Introduction
Immune checkpoint inhibitors (ICIs) have
revolutionized treatment strategies for multiple cancer types, such
as lung cancer (1–4), head and neck squamous cell carcinoma
(5,6), esophageal cancer (7) and metastatic renal cell cancer
(8). Available predictive
immunotherapy biomarkers for treatment responses include programmed
death-ligand 1 (PD-L1) expression, tumor mutation burden (TMB) and
microsatellite instability-high/deficient mismatch repair (dMMR).
Studies have shown that nivolumab, pembrolizumab and atezolizumab
are recommended for the second-line treatment of patients with
non-small cell lung cancer (NSCLC) and PD-L1 expression ≥1% (tumor
proportion score) (9–11), but that the objective response rate
(ORR) is <21.2%. Pembrolizumab is recommended as a second-line
treatment for patients with dMMR/TMB-high gastric cancer, with an
ORR of ~46.7%. However, no additional markers are available to
predict prognosis, and the positive rate of the aforementioned
markers is low (12). In addition,
immunotherapy is less effective in patients who have not been
tested for immunotherapy biomarkers. The clinical application of
PD-L1 and genetic testing are limited by unusable specimens and
high cost. No other reliable biomarker for effectively selecting
responsive patients has been identified to date, especially
effective markers for pan-cancer survival. Identifying new,
reliable and clinically accessible biomarkers for patients with
cancer treated with ICIs as second-line therapy is essential for an
improved response.
It has been recognized that hypercoagulability is
relevant to the poor prognosis of patients with cancer (13). Fibrinogen (FIB) is an important
member of the coagulation system, and also plays an important role
in the inflammatory response and tumor progression (14,15).
Several studies have shown that elevated pretreatment or
preoperative FIB levels are associated with poor outcomes in
numerous types of cancer, such as breast, lung, colorectal and
gastric cancer (16–19). The cut-off values of FIB in these
studies were determined to be 2.83, 4.0, 3.64 and 4.0 g/l,
respectively. However, only a few studies have indicated the
relationship between FIB and prognosis in patients with cancer
treated with ICIs. Some studies have applied the combination of FIB
and other clinical factors, such as the FIB-albumin ratio (FAR),
for predicting immunotherapy prognosis and obtained positive
outcomes (20,21), but the results of the different
studies were not consistent. Yuan et al (20) showed that an increased FAR (≥0.145)
was an independent prognostic factor of progression-free survival
(PFS) and overall survival (OS) for patients with NSCLC treated
with ICIs as first-line therapy, but Guo and Liang (21) showed that FAR could not be an
independent prognostic factor of OS for patients with cancer,
indicating that FAR was not an accurate predictor of OS/DFS. In
addition, the prognostic value of FIB and albumin were not analyzed
individually in either of the two aforementioned research
studies.
Nevertheless, the association between FIB and its
prognostic role in patients with cancer treated with immunotherapy
remains unknown. The aim of the present retrospective clinical
study was to investigate the association between FIB and the
prognosis of patients with cancer treated with ICIs as second-line
therapy.
Materials and methods
Patients
From February 2015 to February 2022, a total of 61
patients with various types of stage III–IV malignant tumors
(according to the 8th edition of the American Joint Committee on
Cancer Staging) (22) treated with
ICIs as a second-line treatment in Hainan Hospital of the Chinese
People's Liberation Army (PLA) General Hospital (Sanya, China) were
studied retrospectively. Among them, 2 patients had received
systemic therapy that included ICIs as first-line treatment. The
inclusion criteria for the patients were as follows: i) Age >18
years; ii) stage IV or unresectable stage III malignancy confirmed
by histology and imaging; iii) available laboratory assays before
and after immunotherapy; and iv) anti-programmed cell death protein
1 (PD-1) monotherapy or combination therapy with chemotherapy or
targeted therapies as second-line treatment. Patients meeting any
of the following criteria were excluded: i) Other malignant tumors;
ii) chronic inflammatory diseases; iii) current treatment with
glucocorticoids; iv) acute infection; v) vein thrombosis; and vi)
disseminated intravascular coagulation or treatment with
anticoagulant or procoagulant drugs within 1 month of second-line
treatment.
Clinicopathological parameters of the patients
included sex, age (<60 or ≥60 years old), smoking history,
Eastern Cooperative Oncology Group performance status (23), pathological histology, surgical
history, number of metastatic sites, PD-L1 testing results and the
administration of locoregional therapy (radiotherapy or
interventional therapy) during second-line therapy. The study was
approved by the Ethics Committee of Hainan Hospital of the Chinese
PLA General Hospital (approval no. 301HLFYLS15). Written informed
consent was waived by the committee due to the retrospective nature
of the study.
Determination of pretreatment FIB
levels
The baseline coagulation (normal FIB range,
2.38-4.98 g/l) of the patients was assessed before the second-line
treatment (on the day before receiving immunotherapy or within 7
days before the start of immunotherapy). The samples (5 ml venous
blood) were collected in tubes with sodium citrate in and were
processed in the hospital laboratory within 6 h to detect FIB.
Follow-up procedure and definition of
response
Patient information was obtained through electronic
medical records or by telephone. Imaging review was performed every
6–8 weeks to assess the response to treatment. The evaluation
criteria were based on those outlined in The Response Evaluation
Criteria in Solid Tumors (version 1.1) (24). The ORR included complete response
(CR) and partial response (PR). Disease control rate (DCR) included
CR, PR and stable disease (SD). PFS time was defined as the time
from the beginning of second-line treatment to disease progression,
death or last follow-up. OS time was calculated as the time from
initial diagnosis to death or censoring. OS2 time was defined as
the time from the beginning of second-line treatment to death or
last follow-up. The last follow-up date was March 1, 2022, and the
median follow-up time (from the beginning of second-line treatment)
was 17 months (range, 2–51 months).
Statistical analysis
Statistical analyses were performed using SPSS
(version 21.0; IBM Corp.). Receiver operating characteristic curve
analysis was used to determine the optimal cut-off value for FIB.
The relationship between pretreatment FIB and other
clinicopathological parameters was calculated using the
χ2 test or Fisher's exact test when appropriate.
Kaplan-Meier plots show PFS and OS survival curves, and the
log-rank test was used to compare survival outcomes of patients
with cancer separated by FIB. Univariate and multivariate analyses
were conducted using Cox's regression test. All P-values were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline patient characteristics and
the treatment response
The clinicopathological characteristics of the 61
patients with cancer included in this study are listed in Table I. In total, 15 female and 46 male
patients were included. The mean age of the patients was 58.54
years old (range, 25–79 years old). The most common tumor types
were lung, head and neck, and esophageal cancer (Table II). The ICIs included atezolizumab,
durvalumab, camrelizumab, pembrolizumab, toripalimab, tislelizumab
and sintilimab (Table III).
 | Table I.Differences in pretreatment FIB among
different clinicopathological parameters in 61 patients. |
Table I.
Differences in pretreatment FIB among
different clinicopathological parameters in 61 patients.
|
|
| Pretreatment FIB
level, n |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients, n
(%) | Low | High | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 15 (24.59) | 6 | 9 | 0.93 |
|
Male | 46 (75.41) | 19 | 27 |
|
| Age, years |
|
|
|
|
|
<60 | 31 (50.82) | 17 | 14 | 0.03a |
|
≥60 | 30 (49.18) | 8 | 22 |
|
| Smoking
history |
|
|
|
|
|
Yes | 29 (47.54) | 12 | 17 | 0.95 |
| No | 32 (52.46) | 13 | 19 |
|
| ECOG score |
|
|
|
|
|
0-1 | 54 (88.52) | 22 | 32 | >0.99 |
| ≥2 | 7 (11.48) | 3 | 4 |
|
| Surgical
history |
|
|
|
|
|
Yes | 31 (50.82) | 11 | 20 | 0.38 |
| No | 30 (49.18) | 14 | 16 |
|
| Metastatic sites,
n |
|
|
|
|
|
<2 | 25 (40.98) | 11 | 14 | 0.69 |
| ≥2 | 36 (59.02) | 14 | 22 |
|
| Locoregional
therapy |
|
|
|
|
|
Yes | 9 (14.75) | 5 | 4 | 0.55 |
| No | 52 (85.25) | 20 | 32 |
|
| PD-L1
expression |
|
|
|
|
|
Positive | 7 (11.48) | 1 | 6 | 0.16 |
|
Negative | 2 (3.28) | 0 | 2 |
|
|
Missing | 52 (85.25) | 24 | 28 |
|
| Pretreatment FIB,
g/lb |
| 2.84±0.44 | 4.63±1.06 |
|
 | Table II.Tumor types among the entire patient
cohort (n=61). |
Table II.
Tumor types among the entire patient
cohort (n=61).
| Tumor types | Patients, n
(%) |
|---|
| Lung cancer | 16 (26.23) |
| Head and neck
cancer | 12 (19.67) |
| Esophageal
cancer | 6 (9.84) |
| Gastric cancer | 5 (8.20) |
| Hepatocellular
carcinoma | 5 (8.20) |
| Biliary tract
carcinoma | 5 (8.20) |
| Urinary system
carcinoma | 5 (8.20) |
| Gynecological
carcinoma | 4 (6.56) |
| Others | 3 (4.92) |
 | Table III.Application of immune checkpoints in
the cohort (n=61). |
Table III.
Application of immune checkpoints in
the cohort (n=61).
| PD-1/PD-L1
inhibitor | Patients, n
(%) |
|---|
| Atezolizumab | 2 (3.28) |
| Durvalumab | 1 (1.64) |
| Camrelizumab | 8 (13.11) |
| Tislelizumab | 8 (13.11) |
| Sintilimab | 2 (3.28) |
| Pembrolizumab | 21 (34.43) |
| Toripalimab | 19 (31.15) |
The treatment response was as follows (Table IV): CR, 0 (0%); PR, 16 (26.23%);
SD, 23 (37.70%); and PD, 22 (36.07%). The median OS and PFS times
were 36.42 months (95% CI, 28.81–44.02) and 6.29 months (95% CI,
5.04–7.54), respectively.
 | Table IV.Short-term efficacy in the low
pretreatment (n=52) and high pretreatment (n=9) groups of
patients. |
Table IV.
Short-term efficacy in the low
pretreatment (n=52) and high pretreatment (n=9) groups of
patients.
|
| Pretreatment
fibrinogen level |
|---|
|
|
|
|---|
| Response | <3.47 g/l | ≥3.47 g/l |
|---|
| CR, n | 0 | 0 |
| PR, n | 9 | 7 |
| SD, n | 9 | 14 |
| PD, n | 7 | 15 |
| ORR, n (%) | 9 (36.00) | 7 (19.44) |
| DCR, n (%) | 18 (72.00) | 21 (58.33) |
Predictive value of FIB for PFS and
OS
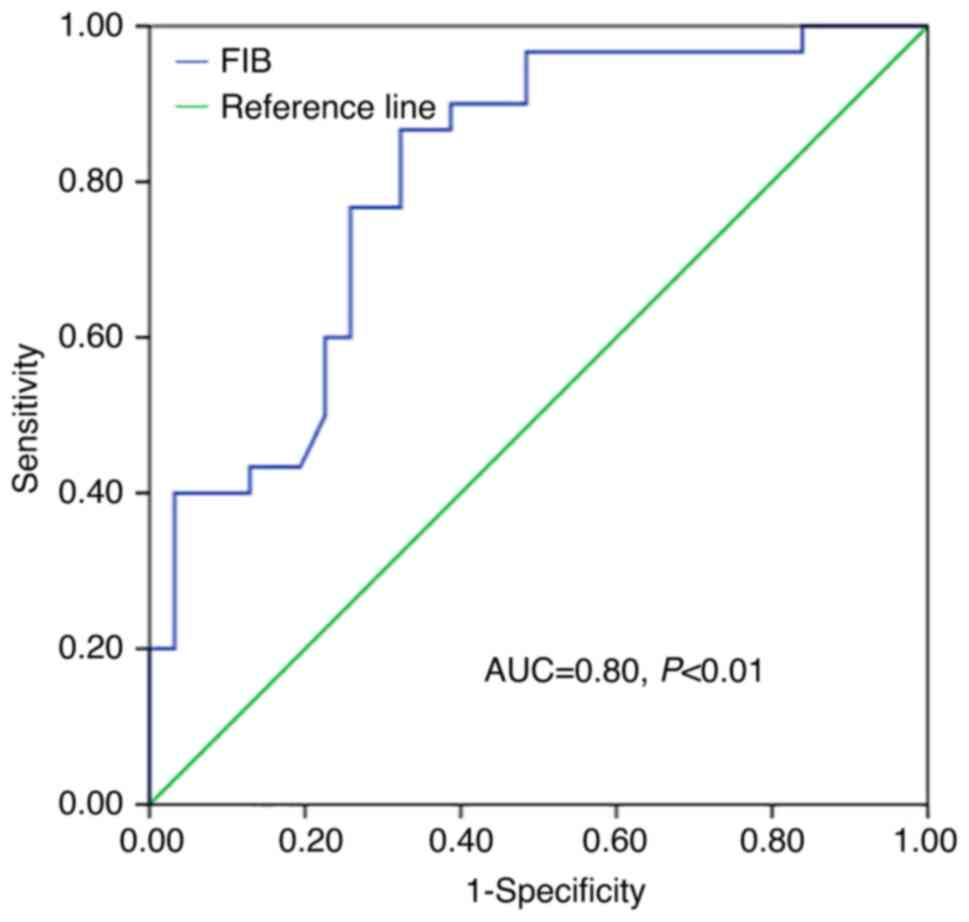
The predictive pretreatment FIB cut-off value for OS
was 3.47 (area under the curve, 0.80; sensitivity, 0.87;
specificity, 0.68; Fig. 1).
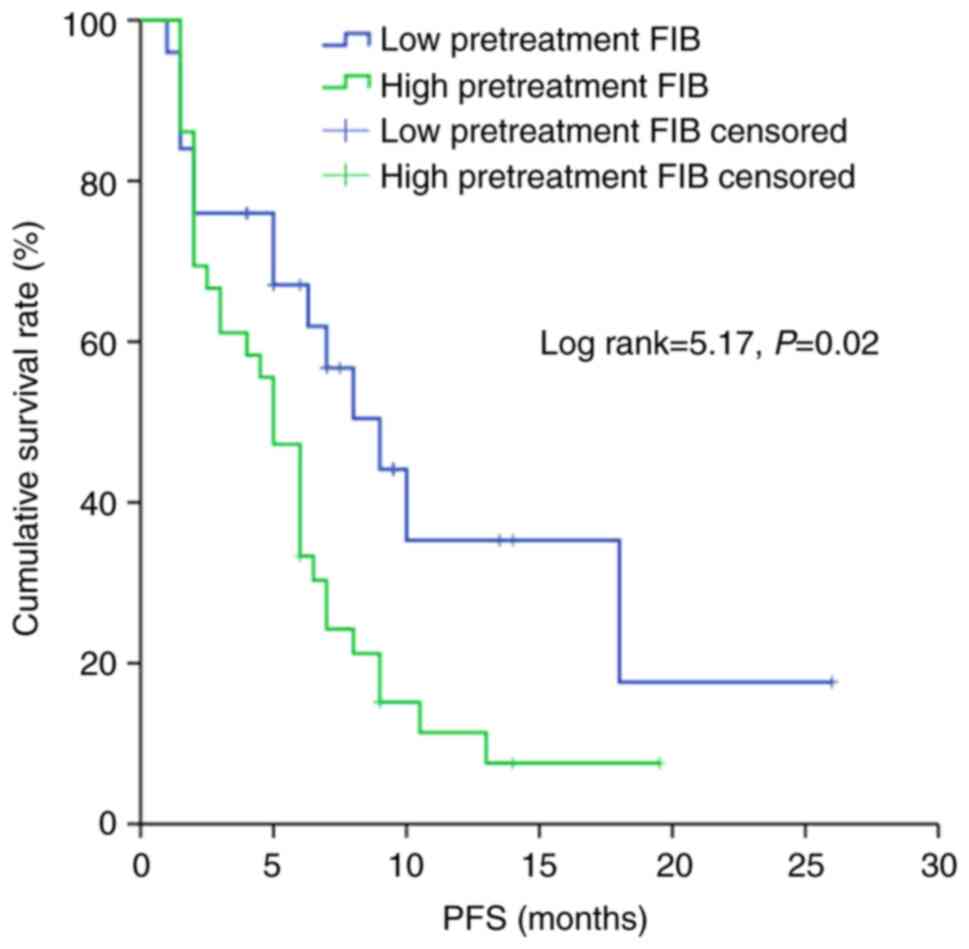
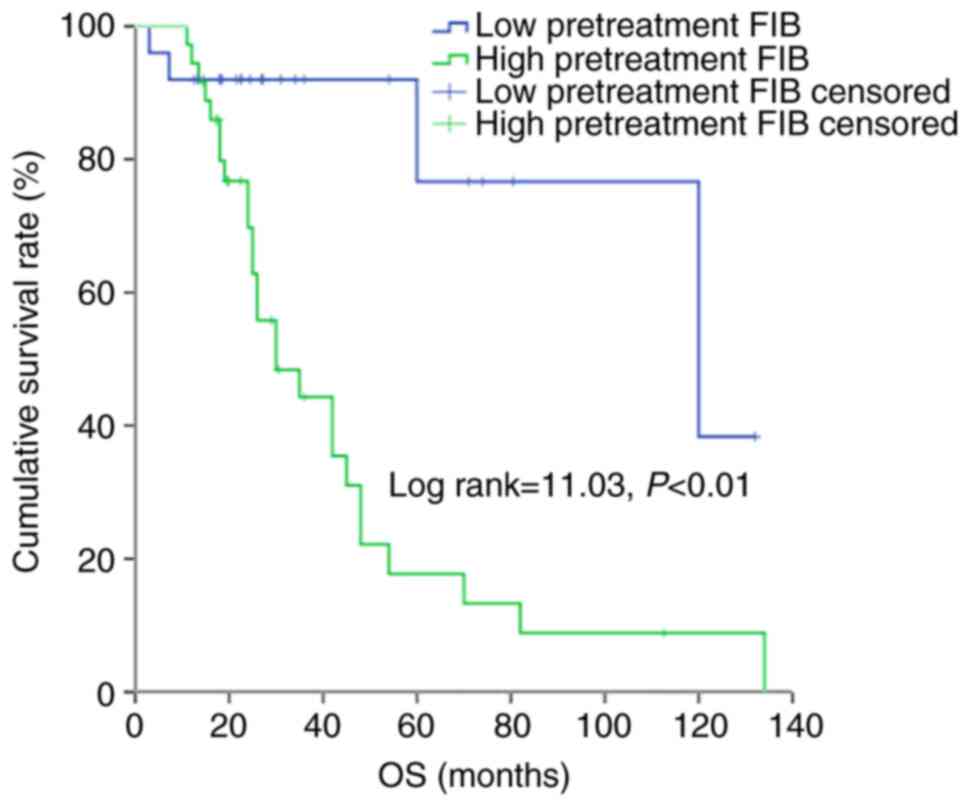
According to the Kaplan-Meier analysis based on a cut-off point of
3.47 g/l, patients with a low pretreatment FIB exhibited
significantly higher PFS and OS times compared with those with a
high pretreatment FIB (P<0.05 and P<0.01, respectively)
(Figs. 2 and 3).
Univariate and multivariate analyses
for PFS, OS and OS2
According to univariate analysis, high pretreatment
FIB levels and high FAR were associated with shorter PFS (P=0.03
and P<0.01, respectively) (Table
V). Multivariable analysis showed that in contrast to FAR
(P=0.02), the pretreatment FIB levels were not an independent
predictor of PFS. Male sex and high pretreatment FIB levels were
associated with shorter OS time and were also found to be
independent prognostic factors of OS (P=0.01 and P=0.04,
respectively) (Table VI). Only the
pretreatment FIB level was an independent predictor of OS2 (P=0.02)
(Table VII). According to the
hazard ratios obtained, a lower FIB level was a protective factor
for PFS, OS and OS2.
 | Table V.Progression-free survival
analysis. |
Table V.
Progression-free survival
analysis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | Reference |
|
|
|
|
|
|
Female | 1.07 | 0.55-2.08 | 0.84 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
<60 | Reference |
|
|
|
|
|
|
≥60 | 0.97 | 0.54-1.74 | 0.92 |
|
|
|
| Smoking
history |
|
|
|
|
|
|
|
Yes | 1.20 | 0.67-2.15 | 0.54 |
|
|
|
| No | Reference |
|
|
|
|
|
| Surgery
history |
|
|
|
|
|
|
|
Yes | 0.79 | 0.44-1.42 | 0.43 |
|
|
|
| No | Reference |
|
|
|
|
|
| Metastatic
sites |
|
|
|
|
|
|
|
<2 | Reference |
|
|
|
|
|
| ≥2 | 1.24 | 0.68-2.25 | 0.49 |
|
|
|
| Pretreatment
fibrinogen, g/l |
|
|
|
|
|
|
|
<3.47 | Reference |
|
|
|
|
|
|
≥3.47 | 1.99 | 1.06-3.74 | 0.03a |
|
|
|
| FAR |
|
|
|
|
|
|
|
<0.09 | Reference |
|
|
|
|
|
|
≥0.09 | 3.15 | 1.58-6.31 |
<0.01a | 3.48 | 1.22-9.91 | 0.02a |
 | Table VI.Overall survival analysis. |
Table VI.
Overall survival analysis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | Reference |
|
|
|
|
|
|
Female | 0.34 | 0.13-0.93 | 0.03a | 0.24 | 0.08-0.72 | 0.01a |
| Age, years |
|
|
|
|
|
|
|
<60 | Reference |
|
|
|
|
|
|
≥60 | 1.74 | 0.80-3.79 | 0.16 |
|
|
|
| Smoking
history |
|
|
|
|
|
|
|
Yes | 1.44 | 0.66-3.15 | 0.36 |
|
|
|
| No | Reference |
|
|
|
|
|
| Surgery
history |
|
|
|
|
|
|
|
Yes | 0.66 | 0.31-1.41 | 0.28 |
|
|
|
| No | Reference |
|
|
|
|
|
| Metastatic
sites |
|
|
|
|
|
|
|
<2 | Reference |
|
|
|
|
|
| ≥2 | 1.79 | 0.83-3.88 | 0.14 |
|
|
|
| Pretreatment
fibrinogen, g/l |
|
|
|
|
|
|
|
<3.47 | Reference |
|
|
|
|
|
|
≥3.47 | 5.02 | 1.73-14.53 |
<0.01a | 4.84 | 1.09-21.5 | 0.04a |
| FAR |
|
|
|
|
|
|
|
<0.09 | Reference |
|
|
|
|
|
|
≥0.09 | 3.23 | 1.23-8.52 | 0.02a |
|
|
|
 | Table VII.Overall survival2 analysis. |
Table VII.
Overall survival2 analysis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | Reference |
|
|
|
|
|
|
Female | 0.63 | 0.27-1.50 | 0.30 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
<60 | Reference |
|
|
|
|
|
|
≥60 | 1.92 | 0.90-4.12 | 0.09 |
|
|
|
| Smoking
history |
|
|
|
|
|
|
|
Yes | 0.90 | 0.43-1.88 | 0.77 |
|
|
|
| No | Reference |
|
|
|
|
|
| Surgery
history |
|
|
|
|
|
|
|
Yes | 0.94 | 0.45-1.97 | 0.87 |
|
|
|
| No | Reference |
|
|
|
|
|
| Metastatic
sites |
|
|
|
|
|
|
|
<2 | Reference |
|
|
|
|
|
| ≥2 | 1.31 | 0.61-2.82 | 0.48 |
|
|
|
| Pretreatment
fibrinogen, g/l |
|
|
|
|
|
|
|
<3.47 | Reference |
|
|
|
|
|
|
≥3.47 | 4.06 | 1.41-11.67 |
0.01a | 3.69 | 1.28-10.63 |
0.02a |
| FAR |
|
|
|
|
|
|
|
<0.09 | Reference |
|
|
|
|
|
|
≥0.09 | 3.27 | 1.25-8.55 |
0.02a |
|
|
|
Discussion
In this study, it was shown that low pretreatment
FIB levels predicted longer PFS and OS times than high pretreatment
FIB levels for patients with cancer treated with ICIs as
second-line treatment. The ORR and DCR of the low pretreatment FIB
group were better than those of the high pretreatment FIB group.
Multivariate analysis demonstrated that FIB was independently
associated with OS and OS2.
Pretreatment FIB has been reported to play a notable
prognostic role in numerous types of cancer. However, only a few
studies have shown the relationship between FIB and immunotherapy.
Shen et al (25) conducted a
study on 57 patients with unresectable hepatocellular carcinoma who
were treated with lenvatinib and ICI, and showed that high FIB was
significantly associated with poor survival (P=0.024), and the
cut-off value of FIB was 2.83 g/l. Nenclares et al (26) showed that on-treatment FIB level
(day 28) was a reliable biomarker to predict both disease
progression and mortality for 100 patients with HNSCC treated with
immunotherapy (P=0.008). Among them, 55 enrolled patients were
treated with ICIs as first-line treatment, and 36 patients were
treated with second-line therapy. The cut-off value for
on-treatment FIB levels was 4 g/l. The outcome of the current study
was consistent with these studies, with the exception of the
cut-off levels reported, indicating that there are still
limitations that need to be explored in depth in the future.
Previous studies indicated that FIB levels were associated with
age, and that the FIB level increased with increasing age (18,27),
which was consistent with the findings of the present study. Other
relevant indicators for FIB include tumor differentiation, tumor
location, pathological tumor (pT) category, pathological nodal (pN)
status and Tumor-Node-Metastasis stage (18,25,26).
An appropriate predictive value of FIB in clinical practice may
need to be selected in combination with other indicators for a
comprehensive analysis. The results of FAR in the present study
showed that it was an independent prognostic factor of PFS, but it
could not be independently associated with OS in patients with
cancer. Following combination of these results with those from
previous studies such as the one carried out by Guo and Liang
(21), it is hypothesized that FAR
is not a suitable biomarker for evaluating prognosis in patients
with cancer treated with ICIs.
As a potentially notably predictor of immunotherapy,
the underlying mechanism of FIB has not been thoroughly clarified.
Patients with malignant tumors tend to have varying degrees of
hypercoagulability (28,29). Based on the recognized mechanisms
for FIB and tumor progression, four hypotheses have been proposed.
Firstly, FIB can bind or interact with growth factors such as
fibroblast growth factor-2 (FGF-2), platelet-derived growth factor
(PDGF) and transforming growth factor-β (TGF-β) (30,31).
Derynck et al (32)
demonstrated that TGF-β could release immunosuppressive cytokines
and generate an immunosuppressive environment, thus weakening the
effect of immunotherapy. Binding of FIB with FGF-2 or PDGF can
enhance their ability to promote cancer cell proliferation,
metastasis and angiogenesis (30,31).
Second, FIB is mainly synthesized upon inflammatory stimulation by
IL-6 and IL-1β, as well as by cancer cells (33–35).
Increased FIB levels promote the migration of cancer cells and
protect them from the innate immune surveillance system by
promoting platelet binding (36,37).
Third, a high concentration of FIB can induce the
epithelial-mesenchymal transition (EMT) (38). Zhang et al (39) demonstrated that EMT can increase
PD-L1 expression in tumors, and the interaction of PD-1 and PD-L1
can decrease cytotoxic T-cell activity, which leads to resistance
to immunotherapy in colorectal cancer. Fourth, fibrinogen-like
protein 1 (FGL1) belongs to the FIB superfamily, with high amino
acid homology to the carboxyl terminus of the FIB β- and γ-subunits
(40). FGL1 is a major immune
inhibitory ligand of lymphocyte-activation gene-3 (LAG-3), and the
FGL1/LAG3 interaction can cause immune suppression (41). Whether high FIB is related to the
FGL1/LAG3 interaction needs to be further explored. The
aforementioned factors may be the cause of poor immunotherapy
effects in patients with high FIB.
A number of studies have reported that high FIB or
other coagulation indices are associated with tumor progression,
such as that in breast, pancreatic and esophageal cancer (42–44).
Izuegbuna et al (42) and
Wang et al (43) showed that
patients with breast cancer and patients with pancreatic cancer
with a higher concentration of FIB, had a worse tumor stage.
Kołodziejczyk et al (45)
reported that the products of FIB degradation were associated with
disease progression and metastasis. Liu et al (46) conducted a study that included 176
patients with metastatic breast cancer and showed that the FIB
levels significantly increased after first-line therapy in patients
with disease progression. Therefore, we hypothesize that FIB has a
higher predictive value for the efficacy of second- or third-line
therapy. Further research is needed to provide evidence of this
predictive index in first-line therapy.
Although the present study provided evidence to
support the prognostic significance of elevated FIB in patients
with cancer, there were still limitations. First, this study was a
retrospective analysis and included only 61 patients. Consequently,
selection bias was unavoidable. Second, only 13 patients underwent
genetic testing, and only 9 patients had PD-L1 expression tested in
the present study. Thus, the interaction between relevant genetic
information and the therapeutic effect of ICIs was not described.
Third, due to the nature of this retrospective study, it was not
feasible to explore the mechanism of FIB in the context of
immunotherapy in depth. Nonetheless, further prospective trials and
primary research studies are needed to confirm the predictive value
of FIB in patients with immunotherapy.
Overall, to the best of our knowledge, this study is
the first observation concerning the prognostic role of FIB across
cancer types, particularly in patients treated with ICIs. FIB is a
promising prognostic factor for predicting the prognosis of
patients undergoing immunotherapy.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81872009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RX, BY, FL and QZ conceived and designed the study.
RX and TY acquired the data and drafted the manuscript. RX and JY
analyzed and interpreted the data. BY, JY and FL checked the data,
and performed critical revision of the manuscript. QZ supervised
the study. RX, TY and QZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hainan Hospital of the Chinese People's Liberation Army (PLA)
General Hospital (approval no. 301HLFYLS15), and written informed
consent was waived by the Ethics Committee of Hainan Hospital of
the PLA General Hospital due to the retrospective nature of the
study. All methods were carried out in accordance with relevant
guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FIB
|
fibrinogen
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
ICIs
|
immune checkpoint inhibitors
|
|
dMMR
|
deficient mismatch repair
|
|
TMB
|
tumor mutation burden
|
|
ORR
|
objective response rate
|
|
HR
|
hazard ratio
|
|
DCR
|
disease control rate
|
|
FGF-2
|
fibroblast growth factor-2
|
|
PDGF
|
platelet-derived growth factor
|
|
TGF-β
|
transforming growth factor-β
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FGL1
|
fibrinogen-like protein 1
|
|
LAG-3
|
lymphocyte-activation gene-3
|
References
|
1
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
Non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Lu S, Yu XM, Hu YP, Sun YP, Wang
ZJ, Zhao J, Yu Y, Hu CH, Yang K, et al: Tislelizumab plus
chemotherapy vs chemotherapy alone as first-line treatment for
advanced squamous Non-small-cell lung cancer: A phase 3 randomized
clinical trial. JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
Extensive-stage Small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen EEW, Soulières D, Tourneau CL, Dinis
J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al:
Pembrolizumab versus methotrexate, docetaxel, or cetuximab for
recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YL, Lu S, Cheng Y, Zhou CC, Wang J, Mok
T, Zhang L, Tu HY, Wu L, Feng J, et al: Nivolumab Versus Docetaxel
in a Predominantly Chinese Patient Population With Previously
Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical
Trial. J Thorac Oncol. 14:867–875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, Pawel JV, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shitara K, Özgüroğlu M, Bang YJ,
Bartolomeo MD, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palumbo JS and Degen JL: Mechanisms
coupling the hemostatic system to colitis-associated cancer. Thromb
Res. 125 (Suppl 2):S39–S43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Wang Y, Sun P, Wang ZQ, Wang DS,
Zhang DS, Wang FH, Fu JH, Xu RH and Li YH: Fibrinogen promotes
malignant biological tumor behavior involving epithelial
mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal
squamous cell carcinoma. J Cancer Res Clin Oncol. 143:2413–2424.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steinbrecher KA, Horowitz NA, Blevins EA,
Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ,
Pinkerton MD, Talmage KE, et al: Colitis associated cancer is
dependent on the interplay between the hemostatic and inflammatory
systems and supported by integrin alpha(M)beta(2) engagement of
fibrinogen. Cancer Res. 70:2634–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen J, Yang Y, Ye F, Huang X, Li S, Wang Q
and Xie X: The preoperative plasma fibrinogen level is an
independent prognostic factor for overall survival of breast cancer
patients who underwent surgical treatment. Breast. 24:745–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohara S, Suda K, Tomizawa K, Takemoto T,
Fujino T, Hamada A, Koga T, Nishino M, Chiba M, Sato K, et al:
Prognostic value of plasma fibrinogen and d-dimer levels in
patients with surgically resected non-small cell lung cancer. Surg
Today. 50:1427–1433. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Han W, Song Y, Gao P, Yang Y, Yu D,
Wang Y and Wang Z: Prognostic value of preoperative fibrinogen for
predicting clinical outcome in patients with nonmetastatic
colorectal cancer. Cancer Manag Res. 12:13301–13309. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin Y, Liu ZH, Qiu YF, Wu HN, Liang R,
Chen GY, Qin G, Li YQ and Zou DH: Clinical significance of plasma
D-dimer and fibrinogen in digestive cancer: A systematic review and
meta-analysis. Eur J Surg Oncol. 44:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan CL, Huang MF, Wang HL, Jiang W, Su CY
and Zhou SZ: Pretreatment Fibrinogen-Albumin Ratio (FAR) associated
with treatment response and survival in advanced Non-small cell
lung cancer patients treated with first-line Anti-PD-1 therapy plus
Platinum-based combination chemotherapy. Cancer Manag Res.
14:377–386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo ZW and Liang J: Fibrinogen-Albumin
Ratio Index (FARI) as a certain prognostic biomarker in pretreated
patients with immunotherapy. Cancer Manag Res. 13:4169–4180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen YJ, Wang HG, Wei JY and Li WD: Early
prediction of objective response of fibrinogen in a real-world
cohort of hepatocellular carcinoma cases treated by programmed cell
death receptor-1 and lenvatinib. Onco Targets Ther. 14:5019–5026.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nenclares P, Gunn L, Soliman H, Bover M,
Trinh A, Leslie I, Wong KH, Melcher A, Newbold K, Nutting CM, et
al: On-treatment immune prognostic score for patients with relapsed
and/or metastatic head and neck squamous cell carcinoma treated
with immunotherapy. J Immunother Cancer. 9:e0027182021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mei Y, Zhao S, Lu XF, Liu HX, Li XY and Ma
R: Clinical and prognostic significance of preoperative plasma
fibrinogen levels in patients with operable breast cancer. PLoS
One. 11:e01462332016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goad KE and Gralnick HR: Coagulation
disorders in cancer. Hematol Oncol Clin North Am. 10:457–484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Falanga A, Marchetti M and Vignoli A:
Coagulation and cancer: Biological and clinical aspects. J Thromb
Haemost. 11:223–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martino MM, Briquez PS, Ranga A, Lutolf MP
and Hubbell JA: Heparin-binding domain of fibrin(ogen) binds growth
factors and promotes tissue repair when incorporated within a
synthetic matrix. Proc Natl Acad Sci USA. 110:4563–4568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahni A, Simpson-Haidaris PJ, Sahni SK,
Vaday GG and Francis CW: Fibrinogen synthesized by cancer cells
augments the proliferative effect of fibroblast growth factor-2
(FGF-2). J Thromb Haemost. 6:176–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Derynck R, Turley SJ and Akhurst RJ: TGFβ
biology in cancer progression and immunotherapy. Nat Rev Clin
Oncol. 18:9–34. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tennent GA, Brennan SO, Stangou AJ,
O'Grady J, Hawkins PN and Pepys MB: Human plasma fibrinogen is
synthesized in the liver. Blood. 109:1971–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Falanga A and Marchetti M: Hemostatic
biomarkers in cancer progression. Thromb Res. 164:54–61. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simpson-Haidaris PJ and Rybarczyk B:
Tumors and fibrinogen: The role of fibrinogen as an extracellular
matrix protein. Ann N Y Acad Sci. 936:406–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng S, Shen J, Jiao Y, Liu Y, Zhang CM,
Wei M, Hao S and Zeng XL: Platelets and fibrinogen facilitate each
other in protecting tumor cells from natural killer cytotoxicity.
Cancer Sci. 100:859–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: Clinical and
prognostic significance of preoperative plasma hyperfibrinogenemia
in gallbladder cancer patients following surgical resection: A
retrospective and in vitro study. BMC Cancer. 14:5662014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang N, Ng AS, Cai S, Li Q, Yang L and
Kerr D: Novel therapeutic strategies: Targeting
epithelial-mesenchymal transition in colorectal cancer. Lancet
Oncol. 22:e358–e368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamamoto T, Gotoh M, Sasaki H, Terada M,
Kitajima M and Hirohashi S: Molecular cloning and initial
characterization of a novel fibrinogen-related gene, HFREP-1.
Biochem Biophys Res Commun. 193:681–687. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Sanmamed MF, Datar I, Su TT, Ji L,
Sun J, Chen L, Chen Y, Zhu G, Yin W, et al: Fibrinogen-like Protein
1 is a major immune inhibitory ligand of LAG-3. Cell. 176:334–347.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Izuegbuna OO, Agodirin OS, Olawumi HO and
Olatoke SA: Plasma D-Dimer and fibrinogen levels correlates with
tumor size and disease progression in nigerian breast cancer
patients. Cancer Invest. 39:597–606. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Gao JB, Bai M, Liu R, Li H, Deng
T, Zhou L, Han R, Ge S, Huang D and Ba Y: The pretreatment platelet
and plasma fibrinogen level correlate with tumor progression and
metastasis in patients with pancreatic cancer. Platelets.
25:382–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takeuchi H, Ikeuchi S, Kitagawa Y, Shimada
A, Oishi T, Isobe Y, Kubochi K, Kitajima M and Matsumoto S:
Pretreatment plasma fibrinogen level correlates with tumor
progression and metastasis in patients with squamous cell carcinoma
of the esophagus. J Gastroenterol Hepatol. 22:2222–2227. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kolodziejczyk J and Ponczek MB: The role
of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs)
in tumor progression. Contemp Oncol (Pozn). 17:113–119.
2013.PubMed/NCBI
|
|
46
|
Liu Q, Fang S, Liang S, Lv J, Wang G, Tang
R, Ji X, Zhao T, Li J, Xu L, et al: The prognostic role of a
combined fibrinogen and inflammation-based index in patients with
metastatic breast cancer. Transl Cancer Res. 9:7065–7078. 2020.
View Article : Google Scholar : PubMed/NCBI
|