Introduction
Limited evidence is currently available regarding
the clinical outcomes of older adult patients (≥70 years) with
advanced non-small cell lung cancer (NSCLC) who receive
immunotherapy, because this patient population was largely
underrepresented in clinical trials (1). Our group recently reported the results
of a retrospective, single-institution study of the clinical
outcomes of older adult patients with advanced-stage NSCLC who
received immunotherapy (2), and we
found that immunotherapy is effective and well tolerated in these
patients. Despite an initial response to treatment, most patients
(of all ages) with advanced NSCLC develop resistance to
immunotherapy (3–9). However, during our retrospective
review, we identified a subset of older adult patients who
continued to receive immunotherapy beyond documented radiographic
disease progression (as defined by Response Evaluation Criteria in
Solid Tumors, version 1.1 [RECIST v1.1]) owing to perceived
clinical benefit. The current case series reviews the outcomes of
this subset of older adult patients who received immunotherapy
beyond radiographic progression to highlight a potential treatment
strategy that may be of benefit to select patients.
Materials and methods
Patients
Using The University of Texas MD Anderson Cancer
Center Gemini Lung Cancer database, our group performed a
retrospective review of the clinical outcomes of older adult
patients (age ≥70 years) with advanced stage III/IV (per American
Joint Committee on Cancer 8th Edition) NSCLC treated with
anti-PD-(L)1 monotherapy from March 2015 through April 2019.
Clinical therapy responses were evaluated by a clinical radiologist
using RECIST v1.1 criteria, with radiographic progression of
disease defined as one or more of the following: i) ≥20% increase
in sum of longest diameters of target lesions; ii) progression of
non-target lesions; or iii) new lesions (10). Toxicities were assessed using Common
Terminology Criteria for Adverse Events version 5. Patients treated
beyond disease progression were defined as individuals who received
immunotherapy for a minimum of 8 weeks prior to documentation of
progression and then subsequently continued immunotherapy for at
least 6 weeks.
Statistical analysis
Regarding statistical analysis, the categorical and
continuous characteristics were summarized, and analyses of overall
survival and duration of immunotherapy beyond progression were
performed. The distributions of overall survival and duration of
therapy beyond progression were estimated by the Kaplan-Meier
method. Regression analysis based on the Cox proportional hazard
(PH) model were conducted on overall survival and duration of
therapy beyond progression. A two-sided P-value of 0.05 was
considered significant. All analyses were performed in SAS 9.4
software.
Results
Patient demographics
Of the 159 patients who met the initial inclusion
criteria, 33 (21%) received immunotherapy beyond radiographically
indicated disease progression. Of these 33 patients, 12 (36%) were
female and 21 (64%) were male, 17 (52%) were 70–74 years of age, 30
(91%) were former or current smokers, and 26 (79%) had
histologically indicated adenocarcinoma. As this is a real-world
patient cohort, immunotherapy was prescribed for patients per FDA
label as standard of care. Thirty of the thirty-three (91%)
patients received immunotherapy alone in the second-line and beyond
treatment setting, as most of the patients in our retrospective
study were treated prior to FDA approval in August 2018 and October
2018 of combination chemoimmunotherapy in the first-line setting of
metastatic non-squamous and squamous NSCLC per KEYNOTE-189 and
KEYNOTE-407, respectively (11,12).
PD-L1 expression was available for 10 of 33 patients; 8 patients
had PD-L1 positive disease (i.e. TPS 1% or higher), while 2
patients had PD-L1 negative disease (i.e. TPS <1%) (Table I).
 | Table I.Baseline patient characteristics of 33
patients who received immune checkpoint inhibitors to treat NSCLC
beyond radiographic disease progression. |
Table I.
Baseline patient characteristics of 33
patients who received immune checkpoint inhibitors to treat NSCLC
beyond radiographic disease progression.
| Characteristic | No. (%) |
|---|
| Sex |
|
|
Female | 12 (36) |
| Male | 21 (64) |
| Age, years |
|
|
70-74 | 17 (52) |
|
75-79 | 12 (36) |
|
80-85 | 4 (12) |
| NSCLC type |
|
|
Adenocarcinoma | 26 (79) |
| Not
otherwise specified | 2 (6) |
| Squamous
cell carcinoma | 5 (15) |
| Immunotherapy |
|
|
Nivolumab | 29 (88) |
|
Pembrolizumab | 4 (12) |
| Immunotherapy as
first-line treatment |
|
| No | 30 (91) |
| Yes | 3 (9) |
| Immunotherapy as
second-line or later treatment |
|
| Second
line | 18 (60) |
| Later
than second line | 12 (40) |
| Smoking status |
|
|
Current | 2 (6) |
|
Former | 28 (85) |
|
Never | 3 (9) |
| PD-L1 expression
level |
|
|
<1% | 2 (6) |
|
1–49% | 4 (12) |
| ≥50% | 4 (12) |
|
Unknown | 23 (70) |
Outcomes of patients who continued on
immunotherapy beyond disease progression
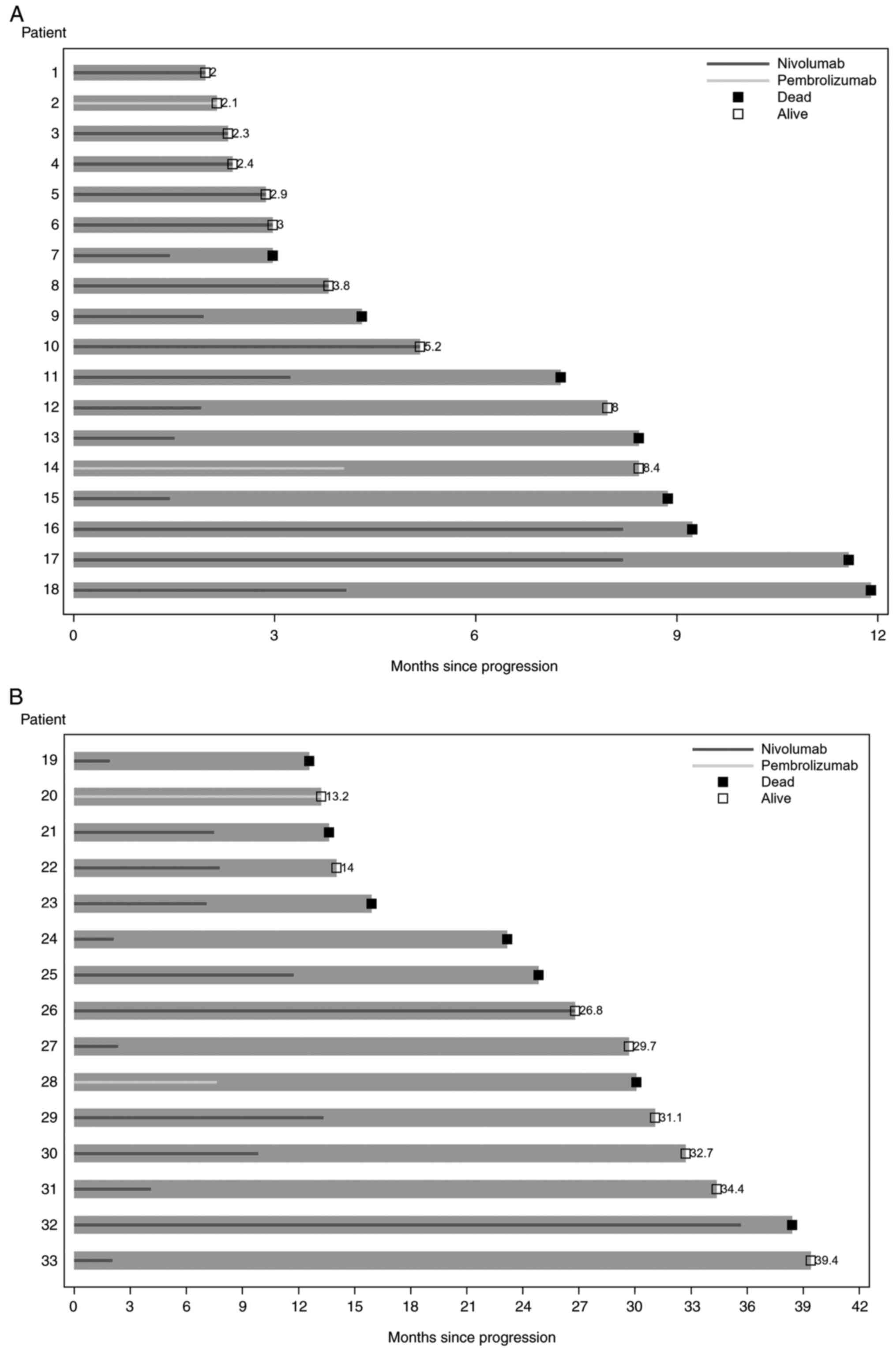
Among the 33 patients, the median duration of
immunotherapy beyond disease progression, i.e., the time from
disease progression to immunotherapy end, death, or last follow-up,
was 7.1 months (95% CI 3.0-8.2 months; Fig. 1). With a median follow-up period of
30.1 months, the median overall survival, defined as the time from
the start of immunotherapy to death or last follow-up, was 31.5
months (95% CI 16.5 months to not reached; Table II). Eight patients (24%) received
local consolidative radiotherapy, with a median duration of
immunotherapy beyond disease progression of 8.2 months (95% CI
1.9-13.3 months). Twenty-five patients (76%) did not receive local
consolidative therapy, and these patients had a median duration of
immunotherapy beyond disease progression of 4.1 months (95% CI
2.3-7.8 months; Table II).
 | Table II.Outcomes for patients who received ICI
BDP, by patient subtype. |
Table II.
Outcomes for patients who received ICI
BDP, by patient subtype.
| Patient subtype | Median duration of
ICI BDP, months | Median overall
survival, months |
|---|
| Pseudo-progression,
n=6 | 11.7 (95% CI
7.1-35.7) | 26.2 (95% CI
16.5-40) |
| Local consolidative
therapy + | 8.2 (95% CI
1.9-13.3) | Not reached |
| ICI BDP, n=8 |
|
|
| ICI BDP alone/no
local | 4.1 (95% CI
2.3-7.8) | 31.5 (95% CI 16.5 to
not reached) |
| consolidative
therapy, n=25 |
|
|
Outcomes of patients who received
local consolidative radiotherapy and continued on immunotherapy
beyond progression
A dedicated radiology review was conducted on the
subset of 8 patients who received local consolidative radiotherapy
and subsequently continued with immunotherapy beyond documented
radiographic disease progression (Table III). Decision to pursue
combined-modality therapy for these 8 patients was up to the
discretion of the treating medical oncologist and radiation
oncologist. Ongoing studies are evaluating a combination of
stereotactic body radiotherapy with checkpoint inhibitors in
oligoprogressive NSCLC to overcome acquired resistance and will
help us gain insight into determining which patients would be most
suitable for this combination treatment approach (13,14).
At time of first radiographic progression, 3 of the 8 patients
(38%) showed no response to treatment (i.e., imaging was notable
for progressive metastatic lesions without any sites of tumor
regression), and five patients (63%) showed a mixed response to
systemic therapy (i.e., imaging was notable for simultaneous
regression and progression in the metastatic lesions) (15). At time of local consolidative
therapy, 5 of the 8 patients had oligometastatic disease, defined
as 3 or fewer sites of progression, the other 3 patients had
polymetastatic disease; 3 patients received complete consolidation
and 5 patients received incomplete consolidation. After receiving
local consolidative therapy and resuming immunotherapy, 7 of the 8
patients ultimately had disease progression, and the other patient
did not show evidence of progression upon subsequent follow-up. Of
the 7 patients who had disease progression, the median time to
second objective disease progression after local consolidative
radiotherapy was administered was 5.8 months (95% CI 1.2-10.0
months).
 | Table III.Clinical characteristics and outcomes
of patients who received local consolidative therapy at the time of
first radiographic progression. |
Table III.
Clinical characteristics and outcomes
of patients who received local consolidative therapy at the time of
first radiographic progression.
| Patient | Duration of
immunotherapy prior to first radiographic progression | Progression pattern
(progression or mixed response) | Site of progression
(oligometastatic or polymetastatic) | Sites of active
disease at initiation of local consolidative therapy | Complete or
incomplete consolidation | Progression during
subsequent follow-up | Time to second
objective disease progression after local consolidative
therapy |
|---|
| 1 | 3.6 months | Progression | Oligometastatic | Left chest wall,
right pelvic mass | Complete | Yes | 10 months |
| 2 | 23.7 months | Progression | Polymetastatic | Retroperitoneal
lymph node, paraaortic lymph node, right iliac, acetabular and
ischial bone | Incomplete | Yes | 1.2 months |
| 3 | 7.3 months | Mixed response |
Oligometastatic | Right lower | Complete lobe | No | Not applicable |
| 4 | 11.1 months | Mixed response | Polymetastatic | Multiple pulmonary
nodules in mediastinal lymph nodes, retroperitoneal soft
tissue | Incomplete | Yes | 6.5 months |
| 5 | 4.1 months | Mixed response |
Oligometastatic | Axillary lymph
node, brain metastases | Incomplete | Yes | 3.4 months |
| 6 | 21.4 months | Mixed response |
Oligometastatic | Pleural metastases,
left supraclavicular lymph node | Complete | Yes | 6.7 months |
| 7 | 11.6 months | Mixed response |
Oligometastatic | Adrenal gland,
supraclavicular lymph node, brain metastases | Incomplete | Yes | 2.6 months |
| 8 | 9.5 months | Progression | Polymetastatic | Brain, pulmonary
metastases, mediastinal lymph node, paraaortic lymph nodes,
liver | Incomplete | Yes | 5.8 months |
Outcomes of patients who experienced
pseudo-progression
Six of the 33 patients (18%) exhibited
pseudo-progression, defined as a delayed response to immunotherapy
with decreased tumor burden in subsequent radiologic studies
(16), 4 achieved stable disease as
the best response (with a return of their tumor burden to
baseline), and 2 achieved a partial response. The median duration
of immunotherapy continued beyond pseudo-progression was 11.7
months (95% CI 7.1-35.7 months), and the median overall survival
for this group was 26.2 months (95% CI 16.5-40.0 months).
Safety
Patients who received immunotherapy beyond disease
progression most commonly experienced fatigue (n=6, 18%),
pneumonitis (n=4, 12%), rash (n=3, 9%), and hypothyroidism (n=3,
9%). Three patients (9%) had grade 3 or higher toxicities. One
patient had grade 3 arthralgias, and 2 patients had grade 3
pneumonitis resulting in discontinuation of therapy. Four of
thirty-three patients (12%) were treated with pembrolizumab; one
patient on pembrolizumab experienced grade 1 rash and grade 1
diarrhea, while one patient on pembrolizumab experienced grade 1
fatigue. All other toxicities described occurred in the twenty-nine
patients treated with nivolumab.
Discussion
Immunotherapy continued beyond disease progression
(defined by RECIST v1.1) in older adults with advanced NSCLC may be
of benefit to a select group of patients. Additionally, local
consolidative therapy with radiation may allow prolonged duration
of immunotherapy.
Real-world outcomes of this treatment strategy in
the management of NSCLC and other tumor types, such as
advanced-stage melanoma, have been retrospectively studied by
previous groups, and select patients had durable progression-free
survival benefit despite discordant responses to immunotherapy
(17–22). For example, one retrospective study
analyzed the clinical outcomes of 208 NSCLC patients treated with
immunotherapy and found that oligoprogression was the major pattern
of progression after acquired resistance from immunotherapy
(17). The most common treatment
used for management of oligoprogression was a combination of local
radiotherapy and continued immunotherapy (33%, n=38 patients). This
resulted in significantly longer second progression-free survival
(PFS) (12.0 months vs. 10 months, P=0.006) and overall survival
(26.3 months vs. 18.5 months, P=0.001) compared to other treatment
strategies (17). Reinhorn et
al previously evaluated real-life practice and outcomes related
to immunotherapy beyond progression in advanced NSCLC patients
treated with immunotherapy (18).
Of 207 patients, 22% received immunotherapy beyond progression, and
36% achieved a clinical benefit. 27% of patients had a
progression-free interval over 6 months after receiving
immunotherapy beyond progression (18). A retrospective study of 125 Chinese
patients with advanced NSCLC who experienced progressive disease
after receiving monotherapy or combination therapy with PD-1/PD-L1
inhibitors by Ge et al found that patients who were treated
with immunotherapy for more than 6 weeks after PD (n=39) had longer
overall survival (26.6 months vs. 9.5 months; P<0.001) and PFS
(PFS, 8.9 months vs. 4.1 months; P<0.001), compared to those who
did not receive immunotherapy beyond progression (19). Subgroup analysis showed significant
benefits for overall survival and PFS in the overall population and
particularly for overall survival in males, squamous histology, no
brain or liver metastases, any age, not beyond ≥ the third
treatment line, with partial response to previous immunotherapy and
monotherapy as previous immunotherapy (19).
Our retrospective case series is unique given the
limited dedicated study of the continuation of immunotherapy beyond
radiographic progression specifically in patients aged 70 years or
older. Our findings are of clinical significance and potentially
address an unmet need in standard clinical practice for these
patients, who are likely to be more frail and potentially more
vulnerable (23).
Exploratory analysis of selected population
subgroups in our retrospective study revealed no statistically
significant interaction between duration of immunotherapy
administered beyond radiographic progression and patient sex, age,
NSCLC subtype, immunotherapy agent used, smoking status, or if
immunotherapy was used in the first line setting or beyond (data
not shown). However, we acknowledge that the relatively smaller
sample sizes may not provide adequate power for subgroup analysis.
A prospective study with a larger sample size is needed to draw
further conclusions regarding clinical characteristics and
biomarkers that would clarify which patients would benefit most
from such a treatment strategy.
Due to small sample sizes, results from our
retrospective study are primarily hypothesis-generating with
regards to determining which patients may benefit most from a
combined modality treatment approach. However, previous studies
suggest that a combination of radiation therapy and immune
checkpoint inhibition may act at various stages of the antitumor
response to induce synergy between the two treatment modalities
(24); radiotherapy may enhance the
immunotherapeutic effects of PD-1/PD-L1 inhibitors, as it can prime
antigen release and improve antigen processing to result in
enhanced T-cell killing. Welsh et al in a phase I/II trial
of pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer found that combined immunoradiotherapy
was generally safe, with only a few high-grade adverse events
observed; exploratory findings from this study suggested that RT
may be more beneficial for patients with low PD-L1 expression
(25).
In conclusion, our retrospective study demonstrates
that treatment with immunotherapy beyond radiographic progression
may be safe and feasible in a selected subset of older adult
patients with metastatic NSCLC. Future studies are needed to
prospectively validate the safety and efficacy of this treatment
strategy in different clinical and histopathologic subsets of
patients with metastatic NSCLC, including individuals of different
age groups.
Acknowledgements
Preliminary findings of this work were presented in
poster format at the 2021 Society for Immunotherapy of Cancer
annual meeting, November 10–14, 2021, Washington D.C., USA
Funding
Supported by generous philanthropic contributions to The
University of Texas MD Anderson Cancer Center Lung Moon Shot
Program and the MD Anderson Cancer Center Support (grant no. P30
CA01667). CJP is supported by The National Institute of Aging
(grant no. R03 AG064374).
Availability of data and materials
The datasets used and/or analyzed during the current
study are not publicly available due to patient security but are
available from the corresponding author on reasonable request.
Authors' contributions
EKS and MA confirm the authenticity of all the raw
data. EKS performed conceptualization, data curation (i.e.
organization and management of the raw data set), investigation,
and preparation of the original draft of the manuscript. FM
performed conceptualization, data curation, and review and editing
of the manuscript. MW performed data curation, investigation, and
review and editing of the manuscript. CHL performed formal analysis
with statistical software. JJL performed formal analysis, software
use, and supervision. BC performed data curation and investigation.
CJP performed conceptualization and review and editing of the
manuscript. JVH performed supervision, conceptualization, and
review and editing of the manuscript. MA performed
conceptualization, data curation, supervision, investigation, and
original draft preparation of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved and conducted in
accordance with the institutional review board at The University of
Texas MD Anderson Cancer Center (approval no. PA13-0589). All
patients provided written informed consent/authorization for
participation in research.
Patient consent for publication
All identifying information of patients has been
removed. All patients when consenting were made aware that study
information and study analyses may be published in scientific
literature and/or other public scientific resources.
Competing interests
MW is an employee of Medscape, LLC, New York, NY.
JVH has served on the scientific advisory boards for AstraZeneca,
EMD Serono, Boehringer-Ingelheim, Catalyst, Genentech,
GlaxoSmithKline, Hengrui Therapeutics, Eli Lilly, Spectrum, Sanofi,
Takeda, Mirati Therapeutics, BMS, BrightPath Biotherapeutics,
Janssen Global Services, Nexus Health Systems, Pneuma Respiratory,
Kairos Venture Investments, Roche, Leads Biolabs, RefleXion, and
Chugai Pharmaceuticals. He receives research support from
AstraZeneca, Bristol-Myers Squibb, Spectrum, and Takeda and
royalties and licensing fees from Spectrum outside of the submitted
work. MA received research funding (to institution) from Genentech,
Nektar Therapeutics, Merck, GlaxoSmithKline, Novartis, Jounce
Therapeutics, Bristol Myers Squibb, Eli Lilly, Adaptimmune,
Shattuck Lab, and Gilead and receives consultant and advisor fees
from GlaxoSmithKline, Shattuck Lab, Bristol Myers Squibb, and
AstraZeneca. He receives speaker fees from AstraZeneca, Nektar
Therapeutics, and SITC and acknowledges participation in safety
review committees for Nanobiotix-MDA alliance and Hengenix outside
of the submitted work. The remaining authors declare no conflict of
interest.
References
|
1
|
Luciani A, Marra A, Toschi L, Cortinovis
D, Fava S, Filipazzi V, Tuzi A, Cerea G, Rossi S, Perfetti V, et
al: Efficacy and safety of Anti-PD-1 immunotherapy in patients aged
≥ 75 years with non-small-cell lung cancer (NSCLC): An Italian,
multicenter, retrospective study. Clin Lung Cancer. 21:e567–e571.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altan M, Singhi EK, Worst M, Carter BW,
Leung CH, Lee JJ, Presley CJ, Lewis J, Rinsurongkawong W,
Rinsurongkawong V, et al: Clinical effectiveness and safety of
anti-PD-(L)1 therapy among older adults with advanced non-small
cell lung cancer. Clin Lung Cancer. 23:236–243. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fossella FV, DeVore R, Kerr RN, Crawford
J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small-cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 non-small cell lung cancer study group. J Clin Oncol.
18:2354–2362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson H, Hopwood P, Stephens RJ,
Thatcher N, Cottier B, Nicholson M, Milroy R, Maughan TS, Falk SJ,
Bond MG, et al: Gemcitabine plus best supportive care (BSC) vs. BSC
in inoperable non-small cell lung cancer-a randomized trial with
quality of life as the primary outcome. UK NSCLC Gemcitabine Group.
Non-small cell lung cancer. Br J Cancer. 83:447–453. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciuleanu T, Brodowicz T, Zielinski C, Kim
JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: A
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ardizzoni A, Tiseo M, Boni L, Vincent AD,
Passalacqua R, Buti S, Amoroso D, Camerini A, Labianca R,
Genestreti G, et al: Pemetrexed versus pemetrexed and carboplatin
as second-line chemotherapy in advanced non-small-cell lung cancer:
Results of the GOIRC 02–2006 randomized phase II study and pooled
analysis with the NVALT7 trial. J Clin Oncol. 30:4501–4507. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pérol M, Chouaid C, Pérol D, Barlési F,
Gervais R, Westeel V, Crequit J, Léna H, Vergnenègre A, Zalcman G,
et al: Randomized, phase III study of gemcitabine or erlotinib
maintenance therapy versus observation, with predefined second-line
treatment, after cisplatin-gemcitabine induction chemotherapy in
advanced non-small-cell lung cancer. J Clin Oncol. 30:3516–3524.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawaguchi T, Ando M, Asami K, Okano Y,
Fukuda M, Nakagawa H, Ibata H, Kozuki T, Endo T, Tamura A, et al:
Randomized phase III trial of erlotinib versus docetaxel as second-
or third-line therapy in patients with advanced non-small-cell lung
cancer: Docetaxel and Erlotinib lung cancer trial (DELTA). J Clin
Oncol. 32:1902–1908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schoenfeld JD, Giobbie-Hurder A,
Ranasinghe S, Kao KZ, Lako A, Tsuji J, Liu Y, Brennick RC, Gentzler
RD, Lee C, et al: Durvalumab plus tremelimumab alone or in
combination with low-dose or hypofractionated radiotherapy in
metastatic non-small-cell lung cancer refractory to previous
PD(L)-1 therapy: An open-label, multicentre, randomised, phase 2
trial. Lancet Oncol. 23:279–291. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ochoa-de-Olza M, Bourhis J, Coukos G and
Herrera FG: Low-dose irradiation for reversing immunotherapy
resistance: How to translate? J Immunother Cancer. 10:e0049392022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rauwerdink DJW, Molina G, Frederick DT,
Sharova T, van der Hage J, Cohen S and Boland GM: Mixed response to
immunotherapy in patients with metastatic melanoma. Ann Surg Oncol.
27:3488–3497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shroff GS, Strange CD, Altan M, Carter BW,
Ahuja J, Godoy MCB, Truong MT and Vlahos I: Post-immunotherapy
imaging in lung cancer. Clin Radiol. 77:44–57. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Li H and Fan Y: Progression
patterns, treatment, and prognosis beyond resistance of responders
to immunotherapy in advanced non-small cell lung cancer. Front
Oncol. 11:6428832021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reinhorn D, Jacobi O, Icht O, Dudnik E,
Rotem O, Zer A and Goldstein DA: Treatment beyond progression with
immune checkpoint inhibitors in non-small-cell lung cancer.
Immunotherapy. 12:235–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge X, Zhang Z, Zhang S, Yuan F, Zhang F,
Yan X, Han X, Ma J, Wang L, Tao H, et al: Immunotherapy beyond
progression in patients with advanced non-small cell lung cancer.
Transl Lung Cancer Res. 9:2391–2400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Enomoto T, Tamiya A, Matsumoto K, Adachi
Y, Azuma K, Inagaki Y, Kouno S, Taniguchi Y, Saijo N, Okishio K, et
al: 79P-Nivolumab treatment beyond progression disease in advanced
non-small cell lung cancer. Ann Oncol. 30:xi282019. View Article : Google Scholar
|
|
21
|
Czarnecka AM, Sobczuk P, Rogala P, Świtaj
T, Placzke J, Kozak K, Mariuk-Jarema A, Spałek M, Dudzisz-Śledź M,
Teterycz P, et al: Efficacy of immunotherapy beyond RECIST
progression in advanced melanoma: A real-world evidence. Cancer
Immunol Immunother. 71:1949–1958. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klemen ND, Wang M, Feingold PL, Cooper K,
Pavri SN, Han D, Detterbeck FC, Boffa DJ, Khan SA, Olino K, et al:
Patterns of failure after immunotherapy with checkpoint inhibitors
predict durable progression-free survival after local therapy for
metastatic melanoma. J Immunother Cancer. 7:1962019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corbaux P, Maillet D, Boespflug A,
Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M,
Kiakouama-Maleka L, Dalle S, Falandry C and Péron J: Older and
younger patients treated with immune checkpoint inhibitors have
similar outcomes in real-life setting. Eur J Cancer. 121:192–201.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nabrinsky E, Macklis J and Bitran J: A
review of the abscopal effect in the era of immunotherapy. Cureus.
14:e296202022.PubMed/NCBI
|
|
25
|
Welsh J, Menon H, Chen D, Verma V, Tang C,
Altan M, Hess K, de Groot P, Nguyen QN, Varghese R, et al:
Pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer: A randomized phase I/II trial. J
Immunother Cancer. 8:e0010012020. View Article : Google Scholar : PubMed/NCBI
|