Introduction
With estimated 900,000 new cases and 830,000 deaths
in 2020, hepatocellular carcinoma (HCC) is the sixth most frequent
neoplasm and third leading cause of cancer-related deaths globally
(1,2). Recent progress in systemic
chemotherapy for advanced HCC, such as immune checkpoint inhibitors
(ICIs) and molecular targeted agents, have improved patient
outcomes (3–7).
The results of the IMbrave150 study indicated that a
combination of atezolizumab and bevacizumab, monoclonal antibodies
against programmed death ligand 1 (PD-L1) and vascular endothelial
growth factor (VEGF), can extend progression-free survival (PFS)
and overall survival (OS) in advanced HCC patients compared with
sorafenib, a multiple-target tyrosine kinase inhibitor (TKI),
through anti-angiogenesis and anti-proliferation effects (3). As a result, atezolizumab plus
bevacizumab has become the first-line systemic chemotherapy regimen
for advanced HCC.
Lenvatinib is an oral multi-kinase inhibitor of VEGF
receptors 1–3, fibroblast growth factor (FGF) receptors 1–4,
platelet-derived growth factor (PDGF) receptor α, rearranged during
transfection (RET), and stem cell factor receptor (KIT) (8,9). A
global, randomized, multi-center, open-label trial to evaluate the
non-inferiority of sorafenib (REFLECT; NCT01761266) revealed that
lenvatinib significantly improved PFS compared with sorafenib in
patients with previously untreated, metastatic, or advanced HCC
(4). Lenvatinib is now approved for
the treatment of HCC (3).
Despite these advancements in treatment methods,
patients with advanced HCC continue to have a poor prognosis. The
appropriate choice of chemotherapy may further improve patient
prognosis. As a result, it is critical to choose agents that are
appropriate for personalized HCC treatment. Therefore, potential
predictive biomarkers and an increased knowledge of the mechanisms
of response or resistance to systemic chemotherapies are
required.
However, no established biomarkers have been
identified to predict responsiveness to systemic chemotherapy in
HCC. Previous studies have reported CD8+ tumor-infiltrating
lymphocytes (TILs) as biomarkers for systemic chemotherapy
responsiveness in HCC and other cancers (10–12).
Recent gene expression profiling data of liver tumor biopsy samples
have shown that CD8+ TILs are potentially associated
with clinical response to atezolizumab plus bevacizumab, although
further research is needed to confirm these findings (13). No reports have evaluated
CT8+ TILs as a biomarker for systemic chemotherapy in
HCC using only immunostaining procedures. In this study, we
investigated whether CD8+ TILs identified by
immunostaining of liver tumor biopsy tissues could be a useful
biomarker for predicting responses to systemic chemotherapy in
HCC.
Materials and methods
Patients
This single-center prospective study analyzed the
efficacy of atezolizumab plus bevacizumab and lenvatinib alone in
HCC patients with and without CD8+ TILs at Aso Iizuka
Hospital between December 2018 and September 2022. A total of 63
patients received combination therapy with atezolizumab and
bevacizumab and 92 patients received lenvatinib for advanced HCC.
Of these individuals, we excluded 102 patients who did not undergo
liver tumor biopsy prior to chemotherapy and 14 patients who were
followed up within 6 weeks before the evaluation of treatment
response. In total, we evaluated 39 patients for this study. This
study was conducted in accordance with the guidelines of the
Declaration of Helsinki and was approved by the Ethics Committee of
Aso Iizuka Hospital. The study of lenvatinib was approved in
approval No. 18070 and the study of atezolizumab plus bevacizumab
was approved in approval No. 22008. The opt-out method was used to
obtain consent for this study.
Albumin-bilirubin (ALBI) score
Liver function was assessed using the ALBI score.
ALBI scores were calculated as follows: ALBI score=log10 (T-Bil
[mg/dl]x17.1)x0.66 + (ALB [g/dl]x10)x-0.085, where T-Bil is total
bilirubin and ALB is the serum albumin level (14).
Treatment protocol
Patients received atezolizumab (1200 mg) and
bevacizumab (7.5 mg/kg) intravenously every 3 weeks. The IMbrave150
study protocol was defined by Chugai Co., Ltd. (Tokyo, Japan)
(3). Treatment was continued until
disease progression or intolerable side effects.
Patients received lenvatinib based on body weight (8
mg/day for those weighing less than 60 kg and 12 mg/day for those
weighing ≥60 kg) (Eisai Co., Ltd., Tokyo, Japan). Dose interruption
followed by dose reduction (8 mg/day, 4 mg/day, or 4 mg every other
day) was allowed if a patient developed a lenvatinib-related
adverse event. The protocol for the REFLECT study was provided by
Eisai Co., Ltd. (4).
Evaluation of efficacy
Computed tomography (CT) or magnetic resonance
imaging (MRI) was used to determine treatment response every 6 to
12 weeks after treatment initiation. Antitumor response was
assessed by the treating physician on the basis of modified RECIST
version 1.1 (15). The disease
control rate (DCR) was defined as complete response (CR), partial
response (PR), or stable disease (SD) lasting at least 4 months.
The objective response rate (ORR) was defined as PR + CR. The
patient was followed up every 3 weeks and treatment was continued
until disease progression or intolerable side effects occurred.
Immunohistochemistry (IHC)
Liver tumor biopsy specimens fixed in 10% formalin
were embedded in paraffin for 10–48 h at room temperature. Serial
sections (5 µm) were cut from paraffin blocks and stained with
hematoxylin and eosin. The presence of CD8+ T cells was
determined by IHC using the following primary antibody: mouse
anti-human monoclonal CD8 (clone C8/144B; 1:50; DAKO, Agilent,
Santa Clara, CA, USA). After incubation with secondary antibodies,
staining reactions were performed using the Bond Polymer System
(Leica Biosystems, Buffalo Grove, IL, USA).
IHC staining of CD8+ cell infiltration
was assessed on the basis of the number of positively stained
CD8+ TILs by examining high-power fields (HPFs) selected
with the most confluent areas of CD8+ TILs at 400×
magnification. An optimal cutoff was obtained using the mean value
(CD8: 15.9 cells/HPF).
Statistical analysis
JMP Pro version 11 statistical software (SAS
Institute Inc. Cary, NC, USA) was used for all analyses. Data are
presented as medians (interquartile ranges). Significant
differences between groups were examined by the χ-test.
Kaplan-Meier (KM) analysis was performed for statistical analysis
of PFS. Significant differences in PFS were determined by log-rank
analysis. Statistical significance was determined at P<0.05.
Results
Patient characteristics
The characteristics of the 24 patients who received
atezolizumab plus bevacizumab and 15 patients who received
lenvatinib are shown in Tables I
and II, respectively. We
classified the enrolled patients into high-level and low-level
CD8+ TILs groups by IHC staining of CD8+ TILs
in liver tumor biopsy samples.
 | Table I.Baseline characteristics of patients
who received atezolizumab plus bevacizumab. |
Table I.
Baseline characteristics of patients
who received atezolizumab plus bevacizumab.
|
Characteristics | All | High-level
CD8+ TILs | Low-level
CD8+ TILs | P-value |
|---|
| Number | 24 | 12 | 12 |
|
| Age, years | 77.5
(21.0-85.3) | 81.5
(72.3-86.8) | 74.5
(66.3-79.8) | 0.132 |
| Sex, n
(male/female) | 19/5 | 8/4 | 11/1 | 0.121 |
| MVI positive,
n | 6 | 2 | 4 | 0.480 |
| EHS positive,
n | 3 | 2 | 1 | 0.742 |
| Intrahepatic max
tumor size, cm | 5.0 (3.5-8.3) | 6.0 (3.0-8.9) | 4.1 (3.6-7.6) | 0.568 |
| Numbers of tumors
>5 | 13 | 5 | 8 | 0.217 |
| Etiology |
|
|
| 0.152 |
| HBV | 3 | 0 | 3 |
|
| HCV | 12 | 8 | 4 |
|
| NBNC | 9 | 4 | 5 |
|
| Child-Pugh |
|
|
| 0.614 |
|
Child-Pugh score A | 19 | 10 | 9 |
|
|
Child-Pugh score B/C | 5 | 2 | 3 |
|
| Alb, g/dl | 3.5 (3.2-3.8) | 3.75 (3.2-3.8) | 3.35
(3.12-3.6) | 0.295 |
| T.Bil, g/dl | 0.9 (0.6-1.5) | 0.80
(0.53-1.0) | 1.15
(0.65-1.18) | 1.000 |
| ALBI score | −2.20 (−2.54 to
−1.73) | −2.18 (−2.24 to
−1.72) | −2.46 (−2.60 to
−1.91) | 0.322 |
| BCLC stage |
|
|
| 0.406 |
| A | 0 | 0 | 0 |
|
| B | 14 | 8 | 6 |
|
| C | 10 | 4 | 6 |
|
| Tumor marker |
|
|
|
|
| AFP,
ng/ml | 81.1
(5.5-1563.5) | 190.5
(7.2-12670) | 45.3
(4.3-428.2) | 0.309 |
|
PIVKA-II, mAU/ml | 1,693.5
(103.8-7713.3) | 3,162.0
(86.5-14698) | 956.5
(103.8-5564.0) | 0.680 |
 | Table II.Baseline characteristics of patients
who received lenvatinib. |
Table II.
Baseline characteristics of patients
who received lenvatinib.
|
Characteristics | All | High-level
CD8+ TILs | Low-level
CD8+ TILs | P-value |
|---|
| Number | 15 | 5 | 10 |
|
| Age, years | 73 (62.0-80.0) | 74.5
(61.3-82.3) | 71.0
(64.5-74.5) | 0.759 |
| Sex, n
(male/female) | 13/2 | 4/1 | 9/1 | 0.600 |
| Etiology |
|
|
| 0.069 |
| HBV | 4 | 2 | 2 |
|
| HCV | 4 | 3 | 1 |
|
| NBNC | 7 | 0 | 7 |
|
| MVI positive,
n | 3 | 2 | 1 | 0.182 |
| EHS positive,
n | 2 | 1 | 1 | 0.600 |
| Intrahepatic max
tumor size, cm | 3.0 (1.7-6.5) | 5.3 (1.8-8.3) | 3.0 (1.7-6.2) | 0.389 |
| Numbers of tumors
>5 | 9 | 3 | 6 | 0.465 |
| Child-Pugh |
|
|
| 0.394 |
|
Child-Pugh score A | 13 | 4 | 9 | 0.600 |
|
Child-Pugh score B/C | 2 | 1 | 1 |
|
| Alb, g/dl | 3.8 (3.1-4.4) | 4.2 (3.4-4.4) | 3.75
(3.48-4.4.1) | 0.723 |
| T.Bil, g/dl | 0.9 (0.7-1.1) | 1.0 (0.9-1.35) | 0.75
(0.68-1.1) | 0.275 |
| ALBI score | −2.56
(−3.03-2.18) | −2.75
(−2.96-1.99) | −2.44
(−3.05-2.13 | 0.977 |
| BCLC stage |
|
|
| 0.061 |
| A | 1 | 0 | 1 |
|
| B | 12 | 3 | 9 |
|
| C | 2 | 2 | 0 |
|
| Tumor marker |
|
|
|
|
| AFP,
ng/ml | 6.7 (3.3-23.7) | 2,317
(6.6-6080.7) | 6.2 (2.8-7.1) | 0.657 |
|
PIVKA-II, mAU/ml | 138 (42–1135) | 77
(38.5-8650.5) | 266
(38.8-2571.3) | 0.575 |
There were 12 patients with high-level
CD8+ TILs and 12 with low-level CD8+ TILs. In
the high-level group, eight patients were categorized as Barcelona
Clinic Liver Cancer (BCLC) stage B and four were BCLC stage C; in
the low-level group, six patients were BCLC stage B and six were
BCLC stage C (P=0.460). Age, sex, etiology, Child-Pugh grade, ALBI
score, tumor size, number of intrahepatic lesions, microvascular
invasion, extrahepatic spread, serum α-fetoprotein (AFP) levels,
and protein induced by vitamin K absence or antagonist-II
(PIVKA-II) levels were similar between the two groups.
There were five patients with high-level
CD8+ TILs and 10 with low-level CD8+ TILs. In
the high-level group, three patients were categorized as BCLC stage
B and two were BCLC stage C; in the low-level group, one patient
was BCLC stage A and nine were BCLC stage B (P=0.608). Age, sex,
etiology, Child-Pugh grade, ALBI score, tumor size, number of
intrahepatic lesions, microvascular invasion, extrahepatic spread,
serum AFP levels, and PIVKA-II levels were similar between the two
groups.
IHC for CD8+ TILs in HCC
tissues
CD8 +TIL levels were assessed by IHC prior to
atezolizumab plus bevacizumab treatment. Typical cases are shown in
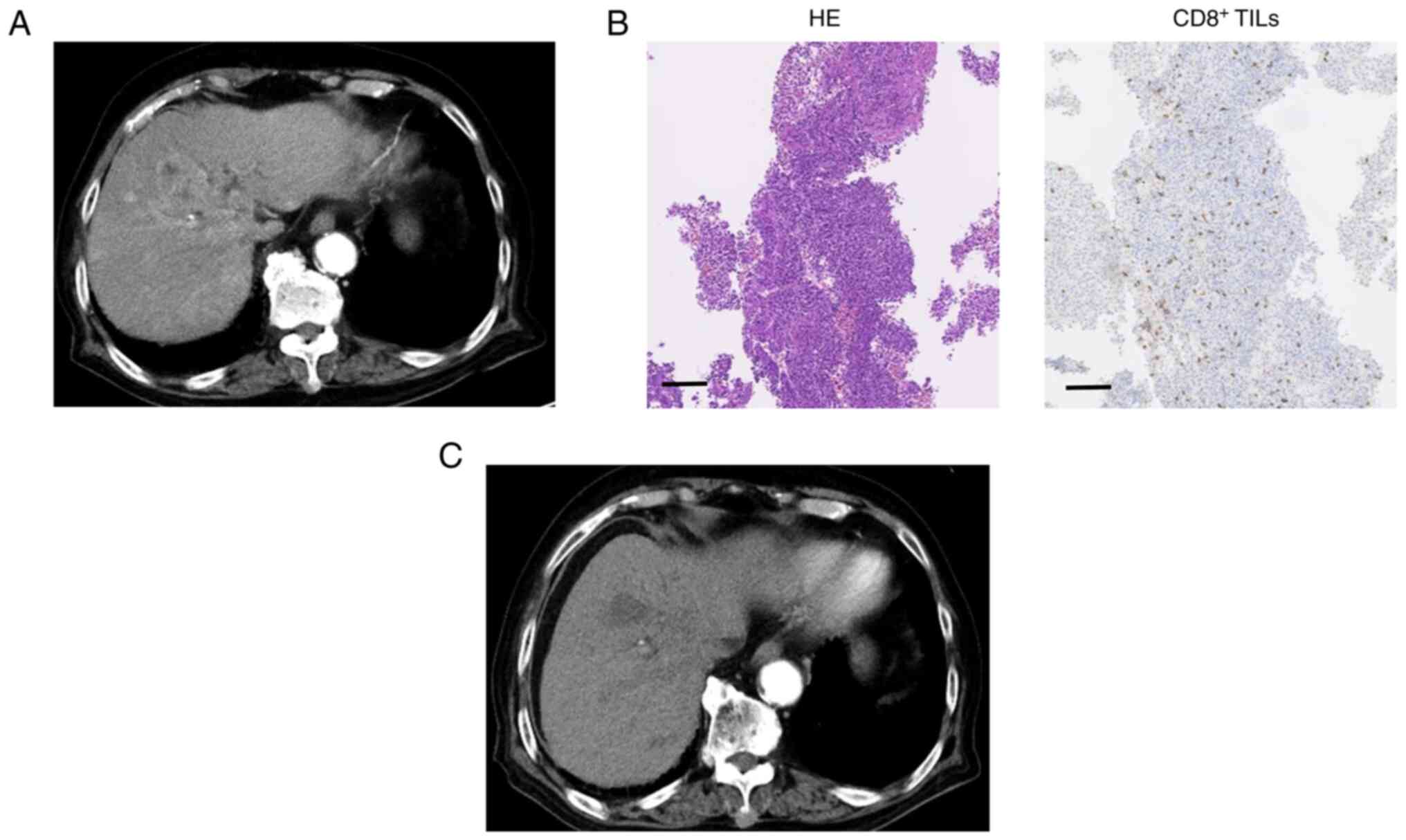
Figs. 1 and 2. Case 1 was an 88-year-old man with
unresectable multiple HCC related to hepatitis C virus infection
(Fig. 1). IHC staining of this
poorly differentiated HCC indicated high levels of CD8+
TILs. Following administration of atezolizumab plus bevacizumab,
liver CT images showed a reduction in the size and enhancement of
the arterial stage of the tumor, indicating a PR. The patient's
serum AFP level was 1,779.7 ng/ml before treatment, which decreased
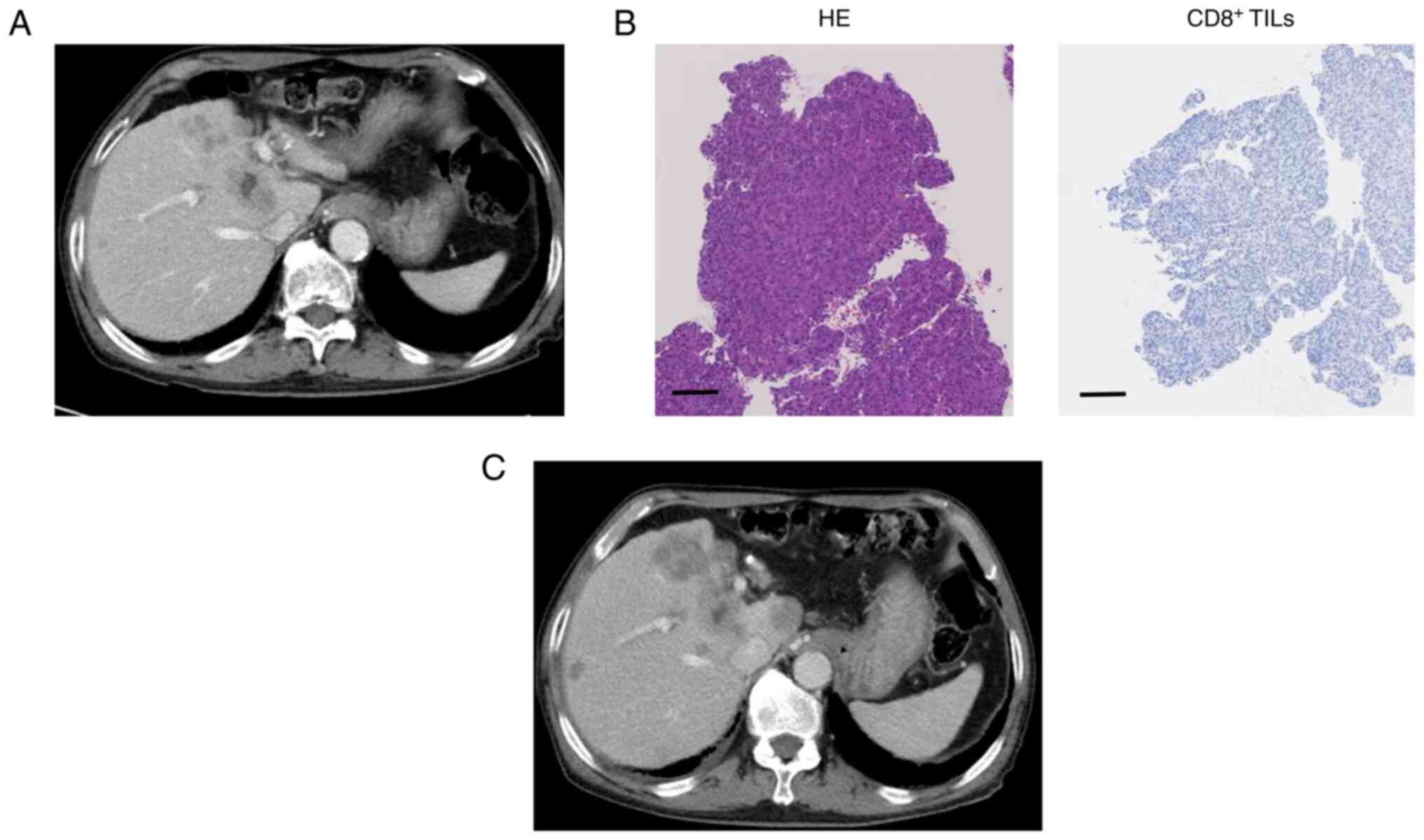
to 52.5 ng/ml after four cycles. Case 2 was a 78-year-old man with
hepatitis C virus-related advanced HCC (Fig. 2). The specimen was diagnosed as
poorly differentiated HCC, and IHC staining showed low
CD8+ TIL levels. Post-treatment CT images showed an
increase in tumor size, indicating disease progression. The
patient's serum AFP level was 3,337.6 ng/ml before treatment, which
increased to 11,028.0 ng/ml after four cycles.
Efficacies of atezolizumab plus
bevacizumab and lenvatinib in the high-level vs. low-level
CD8+ TILs groups
Atezolizumab plus bevacizumab
Among the patients who received atezolizumab plus
bevacizumab, the ORR (CR+PR) was 8/12 (66.6%) in the high-level
CD8+ TILs group and 4/12 (33.3%) in the low-level
CD8+ TILs group (P=0.012). The DCRs (CR+PR+SD) were
10/12 (83.3%) and 6/12 (50.0%) in the high-level and low-level
CD8+ TILs groups, respectively (P=0.031) (Table III). Therefore, there was a higher
response rate in the high-level CD8+ TILs group compared
with the low-level CD8+ TILs group following
atezolizumab plus bevacizumab therapy.
 | Table III.Comparison of responses to
atezolizumab plus bevacizumab between the high-level and low-level
CD8+ tumor infiltrating lymphocytes groups. |
Table III.
Comparison of responses to
atezolizumab plus bevacizumab between the high-level and low-level
CD8+ tumor infiltrating lymphocytes groups.
| Response | All (n=24)% | High-level
CD8+ TILs (n=12)% | Low-level
CD8+ TILs (n=12)% | P-value |
|---|
| Overall
Response |
|
|
| 0.189 |
| CR | 0 (0) | 0 (0) | 0 (0) |
|
| PR | 12 (50) | 8 (66.6) | 4 (33.3) |
|
| SD | 4 (16.7) | 2 (16.7) | 2 (16.7) |
|
| PD | 8 (33.3) | 2 (16.7) | 6 (50) |
|
| ORR (CR+PR) | 12 (50) | 8 (66.6) | 4 (33.3) | 0.012 |
| DCR (CR+PR+SD) | 16 (66.7) | 10 (83.3) | 6 (50) | 0.031 |
Lenvatinib
Among the patients who received lenvatinib, the ORR
(CR+PR) was 2/5 (40.0%) in the high-level CD8+ TILs
group and 2/10 (20.0%) in the low-level CD8+ TILs group
(P=0.417). The DCRs (CR+PR+SD) were 2/5 (40.0%) and 8/10 (80.0%) in
the high-level and low-level CD8+ TILs groups,
respectively (P=0.121) (Table IV).
The CD8+ TIL levels had no effect on the efficacy of
lenvatinib.
 | Table IV.Comparison of responses to lenvatinib
between the high-level and low-level CD8+ tumor
infiltrating lymphocytes groups. |
Table IV.
Comparison of responses to lenvatinib
between the high-level and low-level CD8+ tumor
infiltrating lymphocytes groups.
| Response | All (n=15)% | High-level
CD8+ TILs (n=5)% | Low-level
CD8+ TILs (n=10)% | P-value |
|---|
| Overall
Response |
|
|
| 0.086 |
| CR | 1 (6.7) | 0 (0) | 1 (10) |
|
| PR | 3 (20.0) | 2 (40) | 1 (10) |
|
| SD | 6 (40) | 0 (0) | 6 (60) |
|
| PD | 5 (33.3) | 3 (60) | 2 (20) |
|
| ORR (CR + PR) | 4 (26.7) | 2 (40) | 2 (20) | 0.417 |
| DCR (CR + PR +
SD) | 10 (66.7) | 2 (40) | 8 (80) | 0.121 |
PFS
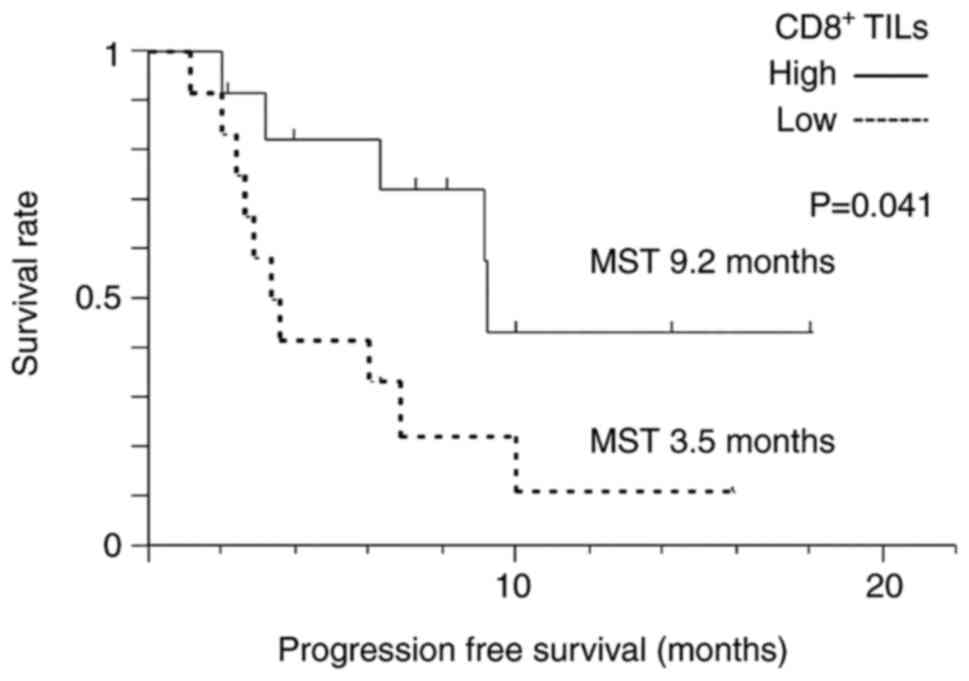
Atezolizumab plus bevacizumab
The median PFS of all patients who were given
atezolizumab plus bevacizumab therapy was 6.9 months. KM analysis
revealed that the median PFS in the high-level CD8+ TILs
group (not reached) was increased compared with the low-level
CD8+ TILs group (4.7 months) (P=0.047) (Fig. 3).
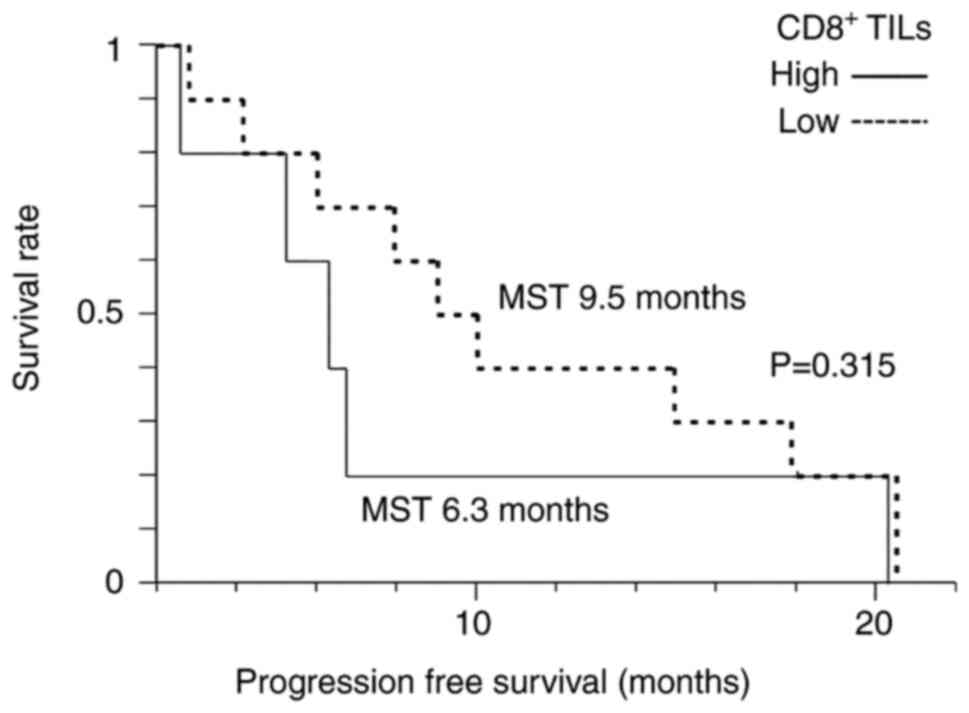
Lenvatinib
The median PFS of all patients who received
lenvatinib therapy was 7.9 months. KM analysis showed no
significant differences in median PFS between the high-level
CD8+ TILs group (6.3 months) and low-level
CD8+ TILs group (9.5 months) (P=0.315) (Fig. 4).
Discussion
The immune response potentially plays an important
role in cancer progression. The most recent immunogenomic
classification of HCC was published in 2022 (16). The study reported that 65% of HCC
cases in the non-inflammatory group and 35% of those in the
inflammatory group were more likely to respond to ICI treatment.
The inflamed group can be further classified into the active,
exhausted, and immune-like subclasses. The inflamed class is
characterized by strong interferon signaling and cytolytic
activity, upregulation of effector molecules of cytotoxic T cells,
and increased levels of checkpoint molecules and CD8+ T
cells.
Recently, the combination of an ICI with a VEGF
inhibitor using atezolizumab plus bevacizumab, as well as the
multi-kinase angiogenesis inhibitor lenvatinib, were approved as
systemic therapy options for patients with advanced HCC (3,4). Gene
expression profiling of immune-related transcripts has recently
been correlated with objective tumor response and survival in HCC
patients who have received nivolumab. It was reported that
non-inflamed HCC cases with immune exclusion are resistant to ICIs
(17–20). Therefore, it is important to assess
the immune conditions of HCC tumors prior to chemotherapy.
Appropriate chemotherapy selection could further improve prognosis.
As a result, predictive biomarkers are needed for each therapy.
Recent studies across several cancer types have
shown that biomarkers for response to ICI therapy include tumor
mutation burden (TMB) and PD-L1 expression levels in the tumor
microenvironment (TME) (21–25).
However, these have proved difficult for use as HCC predictive
biomarkers because of the low incidence of high TMB and high PD-L1
expression observed in HCC tumors (26,27).
In this study, we evaluated whether IHC staining of
liver tumor biopsies to assess CD8+ T cell infiltration
in HCC tumor tissues could predict patient response to systemic
chemotherapy. TILs reflect the local immune response and are
potentially key for controlling tumor progression (28,29).
TILs were previously characterized to be predominantly T cells, the
majority of which have a cytotoxic effector phenotype
(CD8+) (30–32). Immune responses mediated by
CD8+ T cells can promote the accumulation of distinct
endogenous CD8+ and CD4+ T cells that support
antitumor activities in the TME (33–35).
Correlations between CD8+ T cell levels in the TME and
response to ICI therapy have been reported in various cancer types
(36,37). Recent studies have indicated that
HCC cases with immune exclusion, which are associated with
decreased CD8+ T cell infiltration, show resistance to
ICIs. Furthermore, gene expression profiling has suggested that
CD8+ TILs are potentially correlated with clinical
response to atezolizumab plus bevacizumab treatment (13,18–20,38).
We evaluated CD8+ TIL levels using IHC staining of liver
tumor biopsy tissues. In our study, HCC patients with high-level
CD8+ TILs had better treatment responses and longer PFS
compared with those who had low-level CD8+ TILs. Thus,
we used liver tumor biopsy tissues to identify CD8+ T
cell infiltration as a predictive marker for response to
atezolizumab plus bevacizumab therapy.
Conversely, we observed no significant differences
between treatment responses to lenvatinib therapy between HCC
patients with high-level and low-level CD8+ TILs. A
recent preclinical study showed that HCC cells with β-catenin
activation, which indicates a non-inflammatory tumor, were more
sensitive to sorafenib than those without β-catenin activation
(39). Like sorafenib, lenvatinib
might be effective for non-inflamed HCC cases. Further studies with
more cases per subpopulation are needed to evaluate the
heterogeneity of CD8+ T cells.
The limitations of this study include the small
number of HCC patients who underwent liver tumor biopsy because of
its single-center design. This study also included advanced HCC
cases of different stages. Ideally, groups could be matched
according to liver function and tumor stage, but this is difficult
when analyzing a small number of cases. Additionally, it is not
clear whether the CD8+ TILs of one tumor can reflect the
status of other tumors, especially given the heterogeneous nature
of HCC with multiple lesions. The difference between atezolizumab
plus bevacizumab and lenvatinib treatments on the basis of the
degree of CD8+ TILs was not studied because of the small
number of cases and varied tumor backgrounds. Additionally, we did
not evaluate OS because liver function at the start of chemotherapy
was not matched because of the small number of cases.
In conclusion, these findings suggest that
CD8+ TILs, as evaluated by IHC staining of liver tumor
biopsy tissues, may be a useful biomarker for predicting HCC
patient response to lenvatinib and atezolizumab plus bevacizumab
treatments. Our findings indicate the recommendation of
atezolizumab plus bevacizumab for HCC cases with high-level
CD8+ TILs by conducing IHC staining of liver tumor
biopsy tissues before treatment decisions. Further studies
regarding the selection of chemotherapy are needed to improve the
prognosis of advanced HCC patients.
Acknowledgements
The authors would like to thank Mrs Yukie Ishibashi
(Department of Hepatology, Iizuka Hospital, Iizuka, Japan) for
assistance with manuscript preparation. The authors would also like
to thank Dr James Mahaffey and Dr Joseph Iacona for editing a draft
of this manuscript.
Funding
This research was conducted with the assistance of an Aso Iizuka
Hospital Clinical Research Grant (grant no. 22008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, MY, AM and KM designed the study. AK, KK, KT,
and YM assisted with data analyses. YM and YO performed
pathological examinations, including immunostaining. AK wrote the
initial draft of the manuscript. MY contributed to the analysis and
interpretation of the data. MY, AM, and KM assisted in the
preparation and critical review of the manuscript. AK and MY
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript, and agreed to be
accountable for all aspects of the work.
Ethics approval and consent to
participate
This research and study protocol were performed in
accordance with the principles and ethical guidelines of the 1975
Declaration of Helsinki. This study received approval from the Aso
Iizuka Hospital Ethics Committee (approval nos. 18070 and 22008).
We applied the opt-out method to obtain consent for this study.
Patient consent for publication
Written informed consent was obtained from two
patients to using their images for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
ICI
|
immune checkpoint inhibitor
|
|
PD-L1
|
programmed death ligand 1
|
|
VEGF
|
vascular endothelial growth factor
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
ALBI score
|
albumin-bilirubin score
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
DCR
|
disease control rate
|
|
ORR
|
objective response rate
|
|
IHC
|
immunohistochemistry
|
|
HFP
|
high-power field
|
|
BCLC
|
Barcelona Clinic liver cancer
|
|
AFP
|
α-fetoprotein
|
|
PIVKA-II
|
protein induced by vitamin K absence
or antagonist-II
|
|
TMB
|
tumor mutation burden
|
References
|
1
|
Caldwell S and Park SH: The epidemiology
of hepatocellular cancer: From the perspectives of public health
problem to tumor biology. J Gastroenterol. 44:96–101. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eso Y and Marusawa H: Novel approaches for
molecular targeted therapy against hepatocellular carcinoma.
Hepatol Res. 48:597–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurkmans DP, Kuipers ME, Smit J, van
Marion R, Mathijssen RHJ, Postmus PE, Hiemstra PS, Aerts JGJV, von
der Thüsen JH and van der Burg SH: Tumor mutational load, CD8+ T
cells, expression of PD-L1 and HLA class I to guide immunotherapy
decisions in NSCLC patients. Cancer Immunol Immunother. 69:771–777.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Li C, Cai X, Xie Z, Zhou L, Cheng B,
Zhong R, Xiong S, Li J, Chen Z, et al: The association between
CD8+ tumor-infiltrating lymphocytes and the clinical
outcome of cancer immunotherapy: A systematic review and
meta-analysis. EClinicalMedicine. 41:1011342021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rimini M, Rimassa L, Ueshima K, Burgio V,
Shigeo S, Tada T, Suda G, Yoo C, Cheon J, Pinato DJ, et al:
Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in
non-viral unresectable hepatocellular carcinoma: An international
propensity score matching analysis. ESMO Open. 7:1005912022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu AX, Abbas AR, de Galarreta MR, Guan Y,
Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, et al: Molecular
correlates of clinical response and resistance to atezolizumab in
combination with bevacizumab in advanced hepatocellular carcinoma.
Nat Med. 28:1599–1611. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montironi C, Castet F, Haber PK, Pinyol R,
Torres-Martin M, Torrens L, Mesropian A, Wang H, Puigvehi M, Maeda
M, et al: Inflamed and non-inflamed classes of HCC: A revised
immunogenomic classification. Gut. 72:129–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, et al:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinyol R, Sia D and Llovet JM: Immune
Exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies
in HCC. Clin Cancer Res. 25:2021–2023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Cancer Res.
25:2116–2126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Zhou Q, Liu J and Zhang W: CTNNB1
alternation is a potential biomarker for immunotherapy prognosis in
patients with hepatocellular carcinoma. Front Immunol.
12:7595652021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai R, Lv Z, Xu D and Cui J: Predictive
biomarkers for cancer immunotherapy with immune checkpoint
inhibitors. Biomarker Res. 8:342020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus Ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cristescu R, Mogg R, Ayers M, Albright A,
Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al:
Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based
immunotherapy. Science. 362:eaar35932018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship with clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanasekaran R, Nault JC, Roberts LR and
Zucman-Rossi J: Genomic medicine and implications for
hepatocellular carcinoma prevention and therapy. Gastroenterology.
156:492–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuta K, Ishii G, Kim E, Shiono S,
Nishiwaki Y, Endoh Y, Kodama T and Nagai K and Nagai K: Primary
lung adenocarcinoma with massive lymphocyte infiltration. Am J Clin
Pathol. 123:547–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Canna K, McArdle PA, McMillan DC, McNicol
AM, Smith GW, McKee RF and McArdle CS: The relationship between
tumour T-lymphocyte infiltration, the systemic inflammatory
response and survival in patients undergoing curative resection for
colorectal cancer. Br J Cancer. 92:651–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schondorf T, Engel H, Lindemann C,
Kolhagen H, von Rucker AA and Mallmann P: Cellular characteristics
of peripheral blood lymphocytes and tumour-infiltrating lymphocytes
in patients with gynaecological tumours. Cancer Immunol Immunother.
44:88–96. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben-Hur H, Cohen O, Schneider D, Gurevich
P, Halperin R, Bala U, Mozes M and Zusman I: The role of
lymphocytes and macrophages in human breast tumorigenesis: An
immunohistochemical and morphometric study. Anticancer Res.
22:1231–1238. 2002.PubMed/NCBI
|
|
32
|
Leong PP, Mohammad R, Ibrahim N, Ithnin H,
Abdullah M, Davis WC and Seow HF: Phenotyping of lymphocytes
expressing regulatory and effector markers in infiltrating ductal
carcinoma of the breast. Immunol Lett. 102:229–236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schillaci R, Salatino M, Cassataro J,
Proietti CJ, Giambartolomei GH, Rivas MA, Carnevale RP, Charreau EH
and Elizalde PV: Immunization with murine breast cancer cells
treated with antisense oligodeoxynucleotides to type I insulin-like
growth factor receptor induced an antitumoral effect mediated by a
CD8+ response involving Fas/Fas ligand cytotoxic
pathway. J Immunol. 176:3426–3437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dobrzanski MJ, Reome JB, Hylind JC and
Rewers-Felkins KA: CD8-mediated type 1 antitumor responses
selectively modulate endogenous differentiated and
nondifferentiated T cell localization, activation, and function in
progressive breast cancer. J Immunol. 177:8191–8201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huh JW, Lee JH and Kim HR: Prognostic
significance of tumor-infiltrating lymphocytes for patients with
colorectal cancer. Arch Surg. 147:366–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tokito T, Azuma K, Kawahara A, Ishii H,
Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M, et al:
Predictive relevance of PD-L1 expression combined with
CD8+ TIL density in stage III non-small cell lung cancer
patients receiving concurrent chemoradiotherapy. Eur J Cancer.
55:7–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu S, Lachapelle J, Leung S, Gao D,
Foulkes WD and Nielsen TO: CD8+ lymphocyte infiltration is an
independent favorable prognostic indicator in basal-like breast
cancer. Br Cancer Res. 14:R482012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu CL, Ou DL, Bai LY, Chen CW, Lin L,
Huang SF, Cheng AL, Jeng YM and Hsu C: Exploring markers of
exhausted CD8 T cells to predict response to immune checkpoint
inhibitor therapy for hepatocellular carcinoma. Liver Cancer.
10:346–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sohn BH, Park IY, Shin JH, Yim SY and Lee
JS: Glutamine synthetase mediates sorafenib sensitivity in
β-catenin-active hepatocellular carcinoma cells. Exp Mol Med.
50:e4212018. View Article : Google Scholar : PubMed/NCBI
|