Colorectal cancer (CRC) occurs in the colon or
rectum, and has the third highest incidence and second highest
mortality rate among types of cancer worldwide (1–3), thus
it is associated with a heavy disease burden. According to the 2019
China Cancer Center report, China ranked second in CRC incidence
and fourth in CRC mortality in 2015 (4). Early CRC (ECC) refers to CRC confined
to the mucosal and submucosal layers, with or without lymph node
metastasis (5,6). The 5-year survival rates of patients
with ECC are ≥90% (7), whereas the
5-year survival rate of patients with progressive CRC is markedly
lower, at <10% (8,9). Previous studies have reported that
colonoscopy and lesion resection can reduce CRC incidence by 76–90%
and mortality by 53% (10,11). The presence of colonic adenomas,
particularly progressive adenomas, is associated with significantly
increased CRC incidence and mortality, and resection of adenomas
significantly reduces CRC incidence [standardized incidence ratio
(SIR), 0.24-0.65] and mortality (SIR, 0.26-0.80) (12). Early detection and treatment of
cancer are critical to patient prognosis. Current treatments for
ECC are primarily surgery, endoscopic submucosal dissection (ESD)
and endoscopic mucosal resection (EMR) (13). The advantages and disadvantages of
ESD and EMR in different parts of the GI tract are presented in
Table I. Surgery was once
considered the standard method for treating early-stage
gastrointestinal cancer; however, although surgery can completely
remove a lesion, it has a number of disadvantages, including the
associated trauma, slow recovery and high complication rates.
Compared with traditional surgery, ESD preserves normal bowel
function, and results in less trauma and faster postoperative
recovery (14). Moreover, patients
who undergo ESD for the treatment of ECC and precancerous lesions
have a higher overall survival rate, with a long-term prognosis
similar to that of conventional surgery (15). Compared with EMR, ESD has a
relatively higher risk of complications, due to its greater
surgical difficulty and longer operative time (16), but can be used to treat larger
lesions, reduce the postoperative residual and recurrence rates,
and yields a more accurate pathological histology report;
therefore, ESD is gradually becoming the main treatment modality
for early gastrointestinal cancer and precancerous lesions
(17–20) (Fig.
1). Although ESD can achieve relatively good efficacy in the
treatment of ECC, the incidence of post-ESD complications,
including fever, bleeding, perforation, electrocoagulation syndrome
and stricture, is relatively high. The relatively high complication
rate is due to the limits of endoscopic operation and anatomical
factors, such as the rich vascularity of the colorectal mucosa, the
thin intestinal wall and the substantial curvature of the
intestine. Therefore, understanding and controlling the occurrence
of common complications after colorectal ESD surgery, related risk
factors, and their prevention and control measures, are important
topics for clinicians. The present review provides an overview of
the occurrence of common postoperative complications after
colorectal ESD, related risk factors, and prevention and control
measures, to serve as a reference for clinical treatment.
Risk factors for fever after ESD include age, lesion
size, postoperative bleeding or perforation, and surgery duration.
Advanced age is often associated with numerous underlying diseases
and immune deficiency, which, together with longer ESD surgery
duration, increase the risk of postoperative infection (28). Nakanishi et al (29) also reported that age was a risk
factor for fever in patients following colorectal ESD, as was
lesion size (21). Usually ESD
resection depth is limited to the mucosal and submucosal layers,
but sometimes it penetrates the muscular layer or all layers
(30). Deeply infiltrated ulcers
can form soon after ESD and the inflammatory response during their
healing is another cause of fever (31). Furthermore, deep/large ulcers after
ESD are associated with postoperative bleeding and perforation and,
if postoperative bleeding requiring re-electrocoagulation occurs,
burns to the intestinal wall can cause an inflammatory response in
the plasma membrane (32). Complete
intraoperative clamping of the wound in ESD can reduce perforation
and various adverse events, particularly fever (33), supported by the fact that mucosal
defect closure accelerates wound healing (34). Postoperative perforation combined
with fever and elevated infection indicators, such as white blood
cells, may indicate secondary infection (35), and surgical intervention may be
required in severe cases of secondary infection (36,37).
The consensus among Chinese experts is that
postoperative antimicrobials should be used for patients with
advanced age, extensive underlying disease, large resection area,
long operative time, and postoperative bleeding or perforation
(38,39). Routine use of antibiotics after ESD
for possible postoperative free abdominal, mediastinal,
retroperitoneal or systemic infection is recommended (40). Since post-ESD bleeding or
perforation can cause fever, preoperative anticoagulation and
antiplatelet drugs should be avoided. Submucosal injection of
epinephrine-saline and hemostatic clips can achieve intraoperative
hemostasis; to prevent perforation, adequate submucosal injection
should be ensured. Cooling treatment should be given for
postoperative absorption fever, while for electrocoagulation
syndrome, cooling, analgesia and antibiotics are recommended For
intraoperative perforation, timely clamping and antibiotics should
be used. In patients with large lesion diameters and long
postoperative fasting time, clinicians must be alert for
postoperative fever (41).
Shortening operation time and reducing serious postoperative
complications, such as bleeding and perforation, are important for
fever prevention after ESD.
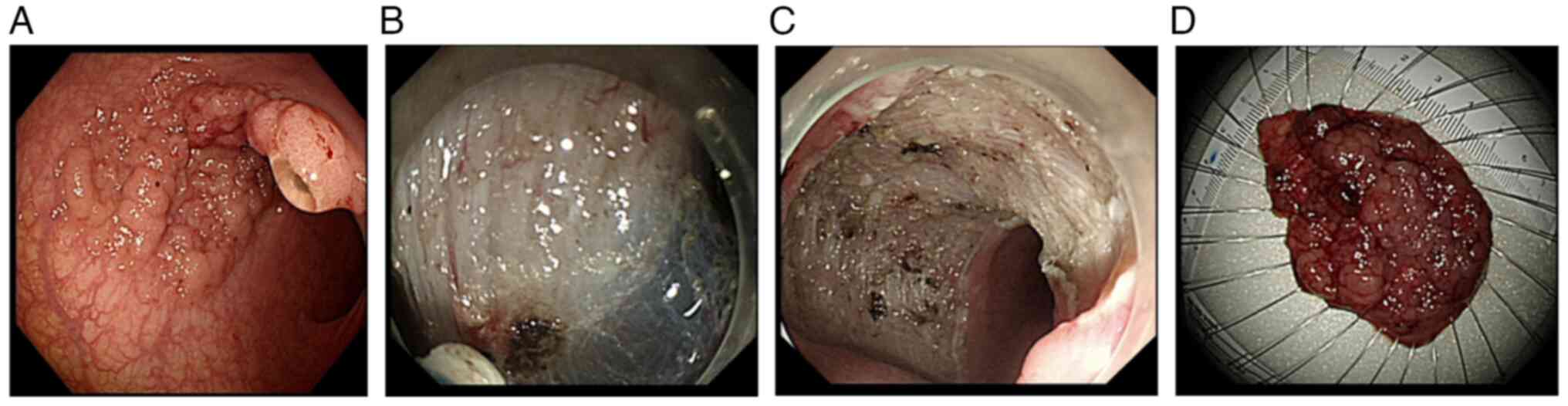
Bleeding is a common complication after ESD, and
some bleeding associated with the procedure is almost unavoidable,
including intraoperative and late postoperative bleeding (Fig. 2). Intraoperative bleeding is defined
as active bleeding during ESD. Postoperative delayed bleeding
refers to bleeding occurring >6 h after ESD, mainly manifesting
as obvious blood in the stool or a decrease in hemoglobin of >20
g/l relative to the preoperative period (42–44),
and is an indication for emergency endoscopic hemostasis (45). Bleeding occurs after 0–11.11% of
colorectal ESD procedures (42,46–49).
Li et al (50) reported that
most late bleeding after colorectal ESD occurred within 5 days
following surgery. Furthermore, the incidence of immediate and
delayed postoperative bleeding has been reported to be 0.39% (95%
CI, 0.11-1.3%) and 1.8% (95% CI, 1.4-2.4%) in Asian countries, and
3.3% (95% CI, 1.4-7.6%) and 3.9% (95% CI, 2.5-5.8%) in European and
North American countries, respectively. Bleeding rates post-ESD are
lower in Asia than in Europe (51),
which may be related to the fact that ESD was performed relatively
earlier and with more advanced techniques in Asian countries.
Risk factors affecting post-ESD bleeding include
duration of surgery, lesion size and location, histological type,
intraoperative mucosal lift, and preoperative administration of
antiplatelet or anticoagulant medications. Yamamoto et al
(52) reported that operative time,
lesion location and histological type were independent risk factors
for postoperative bleeding in ESD. Furthermore, the risk of delayed
bleeding is higher in cecum lesions than those at other sites
(53). Chiba et al (54) reported an increased risk of delayed
bleeding after ESD when lesions were in the rectum and ≥40 mm.
Inadequate submucosal injection volume and high fibrosis at the
lesion site are risk factors for delayed bleeding after colorectal
ESD (50,55), as well as a major cause of poor
separation of the mucosal layer from the mucosal muscle layer.
Therefore, more caution is needed for poorly elevated colorectal
lesions. A higher risk of postoperative bleeding has been reported
in hypertensive patients treated with ESD (56–58);
however, other studies reported no correlation between hypertension
and postoperative bleeding after ESD (50,59). A
previous study reported that the effect of lesion size on delayed
bleeding was not statistically significant (60); however, some scholars take the
opposite view (59). Previous
reports stated that antiplatelet agents should be discontinued
before ESD treatment (56,61,62),
unless patients are at high risk of combined thromboembolism;
however, certain studies disagree (47,59).
Therefore, risk factors for postoperative bleeding after ESD to
date are controversial, and further research is needed.
Most bleeding from small vessels after ESD can be
stopped by electrocoagulation (14). Bleeding from larger vessels requires
hemostasis by metal clip closure combined with electrocoagulation
(14). During ESD, effective
identification of submucosal vessels, timely electrocoagulation and
careful treatment of trauma are essential. Furthermore, local
cleaning with ice-cold saline containing norepinephrine and
hemostatic drugs, such as hemagglutinin, can achieve a hemostatic
effect and effectively reduce postoperative bleeding risk (63), while prophylactic clamping of trauma
reduces delayed bleeding risk after colorectal ESD (64) by reducing irritation from intestinal
contents and accelerating incision healing. Therefore, for larger
colorectal lesions, clamping of trauma is beneficial to promote
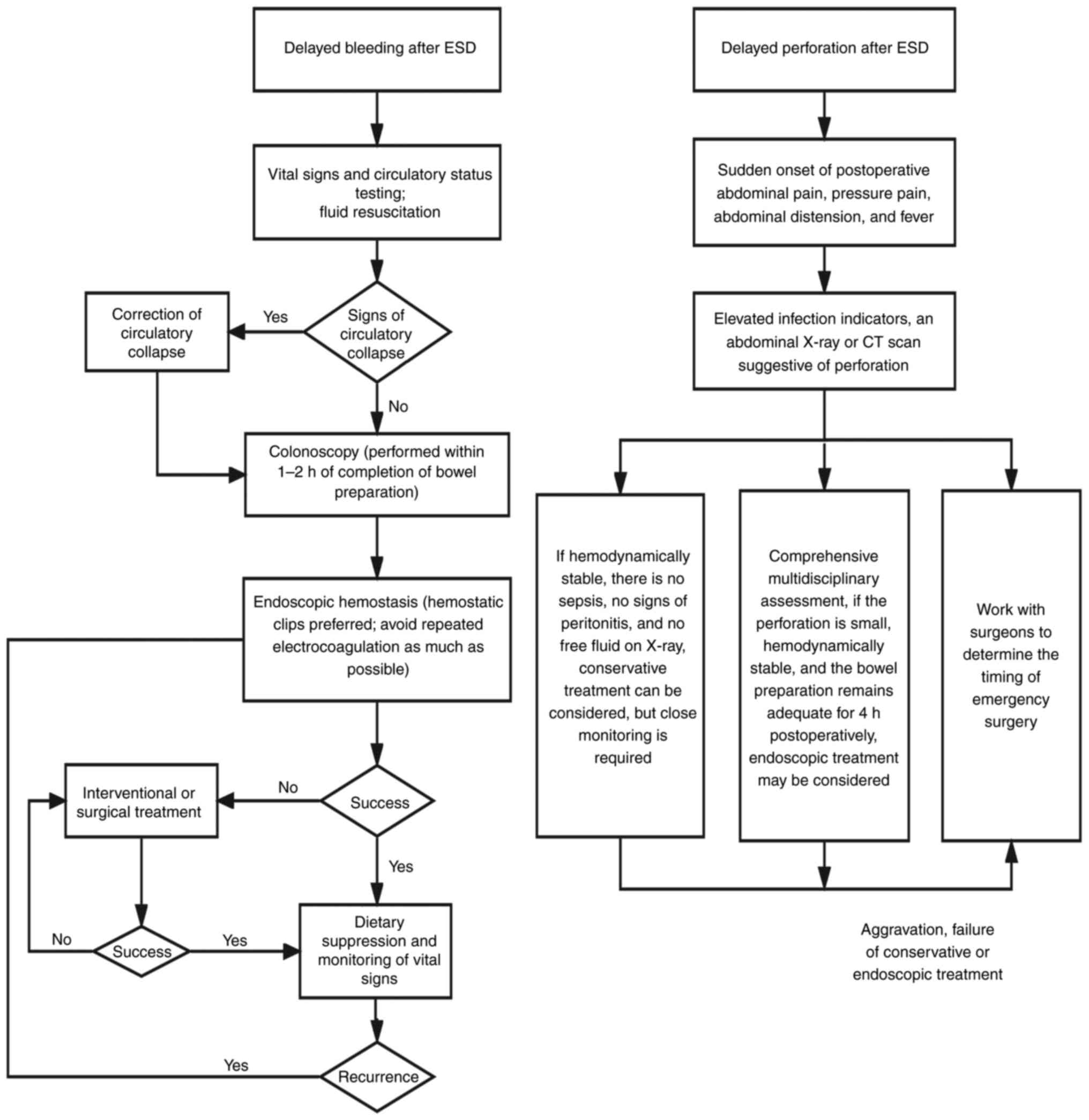
incisional healing and prevent delayed bleeding. Emergency
management measures related to postoperative bleeding in ESD are
shown in Fig. 3. Huang et al
(65) reported that patients with
colorectal lesions who received traction ring-assisted ESD
experienced significantly less intraoperative bleeding than those
who did not.
The risk of perforation is elevated after colorectal
ESD because the muscular layer of the intestinal wall is thinner
than that in the gastric wall (51,66).
The main goal of ESD treatment is complete resection without
perforation (67) and reported
perforation rates after colorectal ESD are 0–10.7% (44,68).
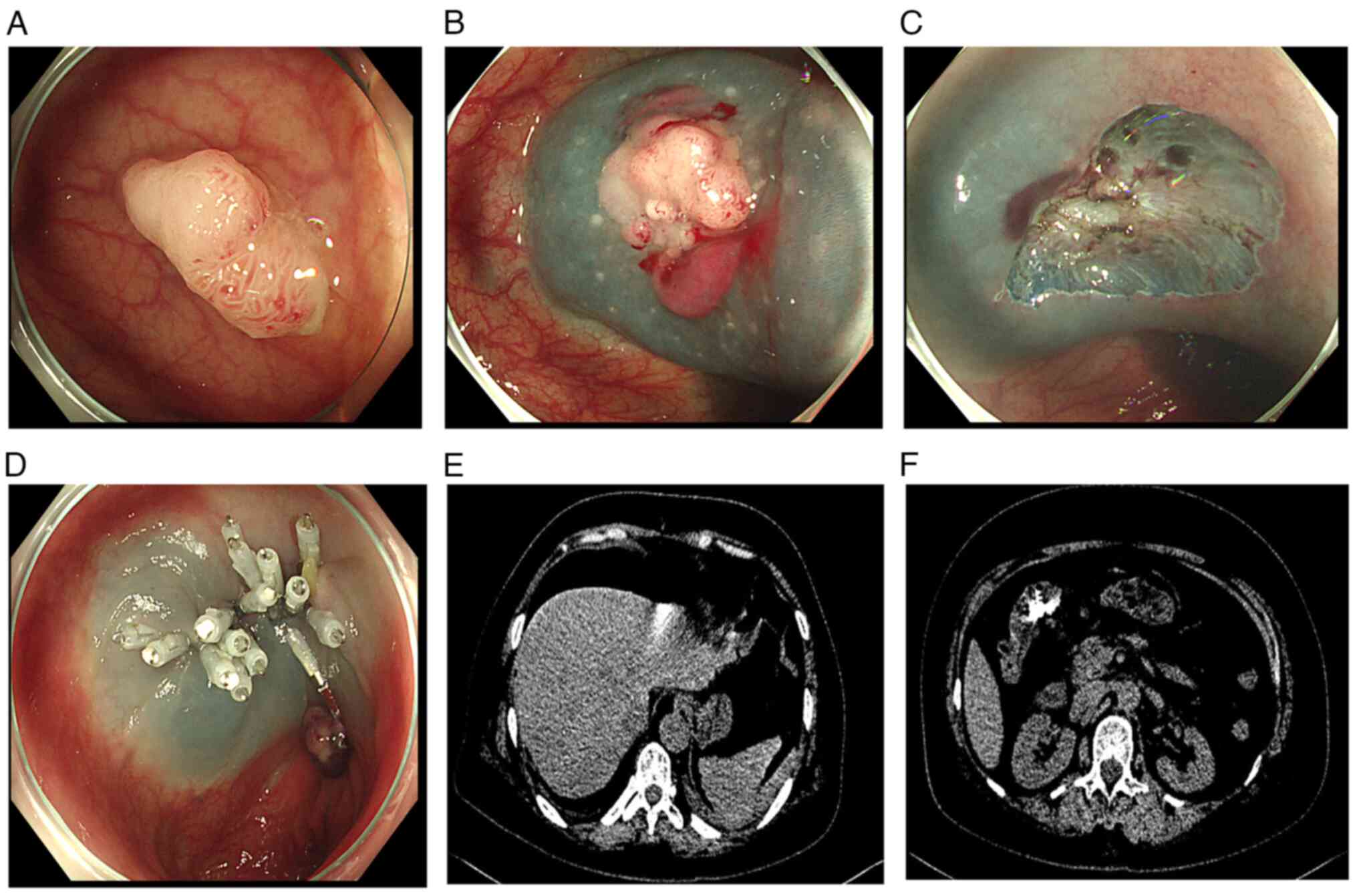
Perforations include intraoperative and delayed perforations.
Intraoperative perforation (69) is
defined as an intraoperative, endoscopically confirmed bowel wall
defect, most of which can be successfully clamped endoscopically.
Delayed perforation (70) is
defined as perforation occurring after ESD, with postoperative
peritonitis, and can be confirmed by abdominal X-ray or computed
tomography (CT) (Fig. 4). Most
delayed perforations occur within 14 h of surgery (44) and, although incidence is very low,
when it occurs, spillage of bowel contents causes severe
peritonitis, which often requires surgery (71,72),
indirectly illustrating the importance of preoperative bowel
cleansing. Similar to postoperative bleeding, immediate and delayed
perforation rates were reported to be 3.8% (95% CI, 3.1-4.6%) and
0.18% (95% CI, 0.08-0.42%) in Asian countries, and 6.6% (95% CI,
4.6-9.4%) and 1.2% (95% CI, 0.29-4.6%) in European and North
American countries, respectively. These findings indicated that the
incidence of postoperative colorectal ESD perforation is lower in
Asia than in Europe (51).
Risk factors influencing perforation after
colorectal ESD include duration of surgery, lesion size, lesion
location and submucosal fibrosis. Su et al (73) reported that the perforation rate in
patients with colorectal lesions >4 cm in diameter (7.04%) was
higher than that in patients with lesion diameter <4 cm (5.2%),
there was a significant difference between the two groups. Hong
et al (74) reported that
lesion size, submucosal fibrosis and duration of surgery were
associated with postoperative perforation after colorectal ESD.
Another study reported tumor size, site, submucosal fibrosis and
operator experience as potential risk factors for postoperative
perforation (75). Furthermore, a
Japanese study reported that longer operative times were more
likely to result in postoperative perforation of the colorectal ESD
(52). Prolonged operative time is
related to various factors, such as operator experience and the
difficulty of the ESD procedure. Another study reported a higher
incidence of perforation following left hemicolectomy (73). Further studies are needed in the
future regarding the relationship between the lesion site and
postoperative perforation of colorectal ESD.
Perforation post-colorectal ESD is a serious
complication that can cause extensive peritonitis, infectious shock
and even be life-threatening. Adequate preoperative preparation,
such as water fasting and bowel cleansing, is necessary. The
colorectal wall is relatively weak and thermal damage from
excessive electrocoagulation may lead to perforation; therefore,
avoiding excessive intraoperative electrocoagulation can reduce
postoperative perforation incidence (72,76).
Adequate submucosal injection reduces perforation due to disruption
of the muscularis during dissection caused by electrocoagulation.
Furthermore, timely perforation closure is closely related to
prognosis and can reduce peritonitis occurrence (77). If intraoperative perforation occurs,
it can generally be detected and smaller perforations can be closed
with metal clips. For larger perforations, metal clips combined
with nylon rope wrap, alongside fasting and gastrointestinal
decompression, the use of intravenous antibiotics and other means,
may be required; generally, the treatment is effective (78). Surgical laparoscopic exploration and
repair are preferable in cases of delayed perforation that cannot
be completely closed endoscopically or for cases in which
conservative treatment is ineffective (14) (Fig.
3).
PEECS refers to a group of inflammatory response
syndromes, including limited peritonitis, which can are caused by
electrocoagulation damage to the intestinal wall during ESD
(72,79). The reported incidence of PEECS is
4.8-14.2% (22,80–83).
Typical clinical manifestations of PEECS include abdominal pain,
limited peritoneal irritation, fever and chills, among others.
Laboratory tests show elevated leukocytes and C-reactive protein,
and abdominal X-ray or computed tomography scans reveal no signs of
perforation. PEECS does not usually require surgical treatment, but
can result in delayed perforation in severe cases (84). Based on analysis of relevant
domestic and international literature, the mechanism underlying
PEECS remains unclear.
Research on risk factors associated with PEECS has
gradually increased in recent years. Age, lesion diameter, combined
malignancy and lesion location have been reported as independent
risk factors for PEECS development (80). Female sex, lesion site, lesion size
and submucosal fibrosis have also been reported to be associated
with PEECS occurrence (22,85,86).
Sun et al (81) reported a
higher incidence of PEECS after colonic ESD than rectal ESD, and
that larger lesions were associated with a higher incidence of
PEECS. Notably, Yamasaki et al (87) reported that line-assisted complete
clip closure was effective in reducing PEECS incidence. This
subject warrants further study in the future.
Compared to bleeding and perforation, PEECS are less
severe and have a better prognosis, but there remains a risk of
delayed perforation. Once diagnosed, immediate treatment, such as
fasting, keeping the bowels open and intravenous administration of
broad-spectrum antibiotics, can alleviate symptoms; however, in
rare cases where conservative treatment is ineffective or the
condition worsens, the possibility of delayed perforation should be
considered. If intestinal perforation is confirmed, immediate
surgical repair is required. Li et al (84) reported that placement of anal canal
drainage and decompression after ESD reduced the incidence of
postoperative PEECS, possibly because of a reduced risk of
infection, and delayed perforation, due to reduced exposure of the
surgical wound to intestinal bacteria and fecal matter. A study in
the United States reported that prophylactic antibiotics reduced
the risk of PEECS and effectively relieved abdominal pain (88). Furthermore, prolonging mucosal
augmentation time at the lesion may reduce the risk of intestinal
wall damage caused by electrocoagulation, thus reducing the risk of
PEECS (89).
Stenosis is a less frequent complication after
colorectal ESD, generally defined as the inability of a normal
endoscope to pass successfully through the postoperative bowel
lumen. A study by Hayashi et al (90) reported that the incidence of
colorectal stenosis was 0.49% (4/822 cases) and that of
postoperative stenosis was 11% (2/18) for 90–100% circumferential
lesions. Another study (91)
reported that the stenosis rate after rectal ESD was 19.7% (12/61),
with that of stenosis after total circumferential resection 71.4%
(5/7), and that of stenosis after circumferential >90% lesion
resection 43.8% (7/16). The results of these studies suggested that
circumferential resection of large lesions is a risk factor for
postoperative stenosis. General endoscopic balloon dilatation
treatment may improve postoperative stenosis to a certain extent,
while local hydrocortisone enemas may also help to prevent
postoperative stenosis (92).
Due to its anatomical location, perforation of the
lower rectum after surgery can easily cause mediastinal or
subcutaneous emphysema, and it is challenging to confine the
infected lesion to a single location. Therefore, such lesions can
easily spread along the loose tissue to the buttocks and abdomen,
causing multiple muscle necrosis and inflammation of the
surrounding fascia. Such fulminant necrotizing fasciitis
(Fournier's syndrome) is rare clinically, and has few reports to
date. Once present, the condition can easily cause sepsis and
diffuse intravascular coagulation with serious consequences.
Fournier's syndrome is exceptionally aggressive, with reports of
mortality rates as high as 20–40%. Therefore, prompt treatment with
broad-spectrum antibiotics and surgery should be used following
detection (44).
In summary, ESD has both advantages and
disadvantages for the treatment of ECC, and reducing the occurrence
of postoperative complications is an important safeguard for the
successful implementation of colorectal ESD surgery. Therefore,
endoscopists should strictly grasp the indications for ESD surgery,
understand the relevant risk factors affecting postoperative
complications, and take immediate and effective measures to
intervene once they occur, which will effectively improve the
safety of colorectal ESD treatment. Furthermore, the clinical
application of colorectal ESD could be further expanded, so that
more patients with early-stage colorectal cancer can benefit from
ESD technology in the future.
Not applicable.
Funding for the present study was received from The Kunming
Medical Joint Special Project-Surface Project (grant no.
202101AC070802) and The Yunnan Provincial Clinical Medical Research
Center for Digestive Diseases-Research and Application of Difficult
and Critical Digestive Diseases in Yunnan Province (grant no.
202002AA100205).
Not applicable.
JS was responsible for research design,
conceptualization and writing the first draft. XX, DZ and XH were
responsible for conceptualization, organization, investigation and
research design. YL and GY were responsible for supervision,
conceptualization and revision of the manuscript. QN was
responsible for research supervision and revision of the
manuscript. Data authentication is not applicable. All authors
contributed to the article, and read and approved the final
manuscript.
The publication of the images in the figures was
approved by the Ethics Committee of The First Affiliated Hospital
of Kunming Medical University (Kunming, China).
Written informed consent for publication of the
images in the figures was obtained from the patients.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinese Society Of Clinical Oncology Csco
Diagnosis and Treatment Guidelines For Colorectal Cancer Working
Group, . Chinese society of clinical oncology (CSCO) diagnosis and
treatment guidelines for colorectal cancer 2018 (English version).
Chin J Cancer Res. 31:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng RS, Sun KX, Zhang S, Zeng HM, Zou
XN, Chen R, Gu XY, Wei WW and He J: Analysis of malignant tumor
prevalence in China in 2015. Chin J Oncol. 41:19–28. 2019.(In
Chinese).
|
|
5
|
McGill SK, Soetikno R and Kaltenbach T:
Optical diagnosis of early colorectal cancer: Riding the highs and
lows of the Japanese narrow-band imaging expert team
classification. Gastrointest Endosc. 86:710–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin JW, Han KS, Hyun JH, Lee SJ, Kim B,
Hong CW, Kim BC, Sohn DK, Chang HJ, Kim MJ, et al: Risk of
recurrence after endoscopic resection of early colorectal cancer
with positive margins. Endoscopy. 50:241–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Courtney RJ, Paul CL, Carey ML,
Sanson-Fisher RW, Macrae FA, D'Este C, Hill D, Barker D and Simmons
J: A population-based cross-sectional study of colorectal cancer
screening practices of first-degree relatives of colorectal cancer
patients. BMC Cancer. 13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao L, Shen XF and Chen LG: Analysis of
risk factors for late bleeding after transendoscopic submucosal
dissection for colorectal cancer. Zhejiang Trauma Surgery.
27:476–477. 2022.
|
|
9
|
Jiang YQ: Clinical study of endoscopic
submucosal dissection for early colorectal cancer and precancerous
lesions. South China University; 2021
|
|
10
|
Miller KD, Sauer AG, Ortiz AP, Fedewa SA,
Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A and Siegel
RL: Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J
Clin. 68:425–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C: Analysis of risk factors for late
bleeding after endoscopic mucosal resection of colorectal polyps.
China Medical University; 2021
|
|
12
|
Nishihara R, Wu K, Lochhead P, Morikawa T,
Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, et al:
Long-term colorectal-cancer incidence and mortality after lower
endoscopy. N Engl J Med. 369:1095–1105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai Y, Yang F, Ma D and Zou W: Guidelines
for early colorectal cancer screening and endoscopic management in
China (Beijing, 2014). Chin J Gastrointestinal Endoscopy.
32:341–360. 2015.
|
|
14
|
Zhou PH, Cai MY and Yao LQ: Expert
consensus on the treatment of gastrointestinal mucosal lesions by
endoscopic submucosal dissection. Chin J Gastrointestinal Surg.
1083–1086. 2012.
|
|
15
|
Wang H, Lu PX, Zhang DX, Han HC, Zhong YS
and Yao LQ: Efficacy of endoscopic treatment or surgery in patients
with early colorectal cancer and precancerous lesions. J Pract Med.
35:1639–1643. 2019.
|
|
16
|
Yao DH: Advances in the clinical
application of endoscopic submucosal dissection. Southwest Defense
Med. 20:215–217. 2010.
|
|
17
|
Li P, Wang CJ, Chen GY and Xu CQ: Chinese
consensus on screening and diagnosis and treatment of early stage
esophageal squamous cell carcinoma and precancerous lesions
(Beijing, 2015). Chin J Gastrointest Endoscopy. 33:3–18. 2016.
|
|
18
|
Expert group of the major project of
Beijing Municipal Commission of Science and Technology ‘Research on
the standardization of early gastric cancer treatment’, . Expert
consensus opinion on standardized endoscopic resection of early
gastric cancer (2018, Beijing). Chin J Gastrointestinal Endoscopy.
36:381–392. 2019.
|
|
19
|
Yamamoto Y, Kikuchi D, Nagami Y, Nonaka K,
Tsuji Y, Fujimoto A, Sanomura Y, Tanaka K, Abe S, Zhang S, et al:
Management of adverse events related to endoscopic resection of
upper gastrointestinal neoplasms: Review of the literature and
recommendations from experts. Dig Endosc. 31 (Suppl 1):S4–S20.
2019. View Article : Google Scholar
|
|
20
|
Komeda Y, Watanabe T and Kudo M:
Requirement of additional surgery after non-curative endoscopic
submucosal dissection for early colorectal cancer. J Invest Surg.
34:895–896. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izumi K, Osada T, Sakamoto N, Kodani T,
Higashihara Y, Ritsuno H, Shibuya T, Nagahara A, Ogihara T, Kikuchi
K and Watanabe S: Frequent occurrence of fever in patients who have
undergone endoscopic submucosal dissection for colorectal tumor,
but bacteremia is not a significant cause. Surg Endosc.
28:2899–2904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito S, Hotta K, Imai K, Yamaguchi Y,
Kishida Y, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M,
et al: Risk factors of post-endoscopic submucosal dissection
electrocoagulation syndrome for colorectal neoplasm. J
Gastroenterol Hepatol. 33:2001–2006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shichijo S, Takeuchi Y, Shimodate Y,
Yamashina T, Yamasaki T, Hayashi T, Hirasawa K, Fukunaga S,
Yamaguchi S, Asai S, et al: Performance of perioperative
antibiotics against post-endoscopic submucosal dissection
coagulation syndrome: A multicenter randomized controlled trial.
Gastrointest Endosc. 95:349–359. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu JF: Study of risk factors and causes of
fever after endoscopic submucosal dissection of gastrointestinal
tumors. Zhejiang University; 2016
|
|
25
|
Deng CQ and Feng L: Clinical study of
bacteremia and endotoxemia after endoscopic mucosal dissection.
Southwest Military Med. 18:514–516. 2016.
|
|
26
|
Zhang QS, Han B, Xu JH, Gao P and Shen YC:
Antimicrobial prophylaxis in patients with colorectal lesions
undergoing endoscopic resection. World J Gastroenterol.
21:4715–4721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
La Regina D, Mongelli F, Fasoli A, Lollo
G, Ceppi M, Saporito A, Garofalo F, Giuseppe MD and di Tor Vajana
AF: Clinical adverse events after endoscopic resection for
colorectal lesions: A meta-analysis on the antibiotic prophylaxis.
Dig Dis. 38:15–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Shen XJ, Yue M, Zhou SQ, Wang SW
and Zhu JY: Safety of gastric endoscopic mucosal dissection and
risk factors associated with complications in elderly patients.
Chin J Gerontol. 38:3910–3912. 2018.
|
|
29
|
Nakanishi T, Araki H, Ozawa N, Takada J,
Kubota M, Imai K, Onogi F, Ibuka T, Shiraki M, Shimizu M and
Moriwaki H: Risk factors for pyrexia after endoscopic submucosal
dissection of gastric lesions. Endosc Int Open. 2:E141–E147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou PH, Chen WF and He MJ: Standardized
endoscopic diagnosis and treatment of early gastric cancer. Chin J
Pract Surg. 34:604–607. 2014.
|
|
31
|
Zhang LL and Yang Z: Advances in the
pharmacological treatment of artificial ulcers after endoscopic
mucosal dissection. J Clin Military Med. 45:1093–1095. 2017.
|
|
32
|
Cha JM, Lim KS, Lee SH, Joo YE, Hong SP,
Kim TI, Kim HG, Park DI, Kim SE, Yang DH and Shin JE: Clinical
outcomes and risk factors of post-polypectomy coagulation syndrome:
A multicenter, retrospective, case-control study. Endoscopy.
45:202–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harada H, Suehiro S, Murakami D, Nakahara
R, Ujihara T, Shimizu T, Miyama Y, Katsuyama Y, Hayasaka K and
Tounou S: Clinical impact of prophylactic clip closure of mucosal
defects after colorectal endoscopic submucosal dissection. Endosc
Int Open. 5:E1165–E1171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osada T, Sakamoto N, Ritsuno H, Murakami
T, Ueyama H, Matsumoto K, Shibuya T, Ogihara T and Watanabe S:
Closure with clips to accelerate healing of mucosal defects caused
by colorectal endoscopic submucosal dissection. Surg Endosc.
30:4438–4444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang R, Zhang LH, Zhang RC, Luo H, Wang
XP, Tao Q, et al: Analysis of risk factors associated with fever
after endoscopic submucosal dissection. Chin J Gastrointest Endos.
31:72–75. 2014.
|
|
36
|
Suzuki H, Oda I, Sekiguchi M, Abe S,
Nonaka S, Yoshinaga S, Nakajima T and Saito Y: Management and
associated factors of delayed perforation after gastric endoscopic
submucosal dissection. World J Gastroenterol. 21:12635–12643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuda Y, Kataoka N, Yamaguchi T, Tomita
M, Sakamoto K and Makimoto S: Delayed esophageal perforation
occurring with endoscopic submucosal dissection: A report of two
cases. World J Gastrointest Surg. 7:123–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li P, Wang YJ, Chen GY and Xu CQ: Chinese
consensus on screening and diagnosis and treatment of early stage
esophageal squamous cell carcinoma and precancerous lesions
(2015-Beijing). Chin J Pract Intern Med. 36:20–33. 2016.
|
|
39
|
Yang F, Ma D and Zou DW: Expert
recommendations on perioperative medication for endoscopic
submucosal dissection of gastric mucosal lesions (2015, Suzhou).
Chin J Intern Med. 54:905–908. 2015.
|
|
40
|
Zhou PH, Zhong YS, Li QL and Qi ZP: Expert
consensus on endoscopic diagnosis and treatment of gastrointestinal
submucosal tumors in China (2018 edition). Chin J Pract Surg.
38:840–850. 2018.
|
|
41
|
Ren PY, Yin YQ and Gao YH: Progress in the
study of complications of colorectal endoscopic submucosal
dissection. J Gastroenterol Hepatol. 30:341–345. 2021.
|
|
42
|
Xu K, Jin HL, Ding X and Tong C: The value
and safety of endoscopic submucosal dissection for early colorectal
cancer. Application value and safety assessment of endoscopic
submucosal dissection for early colorectal cancer. Chin J Endoscop.
24:17–22. 2018.
|
|
43
|
Tanaka S, Kashida H, Saito Y, Yahagi N,
Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, et al:
Japan gastroenterological endoscopy society guidelines for
colorectal endoscopic submucosal dissection/endoscopic mucosal
resection. Dig Endosc. 32:219–239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanaka S, Kashida H, Saito Y, Yahagi N,
Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, et al:
JGES guidelines for colorectal endoscopic submucosal
dissection/endoscopic mucosal resection. Dig Endosc. 27:417–434.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim DH and Lim SW: Analysis of delayed
postpolypectomy bleeding in a colorectal clinic. J Korean Soc
Coloproctol. 27:13–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen J and Yu HG: Clinical analysis of
endoscopic submucosal dissection for early colorectal cancer and
its precancerous lesions. Chin J Gastrointest Endoscopy.
33:151–154. 2016.
|
|
47
|
Arimoto J, Higurashi T, Chiba H, Misawa N,
Yoshihara T, Kato T, Kanoshima K, Fuyuki A, Ohkubo H, Goto S, et
al: Continued use of a single antiplatelet agent does not increase
the risk of delayed bleeding after colorectal endoscopic submucosal
dissection. Dig Dis Sci. 63:218–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Milano RV, Viale E, Bartel MJ,
Notaristefano C and Testoni PA: Resection outcomes and recurrence
rates of endoscopic submucosal dissection (ESD) and hybrid ESD for
colorectal tumors in a single Italian center. Surg Endosc.
32:2328–2339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tanaka S, Terasaki M, Hayashi N, Oka S and
Chayama K: Warning for unprincipled colorectal endoscopic
submucosal dissection: Accurate diagnosis and reasonable treatment
strategy. Dig Endosc. 25:107–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Yu HG, Li SQ and Zhu XY: Analysis of
risk factors for bleeding after colorectal endoscopic submucosal
dissection. Med Rev. 25:1026–1029+1035. 2019.
|
|
51
|
Akintoye E, Kumar N, Aihara H, Nas H and
Thompson CC: Colorectal endoscopic submucosal dissection: A
systematic review and meta-analysis. Endosc Int Open.
4:E1030–E1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamamoto K, Shimoda R, Ogata S, Hara M,
Ito Y, Tominaga N, Nakayama A, Sakata Y, Tsuruoka N, Iwakiri R and
Fujimoto K: Perforation and postoperative bleeding associated with
endoscopic submucosal dissection in colorectal tumors: An analysis
of 398 lesions treated in Saga, Japan. Intern Med. 57:2115–2122.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Suzuki S, Chino A, Kishihara T, Uragami N,
Tamegai Y, Suganuma T, Fujisaki J, Matsuura M, Itoi T, Gotoda T, et
al: Risk factors for bleeding after endoscopic submucosal
dissection of colorectal neoplasms. World J Gastroenterol.
20:1839–1845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chiba H, Ohata K, Tachikawa J, Arimoto J,
Ashikari K, Kuwabara H, Nakaoka M, Goto T and Nakajima A: Delayed
bleeding after colorectal endoscopic submucosal dissection: When is
emergency colonoscopy needed? Dig Dis Sci. 64:880–887. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Youk EG, Sohn DK, Hong CW, Lee SD, Han KS,
Kim BC, Chang HJ and Kim MJ: Early outcomes of endoscopic
submucosal dissection for colorectal neoplasms according to
clinical indications. Dis Colon Rectum. 59:403–410. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Harada H, Nakahara R, Murakami D, Suehiro
S, Nagasaka T, Ujihara T, Sagami R, Katsuyama Y, Hayasaka K, Tounou
S and Amano Y: The effect of anticoagulants on delayed bleeding
after colorectal endoscopic submucosal dissection. Surg Endosc.
34:3330–3337. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li R, Cai S, Sun D, Shi Q, Ren Z, Qi Z, Li
B, Yao L, Xu M, Zhou P and Zhong Y: Risk factors for delayed
bleeding after endoscopic submucosal dissection of colorectal
tumors. Surg Endosc. 35:6583–6590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jaruvongvanich V, Prasitlumkum N,
Assavapongpaiboon B, Suchartlikitwong S, Sanguankeo A and Upala S:
Risk factors for delayed colonic post-polypectomy bleeding: A
systematic review and meta-analysis. Int J Colorectal Dis.
32:1399–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ogasawara N, Yoshimine T, Noda H, Kondo Y,
Izawa S, Shinmura T, Ebi M, Funaki Y, Sasaki M and Kasugai K:
Clinical risk factors for delayed bleeding after endoscopic
submucosal dissection for colorectal tumors in Japanese patients.
Eur J Gastroenterol Hepatol. 28:1407–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Terasaki M, Tanaka S, Shigita K, Asayama
N, Nishiyama S, Hayashi N, Nakadoi K, Oka S and Chayama K: Risk
factors for delayed bleeding after endoscopic submucosal dissection
for colorectal neoplasms. Int J Colorectal Dis. 29:877–882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Seo M, Song EM, Cho JW, Lee YJ, Lee BI,
Kim JS, Jeon SW, Jang HJ, Yang DH, Ye BD and Byeon JS: A
risk-scoring model for the prediction of delayed bleeding after
colorectal endoscopic submucosal dissection. Gastrointest Endosc.
89:990–998.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xu YQ, Lin ZR, Zhong SS, Lin XL and Wei L:
Meta-analysis of risk factors for late bleeding after endoscopic
resection of colorectal masses. Chin Elect J Colorectal Dis.
9:377–386. 2020.
|
|
63
|
Wang T, Wang DN, Liu WT, Zheng ZQ, Chen X,
Fang WL, Li S, Liang L and Wang BM: Hemostatic effect of topical
hemocoagulase spray in digestive endoscopy. World J Gastroenterol.
22:5831–5836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ogiyama H, Tsutsui S, Murayama Y, Maeda S,
Satake S, Nasu A, Umeda D, Miura Y, Tominaga K, Horiki M, et al:
Prophylactic clip closure may reduce the risk of delayed bleeding
after colorectal endoscopic submucosal dissection. Endosc Int Open.
6:E582–E588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang XY, Ji M, Li P, Niu YL, Meng FD, Yu
L, Wang YJ, Zhao HY, Lv FJ, Zhai HH and Zhang ST: New traction
method (traction ring) for colorectal endoscopic submucosal
dissection. J Dig Dis. 23:318–323. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
ASGE Technology Committee, . Maple JT,
Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Konda V,
Murad FM, Siddiqui UD and Banerjee S: Endoscopic submucosal
dissection. Gastrointest Endosc. 81:1311–1325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Imai K, Hotta K, Ito S, Yamaguchi Y,
Kishida Y, Yabuuchi Y, Yoshida M, Kawata N, Tanaka M, Kakushima N,
et al: A risk-prediction model for en bloc resection failure or
perforation during endoscopic submucosal dissection of colorectal
neoplasms. Dig Endosc. 32:932–939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Du CQ, Yuan WZ, Zhao MW, Jia W, Mi J, Yuan
YD, et al: Efficacy and safety analysis of endoscopic mucosal
dissection for colorectal intramucosal cancer and precancerous
lesions. Chin J Endoscopy. 25:73–77. 2019.
|
|
69
|
Nakajima T, Saito Y, Tanaka S, Iishi H,
Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, et
al: Current status of endoscopic resection strategy for large,
early colorectal neoplasia in Japan. Surg Endosc. 27:3262–3270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Iwatsubo T, Takeuchi Y, Yamasaki Y,
Nakagawa K, Arao M, Ohmori M, Iwagami H, Matsuno K, Inoue S,
Nakahira H, et al: Differences in clinical course of
intraprocedural and delayed perforation caused by endoscopic
submucosal dissection for colorectal neoplasms: A retrospective
study. Dig Dis. 37:53–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Toyonaga T, Man-I M, East JE, Nishino E,
Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, et al:
1,635 Endoscopic submucosal dissection cases in the esophagus,
stomach, and colorectum: Complication rates and long-term outcomes.
Surg Endosc. 27:1000–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hirasawa K, Sato C, Makazu M, Kaneko H,
Kobayashi R, Kokawa A and Maeda S: Coagulation syndrome: Delayed
perforation after colorectal endoscopic treatments. World J
Gastrointest Endosc. 7:1055–1061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Su H, Wang HH, Liu L, Cheng T, He YQ and
Jin P: Comparative analysis of endoscopic submucosal dissection of
early colorectal cancer and precancerous lesions of different
diameters. Chin J Gastrointestinal Endoscopy. 339–343. 2019.
|
|
74
|
Hong SN, Byeon JS, Lee BI, Yang DH, Kim J,
Cho KB, Cho JW, Jang HJ, Jeon SW, Jung SA and Chang DK: Prediction
model and risk score for perforation in patients undergoing
colorectal endoscopic submucosal dissection. Gastrointest Endosc.
84:98–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mizushima T, Kato M, Iwanaga I, Sato F,
Kubo K, Ehira N, Uebayashi M, Ono S, Nakagawa M, Mabe K, et al:
Technical difficulty according to location, and risk factors for
perforation, in endoscopic submucosal dissection of colorectal
tumors. Surg Endosc. 29:133–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kawashima K, Hikichi T, Fujiwara T, Gunji
N, Nakamura J, Watanabe K, Katakura K and Ohira H: Delayed
perforation after endoscopic submucosal dissection for mucosal
colon cancer: A conservatively treated case. Fukushima J Med Sci.
64:157–162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kang DU, Choi Y, Lee HS, Lee HJ, Park SH,
Yang DH, Yoon SM, Kim KJ, Ye BD, Myung SJ, et al: Endoscopic and
clinical factors affecting the prognosis of colorectal endoscopic
submucosal dissection-related perforation. Gut Liver. 10:420–428.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang QE, Zhuang C, Zhang Y, Chen JP and Xu
F: Clinical application of single-clamp endoscopic purse-string
suturing in intestinal perforation. Chin J Gastrointestinal
Endoscopy. 601–603. 2019.
|
|
79
|
Kim ER and Chang DK: Management of
complications of colorectal submucosal dissection. Clin Endosc.
52:114–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang MZ, Tan SY, Luo HS, Li M, Wu PB, Guo
F and Shu Y: Risk factors for electrocoagulation syndrome after
endoscopic submucosal dissection of colorectal lesions. Chinese J
Gen Practitioners. 15:698–6701. 2016.
|
|
81
|
Sun D, Qi ZP, Zhong YS, Shi Q, Cai SL and
Li B: Clinical features and risk factors of electrocoagulation
syndrome after colorectal endoscopic submucosal dissection. Chin J
Pract Surg. 38:662–665. 2018.
|
|
82
|
Arimoto J, Higurashi T, Kato S, Fuyuki A,
Ohkubo H, Nonaka T, Yamaguchi Y, Ashikari K, Chiba H, Goto S, et
al: Risk factors for post-colorectal endoscopic submucosal
dissection (ESD) coagulation syndrome: A multicenter, prospective,
observational study. Endosc Int Open. 6:E342–E349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hong MJ, Kim JH, Lee SY, Sung IK, Park HS
and Shim CS: Prevalence and clinical features of coagulation
syndrome after endoscopic submucosal dissection for colorectal
neoplasms. Dig Dis Sci. 60:211–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li B, Zhou PH, Yao LQ, Xu MD, Ren Z and
Shi QQ: Analysis of the efficacy of endoscopic submucosal
dissection for postoperative anal canal drainage and decompression
of colorectal mucosal lesions. Chin J Pract Surg. 37:802–805.
2017.
|
|
85
|
Yamashina T, Takeuchi Y, Uedo N, Hamada K,
Aoi K, Yamasaki Y, Matsuura N, Kanesaka T, Akasaka T, Yamamoto S,
et al: Features of electrocoagulation syndrome after endoscopic
submucosal dissection for colorectal neoplasm. J Gastroenterol
Hepatol. 31:615–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jung D, Youn YH, Jahng J, Kim JH and Park
H: Risk of electrocoagulation syndrome after endoscopic submucosal
dissection in the colon and rectum. Endoscopy. 45:714–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yamasaki Y, Takeuchi Y, Kato M, Uedo N and
Ishihara R: Line-assisted complete closure of large gastric mucosal
defects by use of multiple clip-and-line technique. VideoGIE.
1:49–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee SP, Sung IK, Kim JH, Lee SY, Park HS,
Shim CS and Ki HK: A randomized controlled trial of prophylactic
antibiotics in the prevention of electrocoagulation syndrome after
colorectal endoscopic submucosal dissection. Gastrointest Endosc.
86:349–357.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yamasaki Y, Takeuchi Y, Iwatsubo T, Kato
M, Hamada K, Tonai Y, Matsuura N, Kanesaka T, Yamashina T, Arao M,
et al: Line-assisted complete closure for a large mucosal defect
after colorectal endoscopic submucosal dissection decreased
post-electrocoagulation syndrome. Dig Endosc. 30:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hayashi T, Kudo SE, Miyachi H, Sakurai T,
Ishigaki T, Yagawa Y, Toyoshima N, Mori Y, Misawa M, Kudo T, et al:
Management and risk factor of stenosis after endoscopic submucosal
dissection for colorectal neoplasms. Gastrointest Endosc.
86:358–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ohara Y, Toyonaga T, Tanaka S, Ishida T,
Hoshi N, Yoshizaki T, Kawara F, Lui KL, Tepmalai K, Damrongmanee A,
et al: Risk of stricture after endoscopic submucosal dissection for
large rectal neoplasms. Endoscopy. 48:62–70. 2016.PubMed/NCBI
|
|
92
|
Ma MX and Bourke MJ: Complications of
endoscopic polypectomy, endoscopic mucosal resection and endoscopic
submucosal dissection in the colon. Best Pract Res Clin
Gastroenterol. 30:749–767. 2016. View Article : Google Scholar : PubMed/NCBI
|