Introduction
Preoperative long-course radiotherapy (LRT) of
typically 50.4 Gy in 28 fractions is the standard treatment for
locally advanced rectal cancer; it gained popularity from the
German CAO/ARO/AIO-94 trial, which reported several advantages of
preoperative chemoradiotherapy (CRT), such as lower local
recurrence, a higher rate of sphincter preservation and lower acute
toxicity than postoperative CRT (1). Along with the ongoing practice of LRT,
clinical trials assessing the change in radiotherapy schedule to
short-course radiotherapy (SRT) consisting of 25 Gy in 5 fractions
were carried out (2–4) which advocated a higher local control
rate in SRT compared with that in surgery alone, as well as SRT
being a simpler treatment than LRT in view of a much shorter
schedule. It is challenging to compare the two schedules due to the
difference in patient selection for each trial. Randomized trials
were performed to compare the relative advantages of LRT and SRT,
which reported no statistically significant difference in
oncological outcomes (5,6) or health-related quality of life
(7,8). However, advocates for SRT emphasize
its lower acute toxicity, lower cost and greater convenience for
patients with cancer compared with LRT (5,9,10).
Meanwhile, advocates for LRT highlight higher rates of pathological
complete remission (pCR), a higher sphincter preservation rate and
lower local recurrence, especially in distant tumors, compared with
SRT (11,12). In the clinical setting, some
patients prefer shorter CRT followed by minimally invasive surgery,
such as local excision for clinical T1-2N0 stages according to the
American Joint Committee on Cancer staging system (AJCC), 8th
edition (13). Without compromising
the advantages of LRT, a shorter radiotherapy schedule would be
preferable for patients who want to avoid multiple hospital visits
for LRT or for those with distant metastases or double primary
cancers requiring early intervention. To optimize the advantages of
LRT and SRT, a phase II multi-institutional clinical trial was
carried out for locally advanced rectal cancer, involving a 2-week
course of preoperative CRT of 33 Gy in 10 fractions, which included
oral capecitabine followed by radical surgery performed 6–8 weeks
after completion of CRT (14). The
long-term outcomes of this 2-week course were recently updated and
they were comparable to historical SRT or LRT in view of survival
rates and acceptable toxicities (15). The radiotherapy of this study was
delivered by a 3D three- or four-field box technique (16). However, in the present study,
intensity-modulated radiotherapy (IMRT) was performed for
hypofractionated preoperative CRT (HPCRT) of 33 Gy or 35 Gy in 10
fractions combined with oral capecitabin in patients in various
stages of rectal cancer. The results of these fractionation
schedules were analyzed to investigate toxicities, tumor responses
and survival outcomes in patients with rectal cancer.
Materials and methods
Patients
Between June 2016 and May 2021, patients who were
diagnosed with rectal adenocarcinoma and had adequate laboratory
data, such as information regarding bone marrow, liver and kidney
function, were eligible for inclusion in this study. Patients were
required to have an Eastern Cooperative Oncology Group (17) performance status of 0–2 and be ≥18
years old. Pretreatment workups included an estimation of
carcinoembryonic antigen (CEA) level, colonoscopy, chest
radiography, computed tomography of the abdomen and pelvis,
magnetic resonance imaging and 18 F-fluorodeoxyglucose positron
emission tomography, if required. The study also included patients
with clinical stage IVA or simultaneous presence of additional
primary cancers who preferentially received HPCRT rather than the
conventional LRT for early intervention. The study was approved by
the Institutional Review Board of Chonnam National University
Hwasun Hospital (Hwasun, South Korea; approval no. CNUHH-2010-009),
and all patients submitted written informed consent upon
enrollment.
Treatment
HPCRT was delivered either by a schedule that
included the following: i) 33 Gy in 10 fractions to the whole
pelvis with IMRT, the protocol of which was performed during the
earlier study period; or ii) 35 Gy in 10 fractions to the primary
bulky tumor via simultaneous integrated boost (SIB) and 33 Gy to
the remaining pelvis with IMRT, the protocol of which was performed
during later study period. In most patients, oral capecitabine was
concurrently administered at a dose of 1,650 mg/m2/day 5
days/week (from Monday to Friday) for up to 10 days during
radiotherapy. Follow-up examinations were repeated before surgery.
Radical surgery was performed 4–8 weeks after the completion of
HPCRT. After surgery, the pathological stage was determined
according to the AJCC staging system. Postoperative chemotherapy
was recommended 4 weeks after surgery, according to the
postoperative pathological stage and patient performance status
(Fig. 1).
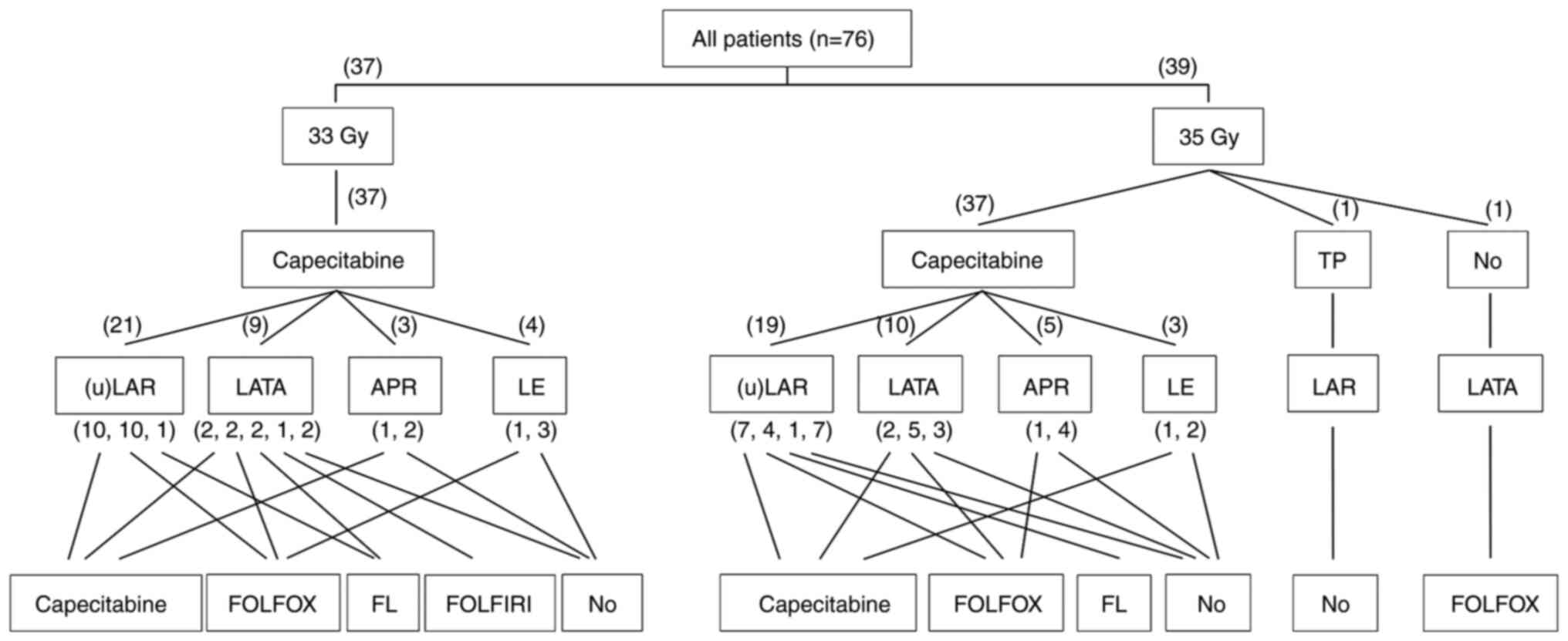 | Figure 1.Detailed treatment characteristics of
HCRT, surgery and postoperative chemotherapy in the patient cohort.
A number in brackets represents the number of each subgroup of
patients. Multiple numbers in one bracket means the corresponding
numbers of patients who received each adjuvant chemotherapy regimen
in order. TP, paclitaxel and cisplatin; (u)LAR, ultra low anterior
resection or low anterior resection; LATA, laparoscopic abdominal
transanal proctocolectomy with coloanal anastomosis; APR,
abdominoperineal resection; LE, local excision (transanal excision,
intersphincteric resection); FOLFOX, fluorouracil, leucovorin and
oxaliplatin; FL, fluorouracil and leucovorin; FOLFIRI,
fluorouracil, leucovorin and irinotecan. |
Evaluation of response and
toxicity
After HPCRT, tumor response was evaluated based on
the pathological finding of tumor and nodal status and tumor
regression grade (TRG), as determined by pathologists. Primary
tumor (T) and nodal (N) downstaging was defined as the lowering of
the T and N stage from clinical staging to postoperative
pathological staging, respectively. Overall downstaging was defined
as overall pathological stage being lower than the initial clinical
stage. TRG was defined as follows: grade 0, no regression; grade I,
minor regression and fibrosis of ≤25%; grade II, moderate
regression and fibrosis of 26–50%; grade III, good regression and
fibrosis of 51–99%; or grade IV, total regression and fibrosis of
100% (18). Acute toxicities of the
gastrointestinal and genitourinary system were assessed from the
initiation of HPCRT and throughout the first 3 months after
surgery. Late toxicities were defined as those that occurred
thereafter. Toxicity was scored according to the National Cancer
Institute Common Terminology Criteria for Adverse Events, version
5.0 (19).
Statistical analysis
Locoregional recurrence was defined as recurrence
within the pelvic cavity and anastomosis site. Distant metastasis
was defined as recurrence outside of the pelvis. Locoregional
failure-free survival (LRFS) was defined as the interval between
the start of HPCRT and the date of locoregional recurrence,
censoring the last follow-up case without locoregional recurrence.
The last follow-up was defined as the date of the last patient
visit to hospital or date of death. Disease-free survival (DFS) was
defined as the interval between the initiation of HPCRT and the
date of any first recurrence, censoring the last follow-up case
without any recurrence. Overall survival (OS) was calculated from
the initiation of HPCRT to the date of death or last follow-up.
Survival for all patients was calculated using the Kaplan-Meier
method. Statistical significance between the groups was analyzed
using a log-rank test. A Cox proportional hazard regression model
was used for multivariate analysis. Statistical analyses were
performed using SPSS (version 25.0; IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient and treatment
characteristics
A total of 116 patients received HPCRT. A total of
40/116 patients were excluded, as they underwent surgery at other
hospital, they refused to receive surgery or they received systemic
chemotherapy for newly developed distant metastases after HPCRT but
before surgery. The remaining 76 patients were included in the
study and patient characteristics are detailed in Table I. The number of patients with
initial clinical stages I, II, III and IVA were 5, 29, 36 and 6,
respectively. By MRI, the distal extent of the tumor ≤5 cm from the
anal verge (AV) and the distal extent of the tumor >5 cm from
the AV were observed in 32 and 44 patients, respectively. A total
of 6 patients had initial distant metastasis, including metastases
of the lung in 3 patients and of the liver 3 patients. In total, 3
patients initially had synchronous double primary cancers, and each
had either lung, gall bladder or colon cancer. Radiotherapy was
performed with a daily fraction size of 3.3 and 3.5 Gy in 37/76
(48.7%) and 39/76 (51.3%) patients, respectively, for a median of
14 days (range, 12–23 days). Concurrent chemotherapy with
capecitabine was administered to most patients; however, a single
patient received Taxol® and cisplatin for the treatment of double
primary lung cancer, and another patient only received radiotherapy
due to immune thrombocytopenia. The median interval between HPCRT
and surgery was 52 days (range, 16–86 days). The surgeries
performed included low anterior resection, laparoscopic abdominal
transanal proctosigmoidectomy with coloanal anastomosis, ultra-low
anterior resection and abdominoperineal resection in 32 (42.1%), 20
(26.3%), 9 (11.8%) and 8 (10.5%) patients, respectively.
Postoperative adjuvant chemotherapy was administered to 52/76
(68.4%) patients according to pathological stage or performance
status. The treatment information is detailed in Table I and Fig. 1.
 | Table I.Patient and treatment
characteristics. |
Table I.
Patient and treatment
characteristics.
| Clinicopathological
features | Value |
|---|
| Age,
yearsa | 70 (42–89) |
| Sex, n (%) |
|
| Male | 42 (55.3) |
|
Female | 34 (44.7) |
| Pre-CRT T-stage, n
(%) |
|
| T1 | 1 (1.3) |
| T2 | 7 (9.2) |
| T3 | 63 (82.9) |
| T4 | 5 (6.6) |
| Pre-CRT N-stage, n
(%) |
|
| N0 | 35 (46.1) |
| N1 | 28 (36.8) |
| N2 | 13 (17.1) |
| Pre-CRT M-stage, n
(%) |
|
| M0 | 70 (92.1) |
|
M1a | 6 (7.9) |
| Pre-CRT overall
stage, n (%) |
|
| I | 5 (6.6) |
| II | 29 (38.2) |
|
III | 36 (47.4) |
|
IVa | 6 (7.9) |
| Pathological
T-stage, n (%) |
|
| 0 | 9 (11.8) |
| T1 | 4 (5.3) |
| T2 | 13 (17.1) |
| T3 | 45 (59.2) |
|
T4a | 5 (6.6) |
| Pathological
N-stage, n (%) |
|
| N0 | 54 (71.1) |
| N1 | 13 (17.1) |
| N2 | 9 (11.8) |
| Pathological
overall stage, n (%) |
|
| 0 | 9 (11.8) |
| I | 13 (17.1) |
| II | 30 (39.5) |
|
III | 18 (23.7) |
|
IVa | 6 (7.9) |
| Pre-CRT distance
from AV, cma | 5.5 (1.3-9.5) |
| Pre-CRT CEA level,
ng/mla | 4.99
(0.90-53.70) |
| Post-CRT/pre-op CEA
level, ng/mla | 3.17
(0.50-35.47) |
| Post-op CEA nadir
level, ng/mla | 1.73
(0.49-10.87) |
| Radiotherapy
duration, monthsa | 14 (12–23) |
| CRT to surgery
interval, daysa | 52 (16–86) |
| CRM,
mma | 6.0 (0–18.0) |
| Surgery, n (%) |
|
|
LAR | 32 (42.1) |
|
LATA | 20 (26.3) |
|
uLAR | 9 (11.8) |
|
APR | 8 (10.5) |
|
TAE | 6 (7.9) |
|
ISR | 1 (1.3) |
Treatment outcomes
A total of 9/76 (11.8%) patients achieved a pCR, and
the treatment characteristics of pCR are shown in Table II. The number of patients in
pathological overall stage 0, I, II, III and IVA was 9 (11.8%), 13
(17.1%), 30 (39.5%), 18 (23.7%) and 6 (7.9%), respectively. The
median post-CRT distal extent of the tumor from the AV by MRI was
5.75 cm (range, 1.8-10.8 cm), which increased from the pre-CRT
extent of 5.5 cm (range, 1.3-9.5 cm). The median post-CRT tumor
length by MRI was 2.0 cm (range, 0–4.0), which decreased from the
pre-CRT length of 4.2 cm (range, 2.0-6.6). Sphincter preservation
was achieved in 24/32 (75.0%) patients with an initial tumor distal
extent ≤5 cm from the AV and in all 44 (100.0%) patients with an
initial distal extent of >5 cm. Prior to HPCRT, 27 patients were
candidates for abdominoperineal resection (≤4 cm from AV; clinical
stage T3-4), and 20 of these patients (74.1%) underwent anal
sphincter-saving surgery. The overall rate of sphincter
preservation was 89.5% (68/76 patients). The median postoperative
CEA nadir level was 1.73 ng/ml (range, 0.49-10.87 ng/ml), which
decreased from the pre-CRT median level of 4.99 ng/ml (range,
0.9-53.7 ng/ml) and post-CRT/preoperative level of 3.17 ng/ml
(range, 0.50-35.5 ng/ml). The other outcomes are presented in
Table I. A total of 28/76 (36.8%)
patients achieved T-downstaging and 25/76 (32.9%) patients achieved
N-downstaging. Overall downstaging was achieved in 34/76 (44.7%)
patients. A detailed breakdown of the downstaging is shown in
Table III, Table IV, Table V. According to the 5-cm cut-off of
tumor distal extent from the AV or the cut off of a median 52 days
between radiotherapy and surgery, there were no marked differences
in TRG or downstaging between the two groups.
 | Table II.Patient and treatment characteristics
of patients who achieved a pathological complete response. |
Table II.
Patient and treatment characteristics
of patients who achieved a pathological complete response.
| Patient | Age, years | Sex | Pre-RT T-stage | Pre-RT N-stage | Pre-RT CEA,
ng/ml | Pre-operative CEA,
ng/ml | Post-operative CEA
nadir, ng/ml | Total dose, Gy | CCTx regimen | Operation type | RT-operation
interval, days | Last follow-up
status | OS time,
months |
|---|
| 1 | 74 | M | 2 | 0 | 2.75 | 1.75 | 1.38 | 35 | Capecitabine | TAE | 59 | Alive | 55 |
| 2 | 68 | F | 3 | 2a | 2.46 | 2.35 | 1.53 | 35 | Capecitabine | LATA | 52 | Alive | 57 |
| 3 | 75 | F | 3 | 0 | 7.8 | 3.44 | 2.08 | 35 | Capecitabine | uLAR | 45 | Alive | 59 |
| 4 | 72 | F | 2 | 0 | 4.42 | 3.74 | 2.68 | 33 | Capecitabine | TAE | 56 | Alive | 27 |
| 5 | 80 | F | 3 | 0 | 2.96 | 2.25 | 1.09 | 35 | Capecitabine | LATA | 58 | Alive | 14 |
| 6 | 83 | M | 3 | 0 | 26.54 | 7.08 | 4.33 | 35 | Capecitabine | LAR | 86 | Alive | 51 |
| 7 | 69 | F | 3 | 0 | 3.32 | 2.4 | 1.61 | 33 | Capecitabine | LATA | 55 | Alive | 59 |
| 8 | 48 | F | 3 | 1 | 6.05 | 0.5 | 0.49 | 33 | Capecitabine | LAR | 54 | Alive | 71 |
| 9 | 71 | M | 3 | 1 | 32.89 | 5.66 | 2.36 | 33 | Capecitabine | LAR | 47 | Alive | 58 |
 | Table III.Changes to T stage pre- and
post-chemoradiotherapy in the 76 patients. |
Table III.
Changes to T stage pre- and
post-chemoradiotherapy in the 76 patients.
|
| Pathological T
stage |
|---|
|
|
|
|---|
| Clinical T
stage | pT0 | pT1 | pT2 | pT3 | pT4 |
|---|
| T1, n | 0 | 1 | 0 | 0 | 0 |
| T2, n | 2 | 3 | 0 | 1 | 1 |
| T3, n | 7 | 0 | 12 | 41 | 3 |
| T4, n | 0 | 0 | 1 | 3 | 1 |
| Total
T-downstaging, n (%) |
|
| 28 (36.8) |
|
|
 | Table IV.Changes to N stage pre- and
post-chemoradiotherapy in the 76 patients. |
Table IV.
Changes to N stage pre- and
post-chemoradiotherapy in the 76 patients.
|
| Pathological N
stage |
|---|
|
|
|
|---|
| Clinical N
stage | pN0 | pN1 | pN2 |
|---|
| N0, n | 31 | 4 | 0 |
| N1, n | 17 | 7 | 4 |
| N2, n | 6 | 2 | 5 |
| Total
N-downstaging, n (%) |
| 25 (32.9) |
|
 | Table V.Changes to overall stage pre- and
post-chemoradiotherapy in the 76 patients. |
Table V.
Changes to overall stage pre- and
post-chemoradiotherapy in the 76 patients.
|
| Pathological
overall stage, n |
|---|
| Clinical overall
stage |
|
|---|
| 0 | I | II | III | IVa |
|---|
| I, n | 2 | 2 | 1 | 0 | 0 |
| II, n | 4 | 6 | 16 | 3 | 0 |
| III, n | 1 | 1 | 20 | 14 | 0 |
| IVA, n | 0 | 0 | 0 | 0 | 6 |
| Total overall
downstaging, n (%)a |
|
| 34 (44.7) |
|
|
Survival and prognostic factors
The follow-up period ranged from 3 to 71 months
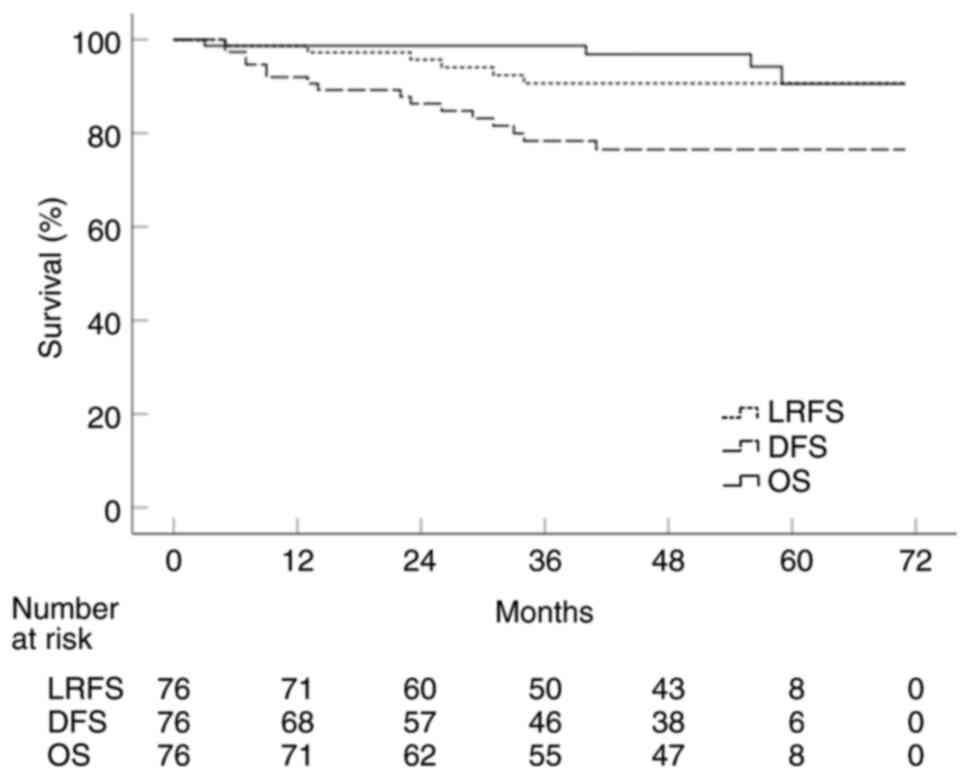
(median, 54). In all 76 patients, the 5-year LRFS, DFS and OS were
90.6, 76.5 and 90.6%, respectively (Fig. 2). The results of univariate analyses
for LRFS, DFS and OS are shown in Table VI. The results of multivariate
analyses are shown in Table VII.
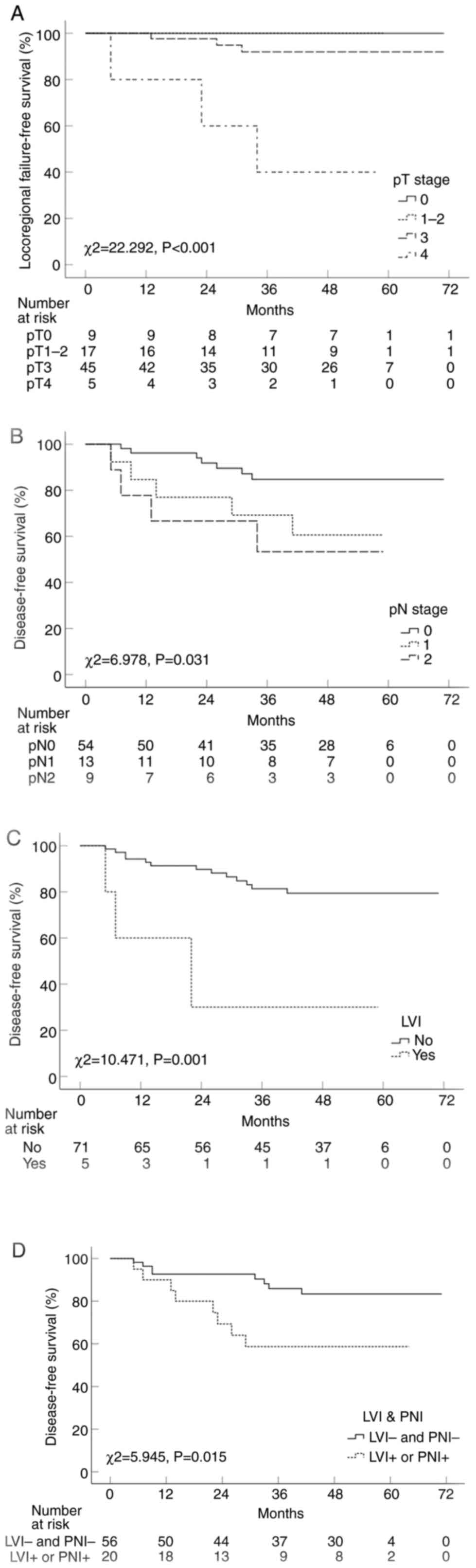
The pT stage was found to be the only significant prognostic factor
for LRFS (P<0.001; Fig. 3A).
However, there was no statistical significance between pT-stage
subgroups, as the hazard ratios for pT3 or pT4 stage were extremely
high compared to those for pT0-2 stage with no locoregional failure
(Tables VI and VII). In the multivariate analysis for
DFS, notable variables included pN stage (Fig. 3B) and lymphovascular space invasion
(LVI; Fig. 3C). The 5-year DFS rate
of patients with negativity for both LVI and PNI (LVI−
and PNI−) versus patients with positivity for either LVI
or PNI (LVI+ or PNI+) was also significantly
higher (P=0.015; Fig. 3D). Upon
multivariate analysis for OS, no significant variables were found.
A total of 6/76 (7.9%) patients with initial stage IVA had one or
more salvage treatments such as surgery, chemotherapy or
stereotactic radiotherapy after completion of HPCRT, and all were
alive at the last follow-up. A single patient with colon cancer and
a single patient with gall bladder cancer out of a total of 3 with
initial double primary tumors underwent surgical resection, and the
other patient with lung cancer was treated with concurrent CRT. All
3 patients were followed up and demonstrated no evidence of disease
or stable disease.
 | Table VI.Univariate analysis for 5-year
survival outcomes. |
Table VI.
Univariate analysis for 5-year
survival outcomes.
|
|
| LRFS | DFS | OS |
|---|
|
|
|
|
|
|
|---|
| Variables | No. of
patients | Survival rate,
% | P-value | Survival rate,
% | P-value | Survival rate,
% | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
≤70 | 39 | 86.2 | 0.185 | 72.1 | 0.575 | 96.2 | 0.091 |
|
>70 | 37 | 97.2 |
| 83.2 |
| 79 |
|
| Sex |
|
|
|
|
|
|
|
|
Female | 34 | 83.5 | 0.215 | 71.1 | 0.268 | 95 | 0.483 |
|
Male | 42 | 95.1 |
| 81.1 |
| 86.5 |
|
| Pre-CRT
T-stage |
|
|
|
|
|
|
|
|
T1-2 | 8 | 100 | 0.481 | 100 | 0.358 | 100 | 0.768 |
| T3 | 63 | 90.6 |
| 73.7 |
| 89.5 |
|
| T4 | 5 | 80 |
| 80 |
| 100 |
|
| Pre-CRT
N-stage |
|
|
|
|
|
|
|
| N0 | 35 | 100 | 0.005a | 87.4 | 0.183 | 78.7 | 0.067 |
| N1 | 28 | 91.1 |
| 72.7 |
| 100 |
|
| N2 | 13 | 69.2 |
| 59.3 |
| 100 |
|
| Pre-CRT
M-stage |
|
|
|
|
|
|
|
| M0 | 70 | 91.7 | 0.48 | 76.3 | 0.728 | 90 | 0.587 |
|
M1a | 6 | 80 |
| 80 |
| 100 |
|
| Pre-CRT overall
stage |
|
|
|
|
|
|
|
| I | 5 | 100 | 0.202 | 100 | 0.323 | 100 | 0.094 |
| II | 29 | 100 |
| 84.8 |
| 77.2 |
|
|
III | 36 | 84.7 |
| 67.6 |
| 100 |
|
|
IVa | 6 | 80 |
| 80 |
| 100 |
|
| Distance from AV,
cm |
|
|
|
|
|
|
|
| ≤5 | 32 | 88.3 | 0.613 | 69.2 | 0.184 | 78.7 | 0.126 |
|
>5 | 44 | 92.2 |
| 81.8 |
| 96.8 |
|
| Pathological
T-stage |
|
|
|
|
|
|
|
| 0 | 9 | 100 |
<0.001a | 100 | 0.054 | 100 | 0.032a |
|
T1-2 | 17 | 100 |
| 85.2 |
| 56.3 |
|
| T3 | 45 | 92 |
| 73.2 |
| 97.8 |
|
| T4 | 5 | 40 |
| 40 |
| 100 |
|
| Pathological
N-stage |
|
|
|
|
|
|
|
| N0 | 54 | 92.9 | 0.005a | 84.7 | 0.031a | 86.6 | 0.422 |
| N1 | 13 | 100 |
| 60.6 |
| 100 |
|
| N2 | 9 | 61 |
| 53.3 |
| 100 |
|
| Pathological
overall stage |
|
|
|
|
|
|
|
| 0 | 9 | 100 | 0.62 | 100 | 0.062 | 100 | 0.008a |
| I | 13 | 100 |
| 80.2 |
| 42.9 |
|
| II | 30 | 88 |
| 81.2 |
| 96.7 |
|
|
III | 18 | 88.1 |
| 54.3 |
| 100 |
|
|
IVa | 6 | 80 |
| 80 |
| 100 |
|
| Pre-CRT CEA,
ng/ml |
|
|
|
|
|
|
|
| ≤5 | 38 | 96.3 | 0.116 | 80.7 | 0.139 | 80.8 | 0.187 |
|
>5 | 38 | 85.5 |
| 67 |
| 96.6 |
|
| Pre-CRT/Post-op
CEA, ng/ml |
|
|
|
|
|
|
|
|
≤2.5 | 24 | 94.7 | 0.398 | 86 | 0.212 | 88.9 | 0.758 |
|
>2.5 | 52 | 88.8 |
| 72.3 |
| 91.6 |
|
| Post-op CEA nadir,
ng/ml |
|
|
|
|
|
|
|
|
≤2.5 | 59 | 91.5 | 0.435 | 78.6 | 0.264 | 94.7 | 0.015a |
|
>2.5 | 17 | 87.5 |
| 68.2 |
| 75.5 |
|
| Radiotherapy
duration, days |
|
|
|
|
|
|
|
|
≤14 | 46 | 89.7 | 0.67 | 72.8 | 0.384 | 85.1 | 0.116 |
|
>14 | 30 | 92 |
| 82.4 |
| 100 |
|
| Fraction size,
Gy |
|
|
|
|
|
|
|
|
3.3 | 37 | 88.5 | 0.515 | 69.4 | 0.164 | 90.7 | 0.647 |
|
3.5 | 39 | 93.3 |
| 85.7 |
| 93 |
|
| CRT to surgery
internal, days |
|
|
|
|
|
|
|
|
≤52 | 44 | 86.9 | 0.232 | 75.4 | 0.758 | 89.6 | 0.429 |
|
>52 | 32 | 96.8 |
| 78.9 |
| 91.7 |
|
| CRM, mm |
|
|
|
|
|
|
|
|
>1 | 45 | 82.3 | 0.889 | 73.8 | 0.655 | 90.4 | 0.537 |
| ≤1 | 31 | 80.4 |
| 80 |
| 90 |
|
| LVI |
|
|
|
|
|
|
|
| No | 71 | 91.6 | 0.124 | 79.4 | 0.001a | 94 | 0.674 |
|
Yes | 5 | 80 |
| 30 |
| 100 |
|
| PNI |
|
|
|
|
|
|
|
| No | 57 | 95.2 | 0.014a | 83.7 | 0.008a | 87.3 | 0.232 |
|
Yes | 19 | 77.3 |
| 56.4 |
| 100 |
|
| TRG |
|
|
|
|
|
|
|
|
1,2 | 28 | 92.1 | 0.817 | 73.3 | 0.634 | 86.1 | 0.719 |
|
3,4 | 48 | 89.5 |
| 78.7 |
| 93 |
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
| No | 24 | 100 | 0.154 | 75.3 | 0.652 | 48.4 |
<0.01a |
|
Yes | 52 | 87.7 |
| 77.5 |
| 100 |
|
 | Table VII.Multivariate analysis for 5-year
survival outcomes. |
Table VII.
Multivariate analysis for 5-year
survival outcomes.
|
| LRFS | DFS | OS |
|---|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Pre-CRT
N-stage |
|
|
|
|
|
|
| N0 |
| 0.260 |
| 0.608 |
| 0.441 |
| N1 |
| 0.937 |
| 0.468 |
| 0.202 |
| N2 |
| 0.928 |
| 0.325 |
| 0.950 |
| Pathological
T-stage |
|
|
|
|
|
|
| 0 | - | 0.044a |
| 0.305 |
| 0.694 |
|
T1-2 | 1.000 | >0.999 |
| 0.505 |
| 0.323 |
|
| (0.000-∞) |
|
|
|
|
|
| T3 | 10416.259 | 0.942 |
| 0.793 |
| 0.989 |
|
| (0.000-∞) |
|
|
|
|
|
| T4 | 106440.674 | 0.927 |
| 0.132 |
| 0.981 |
|
| (0.000-∞) |
|
|
|
|
|
| Pathological
N-stage |
|
|
|
|
|
|
| N0 |
| 0.362 | - | 0.027a |
| 0.799 |
| N1 |
| 0.306 | 3.987
(1.187-13.388) | 0.025a |
| 0.531 |
| N2 |
| 0.287 | 4.157
(1.185-14.580) | 0.026a |
| 0.832 |
| Pathological
overall stage |
|
|
|
|
|
|
| 0 |
| 0.992 |
| 0.598 |
| 0.421 |
| I |
| 0.987 |
| 0.376 |
| 0.323 |
| II |
| 0.914 |
| 0.600 |
| 0.165 |
|
III |
| 0.767 |
| 0.463 |
| 0.504 |
|
IVa |
| 0.838 |
| 0.308 |
| 0.559 |
| Post-op CEA nadir,
ng/ml |
|
|
|
|
|
|
|
≤2.5 |
| 0.045b |
| 0.190 |
| 0.074 |
|
>2.5 |
|
|
|
|
|
|
| LVI |
|
|
|
|
|
|
| No |
| 0.187 | - | 0.002a |
| 0.834 |
|
Yes |
|
| 8.879
(2.201-35.809) |
|
|
|
| PNI |
|
|
|
|
|
|
| No |
| 0.018b |
| 0.059 |
| 0.530 |
|
Yes |
|
|
|
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
| No |
| 0.537 |
| 0.195 |
| 0.398 |
|
Yes |
|
|
|
|
|
|
Patterns of failures and
complications
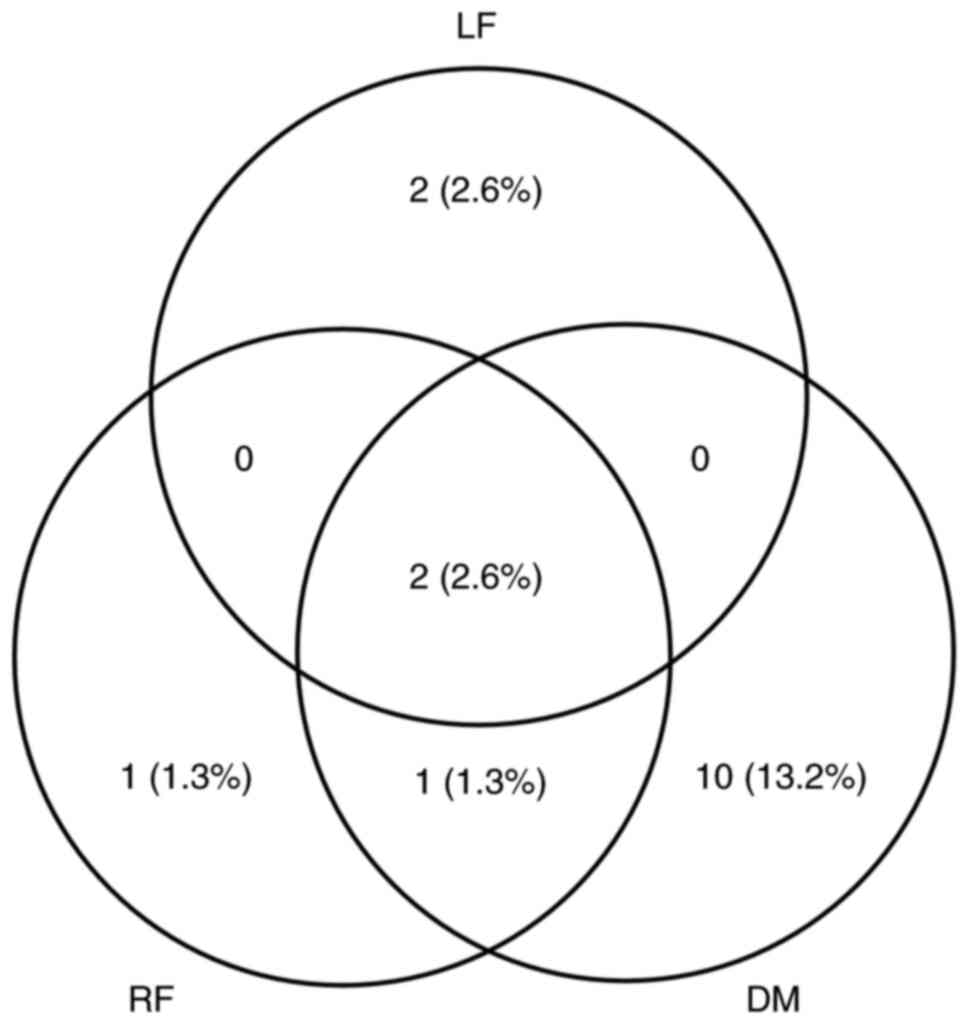
A local failure occurred in 4/76 (5.3%) patients, a
regional failure occurred in 4/76 patients (5.3%) and distant
metastasis occurred in 13/76 patients (17.1%) as a component of
failure. The pattern of failure is illustrated in Fig. 4. Sites of distant metastasis were
identified in the lung, bone, distant lymph nodes and liver in 10
(13.2%), 2 (2.6%), 2 (2.6%) and 1 (1.3%) patients, respectively.
Patients with two or more sites were separately counted. Only 1
patient (1.3%) developed an acute grade 3 complication of
anastomosis leakage, while 3 patients (3.9%) experienced late grade
3 complications such as rectovaginal fistula at anastomosis, anal
bleeding from anastomosis and anastomotic leakage. No grade 4
toxicity was observed. A detailed breakdown of the complications of
all grades is shown in Table
VIII.
 | Table VIII.Gastrointestinal and genitourinary
toxicities. |
Table VIII.
Gastrointestinal and genitourinary
toxicities.
| A, Gastrointestinal
toxicities |
|---|
|
|---|
|
| Grade 3 | Grade 2 | Grade 1 |
|---|
|
|
|
|
|
|---|
| CTCAE | Symptom | n (%) | Symptom | n (%) | Symptom | n (%) |
|---|
| Acute | Anastomosis
leakage | 1 (1.3) | Anal pain | 8 (10.6) | Anal pain | 4 (5.3) |
|
|
|
| Diarrhea | 5 (6.7) | Diarrhea | 3 (4) |
|
|
|
|
|
| Rectal
mucositis | 3 (4) |
| Late | Anal
hemorrhage | 1 (1.3) | Diarrhea | 24 (32) | Anal pain | 5 (6.7) |
|
| Anastomosis
leakage | 1 (1.3) | Anal pain | 4 (5.3) | Constipation | 2 (2.7) |
|
| Rectovaginal
fistula | 1 (1.3) | Anal fissure | 1 (1.3) | Diarrhea | 2 (2.7) |
|
|
|
| Constipation | 1 (1.3) | Anal
hemorrhage | 1 (1.3) |
|
|
|
|
|
| Fecal
incontinence | 1 (1.3) |
|
|
|
|
|
| Rectal
mucositis | 1 (1.3) |
|
| B, Genitourinary
toxicities |
|
|
| Grade 3 | Grade 2 | Grade 1 |
|
|
|
|
|
| CTCAE | Symptom | n (%) | Symptom | n (%) | Symptom | n (%) |
|
| Acute |
|
|
|
| Dysuria | 1 (1.3) |
| Late |
|
| Urinary
retention | 2 (2.7) | Dysuria | 1 (1.3) |
|
|
|
| Urinary
frequency | 1 (1.3) | Urinary
incontinence | 1 (1.3) |
Discussion
In the present study, the rates of T-downstaging,
N-downstaging and overall downstaging were 36.8, 32.9 and 44.7%,
respectively. In total, 1 out of 3 patients with synchronous double
primary cancer achieved overall downstaging of primary rectal
cancer. The pCR rate was 11.8%, and the overall rate of sphincter
preservation was 89.5%. These results were comparable with those of
historical LRT studies (1,6) and similar to the 2-week course KROG
study (14). The comparable
outcomes of the present study could be attributed to dose
prescription of 33 Gy [biological equivalent dose assuming α/β=10
by linear-quadratic model (BED10), 43.9 Gy],or 35 Gy
(BED10, 47.3 Gy), which is between SRT of 25 Gy
(BED10 37.5 Gy) and LRT of 50.4 Gy (BED10,
59.5 Gy). Oral capecitabine was prescribed as the concurrent
chemotherapy agent, followed by delayed surgery with a median
interval of 52 days to allow tumor regression before surgery. This
study showed comparable toxicities to previous LRT results
(1,6). Only 4/76 (5.3%) patients developed
acute or late grade 3 toxicities and there were no grade 4
toxicities in any patients, including the SIB IMRT 35 Gy patient
cohort. A single patient received 35 Gy and was treated with Taxol®
and cisplatin concurrently due to synchronous lung cancer and acute
grade 2 diarrhea. All patients had one or more weekend to rest
without receiving HPCRT, but received IMRT to limit the radiation
dose to normal organs. SIB IMRT of 35 Gy was performed to the gross
tumor to improve the pathological regression. However, in the
groups of patients with 35 Gy versus 33 Gy, the pCR rate was 12.8
and 10.8%, respectively (P=0.786), the sphincter preservation rate
was 87.2 and 91.9%, respectively (P=0.503), and the 5-year DFS was
85.7 and 63.9% (P=0.067), respectively. None of these differences
were statistically significant between the two groups. The 5-year
LRFS, DFS and OS rates were 90.6, 76.5 and 90.6%, respectively.
These survival rates were comparable to previous LRT results
(1,6) or the recently updated 2-week CRT KROG
study (15), despite the fact that
the present study included patients with synchronous double primary
or distant metastasis. A total of 5/76 patients with early clinical
stages, such as T1-2N0, were included. Of these patients, 2 had
synchronous double primary cancers, 2 preferred HPCRT followed by
local excision rather than upfront radical surgery and 1 strongly
demanded SRT due to having to travel long-distances to receive CRT.
A total of 4/5 patients underwent sphincter preservation surgery.
Thus, all the patients had a median radiotherapy duration of 2
weeks, which is 4 weeks shorter than the conventional 6-week LRT,
and due to the shorter CRT duration, patients could receive surgery
4 weeks earlier than those receiving traditional LRT.
In the multivariate analysis for LRFS in this study,
only pT-stage was an independent prognostic factor. It is well
known that patients with sterilized tumors after preoperative CRT
have an excellent prognosis with respect to LRFS or DFS (18,20). A
total of 9 patients who achieved pCR in the current study had
experienced no recurrence at the last follow-up. In the
multivariate analysis for DFS, pN stage and LVI were significant
prognostic variables. The pN stage is a well-known prognostic
factor for survival, and is integral to the AJCC staging system
(21). The prognosis of patients
with remnant lymph node metastasis after CRT is poor, even in cases
of complete primary tumor response (22). Likewise, patients with more advanced
pathological primary tumors (pT3-4) with pN0 stage showed slightly
better recurrence-free survival or OS than those who were pT0-2,
but pN+ (23). It is
well known that the LVI is an independent prognostic factor for
survival. Song et al (24)
reported that LVI was a significant prognostic factor affecting
distant failure-free survival. Saadoun et al (25) developed a nomogram with eight
variables, including pathological stage, LVI and PNI, which
provided individual risk prediction for recurrence. In the current
study, DFS of patients who were both LVI− and
PNI− versus that of patients who were LVI+ or
PNI+ significantly differed, although patients who were
LVI+ or PNI+ received postoperative adjuvant
chemotherapy (5-fluorouracil and oxaliplatin) more frequently than
patients who were LVI− and PNI− (18/20 vs.
34/56; P=0.023). Therefore, more aggressive adjuvant chemotherapy
should be considered to improve DFS for these patients.
A limitation of this study was the small patient
cohort and the slow accrual rate given the study duration. The CRT
option to select patients with a preference for HPCRT or even with
synchronous distant disease or double primary cancer was provided.
However, the treatment protocol was the same, except the total dose
of 33 or 35 Gy, throughout the entire study period, and treatment
consistency was maintained. A randomized controlled study comparing
HPCRT and long-course CRT in a larger patient cohort is required in
the future. Another limitation refers to the heterogenous patient
characteristics, ranging from early to advanced clinical stage (I
to IVA) or to the synchronous double primary cancer. However, most
patients experienced the advantages of HPCRT, which include
convenience, low cost, shorter radiotherapy time and earlier
surgical or other radical treatments for distant disease or double
primary cancer.
In conclusion, HPCRT of 33 or 35 Gy in 10 fractions
showed comparable results to historical conventional LRT studies.
This shorter fractionation scheme may be beneficial, without
reducing oncological outcomes, for patients with early stage
disease, locally advanced rectal cancer, simultaneous distant
metastasis and other double primary cancer requiring early
intervention, or for patients who cannot attend the hospital
multiple times.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TKN conceived and designed the study. IJC, YHK, JYS,
MSY, SJA and SHC acquired data. TKN, IJC and JUJ confirm the
authenticity of all the raw data. IJC and JUJ performed the
statistical analysis. IJC, JUJ and TKN interpreted the results,
analyzed the data and drafted the manuscript. All authors read and
approved the final version of the manuscript.
Ethical approval and consent to
participate
The study was established, according to the ethical
guidelines of the Helsinki Declaration and was approved by
Institutional Review Board of Chonnam National University Hwasun
Hospital (Hwasun, South Korea; approval no. CNUHH-2010-009).
Informed consent was obtained from all patients and/or their legal
guardian(s).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AV
|
anal verge
|
|
BED10
|
biological equivalent dose assuming
α/β=10 by linear-quadratic model
|
|
CRT
|
chemoradiotherapy
|
|
DFS
|
disease-free survival
|
|
HPCRT
|
hypofractionated preoperative CRT
|
|
IMRT
|
intensity-modulated radiotherapy
|
|
LRT
|
long-course radiotherapy
|
|
LRFS
|
locoregional failure-free survival
|
|
LVI
|
lymphovascular space invasion
|
|
N
|
nodal
|
|
OS
|
overall survival
|
|
pCR
|
pathological complete remission
|
|
PNI
|
perineural invasion
|
|
SRT
|
short-course radiotherapy
|
|
SIB
|
simultaneous integrated boost
|
|
T
|
tumor
|
|
TRG
|
tumor regression grade
|
References
|
1
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkesson J, Birgisson H, Pahlman L,
Cedermark B, Glimelius B and Gunnarsson U: Swedish rectal cancer
trial: Long-lasting benefits from radiotherapy on survival and
local recurrence rate. J Clin Oncol. 23:5644–5650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Gijn W, Marijnen CA, Nagtegaal ID,
Kranenbarg EMK, Putter H, Wiggers T, Rutten HJT, Påhlman L,
Glimelius B and van de Velde CJH; Dutch Colorectal Cancer Group, :
Preoperative radiotherapy combined with total mesorectal excision
for resectable rectal cancer: 12-year follow-up of the multicentre,
randomised controlled TME trial. Lancet Oncol. 12:575–582. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sebag-Montefiore D, Stephens RJ, Steele R,
Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint
AS, et al: Preoperative radiotherapy versus selective postoperative
chemoradiotherapy in patients with rectal cancer (MRC CR07 and
NCIC-CTG C016): A multicentre, randomised trial. Lancet.
373:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, Michalski W, Bebenek M and Kryj M: Long-term results of a
randomized trial comparing preoperative short-course radiotherapy
with preoperative conventionally fractionated chemoradiation for
rectal cancer. Br J Surg. 93:1215–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngan SY, Burmeister B, Fisher RJ, Solomon
M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B,
McLachlan SA, et al: Randomized trial of short course radiotherapy
versus long course chemoradiation comparing rates of local
recurrence in patients with T3 rectal cancer: Trans-Tasman
radiation oncology group trial 01.04. J Clin Oncol. 30:3827–3833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pietrzak L, Bujko K, Nowacki MP, Kepka L,
Oledzki J, Rutkowski A, Szmeja J, Kladny J, Dymecki D, Wieczorek A,
et al: Quality of life, anorectal and sexual functions after
preoperative radiotherapy for rectal cancer: Report of a randomised
trial. Radiother Oncol. 84:217–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngan S, Fisher R, Burmeister B, Mackay J,
McLachlan S, Beresford J, McClure B, Goldstein D, Joseph D and
Solomon M: Long-term quality of life in patients treated in TROG
01.04: A randomized trial comparing short course and long course
preoperative radiation therapy for rectal cancer. Int J Radiat
Oncol Biol Phys. 84:S143–S144. 2012. View Article : Google Scholar
|
|
9
|
Initial report from a Swedish multicentre
study examining the role of preoperative irradiation in the
treatment of patients with resectable rectal carcinoma. Swedish
rectal cancer trial. Br J Surg. 80:1333–1336. 1993.PubMed/NCBI
|
|
10
|
Marijnen CAM, Kapiteijn E, van de Velde
CJH, Martijin H, Steup WH, Wiggers T, Kranenbarg EK and Leer JWH;
Cooperative Investigators of the Dutch Colorectal Cancer Group, :
Acute side effects and complications after short-term preoperative
radiotherapy combined with total mesorectal excision in primary
rectal cancer: Report of a multicenter randomized trial. J Clin
Oncol. 20:817–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, Michalski W, Bebenek M, Pudelko M, Kryj M, Oledzki J, Szmeja J,
Słuszniak J, et al: Sphincter preservation following preoperative
radiotherapy for rectal cancer: Report of a randomised trial
comparing short-term radiotherapy vs. conventionally fractionated
radiochemotherapy. Radiother Oncol. 72:15–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerard JP, Chapet O, Nemoz C, Hartweig J,
Romestaing P, Coquard R, Barbet N, Maingon P, Mahe M, Baulieux J,
et al: Improved sphincter preservation in low rectal cancer with
high-dose preoperative radiotherapy: The Lyon R96- 02 randomized
trial. J Clin Oncol. 22:2404–2409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual (8th edition).
Springer International Publishing: American Joint Commission on
Cancer; 2017
|
|
14
|
Lee JH, Kim JG, Oh ST, Lee MA, Chun HG,
Kim DY, Kim TH, Kim SY, Baek JY, Park JW, et al: Two-week course of
preoperative chemoradiotherapy followed by delayed surgery for
rectal cancer: A phase II multi-institutional clinical trial (KROG
11–02). Radiother Oncol. 110:150–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung SY, Kim DY, Jang HS, Kim TH, Park HC,
Chie EK, Nam TK, Kim SH and Lee JH: One-week versus two-week
chemoradiotherapy followed by curative surgery in rectal cancer:
Long-term comparative pooled analysis of two prospective
multicenter phase II trials. Cancer Res Treat. 2023.(Epub ahead of
print). View Article : Google Scholar
|
|
16
|
Halperin EC, Wazer DE, Perez CA and Brady
LW: Perez & Brady's principles and practice of radiation
oncology, 7th ed. Wolters Kluwer; Philadelphia: 2018
|
|
17
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rödel C, Martus P, Papadoupolos T, Füzesi
L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R,
Sauer R and Wittekind C: Prognostic significance of tumor
regression after preoperative chemoradiotherapy for rectal cancer.
J Clin Oncol. 23:8688–8696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
US Department Of Health and Human
Services, . Common terminology criteria for adverse events (CTCAE)
version 5.0. United States: National Cancer Institute; 2017,
https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
|
|
20
|
Gavioli M, Luppi G, Losi L, Bertolini F,
Santantonio M, Falchi AM, D'Amico R, Conte PF and Natalini G:
Incidence and clinical impact of sterilized disease and minimal
residual disease after preoperative radiochemotherapy for rectal
cancer. Dis Colon Rectum. 48:1851–1857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinaux AM, Leijssen L, Bordeianou LG,
Kunitake H, Amri R and Berger DL: Outcomes of persistent lymph node
involvement after neoadjuvant therapy for stage III rectal cancer.
Surgery. 163:784–788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH,
Park W, Choi DH, Nam HR, Kim JS, Cho MJ, et al: Pathological
complete response of primary tumor following preoperative
chemoradiotherapy for locally advanced rectal cancer: Long-term
outcomes and prognostic significance of pathological nodal status
(KROG 09-01). Ann Surg. 252:998–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho E, Park IJ, Hong SM, Lee JL, Kim CW,
Yoon YS, Lim SB, Yu CS and Kim JC: Poorer oncologic outcome of good
responders to PCRT with remnant lymph nodes defies the oncologic
paradox in patients with rectal cancer. Clin Colorectal Cancer.
18:e171–e178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song JH, Yu M, Kang KM, Lee JH, Kim SH,
Nam TK, Jeong JU, Jang HS, Lee JW and Jung JH: Significance of
perineural and lymphovascular invasion in locally advanced rectal
cancer treated by preoperative chemoradiotherapy and radical
surgery: Can perineural invasion be an indication of adjuvant
chemotherapy? Radiother Oncol. 133:125–131. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saadoun JE, Meillat H, Zemmour C, Brunelle
S, Lapeyre A, de Chaisemartin C and Lelong B: Nomogram to predict
disease recurrence in patients with locally advanced rectal cancer
undergoing rectal surgery after neoadjuvant therapy: Retrospective
cohort study. BJS Open. 6:zrac1382022. View Article : Google Scholar : PubMed/NCBI
|