Introduction
In cancer patients there are many mechanisms
contributing to damage of cardiovascular system: breakdown of
coagulation, anemia, exhaustion of organism, release of
cardio-depressive factors such as cytokines, malignant
pericarditis, direct interaction of tumor and heart or vascular
system and anticancer therapy (1).
Natriuretic factors are peptic substances produced
by atrial and ventricular myocardium. Primary stimulus to synthesis
of this factor is intramural pressure of atriums by increasing of
venous return during intravascular hypovolemia (2). Cardiac natriuretic peptides ANP
(atrial natriuretic peptide), BNP (B type natriuretic peptide) and
C-type natriuretic peptide (CNP) are known because of their
compensation effects-systemic arterial vasodilatation, natriuresis,
diuresis, inhibition of system renin-angiotensin-aldosteron and
neuromodulation (3). BNP is
secreted in a form of pro-hormone (pre-pro-BNP) (2). After stimulation of myocardial cells
it is split into two fragments-BNP (biological active peptid) and
inactive N-terminal fragment NT-proBNP. Concentration of BNP and
NT-proBNP are equal in the healthy population. The measurement of
NT-proBNP is considered advantageous compared to BNP due to longer
biological halftime and higher biochemical stability. This is the
reason, why NT-proBNP is biomarker examined in normal conditions
(4). NT-proBNP is used mainly for
diagnosis of heart failure. In addition, it may predict the
development of heart failure and death in patients with
cardiovascular disease. However, NT-proBNP could have predictive
power beyond cardiovascular risk (5). These biomarkers are not exclusively
produced by the heart but are produced by several organs in
response or in association with cardiovascular diseases (6).
However, the interpretation of elevated NT-proBNP
levels remains difficult because of several confounding factors,
such coronary disease, advanced age, renal insufficiency,
respiratory diseases such as pulmonary hypertension leading to
right ventricular dysfunction, thromboembolic disease, atrial
fibrillation, cirrhosis, sepsis, or dysthyroid status could be
responsible for elevation of NT-proBNP (7).
At present, it is well known that natriuretic
peptides may be produced by cancer cells, as well (8). In this regard, small cell lung cancer
may secrete both pro-atrial natriuretic peptide and BNP. Also, BNP
is expressed both in normal adrenal glands and in adrenal tumors,
suggesting that natriuretic peptides may have other roles unrelated
to the cardiovascular system (9).
There are many studies which described the relationship between an
NT-proBNP, and the presence of inflammation with elevation
C-reactive protein and IL-6 cytokine. These studies confirmed a
significant correlation between IL-6, CRP and NT-proBNP (9,10).
A study of Sachico Bando showed a significant
positive correlation between the NT-proBNP and the CRP levels in
cancer patients, which suggested that the plasma BNP levels may
have been elevated due to cancer related inflammation. In addition,
the plasma BNP levels are increased in advanced stages of cancer,
which might be accompanied by systemic inflammation. Furthermore,
the plasma BNP levels tended to decrease after radical surgery in
patients with solid cancers. Similarly, plasma BNP levels were
shown to decrease after chemotherapy in patients with hematological
cancers (3). BNP has been shown to
be upregulated at the transcriptional and translational levels by
proinflammatory cytokines in cardiac myocytes. Proinflammatory
signals are postulated to stimulate members of the
mitogen-activated protein kinase (MAPK) family and c-Jun kinase, as
well as other intracellular signaling cascades, which leads to the
upregulation of the BNP gene expression (3). ProBNP synthesis may be stimulated by
several pro-inflammatory cytokines including tumor necrosis
factor-alfa and several interleukins (11). However, the specific cause of
elevation of natriuretic peptide plasma levels seen in cancer has
not been elucidated. In recent years, it has been demonstrated that
natriuretic peptides, or compounds with similar activity, decrease
the number of several cancer cells in vitro through a
reduction of DNA synthesis and inhibition of C-Fos and c-Jun
proto-oncogenes, inhibit lung metastases and skin carcinogenesis in
animal models, and diminish the expression of vascular endothelial
growth factor and that of its receptor VEGFR2, thus having the
potential to control vasculogenesis (12). A study by Tuňón et al
(13) has shown the opposite
effects of natriuretic peptides on carcinogenesis depending on
their concentrations. In this paper, atrial natriuretic peptide
enhanced proliferation of human gastric cells in vitro at low
concentrations but inhibited their proliferation through cyclic
guanosine 3–5-monophosphate-dependent pathways when it was present
at high concentration (13). Then,
given that most data suggest an anticancer effect of natriuretic
peptides, the possibility exists that their production by cancer
cells represents a negative feedback mechanism to control the tumor
growth (5). In this case the
NT-proBNP elevation would only be a response to the existence of
malignancies. Nevertheless, the fact that natriuretic peptides are
related with mechanism of cancer which are common to multiple
malignancies would agree with these findings (14). Elevated levels of cardiovascular
peptides including BNP/NT-proBNP was reported in patients with
renal cell cancer in a study by Kamai et al (8). This study reported that higher
preoperative serum levels of BNP, NT-proBNP and vascular
endothelial growth factor (VEGF), were associated with worse
performance status, local invasion, distant metastasis and shorter
overall survival. Moreover, serum levels of BNP and NT-proBNP
decreased significantly after tumor resection. This decrease might
be associated with alleviation of stress on the heart (8). Serum measurement of NT-proBNP level in
patient undergoing chemotherapy with anthracyclines is useful for
both, acute and late toxicity. Measurement of Troponin I and
Troponin T is useful for acute toxicity during the chemotherapy,
but not for late toxicity 12 months after chemotherapy, when
Troponin T and I is in normal range (15). A study by Mladosievicova et
al (16) documented that higher
level of NT-proBNP detected in childhood leukemia survivors after
low anthracycline cumulative doses might reflect an initial stage
of anthracycline cardiotoxicity before the development of
echocardiographic abnormalities. NT-pro-BNP is one of the best
available biochemical markers of late anthracycline cardiotoxicity
(16). Study of Pudil et al
(6) reports that cardiac biomarkers
including cardiac troponins and natriuretic peptides are the most
promising clinical tool for both baseline assessment and marker of
early cardiac injury or strain which may predict subsequent changes
in LVEF and development of HF in different cardiotoxic cancer
therapies including anthracyclines, HER-2 antibodies (trastuzumab),
VEGF TKIs and ICIs. Given the fulminant nature of ICI-related
myocarditis, early detection is essential for the best possible
treatment. Elevated troponin was found in 94% of all cases of
ICI-related myocarditis, the diagnostic value of NT-proBNP was
lower with 66% sensitivity (17).
In clinical practice NT-proBNP is useful in
diagnosis of cardiovascular diseases, however, the false positivity
in cancer patients is mostly unknown. Our study aimed to correlate
NT-proBNP with patients/disease characteristics and patient's
outcome in patients without cardiac symptoms.
Our primary mission was to examine the relationship
between levels of NT-proBNP and the status of cancer disease.
Specific aims are correlation of levels NT-proBNP with gravity of
tumor, correlation of levels NT-proBNP with life expectancy, with
clinicopathological variables.
Materials and methods
Patients
This clinical study included consecutive cancer
patients that undergo treatment at the Department of Oncology of
St. Jacob Hospital in Bardejov, between December 2017 and December
2021. Eligible were patients with solid tumor older than 18 years
old, ECOG performance status 0,1 or 2, without symptoms of acute
cardiac failure, acute respiratory failure, that were able to
undergo an outpatient treatment and follow up. Eligible patients
were all patients regardless of the status of their disease,
including non-metastatic patients, metastatic patients with who
were undergoing cancer therapy and achieved disease control
(complete and partial remission and/or stable disease) or
metastatic patients with disease progression.
All patients were thoroughly evaluated with complete
medical history, physical examination, and laboratory and disease
assessment. The study was approved by local ethic committee and all
patients signed informed consent.
NT-pro-BNP measurement
From each patient we obtained peripheral blood for
detection NT-proBNP before therapy, after the end of therapy and 1
year after the first sample. These samples were evaluated in a
biochemical laboratory of St. Jacob Hospital by immunochemical
method using ELESCYS proBNP reagent, with a COBAS e 411 ROCHE
machine.
Level of NT-proBNP is age dependent. The cut-off
value for cardiac failure exclusion is <450 ng/l when patients
are under the age of 50, <900 ng/l for patients 50–75 years old
and <1,800 for patients older than 75 years (18).
Statistical analysis
The patients' characteristics were summarized and
tabulated using the median (range) for continuous variables and
frequency (percentage) for categorical variables. For analysis of
associations between categorical variables, Fisher's exact test was
used. A Kruskal-Wallis test with Dunn's post hoc test was used for
univariate analyses of associations between clinical
characteristics and continuous variables. Patients were divided
into two group dichotomized based NT-pro-BNP cut-off level adjusted
to age (see above) (‘low’ vs. ‘high’). Overall survival was
calculated from the date of initial pro-BNP measurement to the date
of death or last follow-up. Univariate Kaplan-Meier statistical
approach was used to assess outcome of survival data in conjunction
with pro-BNP status in certain populations among our studied group
and long-rank test was used to compare survival between groups. All
p values presented were two-sided, and associations were considered
significant if the p value is less or equal to 0.05. Statistical
analyses were performed using NCSS 2007 software (Hintze J., 2007,
Kaysville, Utah, USA).
Results
Patients' characteristics
The study population consisted of 112 cancer
patients. Sixty-three percent of patients were older than 65 years.
Most patients were metastatic (83.0%). The most common diagnoses
were gastrointestinal malignancies (49.1%), breast cancer (19.6%),
lung cancer (16.1%), genitourinary cancer (9.8%) and gynecological
cancer (5.4%). We monitored serum tumor markers specific for each
diagnosis, when suitable marker was available. Patients'
characteristics are summarized in Table
I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | No. (%) |
|---|
| Age |
|
| Age
<65 years | 41 (36.6) |
| Age ≥65
years | 71 (63.4) |
| Status of
disease |
|
| Present
primary tumor without metastases | 19 (17.0) |
|
Metastatic disease with
disease control (complete or partial remission or stable
disease) | 28 (25.0) |
|
Metastatic disease with
disease progression | 65 (58.0) |
| Chemotherapy |
|
|
Chemotherapy-naive
patients | 68 (60.7) |
|
Pretreated patients | 43 (38.4) |
| Type of cancer |
|
|
Gastrointestinal cancer | 55 (49.1) |
|
Genitourinary cancer | 11 (9.8) |
|
Gynecological cancer | 6 (5.4) |
| Lung
cancer | 18 (16.1) |
| Breast
cancer | 22 (19.6) |
Association between NT-proBNP and
patients/disease characteristics and patients outcome
Mean ± standard error of mean (SEM) value of
NT-pro-BNP in the first, second and third sample was 561.0±75.1,
1,565.4±461.1 and 1,940.7±581.1 ng/l. Differences were
statistically significant between all the samples (P<0.001).
Fifteen (13.4%), 27 (24.1%) and 25 (30.1%) patients had elevated
level of NT-pro-BNP in the first, second and third sample above the
normal value adjusted to age (see cut-off value in Methods
section).
There was a significant elevation of NT-proBNP in
all samples in patients older than 65 years (Table II, Table III, Table IV). Moreover, in the second and
third sample the NT-proBNP level was significantly elevated in
patients with progressive metastatic disease compared to
stationary, or nonmetastatic disease. Within the distinct
diagnoses, all three samples showed the highest NT-proBNP level in
genitourinary cancers, however, the difference was not
statistically significant. In second sample, pro-BNP was also
associated with serum tumor marker level, while in the third sample
it was associated with previous therapy. There were 25 patients
with left and 8 patients with right sided colon cancer, however,
there was no difference in any of NT-proBNP based on tumor
location. Mean ± SEM value of NT-pro-BNP in the first, second and
third sample for left vs. right-sided colon cancer was: 789±238.8
vs. 807.0 vs. 413.6, P=0.76, 971.1±242.1 vs. 929.4±419.3, P=0.95,
1,312.3±406.0 vs. 848.7±907.8, P=0.88.
 | Table II.Association between NT-proBNP-1 and
patient characteristics. |
Table II.
Association between NT-proBNP-1 and
patient characteristics.
|
| NT-proBNP-1 |
|---|
|
|
|
|---|
| Variable | No. | Mean, ng/l | SE, ng/l | P-valuea | Normal, n (%) | Elevated, n (%) | P-valueb |
|---|
| All patients | 112 | 561.0 | 75.1 | NA | 97 (86.6) | 15 (13.4) | NA |
| Age |
|
|
|
|
|
|
|
| <65
years | 41 | 213.6 | 117.5 | <0.01 | 39 (95.1) | 2 (4.9) | 0.05 |
| ≥65
years | 71 | 761.5 | 89.3 |
| 58 (81.7) | 13 (18.3) |
|
| Status of
disease |
|
|
|
|
|
|
|
| Present
primary tumor without metastases | 19 | 547.0 | 183.8 | 0.33 | 18 (94.7) | 1 (5.3) | 0.52 |
|
Metastatic disease with
disease control | 28 | 528.7 | 151.4 |
| 24 (85.7) | 4 (14.3) |
|
|
Progressive metastatic
disease | 65 | 578.9 | 99.4 |
| 55 (84.6) | 10 (15.4) |
|
| Chemotherapy |
|
|
|
|
|
|
|
|
Chemotherapy-naïve
patients | 68 | 619.3 | 96.7 | 0.22 | 56 (82.4) | 12 (17.6) | 0.16 |
|
Pretreated patients | 43 | 480.7 | 121.6 |
| 40 (93.0) | 3 (7.0) |
|
| Type of cancer |
|
|
|
|
|
|
|
|
Gastrointestinal cancer | 55 | 602.3 | 106.9 | 0.13 | 46 (83.6) | 9 (16.4) | 0.22 |
|
Genitourinary cancer | 11 | 805.2 | 239.1 |
| 8 (72.7) | 3 (27.3) |
|
|
Gynecological cancer | 6 | 830.3 | 323.8 |
| 5 (83.3) | 1 (16.7) |
|
| Lung
cancer | 18 | 515.9 | 186.9 |
| 16 (88.9) | 2 (11.1) |
|
| Breast
cancer | 22 | 298.8 | 169.1 |
| 22 (100.0) | 0 (0.0) |
|
| Serum tumor
marker |
|
|
|
|
|
|
|
| Normal
range | 53 | 522.7 | 113.2 | 0.53 | 47 (88.7) | 6 (11.3) | 0.95 |
|
<1.5-fold | 13 | 743.9 | 228.7 |
| 11 (84.6) | 2 (15.4) |
|
|
1.5-10-fold | 19 | 627.7 | 189.1 |
| 16 (84.2) | 3 (15.8) |
|
|
>10-fold | 15 | 455.0 | 212.9 |
| 13 (86.7) | 2 (13.3) |
|
 | Table III.Association between NT-proBNP-2 and
patient characteristics. |
Table III.
Association between NT-proBNP-2 and
patient characteristics.
|
| NT-proBNP-2 |
|---|
|
|
|
|---|
| Variable | No. | Mean, ng/l | SE, ng/l |
P-valuea | Normal, n (%) | Elevated, n
(%) |
P-valueb |
|---|
| All patients | 112 | 1565.4 | 461.1 | NA | 85 (75.9) | 27 (24.1) | NA |
| Age |
|
|
|
|
|
|
|
| <65
years | 41 | 1014.1 | 762.7 | 0.01 | 31 (75.6) | 10 (24.4) | >0.99 |
| ≥65
years | 71 | 1883.8 | 579.6 |
| 54 (76.1) | 17 (23.9) |
|
| Status of
disease |
|
|
|
|
|
|
|
| Present
primary tumor without metastases | 19 | 446.0 | 1106.4 | 0.01 | 18 (94.7) | 1 (5.3) | 0.01 |
|
Metastatic disease with
disease control | 28 | 385.7 | 911.4 |
| 27 (96.4) | 1 (3.6) |
|
|
Progressive metastatic
disease | 65 | 2400.8 | 598.2 |
| 40 (61.5) | 25 (38.5) |
|
| Chemotherapy |
|
|
|
|
|
|
|
|
Chemotherapy-naive
patients | 68 | 1786.3 | 596.1 | 0.33 | 53 (77.9) | 15 (22.1) | 0.50 |
|
Pretreated patients | 43 | 1247.8 | 749.6 |
| 31 (72.1) | 12 (27.9) |
|
| Type of cancer |
|
|
|
|
|
|
|
|
Gastrointestinal cancer | 55 | 1180.4 | 643.4 | 0.27 | 42 (76.4) | 13 (23.6) | 0.31 |
|
Genitourinary cancer | 11 | 4994.2 | 1438.7 |
| 7 (63.6) | 4 (36.4) |
|
|
Gynecological cancer | 6 | 4227.8 | 1948.0 |
| 3 (50.0) | 3 (50.0) |
|
| Lung
cancer | 18 | 875.9 | 1124.7 |
| 16 (88.9) | 2 (11.1) |
|
| Breast
cancer | 22 | 651.6 | 1017.3 |
| 17 (77.3) | 5 (22.7) |
|
| Serum tumor
marker |
|
|
|
|
|
|
|
| Normal
range | 55 | 1260.7 | 692.3 | 0.03 | 47 (85.5) | 8 (14.5) | 0.26 |
|
<1.5-fold | 11 | 1133.7 | 1548.0 |
| 7 (63.6) | 4 (36.4) |
|
|
1.5-10-fold | 18 | 2411.2 | 1210.2 |
| 12 (66.6) | 6 (33.3) |
|
|
>10-fold | 19 | 2151.4 | 1177.9 |
| 14 (73.7) | 5 (26.3) | >0.99 |
 | Table IV.Association between NT-proBNP-3 and
patient characteristics. |
Table IV.
Association between NT-proBNP-3 and
patient characteristics.
|
| NT-proBNP-3 |
|---|
|
|
|
|---|
| Variable | No. | Mean, ng/l | SE, ng/l |
P-valuea | Normal, n (%) | Elevated, n
(%) |
P-valueb |
|---|
| All patients | 83 | 1940.7 | 581.1 | NA | 58 (69.9) | 25 (30.1) | NA |
| Age |
|
|
|
|
|
|
|
| <65
years | 28 | 726.5 | 992.8 | 0.01 | 20 (71.4) | 8 (28.6) | >0.99 |
| ≥65
years | 55 | 2558.8 | 708.4 |
| 38 (69.1) | 17 (30.9) |
|
| Status of
disease |
|
|
|
|
|
|
|
| Present
primary tumor without metastases | 15 | 515.3 | 1321.5 | 0.01 | 14 (3.3) | 1 (6.7) | 0.01 |
|
Metastatic disease with
disease control | 28 | 392.1 | 967.2 |
| 26 (2.9) | 2 (7.1) |
|
|
Progressive metastatic
disease | 40 | 3559.3 | 809.2 |
| 18 (45.0) | 22 (55.0) |
|
| Chemotherapy |
|
|
|
|
|
|
|
|
Chemotherapy-naive
patients | 54 | 2486.1 | 717.5 | 0.05 | 35 (64.8) | 19 (35.2) | 0.21 |
|
Pretreated patients | 29 | 925.2 | 979.1 |
| 23 (79.3) | 6 (20.7) |
|
| Type of cancer |
|
|
|
|
|
|
|
|
Gastrointestinal cancer | 41 | 1218.6 | 809.0 | 0.09 | 32 (78.0) | 9 (22.0) | 0.03 |
|
Genitourinary cancer | 11 | 5486.3 | 1561.9 |
| 6 (4.5) | 5 (45.5) |
|
|
Gynecological cancer | 5 | 2204.2 | 2316.6 |
| 3 (0.0) | 2 (40.0) |
|
| Lung
cancer | 11 | 2985.4 | 1561.9 |
| 4 (6.4) | 7 (63.6) |
|
| Breast
cancer | 15 | 460.4 | 1337.5 |
| 13 (86.7) | 2 (13.3) |
|
| Serum tumor
marker |
|
|
|
|
|
|
|
| Normal
range | 38 | 1897.7 | 902.7 | 0.81 | 30 (78.9) | 8 (21.1).0) | 0.12 |
|
<1.5-fold | 8 | 561.0 | 1967.3 |
| 6 (75.0) | 2 (25.0 |
|
|
1.5-10-fold | 12 | 1446.1 | 1606.3 |
| 6 (50.0) | 6 (50.0) |
|
|
>10-fold | 9 | 1663.9 | 1854.8 |
| 5 (55.6) | 4 (44.4) |
|
In median follow-up of 20.2 months (range 1.3-67.1
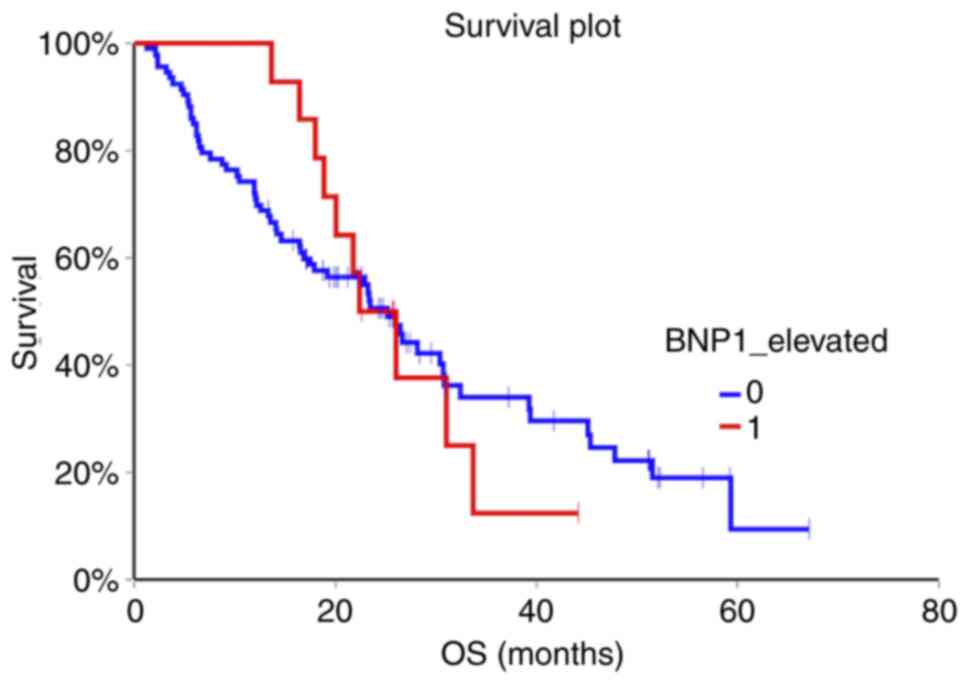
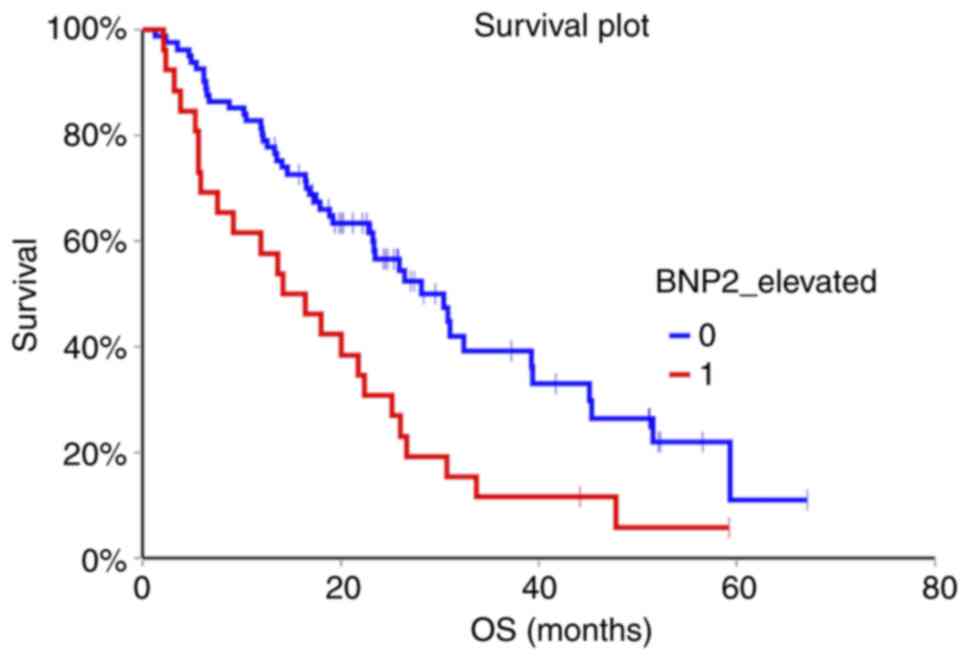
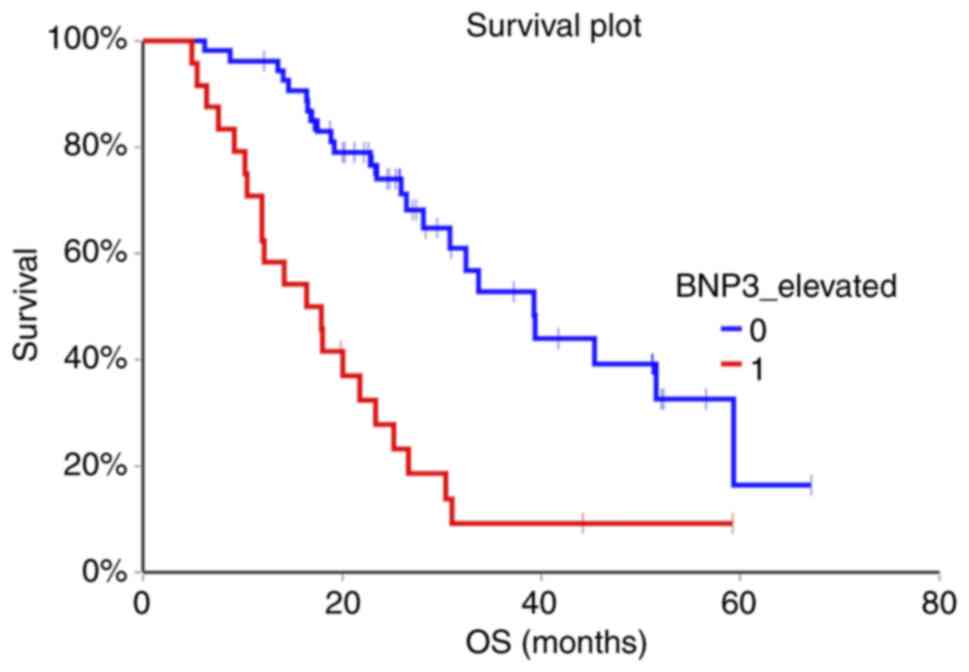
months) 72 (64.3%) of patients died. Patients with non-elevated
NT-proBNP above cut-off value in the second and third sample had
significantly better OS compared to patients with elevated
NT-proBNP (HR=0.47, 95% CI 0.26-0.85, P=0.002 for second sample and
HR=0.29, 95% CI 0.14-0.60, P=0.0000007 for third sample,
respectively). Baseline NT-proBNP value was not prognostic for OS
(HR=0.98, 95% CI 0.50-1.92, P=0.96) (Fig. 1, Fig.
2, Fig. 3).
Discussion
Our findings show that in all samples the NT-proBNP
was elevated in older people. In the second and the third sample,
NT-proBNP was significantly elevated in patients with progressive
metastatic disease compared to nonmetastatic and stationary
metastatic disease. In the second sample there was a significant
elevation of specific serum tumor marker along with NT-proBNP. In
the third sample there was significant elevation of NT-proBNP in
chemotherapy naive patients compare to chemotherapy pretreated
patients.
Finding of Kamai et al (8) in patients with renal cell carcinoma
(RCC) suggested that the preoperative serum levels of
cardiovascular hormones (BNP, NT-proBNP) might be related to
progression of renal cell carcinoma and a worse prognosis. The
author based this interpretation on the fact that the serum levels
of NT-proBNP declined after the nephrectomy. Authors reported that
higher preoperative serum levels of BNP, NT-proBNP and vascular
endothelial growth factor (VEGF), as well as elevated HIF-2 alpha
expression in the primary tumor, were associated with worse
performance status, local invasion, distant metastasis and shorter
overall survival. Possible reason for lower BNP after nephrectomy
may be an indirect production of these hormones by cancer cells
(8). It can be comparable with our
study, where patient with progressive metastatic disease have got
significantly higher levels of NT-proBNP after therapy, and one
year after diagnosis. Antineoplastic therapy with anthracyclines is
often complicated by the development of cardiotoxicity leading to
heart failure (19). In some
instances, it is detected too late with echocardiography, when
significant myocardial damage has already occurred (20). Serum measurement of NT-proBNP level
in patient treated with anthracyclines is useful for both, acute
and late toxicity (15). In our
study, we had 22 patients with breast cancer of whom 16 were
treated with chemotherapy. In our study, there was significant
elevation of NT-proBNP in metastatic patients after therapy and one
year after diagnosis. However, the elevation in several patients
with breast cancer was not significant. There were no patients with
acute cardiac failure in association with chemotherapy. The
association between the NT-proBNP and malignant disease confirms a
study by Papazisis et al (21) who assessed a group of patients with
metastatic renal cell carcinoma treated with sunitinib (a tyrosine
kinase inhibitor). Patients who obtained a clinical benefit 15 days
after treatment had significantly lower NT proBNP compared to
patients without any clinical benefit (a three-fold increase in
patients with progressive disease compared to stable NT-proBNP
levels in patients with clinical benefit, P<0,0001). Median
progression free survival was 12.0 months in patients with less
than 1.5-fold increase (n=22) and 3.9 months in patients with more
than 1.5-fold increases in plasma NT-proBNP (n=13) (Long-rank test,
P=0.001) (21). Similar in our
study patients with good clinical benefit from therapy with stable
metastatic disease had significantly lower levels of NT-proBNP
after treatment and one year after the diagnosis, too. The
elevation of NT-proBNP and CA-125 were markers of shorter survival
in patients with breast cancer treated with trastuzumab. A study by
Rossner et al (22) divided
28 patients with HER-2 positive breast cancer to two group. Group
with NT-proBNP levels <155 pg/ml (n=16, age 57±13 years) vs.
group B with NT-proBNP >155 pg/ml (n=12, age 62±9 years) levels
before and after trastuzumab therapy. Obtained results have shown
NT-proBNP of 65±36 pg/ml vs. 66±33 pg/ml in group A vs. 520±443
pg/ml vs. 498±411 pg/ml in group B. Elevated levels of NT-proBNP,
CA-125 and CA15-3 indicated a higher median three-month-mortality
in trastuzumab-treated patients on long term immunotherapy
(22). In our study, there was
significant elevation of cancer-specific tumor marker in the second
sample of NT-proBNP (after therapy). This is in line with findings
of study by Rossner et al (22). Similar results like antecedent and
our study had two studies in primary and metastatic brain tumor
patients. These studies reported that greater NT-proBNP
concentration was associated with greater mass effect and extent of
perifocal brain edema. Elevated NT-proBNP concentration before
surgery was associated with inferior outcomes at the hospital
discharge and with inferior prognosis of brain tumor patients
(23). Therefore, NT-proBNP can be
considered for perioperative risk stratification, prognostication
and evaluation of cognitive/mental health status in brain tumor
patients. A study by Bunevicius conducted in 245 patients
undergoing craniotomy for brain tumor (mostly meningioma 36% and
high-grade glioma 20%) ascertained that greater NT-proBNP
concentrations were associated with greater 5-year mortality risk
(HR 1.845, 95%, Cl, (1.166-2.920), P=0.009) controlling for
patients age, gender, history of cardiovascular disease,
histological diagnosis and adjuvant therapy. In summary, greater
preoperative NT-proBNP concentration is associated with worse
health status, unfavorable discharge outcome and shorter survival
of brain tumor patients (24). Next
study by authors Pavo et al (10) from 2015 demonstrated that NT-proBNP
is systematically elevated in cancer patients and that it is
likewise associated with long-term mortality independently of age,
gender, tumour entity, tumor stage and manifest cardiac disease at
the first clinical presentation. This study confirmed a significant
correlation between the pro-inflammatory cytokine IL-6 and
inflammatory marker CRP and the hormone NT-proBNP. Whether the
effect on mortality is primarily due to a determinant local
influence on the tumour microenvironment or it is induced by
systemic cardiovascular dysregulation cannot be determined
(10). In our study in accord to
the study by Pavo, there was a significant association between CRP
and NT-proBNP from the second sample (P-value 0.003) and between
CRP and NT-proBNP (P-value 0.0002) from the third sample.
This study has several limitations, including a
relative limited samples size, patients and cancer types of
heterogeneity and this study doesn't stratify patients according to
their clinical and/or molecular subtypes. Moreover, there are other
factors that potentially affect NT-proBNP level including obesity,
anemia and/or ongoing volume therapy. On the other hand, these data
represent pro-BNP measurement in consecutive patients treated in
oncology department in regional hospital in and study results could
help better elucidate clinical utility of NT-proBNP in cancer
patients.
In conclusion we have observed that NT-proBNP was
significantly increased in older patients, in patients with
progressive metastatic disease with poor prognosis, especially in
genitourinary malignancies and lung cancer. The levels of NT-proBNP
were increased in genitourinary and gastrointestinal cancer
compared to other diagnoses, however, not statistically
significant. Our detections and mentioned clinical studies suggest
that level of NT-proBNP shows the extent of oncologic disease.
Higher levels are associated with progression of metastatic disease
in cancer patients and with shorter overall survival. These
findings suggest that subclinical dysfunction of cardiovascular
system is common and has prognostic significance in cancer
patients.
Acknowledgements
The authors would like to thank Mrs. Slávka
Miháliková (Oncologic Department, St. Jacob Hospital Bardejov,
Bardejov, Slovakia), Mrs. Natália Šatanková (Oncologic Department,
St. Jacob Hospital Bardejov, Bardejov, Slovakia) and Mrs. Zdenka
Lazúrová (Oncologic Department, St. Jacob Hospital Bardejov,
Bardejov, Slovakia) for collecting patient data and taking blood
samples.
Funding
The present study was supported by Slovak Research and
Development Agency (contract no. APVV-15-14-0086 and APVV-19-0411
and VEGA 1/0327/19).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JCJr, JCSr, MC and MM were involved in data
collection, analysis, manuscript writing, and study conception and
design. JCJr and MM confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethic committee of
Hospital of St. Jacob in Bardejov, Slovakia. All participants
provided written informed consent.
Patient consent for publication
All study participants provided written informed
consent for the publication of data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poprach A, Petráková K, Vyskocil J, Lakomý
R, Nemecek R, Kocák I, Kocáková I and Vyzula R: Cardiotoxicity of
drugs used in oncology. Klin Onkol. 21:288–293. 2008.(In Czech).
PubMed/NCBI
|
|
2
|
Kinova S, Hulin I, et al: Internal
medicine. Prolitera, ISBN:. 9788097025397:2802013.
|
|
3
|
Bando S, Soeki T, Matsuura T, Tobiume T,
Ise T, Kusunose K, Yamaguchi K, Yagi S, Fukuda D, Iwase T, et al:
Plasma brain natriuretic peptide levels are elevated in patients
with cancer. PLoS One. 12:e01786072012. View Article : Google Scholar
|
|
4
|
Mladosievicova B, et al: Cardiooncology.
Grada, ISBN:. 9788024748382:1332014.
|
|
5
|
Goncalvescová E: Merit of natriuretic
peptides for diagnosis, prognosis, management and therapy of heart
failure. Int Med Practice. 10:428–431. 2006.
|
|
6
|
Pudil R, Mueller C, Čelutkienė J,
Henriksen PA, Lenihan D, Dent S, Barac A, Stanway S, Moslehi J,
Suter TM, et al: Role of serum biomarkers in cancer patients
receiving cardiotoxic cancer therapies: A position statement from
the cardio-oncology study group of the heart failure association
and the cardio-oncology council of the European society of
cardiology. Eur J Heart Fail. 22:1966–1983. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oral I: Brain natriuretic peptide as
prognostic marker of two-year survival in patients with heart
failure in a long-term dialysis programme. Interventional
Cardiology. 3:112–115. 2006.
|
|
8
|
Kamai T, Tokura Y, Uematsu T, Sakamoto K,
Suzuki I, Takei K, Narimatsu T, Kambara T, Yuki H, Betsunoh H, et
al: Elevated serum levels of cardiovascular biomarkers are
associated with progression of renal cancer. Open Heart.
5:e0006662018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aujolett N, Meyer M, Cailliod R, Combier
F, Coignet Y, Campard S, Facy O, Bernard A and Girard C: High
N-terminal Pro-B type natriuretic peptide: A biomarker of lung
cancer? Clin Lung Cancer. 11:341–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pavo N, Raderer M, Hülsmann M, Neuhold S,
Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna
M, et al: Cardiovascular biomarkers in pacients with cancer and
their association with all-cause mortality. Heart. 101:1874–1880.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Popat J, Rivero A, Pratap P and Guglin M:
What is causing extremely elevated amino terminal brain natriuretic
peptide in cancer patients. Congest Heart Fail. 19:143–148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manimala NJ, Frost CD, Lane ML, Higuera M,
Beg R and Vesely DL: Cardiac hormones target nuclear oncogenes
c-Fos and c-Jun in carcinoma cells. Eur J Clin Invest.
43:1156–1162. 2013.PubMed/NCBI
|
|
13
|
Tuňón J, Higueras J, Tarín N, Cristóbal C,
Lorenzo O, Blanco-Colio L, Martín-Ventura JL, Huelmos A, Alonso J,
Aceña A, et al: N-terminal pro-brain natriuretic peptide is
associated with a future diagnosis of cancer in patients with
coronary artery disease. PLoS One. 10:e01267412015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zhao Z, Zu C, Hu H, Shen H, Zhang
M and Wang J: Atrial natriuretic peptide modulates the
proliferation of human gastric cancer cells via KCNQ1 expression.
Oncol Lett. 6:407–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Advani P, Hoyne J, Moreno–Aspita A, Dubin
M, Brock S, Harlow C, Chumsri S, Suter T and Blackshear JL:
High-senzitive troponin T and NT-proBNP kinetics in breast cancer
chemotherapy. Chemotherapy. 62:334–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mladosievicova B, Urbanova D, Radvanska E,
Slavkovsky P and Simkova I: Role of NT-proBNP in detection of
myocardial damage in childhood leukemia survivors treated with and
without anthracyclines. J Exp Clin Cancer Res. 31:862012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michel L, Rassaf T and Totzeck M:
Biomarkers for detection of apparent and subclinical cancer
therapy-related cardiotoxicity. J Thorac Dis. 10:S4282–S4295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Z, Jia Y and Zhu B: BNP and NT-proBNP
as diagnostic biomarkers for cardiac dysfunction in both clinical
and forensic medicine. Int J Mol Sci. 20:18202019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cingelová S: Cardiotoxicity of breast
cancer adjuvant treatment. Klinicka onkologie J. 20:330–334.
2007.
|
|
20
|
Romano S, Fratini S, Ricevuto E,
Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C and
Penco M: NT-proBNP and antracycline-induced cardiotoxicity. Br J
Cancer. 105:1663–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papazisis K, Kontovinis LF, Papandreou CN,
Kouvatseas G, Lafaras C, Antonakis E, Christopoulou M, Andreadis C,
Mouratidou D and Kortsaris AH: Brain natriuretic peptic precursor
(NT-proBNP) level predict for clinical benefit to sunitinib
treatment in patients with metastatic renal cell carcinoma. BMC
Cancer. 10:4892010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossner D, Knobloch K, Lichtinghagen R,
Lichtenberg A, Kuehnle H and Lück HJ: NT-proBNP and Ca 125 as
potential markers of mortality during long-term immunotherapy with
trastuzumab in HER-2-possitive metastatic breast cancer. J Clin
Oncol. 22:804. 2014. View Article : Google Scholar
|
|
23
|
Ruggieri F, Noris A, Beretta L, Mortini P
and Gemma M: Serum B-type natriuretic peptide is affected by
neoplastic edema in patients with brain tumor. World Neurosurg.
85:193–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bunevicius A, Deltuva V, Laws ER, Iervasi
G, Tamsauskas A and Bunevicius R: Preoperative N-terminal
pro-B-type natriuretic peptide concentration and prognosis of brain
tumor patients: A 5-year follow up study. Sci Rep. 7:147752017.
View Article : Google Scholar : PubMed/NCBI
|