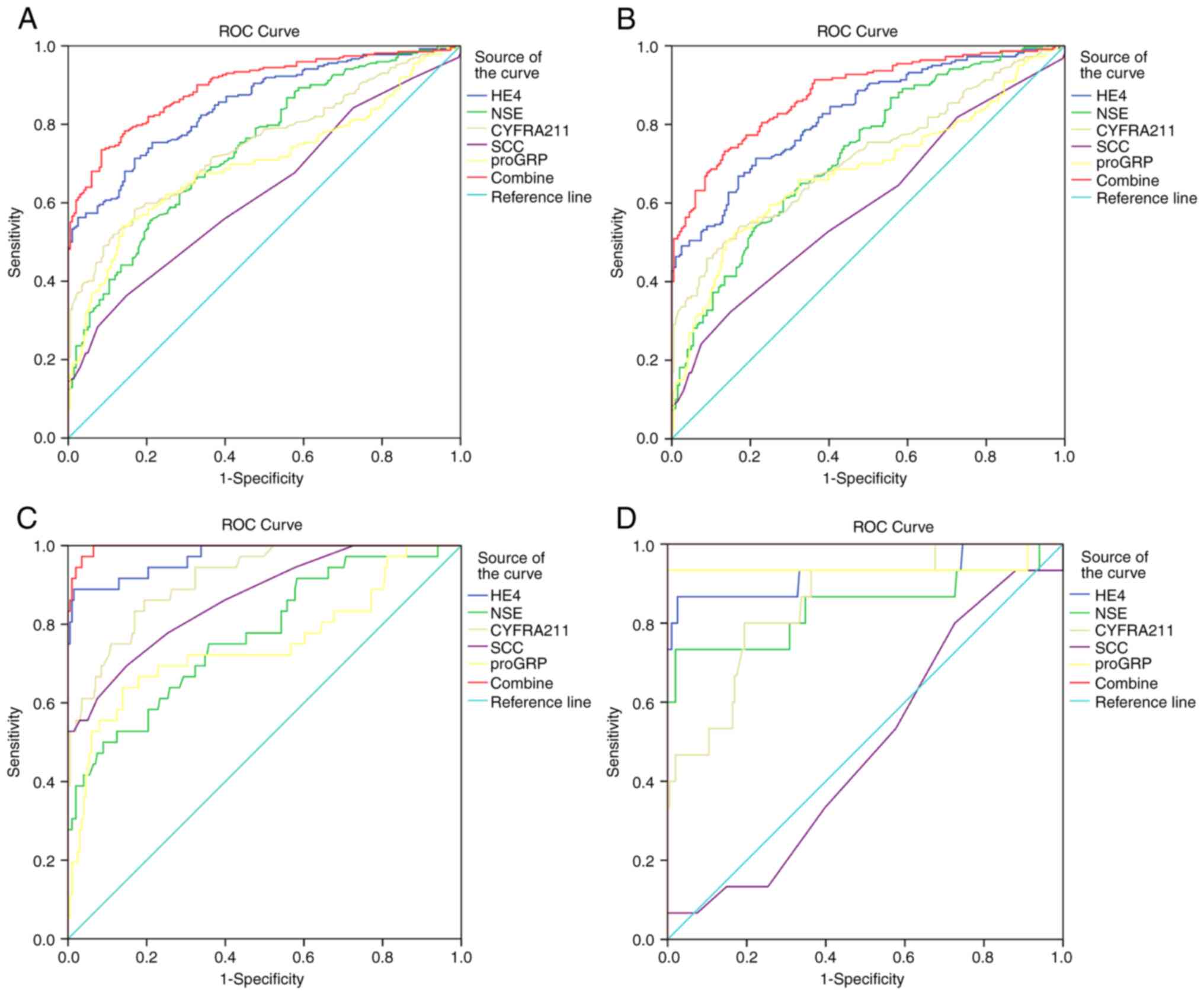 | Figure 3.Sensitivity and specificity of
biomarkers alone or combined in the diagnosis of lung cancer. The
sensitivity and specificity of biomarkers (HE4, NSE, CYFRA21-1,
SCC, proGRP) alone or combined for the diagnosis of (A) all lung
cancer patients, (B) AC, (C) SC, and (D) SCLC was analyzed by
determining the area under the ROC curve. AC, adenocarcinoma; SC,
squamous cell carcinoma; SCLC, small cell lung cancer; BLD, benign
lung diseases; HC, healthy control; NSC, serum neuron-specific
enolase; CYFRA21-1, cytokeratin 19 fragments; SCC, squamous cell
carcinoma antigen; proGRP, progastrin-releasing peptide. |
 | Table IV.Sensitivity and specificity of
biomarkers alone or combined for the diagnosis of LC. |
Table IV.
Sensitivity and specificity of
biomarkers alone or combined for the diagnosis of LC.
| Type | AUC | 95% CI | Cut-off | Sensitivity
(%) | Specificity
(%) | Youden index |
|---|
| Lung cancer |
|
|
|
|
|
|
|
HE4 | 0.851 | 0.818-0.884 | 60.14 | 57.4 | 95.0 | 0.524 |
|
NSE | 0.739 | 0.695-0.783 | 16.01 | 28.3 | 95.0 | 0.233 |
|
CYFRA211 | 0.747 | 0.704-0.790 | 3.14 | 40.1 | 95.0 | 0.351 |
|
SCC | 0.626 | 0.577-0.676 | 1.11 | 21.7 | 95.0 | 0.167 |
|
proGRP | 0.700 | 0.653-0.747 | 54.20 | 32.7 | 95.0 | 0.277 |
|
Combined | 0.896 | 0.868-0.923 | 0.74 | 64.0 | 95.0 | 0.590 |
| Adenocarcinoma |
|
|
|
|
|
|
|
HE4 | 0.826 | 0.787-0.864 | 60.15 | 50.5 | 95.0 | 0.455 |
|
NSE | 0.727 | 0.680-0.775 | 16.01 | 23.6 | 95.0 | 0.187 |
|
CYFRA211 | 0.717 | 0.668-0.765 | 3.14 | 36.4 | 95.0 | 0.314 |
|
SCC | 0.597 | 0.543-0.651 | 1.11 | 16.8 | 95.0 | 0.118 |
|
proGRP | 0.682 | 0.631-0.733 | 54.21 | 27.3 | 95.0 | 0.223 |
|
Combined | 0.878 | 0.846-0.910 | 0.73 | 58.2 | 95.0 | 0.532 |
| Squamous
carcinoma |
|
|
|
|
|
|
|
HE4 | 0.972 | 0.944-0.999 | 60.15 | 88.9 | 95.0 | 0.839 |
|
NSE | 0.772 | 0.683-0.860 | 16.04 | 41.7 | 95.0 | 0.367 |
|
CYFRA211 | 0.914 | 0.868-0.961 | 3.15 | 61.1 | 95.0 | 0.561 |
|
SCC | 0.866 | 0.798-0.934 | 1.11 | 55.6 | 95.0 | 0.506 |
|
proGRP | 0.748 | 0.645-0.852 | 54.20 | 44.4 | 95.0 | 0.395 |
|
Combined | 0.996 | 0.991-1.000 | 0.14 | 97.2 | 95.0 | 0.922 |
| Small-cell lung
carcinoma |
|
|
|
|
|
|
|
HE4 | 0.926 | 0.826-1.000 | 60.15 | 86.7 | 95.0 | 0.817 |
|
NSE | 0.842 | 0.695-0.990 | 16.04 | 73.3 | 95.0 | 0.684 |
|
CYFRA211 | 0.852 | 0.755-0.950 | 3.15 | 46.7 | 95.0 | 0.417 |
|
SCC | 0.482 | 0.342-0.623 | 1.11 | 6.7 | 95.0 | 0.017 |
|
proGRP | 0.939 | 0.824-1.000 | 54.22 | 93.3 | 95.0 | 0.884 |
|
Combined | 1.000 | 1.000-1.000 | 0.00 | 100.0 | 95.0 | 0.950 |
Next, the diagnostic efficacy of serum HE4, NSE,
CYFRA21-1, SCC, and proGRP for AC, SC, and SCLC were analyzed. For
AC, the AUC of HE4 for discriminating AC from healthy controls was
0.826 (95% CI, 0.787-0.864). The cut-off values with a specificity
of 95% were 60.15 pmol/l for HE4. The AUC value of the combination
of serum HE4 with NSE, CYFRA21-1, SCC, and proGRP for cancer
diagnosis was 0.878 (Fig. 3B,
Table IV).
HE4 had the best diagnostic efficacy for SC. The AUC
of HE4 for discriminating SC from healthy controls was AUC 0.972
(95% CI, 0.944-0.999). The cut-off values with a specificity of 95%
were 60.15 pmol/l for HE4. The AUC value of the combination of
serum HE4 with NSE, CYFRA21-1, SCC, and proGRP for cancer diagnosis
was 0.996 (Fig. 3C, Table IV).
proGRP had the best diagnostic efficacy for SCLC.
The AUC of proGRP for discriminating SCLC from healthy controls was
0.939 (95% CI, 0.824-1.000). The cut-off values with a specificity
of 95% were 54.22 pg/ml for proGRP. The AUC value of the
combination of serum HE4 with NSE, CYFRA21-1, SCC, and proGRP for
cancer diagnosis was 1.000 (Fig.
3D, Table IV).
Diagnostic value of serum HE4, NSE,
CYFRA21-1, SCC, and proGRP for early LC
ROC analysis was performed to assess the diagnostic
value of serum HE4, NSE, CYFRA21-1, SCC, and proGRP for early LC. A
total of 183 early LC specimens were included (Table III), including 176 patients with
stage I LC and 7 patients with stage II LC. No limited-stage SCLCs
were collected. The results showed that the AUC values for
discriminating early LC from healthy controls were 0.802 (95% CI,
0.758-0.845) for HE4, 0.728 (95% CI, 0.679-0.778) for NSE, 0.699
(95% CI, 0.646-0.752) CYFRA21-1, 0.605 (95% CI, 0.548-0.662) for
SCC, and 0.685 (95% CI, 0.630-0.739) for proGRP (Fig. 4, Table
V). The cut-off value with a specificity of 95% was 60.15
pmol/l for HE4, 16.01 µg/l for NSE, 3.14 µg/l for CYFRA21-1, 1.11
µg/l for SCC, and 54.20 ng/l for proGRP. Furthermore, the AUC value
of combined serum HE4 with NSE, CYFRA21-1, SCC, and proGRP for
cancer diagnosis was 0.867. The above results indicated that serum
HE4 may serve as a biomarker of early LC and significantly improve
diagnostic efficiency of early LC.
 | Table V.Sensitivity and specificity of
biomarkers alone or combined for the diagnosis of TNM stage I and
II LC patients. |
Table V.
Sensitivity and specificity of
biomarkers alone or combined for the diagnosis of TNM stage I and
II LC patients.
| Marker | AUC | 95% CI | Cut-off | Sensitivity
(%) | Specificity
(%) | Youden's index |
|---|
| HE4 | 0.802 | 0.758-0.845 | 60.15 | 45.1 | 95.0 | 0.401 |
| NSE | 0.728 | 0.679-0.778 | 16.01 | 22.0 | 95.0 | 0.170 |
| CYFRA211 | 0.699 | 0.646-0.752 | 3.14 | 31.3 | 95.0 | 0.263 |
| SCC | 0.605 | 0.548-0.662 | 1.11 | 14.8 | 95.0 | 0.099 |
| proGRP | 0.685 | 0.630-0.739 | 54.20 | 25.8 | 95.0 | 0.208 |
| Combined | 0.867 | 0.831-0.903 | 0.73 | 52.7 | 95.0 | 0.478 |
Discussion
The worldwide incidence and mortality of LC have
been reported to be 56.3 per 100,000 population and 35.0 per
100,000 population, respectively (19), and identifying biomarkers to improve
the diagnostic efficiency is an important research question. The
results of the present study indicated that serum HE4 effectively
improved the diagnostic efficiency of LC, and serum HE4 had good
diagnostic efficiency for early LC. These findings are consistent
with previous research. Iwahori et al (20) reported that the AUC of serum HE4 in
the diagnosis of LC was 0.988 with a cut-off value of 6.56 ng/ml
(sensitivity, 89.8%; specificity, 100%). Liu et al (21) found that the AUC of serum HE4 for LC
diagnosis was 0.85 with a cut-off value of 77.48 pmol/ml
(sensitivity, 67.9%; specificity 93.4). Nagy et al (22) found that the AUC of serum HE4 for LC
diagnosis was 0.848 with a cut-off value of 97.6 pmol/l
(specificity, 64.3%; sensitivity, 95.9%). In the present study, the
AUC of serum HE4 for LC was 0.851 with a cut-off value of 60.14
pmol/ml (sensitivity, 57.4%; specificity, 95%).
SCC, CYFRA211, and NSE, proGRP are commonly used to
diagnose SC and SCLC. Liu et al (23) reported that the AUC of serum SCC and
CYFRA211 for diagnosing lung squamous cell carcinoma were 0.691 and
0.788. Du et al (24)
reported that the AUC of proGRP for the diagnosis of SCLC were
0.855 and 0.905. In the present study, the diagnostic efficiency of
serum HE4 (0.972) was better than that of serum SCC (0.866) and
CYFRA211 (0.914) for SC. The diagnostic efficiency of serum proGRP
(0.939) was better than that of NSE (0.842) and serum HE4 (0.926)
for SCLC. In the present study, the high diagnostic efficiency of
SCC, CYFRA211, NSE, and proGRP for SC and SCLC may have been due to
the small number of patients and the prevalence of advanced-stage
disease. The high diagnostic efficacy of serum HE4 for AC may be
related to the high proportion of cases in LC.
Next, the diagnostic efficacy of serum HE4 for early
LC was investigated. The results showed that serum HE4 was the best
specificity marker for early LC, with a cut-off value of 60.15
pmol/l (sensitivity: 45.1%, specificity: 95.0%), similar to the
results reported by Zeng et al (16). HE4, combined with serum SCC,
CYFRA211, NSE, and proGRP, may further improve the diagnostic
efficiency of early LC.
In the present study, the association between serum
HE4 and clinicopathological features of LC was analyzed, and it was
found that serum HE4 was associated with sex, age, tumor size, T
stage, M stage, and AJCC stage. It has been reported that serum HE4
in LC patients is associated with age and sex. Serum HE4 gradually
increased with age, and there was a significant difference in
levels between males and females (25,26).
Previously, it was shown that serum HE4 was positively correlated
with age in patients with endometrial cancer (27). This characteristic of HE4 expression
requires us to be more careful when interpreting these results, the
differences between sexes regarding HE4 levels may be related to
its tissue source; specifically high HE4 expression in endometrial
tissue may lead to the differences between sexes observed here
(28). Whilst the correlation
between HE4 and age may be related to its own function, it has been
reported that HE4 can promote the proliferation, invasion, and
metastasis of endometrial cancer cell lines (29), and HE4 may have a similar effect in
LC. The incidence of LC has increased in recent years, and with an
increase in age, the risk of developing lung cancer increases,
which may lead to the association with the observed regarding HE4
levels. However, the specific mechanistic differences caused by the
correlation between HE4, sex, and age remain to be further studied.
This suggests that different reference intervals should be
formulated according to age and sex when applying serum HE4 as a
marker for the diagnosis of LC. The present study found that the
larger the tumor diameter, the higher the T and M stage were and
that the higher the AJCC grade was, the higher the serum HE4 levels
were, which indicates that serum H4 may be associated with a poor
prognosis in patients with LC (17).
In conclusion, serum HE4 is a promising biomarker
for LC. Several studies have reported that serum HE4 can be used as
a marker for the diagnosis and prognosis of LC. The present study
further confirmed that serum HE4 could effectively improve the
diagnostic efficiency of LC.
Acknowledgements
The authors would like to thank Professor Jingwu Li
(Department of Oncological Surgery, Tangshan People's Hospital,
Tangshan, Hebei, China) for providing guidance on this subject.
Funding
This study was supported by funding form The Science and
Technology Innovation Team Training Plan Project Fund of Tangshan
(grant no. 19130202C), The Key Research Topics of Medical Science
in Hebei Province (grant no. 20201538), The Key Research Topics of
Medical Science in Hebei Province (grant no. 20220218), and the
Fund of Key Laboratory of Hebei Province (grant no.
SZX2020043).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, YZL and LH conceived and designed the present
study, and acquired, analyzed, and interpreted the data. JL and YFL
drafted the manuscript. QG performed the experiments. AL, SH, HL,
RS, YZ, YFL and XL analyzed the data. JL and LH confirms the
authenticity of all raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
All volunteers signed an informed consent form. The
Ethics Committee at Tangshan People's Hospital approved the
collection and use of serum (approval no. RMYY-LLKS-2019-0620-1;
Tangshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imai H, Kaira K, Suzuki K, Anzai M, Tsuda
T, Ishizuka T, Kuwako T, Naruse I, Nemoto K, Uchino J, et al: A
phase II study of afatinib treatment for elderly patients with
previously untreated advanced non-small-cell lung cancer harboring
EGFR mutations. Lung Cancer. 126:41–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brinkmeyer JK and Moore DC: Necitumumab
for the treatment of squamous cell non-small cell lung cancer. J
Oncol Pharm Pract. 24:37–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horn L, Reck M and Spigel DR: The Future
of immunotherapy in the treatment of small cell lung cancer.
Oncologist. 21:910–921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7:1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickson JL, Horst C, Nair A, Tisi S,
Prendecki R and Janes SM: Hesitancy around low-dose CT screening
for lung cancer. Ann Oncol. 33:34–41. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Zhang Y, Jiang L, Li Y, Li G, Zhou
J, Yang C, Li X, Qu W, Chen Y, et al: New insights into the
diagnostic characteristics and clinical application of serum
biomarkers for lung cancer, and human epididymis protein 4 as a new
biomarker? Neoplasma. 69:729–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Liu X, Shang X, Qi K and Zhang S:
The diagnostic value of the combination of carcinoembryonic
antigen, squamous cell carcinoma-related antigen, CYFRA 21-1,
neuron-specific enolase, tissue polypeptide antigen, and
progastrin-releasing peptide in small cell lung cancer
discrimination. Int J Biol Markers. 36:36–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirchhoff C, Habben I, Ivell R and Krull
N: A major human epididymis-specific cDNA encodes a protein with
sequence homology to extracellular proteinase inhibitors. Biol
Reprod. 45:350–357. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bingle L, Singleton V and Bingle CD: The
putative ovarian tumour marker gene HE4 (WFDC2), is expressed in
normal tissues and undergoes complex alternative splicing to yield
multiple protein isoforms. Oncogene. 21:2768–2773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drapkin R, von Horsten HH, Lin Y, Mok SC,
Crum CP, Welch WR and Hecht JL: Human epididymis protein 4 (HE4) is
a secreted glycoprotein that is overexpressed by serous and
endometrioid ovarian carcinomas. Cancer Res. 65:2162–2169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Wang ZC, Luo L, Mu CY, Xu J, Feng Q,
Li SB, Gu B, Ma P and Lan T: The clinical value of the combined
detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer
diagnosis. Eur Rev Med Pharmacol Sci. 24:604–610. 2020.PubMed/NCBI
|
|
13
|
Wang H, Liu P, Xu H and Dai H: Early
diagonosis of ovarian cancer: Serum HE4, CA125 and ROMA model. Am J
Transl Res. 13:14141–14148. 2021.PubMed/NCBI
|
|
14
|
Plotti F, Guzzo F, Schirò T, Terranova C,
De Cicco Nardone C, Montera R, Luvero D, Scaletta G, Lopez S,
Capriglione S, et al: Role of human epididymis protein 4 (HE4) in
detecting recurrence in CA125 negative ovarian cancer patients. Int
J Gynecol Cancer. ijgc-2019-000211. 2019.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Espiau Romera A, Coronado Martín PJ,
Chóliz Ezquerro M, Cuesta Guardiola T, Adiego Calvo I and Baquedano
Mainar L: Value of preoperative HE4 as predictor of advanced
disease in endometrioid endometrial cancer. Int J Gynaecol Obstet.
153:64–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng Q, Liu M, Zhou N, Liu L and Song X:
Serum human epididymis protein 4 (HE4) may be a better tumor marker
in early lung cancer. Clin Chim Acta. 455:102–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamy PJ, Plassot C and Pujol JL: Serum
HE4: An independent prognostic factor in non-small cell lung
cancer. PLoS One. 10:e01288362015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Ren N, Guo B, Xin H and Yin Y:
Measuring serum human epididymis secretory protein autoantibody as
an early biomarker of lung cancer. Transl Cancer Res. 9:735–741.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwahori K, Suzuki H, Kishi Y, Fujii Y,
Uehara R, Okamoto N, Kobayashi M, Hirashima T, Kawase I and Naka T:
Serum HE4 as a diagnostic and prognostic marker for lung cancer.
Tumour Biol. 33:1141–1149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu W, Yang J, Chi PD, Zheng X, Dai SQ,
Chen H, Xu BL and Liu WL: Evaluating the clinical significance of
serum HE4 levels in lung cancer and pulmonary tuberculosis. Int J
Tuberc Lung Dis. 17:1346–1353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagy B Jr, Bhattoa HP, Steiber Z, Csobán
M, Szilasi M, Méhes G, Müller M, Lázár J, Kappelmayer J and
Antal-Szalmás P: Serum human epididymis protein 4 (HE4) as a tumor
marker in men with lung cancer. Clin Chem Lab Med. 52:1639–1648.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Liu B, Zhu LL, Zhang W and Li Y:
Clinical significance of CYFRA21-1, Scc-Ag and telomerase activity
in serum and pleural effusion of patients with squamous-cell lung
cancer. Bioanalysis. 4:2367–2374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du J, Li Y, Wang L, Zhou Y, Shen Y, Xu F
and Chen Y: Selective application of neuroendocrine markers in the
diagnosis and treatment of small cell lung cancer. Clin Chim Acta.
509:295–303. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ucar EY, Ozkaya AL, Araz O, Akgun M, Meral
M, Kaynar H, Saglam L, Aksoy H and Akcay F: Serum and bronchial
aspiration fluid HE-4 levels in lung cancer. Tumour Biol.
35:8795–8799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hertlein L, Stieber P, Kirschenhofer A,
Krocker K, Nagel D, Lenhard M and Burges A: Human epididymis
protein 4 (HE4) in benign and malignant diseases. Clin Chem Lab
Med. 50:2181–2188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gąsiorowska E, Magnowska M, Iżycka N,
Warchoł W and Nowak-Markwitz E: The role of HE4 in differentiating
benign and malignant endometrial pathology. Ginekol Pol.
87:260–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang SW, Chen H, Dowdy S, Fu A, Attewell
J, Kalogera E, Drapkin R, Podratz K, Broaddus R and Li J: HE4
transcription- and splice variants-specific expression in
endometrial cancer and correlation with patient survival. Int J Mol
Sci. 14:22655–22677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Chen H, Mariani A, Chen D, Klatt E,
Podratz K, Drapkin R, Broaddus R, Dowdy S and Jiang SW: HE4 (WFDC2)
promotes tumor growth in endometrial cancer cell lines. Int J Mol
Sci. 14:6026–6043. 2013. View Article : Google Scholar : PubMed/NCBI
|