Introduction
Drug-induced lung injury (DLI) is a condition
characterized by inflammation, and eventual fibrosis, of the
interstitium of the lungs caused by administration of drugs
(1). Severe DLI has been reported
to occur at a particularly high frequency in patients receiving
sequential treatment with osimertinib following administration of a
programmed cell death ligand 1 (PD-L1) inhibitor (2). However, the relationship of sequential
treatment with an anti-epidermal growth factor receptor (EGFR)
antibodies and PD-L1 blocker with the incidence of DLI remains to
be elucidated. The prognosis and rates of continuation of the
anticancer therapy treatment vary greatly among patients developing
DLI and the efficacy of steroid therapy to treat the DLI has not
yet been established. However, in general clinical practice,
discontinuation of the causative agent and/or steroid treatment are
widely used in patients diagnosed as having significant DLI
(3). The efficacy of treatment
response to treatment of DLI is known to vary depending on its
clinical pattern. In general, the diffuse alveolar damage (DAD)
pattern is poorly responsive to steroids, whereas steroid therapy
appears to be more effective in cases showing the organizing
pneumonia (OP) pattern and somewhat less effective in cases with
the non-specific interstitial pneumonia (NSIP) or hypersensitivity
pneumonitis (HP) pattern (4).
Therefore, in actual clinical practice, both the treatment response
of DLI and the possibility of continuation of anticancer therapy in
patients developing DLI differ depending on the clinical pattern of
the DLI. If treatment resistance of DLI is expected, prompt and
aggressive treatment is warranted; if the DLI is likely to be mild,
however, careful continuation of the current anticancer treatment
may be possible.
Cetuximab, an anti-EGFR monoclonal antibody, and
immune checkpoint inhibitors are often used in the treatment of
head and neck cancer (HNC), although not in the same treatment
line. Cetuximab is often used in combination with cytotoxic
anticancer drugs or radiotherapy, and there are numerous reports of
its efficacy in patients with HNC (5,6). On
the other hand, immune checkpoint inhibitors are widely used as
second- or further-line treatment (7). Immune checkpoint inhibitor therapy has
also been shown to be highly effective when used in combination
with cytotoxic anticancer agents as first-line therapy (8). Meta-analyses have shown that the
incidence of lung injury is ~3% in patients receiving PD-1
inhibitor therapy (9,10), and that the development of DLI could
necessitate treatment discontinuation; the DLI associated with ICI
therapy frequently assumes a relatively less common, but more
severe form of DAD (11). The
purpose of the present study was to determine the incidence of DLI
in HNC patients treated receiving treatment with cetuximab and PD-1
inhibitors. It also attempt to provide useful information for HNC
patients receiving chemotherapy by providing a clinical
classification of DLI.
Materials and methods
Patients
The present study performed a retrospective review
of the medical records of all patients with HNC who received
treatment with cetuximab and/or PD-1 inhibitors (nivolumab or
pembrolizumab) at Chiba University Hospital between September 2014
and December 2020. The incidence and clinical background
characteristics of the patients who developed DLI were analyzed
(age, sex, smoking history, primary site, number of doses
administered of the PD-1 inhibitor and cetuximab, rate of combined
use of cetuximab with paclitaxel, rate of use of radiation therapy
for the head and neck region or the lungs).
Examination of drug-induced lung
injury (DLI)
DLI was defined as any new lung lesion developing
during or within 60 days after the last dose of treatment with a
PD-1 inhibitor and/or cetuximab; patients with other pulmonary
diseases (e.g., infection, pulmonary congestion) diagnosed based on
the medical records (clinical course, radiological findings,
laboratory findings, microbiological findings, etc.) were excluded
from this analysis (1). All the
chest computed tomographic (CT) images of the patients who were
treated for HNC with cetuximab and/or a PD-1 inhibitor were
reviewed independently by three pulmonologists (MA, SK and NS), and
interpreted by consensus among the three pulmonologists. In the
case of discrepancies, the three examiners reviewed the images
together to finalize the diagnosis by consensus. Based on the 2013
ATS guideline, DLI was classified into the following patterns
according to the CT findings: DAD, NSIP, OP, or HP (12).
Study design
The present study was a retrospective study
conducted to analyze the incidence of DLI and laboratory findings
in HNC patients who had received cetuximab and/or PD-1 inhibitor
therapy. Differences in the clinical characteristics of the
patients with and without DLI were also analyzed. The present study
was reviewed and approved by the institutional review board of
graduate school of medicine, Chiba University (approval number
3839). The study is registered in the University Hospital Medical
Information Network (UMIN000046895).
Statistical analysis
Age of patients and number of administration of
drugs were presented as mean ± SD (Table I, Table
II, Table III). All analyses
were performed using the statistical software SPSS 26.0 (SPSS Inc.;
IBM Corp.). One-way ANOVA and Dunnett's test were used to compare
the means of the three groups. When equal variances were
statistically confirmed, unpaired Student's t-test was used to
analyze the comparison of the means of the two groups. When equal
variances were not statistically confirmed, Mann-Whitney U test was
used. In the analysis for contingency table, χ2 was
used. When a contingency table has an expected count ≤5 in ≥20% of
the cells, Fisher's exact test was used. For comparisons of
proportions of three or more groups, the Bonferroni correction was
performed after the Z-test. Univariate and multivariate analyses
were performed to identify factors associated with the risk of DLI.
Factors identified as being significant by univariate analysis with
P-values of less than 0.05 were entered into the model for
multivariate logistic regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
 | Table I.Clinical background characteristics of
the patients. |
Table I.
Clinical background characteristics of
the patients.
| Characteristic | Group C+P (N=43) | Group C (N=101) | Group P (N=35) | P-value, among Group
C+P, Group C, and Group P | P-value, Group C vs.
Group C+P | P-value, Group P vs.
Group C+P |
|---|
| Mean ± SD age, | 60.9±9.6 | 68.2±9.1 | 68.0±9.2 |
<0.01a |
<0.01b |
<0.01b |
| years (range) | (40–78) | (42–84) | (40–80) |
|
|
|
| Sex,
male/female | 38/5 | 82/19 | 31/4 | n.s.c |
|
|
| Smoking,
smoker/ever smoked/never smoked | 33/5/5 | 47/33/21 | 14/13/8 |
<0.05c |
<0.05c |
<0.05c |
| Primary cancer | 2/10/5/2/ | 0/41/27/ | 1/6/4/0/ |
|
|
|
| site,
nasopharynx/ | 6/2/2/1/3/ | 10/3/4/2/ | 7/1/2/2/ |
|
|
|
| oropharynx/ | 1/1/8 | 2/2/0/2/8 | 4/1/0/7 |
|
|
|
| hypopharynx/ |
|
|
|
|
|
|
| larynx/tongue/ |
|
|
|
|
|
|
| buccal mucosa/ |
|
|
|
|
|
|
| maxillary
sinus/ |
|
|
|
|
|
|
|
mandible/parotid |
|
|
|
|
|
|
|
gland/tonsils/ear |
|
|
|
|
|
|
| canal/other |
|
|
|
|
|
|
| Primary cancer
site, | 17 | 68 | 11 |
<0.05c |
<0.05c | n.s.c |
| pharynx | (39.5%) | (67.3%) | (31.4%) |
|
|
|
| Number of | 11.8 | n/a | 7.1 |
|
| 0.04d |
| administrations
of | (1–54) |
| (1–23) |
|
|
|
| the PD-1 inhibitor,
mean (range) |
|
|
|
|
|
|
| Number of | 16.6 | 7.9 | n/a |
|
<0.01d |
|
|
administrations | (1–78) | (1–50) |
|
|
|
|
| of cetuximab, |
|
|
|
|
|
|
| mean (range) |
|
|
|
|
|
|
| Combined use
of | 24 | 3 | n/a |
|
<0.01e |
|
| paclitaxel
with | (55.8%) | (3.0%) |
|
|
|
|
| cetuximab |
|
|
|
|
|
|
| Head and neck | 28 | 91 | (88.6%) |
<0.05c |
<0.05c |
<0.05c |
| lesion
radiation | (65.1%) | (90.0%) |
|
|
|
|
| Lung radiation | 3 (7.0%) | 0 (0%) | 1 (2.9%) | n.s.c |
|
|
| Incidence of | 8 | 8 | 4 | n.s.c |
|
|
| pneumonitis | (18.6%) | (7.9%) | (11.4%) |
|
|
|
 | Table II.Clinical background characteristics
of the patients who received in whom both cetuximab and a PD-1
inhibitor. |
Table II.
Clinical background characteristics
of the patients who received in whom both cetuximab and a PD-1
inhibitor.
|
| Incidence of
drug-induced lung injury |
|
|---|
|
|
|
|
|---|
| Clinical
characteristic | (+) n=8 | (−) n=35 | Statistical
significance |
|---|
| Mean ± SD age,
years | 63.7±7.9 | 59.0±10.8 | n.s.a |
| Sex,
male/female | 6/2 | 32/3 | n.s.b |
| Primary cancer
site, pharynx | 1 (12.5%) | 16 (45.7%) | n.s.b |
| Mean ± SD number of
administrations of the PD-1 inhibitor | 19.1±15.5 | 10.1±10.1 |
P=0.047a |
| Median number of
administrations of cetuximab (range) | 22.5 (9–78) | 13.3±10.611
(1–52) |
P=0.015c |
| Combined use of
paclitaxel with cetuximab | 5 (62.5%) | 19 (54.3%) | n.s.b |
| PD-1 inhibitor
administration prior to cetuximab administration | 3 (37.5%) | 12 (34.3%) | n.s.b |
 | Table III.Differences in the clinical
background characteristics between patients who received cetuximab
and a PD-1 inhibitor in different orders. |
Table III.
Differences in the clinical
background characteristics between patients who received cetuximab
and a PD-1 inhibitor in different orders.
| Clinical
characteristic | PD-1 inhibitor
administration prior to cetuximab administration n=15 | Cetuximab
administration prior to PD-1 inhibitor administration n=28 | P-value |
|---|
| Mean ± SD age,
years | 61.0±11.6 | 60.9±8.6 | n.s.a |
| Sex,
male/female | 13/2 | 25/3 | n.s.b |
| Smoking,
smoker/ever smoked/never smoked | 10/2/3 | 23/3/2 | n.s.b |
| Primary cancer
site, pharynx | 7 (46.7%) | 10 (35.7%) | n.s.c |
| Mean ± SD number of
administrations of the PD-1 inhibitor | 10.8±10.2 | 12.3±12.5 | n.s.a |
| Mean ± SD number of
administrations of cetuximab | 14.6±18.3 | 17.7±14.9 | n.s.a |
| Combined use of
paclitaxel with cetuximab | 13 (86.7%) | 11 (39.3%) | 0.004b |
| Head and neck
lesion radiation | 9 (60.0%) | 19 (67.9%) | n.s.c |
| Lung radiation | 1 (6.7%) | 2 (7.1%) | n.s.b |
| Incidence of
pneumonitis | 3 (20.0%) | 5 (17.9%) | n.s.b |
| Distribution of the
clinical patterns of drug-induced lung injury, diffuse alveolar
damage/non-specific interstitial pneumonia/organizing
pneumonia/hypersensitivity pneumonitis | 1/0/2/0 | 1/2/2/0 | n.s.b |
Results
Frequency of occurrence of DLI
A total of 179 patients with head and neck cancer
received treatment with cetuximab and/or a PD-1 inhibitor at Chiba
University Hospital during the study period. The patients were
divided into three subgroups, as follows, and their outcomes
compared: Patients who had received sequential, not concurrent,
treatment with cetuximab and a PD-1 inhibitor (Group C+P; n=45);
patients who had received cetuximab, but not a PD-1 inhibitor
(Group C; n=101); and patients who had received a PD-1 inhibitor,
but not cetuximab (Group P; n=35; Table
I). The medical condition was accurately assessed and standard
medical treatment was provided. Of the 20 patients with DLI, three
were able to continue treatment without withdrawal, seven required
steroid treatment and the remaining 10 showed improvement only with
withdrawal.
Of the 43 patients in Group C+P, one patient
received pembrolizumab and the remaining 42 received nivolumab. All
35 patients of Group P received nivolumab. The median age of Group
C+P was significantly lower compared with that of the other two
groups. Combined use of paclitaxel with cetuximab was more frequent
in Group C+P than in Group C. The rates of occurrence of DLI in
Group C+P, Group C, and Group P were 18.6, 7.9 and 11.4%,
respectively.
The present study analyzed the background
characteristics of the patients in Group C+P (Table II). The frequency of the pharynx as
primary cancer site was lower and the number of doses of cetuximab
and PD-1 inhibitor administered were higher in the patients who
developed DLI than in those who did not. There was no correlation
between the frequency of DLI and the sequence of administration of
cetuximab and PD-1 inhibitor (Table
III).
Patient factors associated with
DLI
Univariate analysis identified sequential cetuximab
and PD-1 inhibitor therapy as a predictor of the development of
DLI, while use of radiotherapy was associated with a reduced risk
of DLI in the patients with HNC. Multivariate analysis failed to
identify any factors as being significantly associated with the
risk of DLI (Table IV).
 | Table IV.Factors associated with the risk of
drug-induced lung injury. |
Table IV.
Factors associated with the risk of
drug-induced lung injury.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Risk factor | OR (95%CI) | P-value | OR (95%CI) | P-value |
|---|
| Aged >66.4
yearsa | 2.42
(0.62-9.45) | 0.20 |
|
|
| Male sex | 0.82
(0.17-4.03) | 0.81 |
|
|
| Smoking |
|
|
|
|
|
Smoker | 0.74
(0.22-2.52) | 0.63 |
|
|
|
Never-smoked | 1.66
(0.42-6.61) | 0.47 |
|
|
| Primary cancer
site, pharynx | 0.17
(0.02-1.53) | 0.11 |
|
|
| Head and neck
lesion radiationb | 0.20
(0.06-0.71) | 0.01 | 0.29
(0.08-1.10) | 0.07 |
| Sequential
chemotherapy with cetuximab and PD-1 inhibitorb | 4.25
(1.23-14.7) | 0.02 | 2.99
(0.79-11.2) | 0.11 |
|
Cetuximab-containing chemotherapy without
PD-1 inhibitor | 2.53
(0.31-20.5) | 0.38 |
|
|
| PD-1
inhibitor-containing therapy without cetuximab | 2.39
(0.67-8.48) | 0.18 |
|
|
| Number of
administrations of cetuximab >7c | 3.64
(0.93-14.2) | 0.06 |
|
|
| Number of
administrations of the PD-1 inhibitor >5c | 3.06
(0.88-10.6) | 0.08 |
|
|
Clinical types of DLI and
prognosis
The present study determined the clinical types of
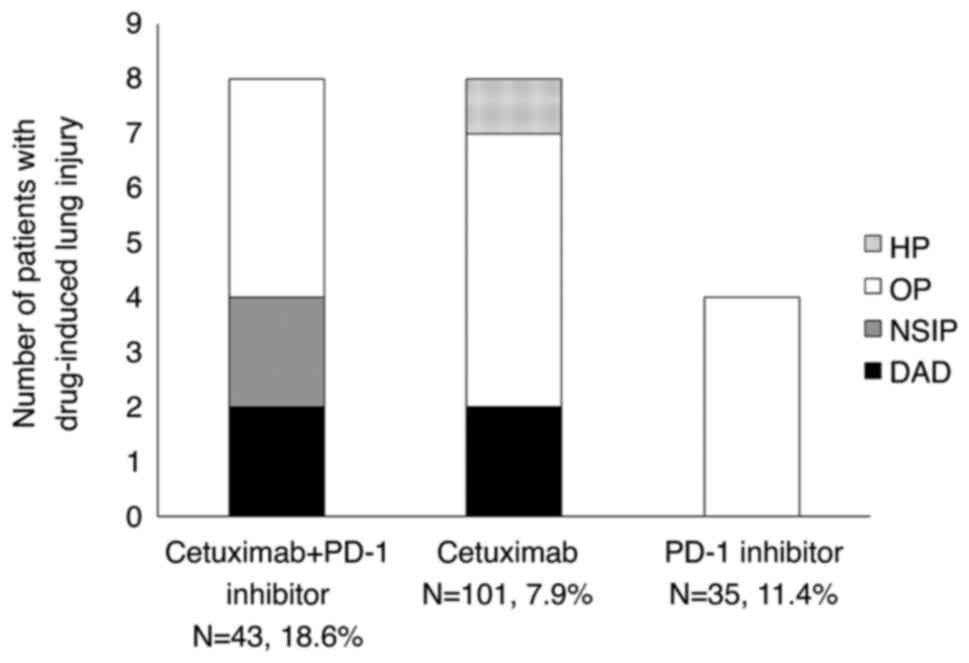
DLI according to the findings on chest CT (Table V and Fig. 1). In Group C+P and Group C, DAD was
observed in two patients each. The cancer treatment was
discontinued in all four patients with pneumonia of DAD, and two of
them succumbed to pneumonia. By contrast, the pattern of DLI in all
the patients of Group P was OP. In regard to the prognosis in
patients with DLI, chemotherapy was discontinued and best
supportive care (BSC) initiated in 6 out of the 8 patients in Group
C+P and all patients in Group P. However, the cancer therapy could
be continued despite the development of DLI in ~50% of the patients
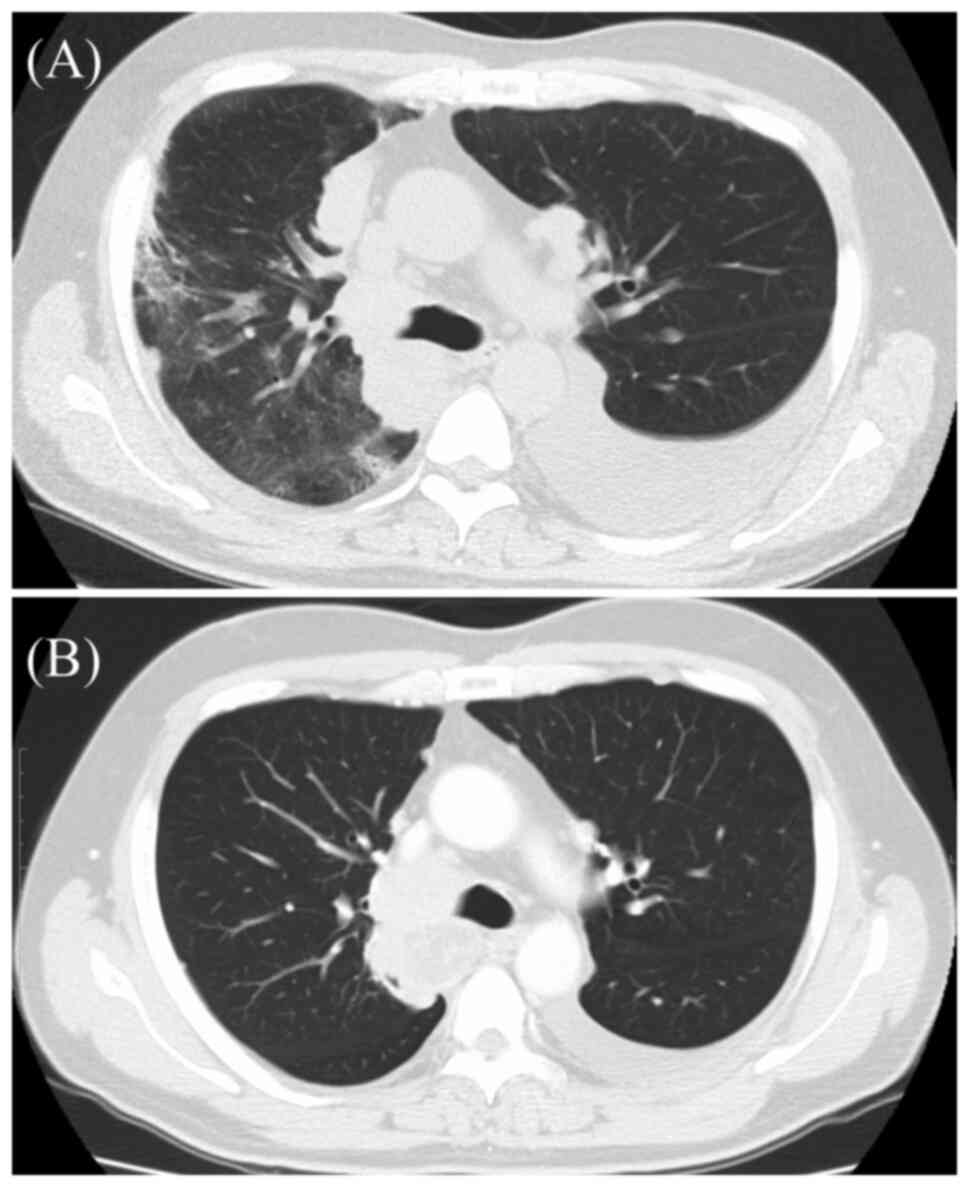
of Group C. A case of DLI in the C+P group, classified as DAD, is
shown in Fig. 2.
 | Table V.Clinical types and outcomes of
DLI. |
Table V.
Clinical types and outcomes of
DLI.
| Clinical type and
outcome | Group C+P n=8 | Group C n=8 | Group P n=4 |
|---|
| Clinical
classification, DAD/NSIP/OP/HP | 2/2/4/0 | 2/0/5/1 | 0/0/4/0 |
| After occurrence of
DLI |
|
|
|
|
Continuation of the anticancer
treatment | 0 (0%) | 4 (50%) | 0 (0%) |
| Best
supportive care due to disease progression | 1 (12.5%) | 0 (0%) | 2 (50%) |
| Best
supportive care due to the development of DLI | 5 (62.5%) | 3 (37.5%) | 2 (50%) |
| Start
of new regimen | 2 (25%) | 1 (12.5%) | 0 (0%) |
Discussion
In patients with HNC, a high incidence of DLI was
observed in patients who received sequential cetuximab and PD-1
inhibitor therapy. A expert opinion of European Society for Medical
Oncology and a guideline of American Society of Clinical Oncology
also comment that DLI due to ICI is rare but can be serious
(13,14). DLI due to ICI should be widely known
to clinicians administering ICIs. The clearly higher rate of DLI in
patients receiving treatment with both an immune checkpoint
inhibitor and cetuximab as compared with that in patients receiving
cetuximab alone is a very important finding clinically. Symptoms of
lung injury vary, but some typical symptoms include easy
fatigability, shortness of breath, dry cough, chest pain, fever and
skin rash. (15,16). In patients with DLI, it is not only
important to investigate the symptoms in detail, but also to
examine the factors associated with the development of the lung
injury (e.g., history of radiation therapy and concomitant
autoimmune disease) (17). Just as
administration of osimertinib following ICI therapy has been
reported as being problematic in patients with non-small cell lung
cancer, the present study showed that it is important to be aware
of the high risk of DLI associated with sequential anti-EGFR
antibody and ICI treatment, regardless of the sequence in which the
two drugs are administered, in patients with HNC. Attention must
also be paid to the previous drug use history. There have been
numerous reports of DLI in patients treated with cetuximab alone
and a PD-1 inhibitor alone. Until now, evaluation of DLI has been
conducted mostly in relation to the use of any drug used alone. In
recent years, however, combinations of drugs with different
mechanisms of action (cytotoxic anticancer drugs, molecular
targeted therapies and immune checkpoint inhibitors) have come to
be widely used, including as first-line therapies. Use of drug
combinations, even as first-line therapy, has become rather common
for patients HNC (8). Therefore,
DLI caused by combined use of drugs also needs to be properly
evaluated. As demonstrated in the present study, use of drug
combinations even sequentially, rather than simultaneously, can
increase the risk of lung injury, although this increase in risk
was not shown to be statistically significant. The incidence of DLI
in Group C+P (18.6%) appeared to be the result of occurrence of DLI
with either a PD-1 inhibitor or cetuximab used alone (Group C,
7.9%; Group P, 11.4%). It was hypothesized that the additive number
of the incidence of DLI in Group C and P might be observed in Group
C+P. As shown in Table II, a
significantly higher cumulative dose of each drug had been
administered in cases of Group C+P that developed DLI. This may
simply indicate that the probability of DLI increases as the number
of doses administered of a drug increases. Although there was a
significant difference in the mean number of doses, as shown in
Table II, the number of cetuximab
doses administered in the DLI group varied in the range of 9–78
(1–52 in the non-DLI group) and the number of PD-1 inhibitor doses
administered varied in the range of 3–42 (2–54 in the non-DLI
group). These ranges were so broad that it was difficult to
determine a safe threshold for the number of doses for either drug.
In the analyses to identify significant factors, the number of
doses administered of either drug was not extracted as a
significant factor, whereas sequential use of the two drugs
was.
Therefore, in patients with HNC, it is important to
pay closer attention to the risk of development of DLI as the
number of doses administered of cetuximab or an ICI increase,
especially in patients with a previous history of use of either
drug. In the present study, pharynx as the primary site of the HNC
was less frequent in patients who developed DLI. By contrast, in
Group C, in which the incidence of DLI was low, pharyngeal cancer
was so common that it accounted for 60% of all the cases. A
possible reason for the low DLI in the pharyngeal cancer cases
might be the high number of pharyngeal cancers in the Group C cases
with low DLI. It is valuable to perform CT at defined intervals to
assess patients for the development of pneumonia. However, the
present study was a retrospective study and there was no fixed
interval between the CT examinations. In the future, evaluation of
DLI by CT performed at fixed intervals would be desirable.
Of the patients included in the present study, there
were 20 cases of DLI. In 50% of these cases (10 cases), the
development of DLI necessitated discontinuation of the anticancer
treatment and initiation of BSC. It is difficult to determine if
the development of DLI might directly worsen the survival prognosis
in patients with advanced cancer, because the survival prognosis is
influenced by a variety of factors. In fact, the estimated mean
survival durations from the date of completion of the latter of the
PD-1 inhibitor and cetuximab treatment were 572±128 and 843±135
days in patients with and without DLI, respectively and this
difference was not statistically significant (P=0.692, log-rank
test). However, most cases of DLI required a change of treatment
and >50% of the cases could receive only BSC with only a few
patients able receive another new treatment (Table V). Therefore, the influence of the
development of DLI on the survival prognosis and quality of life of
patients with advanced cancer is undeniable. Next, the present
study attempted to analyze the factors associated with the
development of DLI in the patients, but unfortunately, it was
unable to identify any significant factors associated with the risk
of DLI. The number of HNC patients in Group C+P who received
radiation therapy was significantly lower compared with that in the
other two groups (28/43 vs. 122/136; P<0.01; χ2
test). Based on the foregoing, radiation to HNC and sequential
chemotherapy with cetuximab and PD-1 inhibitor are likely to be
confounding factors. It is also noteworthy that the prognosis of
the patients differed according to the clinical pattern of DLI. A
total of 20% of patients who received both cetuximab and a PD-1
inhibitor, as well as those who used cetuximab, developed the DAD
pattern of DLI. It is possible that cetuximab use was strongly
associated with DAD, but there have been no reports to date of
cetuximab being profoundly associated with DAD. However, there have
been reports of a poor association between ICI and DAD (18,19).
It was hypothesized that this might be due to the weak association
between ICI use alone and DAD, as previously reported, which is
only an estimation and not supported by statistical analysis. DAD
is considered as being associated with a poor prognosis. In fact,
all four patients who developed DAD required treatment
discontinuation and two of the patients succumbed as a direct
result of DLI. Therefore, the risk of development of DLI should be
clearly understood in HNC patients receiving therapy with both a
PD-1 inhibitor and cetuximab. However, in the cases with the OP
pattern of DLI, treatment discontinuation was necessitated in only
30.7% (4/13) of patients and in 46.2% (6/13) of patients, the
treatment could be completed or the next line of treatment could be
started. In all the four patients of Group C in whom
discontinuation of the anticancer treatment was necessitated, the
common terminology criteria for adverse events grade of DLI was 1,
with no associated clinical symptoms. These findings are consistent
with previous reports of the usefulness of clinical classification
of DLI for determining a patients' prognosis and subsequent course
of treatment (20,21).
Consistent with the findings of the present study,
Matsuo et al (22) also
reported a high incidence of DLI among patients who were treated
with cetuximab and a PD-1 inhibitor. Especially, DLI occurred more
frequently in patients who received PD-1 inhibitor monotherapy
followed by cetuximab-containing chemotherapy than in patients who
received other regimens. As mentioned earlier, in the case of
sequential administration of osimertinib and an ICI, the sequence
of administration was critical for determining the risk of
development of drug-induced lung damage. The present study, on the
other hand, found that the sequence in which the PD-1 inhibitor and
cetuximab were administered had no influence on the frequency of
DLI; this could be because the number of cases was too small.
The present study reviewed the CT images of all the
cases retrospectively and found that in numerous cases of cetuximab
use without a PD-1 inhibitor, the evidence of DLI visualized on the
CT images was not considered as a clinically significant problem
and treatment was continued, in some cases, without the CT findings
of DLI having been detected at all. Therefore, it may be difficult
to evaluate the exact frequency of occurrence and the influence of
the sequence of administration of the culprit drugs by examining
only those cases in which the treatment was interrupted or
terminated due to the obvious development of lung injury.
It is not uncommon for dysphagia to occur during or
following treatment in patients with cancer in the head and neck
region (23,24). Dysphagia leads to undernutrition,
weight loss and prolonged unnatural food intake (tube feeding) and
is a major risk factor for aspiration. Therefore, it is very
important to clearly distinguish between DLI and aspiration
pneumonia in these patients in order to provide appropriate
treatment.
The present study had some limitations. It was a
single-center study, which could have introduced bias in relation
to the patient backgrounds. In particular, cetuximab is used more
often in combination with radiotherapy. The combination of
cetuximab and radiotherapy is now less commonly used because of its
limited efficacy (25). Second, CT
evaluations were not performed at defined intervals, but rather at
the discretion of the attending physician, which may have resulted
in asymptomatic lung damage having been overlooked. However, CT
examinations are usually performed and evaluated once every ~6
months during follow-up after treatment and during treatment the
intervals are even shorter, so that the lack of a defined interval
between CT examinations may have had little impact on the detection
rate of DLI. Third, the present study did not perform
bronchoalveolar lavage fluid examination to diagnose DLI. Although
it is known to be useful in differentiating aspiration pneumonia
from bacterial pneumonia (26), it
was difficult to aggressively perform these tests in advanced
cancer patients who were not necessarily in a conducive physical
condition. The present study showed a high prevalence of DLI
associated with sequential use of anti-EGFR antibodies and ICIs,
but it may have limited clinical impact, as this drug combination
has so far been used only for a limited number of types of cancer.
In view of future expansion of the indications of ICI therapy, it
is suggested that the finding of the present study serves as
potentially important information for the treatment of types of
cancer of other organs in the future. In fact, both anti-EGFR
antibodies and ICIs have already been used in micro satellite
instability-high or tumor mutation burden-high cases of colorectal
cancer. In addition, the mean age was statistically significantly
lower in Group C+P (P<0.01; unpaired t-test) in Table I, although the reason was unclear.
It was hypothesized that the C+P group would tend to be younger
because it is a group of cases that can tolerate multiple
treatments. That is, cases with good general health and few
complications can tolerate multiple treatments. On the other hand,
older patients are presumed to have more complications, making it
difficult for them to progress to second- and third-line treatment,
although there were no results that were accompanied by
statistically significant differences. To prove this hypothesis, it
is necessary to unify attending physician judgment in selecting
regimens that include cetuximab and nivolumab. This is an important
issue for future studies.
There are a number of reports on the occurrence of
DLI with single agents (13,27).
Furthermore, as multiple drugs are increasingly used in
combination, there are more reports on the occurrence of DLI in
such cases (28–30). However, DLI with anticancer drugs
used at different times rather than simultaneously has not yet been
adequately studied. A report of DLI with osimertinib and ICI
(2), as noted, indicates that
future attention should be paid to DLI with cetuximab and an ICI as
well. Matsuo et al (22)
reported similar results; in addition, the present study classified
clinical types of DLI and showed its frequency of occurrence and
the effect on prognosis or treatment strategy. This will be useful
for patient management. Unfortunately, the present study was unable
to find differences in the frequency of DLI occurrence due to
differences in the order of drug administration. It is hoped that
the accumulation of cases will clarify this issue. In conclusion,
DLI is relatively more common in patients who receive sequential
therapy with cetuximab and a PD-1 inhibitor. Furthermore, DLI in
these patients often assumes a serious pattern (DAD). The sequence
in which the two classes of drugs were administered seemed to have
no influence on the risk of DLI according to the analysis in the
present study. Further accumulation of cases is required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MaA, MiA and YT conceived and designed the study,
and confirm the authenticity of all the raw data. MaA wrote the
paper. SK and NS evaluated CT images. IO, KT, CI, HS, TS, KU, and
TH analyzed the clinical data and diagnosed the relation between
drug and diseases. YT critically revised the manuscript. All
authors were involved in the interpretation of the data and
preparation of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Ethical Committee of the Graduate School of Medicine, Chiba
University (approval no. 3839) and conducted in accordance with the
ethical guidelines for medical research in humans in Japan.
The present study did not involve any invasion or
intervention on the patient and used only information such as
medical information. A number of the patients had either succumbed
or had completed their hospital visits. The authors publicly
announced the research plan and guaranteed the opportunity to
refuse as much as possible. The ethical committee of the Graduate
School of Medicine, Chiba University permitted this opt-out
method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD-L1
|
programmed cell death ligand 1
|
|
DLI
|
drug-induced lung injury
|
|
EGFR
|
epidermal growth factor receptor
|
|
CT
|
compute tomography
|
|
DAD
|
diffuse alveolar damage
|
|
NSIP
|
nonspecific interstitial pneumonia
|
|
OP
|
organizing pneumonia
|
|
HP
|
hypersensitivity pneumonitis
|
|
HNC
|
head and neck cancer
|
|
BSC
|
best supportive care
|
References
|
1
|
Kubo K, Azuma A, Kanazawa M, Kameda H,
Kusumoto M, Genma A, Saijo Y, Sakai F, Sugiyama Y, Tatsumi K, et
al: Consensus statement for the diagnosis and treatment of
drug-induced lung injuries. Respir Investig. 51:260–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oxnard GR, Yang JC, Yu H, Kim SW, Saka H,
Horn L, Goto K, Ohe Y, Mann H, Thress KS, et al: TATTON: A
multi-arm, phase Ib trial of osimertinib combined with selumetinib,
savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol.
31:507–516. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrelli F, Signorelli D, Ghidini M,
Ghidini A, Pizzutilo EG, Ruggieri L, Cabiddu M, Borgonovo K,
Dognini G, Brighenti M, et al: Association of steroids use with
survival in patients treated with immune checkpoint inhibitors: A
systematic review and meta-analysis. Cancers (Basel). 12:5462020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bradley B, Branley HM, Egan JJ, Greaves
MS, Hansell DM, Harrison NK, Hirani N, Hubbard R, Lake F, Millar
AB, et al: Interstitial lung disease guideline: The British
thoracic society in collaboration with the thoracic society of
Australia and New Zealand and the Irish thoracic society. Thorax.
63 (Suppl 5):v1–v58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Licitra L, Mesia R, Rivera F, Remenár É,
Hitt R, Erfán J, Rottey S, Kawecki A, Zabolotnyy D, Benasso M, et
al: Evaluation of EGFR gene copy number as a predictive biomarker
for the efficacy of cetuximab in combination with chemotherapy in
the first-line treatment of recurrent and/or metastatic squamous
cell carcinoma of the head and neck: EXTREME study. Ann Oncol.
22:1078–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrington KJ, Ferris RL, Blumenschein G
Jr, Colevas AD, Fayette J, Licitra L, Kasper S, Even C, Vokes EE,
Worden F, et al: Nivolumab versus standard, single-agent therapy of
investigator's choice in recurrent or metastatic squamous cell
carcinoma of the head and neck (CheckMate 141): Health-related
quality-of-life results from a randomised, phase 3 trial. Lancet
Oncol. 18:1104–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi FS: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
systematic review and meta-analysis. JAMA Oncol. 2:1607–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khunger M, Rakshit S, Pasupuleti V,
Hernandez AV, Mazzone P, Stevenson J, Pennell NA and Velcheti V:
Incidence of pneumonitis with use of programmed death 1 and
programmed death-ligand 1 inhibitors in non-small cell lung cancer:
A systematic review and meta-analysis of trials. Chest.
152:271–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito Y, Sasaki S, Oikado K, Tominaga J,
Sata M, Sakai F, Kato T, Iwasawa T, Kenmotsu H, Kusumoto M, et al:
Radiographic features and poor prognostic factors of interstitial
lung disease with nivolumab for non-small cell lung cancer. Cancer
Sci. 112:1495–1505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rochester CL, Vogiatzis I, Holland AE,
Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi
R, Clini EM, et al: An official American thoracic society/European
respiratory society policy statement: Enhancing implementation,
use, and delivery of pulmonary rehabilitation. Am J Respir Crit
Care Med. 192:1373–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conte P, Ascierto PA, Patelli G, Danesi R,
Vanzulli A, Sandomenico F, Tarsia P, Cattelan A, Comes A, De
Laurentiis M, et al: Drug-induced interstitial lung disease during
cancer therapies: Expert opinion on diagnosis and treatment. ESMO
Open. 7:1004042022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rossi SE, Erasmus JJ, McAdams HP, Sporn TA
and Goodman PC: Pulmonary drug toxicity: Radiologic and pathologic
manifestations. Radiographics. 20:1245–1259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohnishi H, Yokoyama A, Yasuhara Y,
Watanabe A, Naka T, Hamada H, Abe M, Nishimura K, Higaki J, Ikezoe
J and Kohno N: Circulating KL-6 levels in patients with drug
induced pneumonitis. Thorax. 58:872–875. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camus P, Fanton A, Bonniaud P, Camus C and
Foucher P: Interstitial lung disease induced by drugs and
radiation. Respiration. 71:301–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balaji A, Hsu M, Lin CT, Feliciano J,
Marrone K, Brahmer JR, Forde PM, Hann C, Zheng L, Lee V, et al:
Steroid-refractory PD-(L)1 pneumonitis: Incidence, clinical
features, treatment, and outcomes. J Immunother Cancer.
9:e0017312021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chuzi S, Tavora F, Cruz M, Costa R, Chae
YK, Carneiro BA and Giles FJ: Clinical features, diagnostic
challenges, and management strategies in checkpoint
inhibitor-related pneumonitis. Cancer Manag Res. 9:207–213. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travis WD, Matsui K, Moss J and Ferrans
VJ: Idiopathic nonspecific interstitial pneumonia: Prognostic
significance of cellular and fibrosing patterns: Survival
comparison with usual interstitial pneumonia and desquamative
interstitial pneumonia. Am J Surg Pathol. 24:19–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kambouchner M, Levy P, Nicholson AG,
Schubel K, Magois E, Feuillet S, Valeyre D, Bernaudin JF and Nunes
H: Prognostic relevance of histological variants in nonspecific
interstitial pneumonia. Histopathology. 65:549–560. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuo M, Yasumatsu R, Masuda M, Toh S,
Wakasaki T, Hashimoto K, Uchi R, Jiromaru R, Sato K, Manako T and
Nakagawa T: Drug-induced interstitial lung disease in recurrent
and/or metastatic head and neck cancer patients treated with
cetuximab and/or nivolumab. Oral Oncol. 113:1051292021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McConnel FM and O'Connor A: Dysphagia
secondary to head and neck cancer surgery. Acta Otorhinolaryngol
Belg. 48:165–170. 1994.PubMed/NCBI
|
|
24
|
Platteaux N, Dirix P, Dejaeger E and Nuyts
S: Dysphagia in head and neck cancer patients treated with
chemoradiotherapy. Dysphagia. 25:139–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korpics MC, Turchan WT, Koshy M and
Spiotto MT: Decreased overall survival in patients with locally
advanced head and neck cancer receiving definitive radiotherapy and
concurrent cetuximab: National cancer database analysis. Head Neck.
44:1528–1544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raghu G, Remy-Jardin M, Myers JL, Richeldi
L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F,
et al: Diagnosis of idiopathic pulmonary fibrosis. An official
ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care
Med. 198:e44–e68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skeoch S, Weatherley N, Swift AJ, Oldroyd
A, Johns C, Hayton C, Giollo A, Wild JM, Waterton JC, Buch M, et
al: Drug-induced interstitial lung disease: A systematic review. J
Clin Med. 7:3562018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spagnolo P, Bonniaud P, Rossi G,
Sverzellati N and Cottin V: Drug-induced interstitial lung disease.
Eur Respir J. 60:21027762022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baumhäkel M, Kasel D, Rao-Schymanski RA,
Böcker R, Beckurts KT, Zaigler M, Barthold D and Fuhr U: Screening
for inhibitory effects of antineoplastic agents on CYP3A4 in human
liver microsomes. Int J Clin Pharmacol Ther. 39:517–528. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilbert CJ, Petros WP, Vredenburgh J,
Hussein A, Ross M, Rubin P, Fehdrau R, Cavanaugh C, Berry D,
McKinstry C and Peters WP: Pharmacokinetic interaction between
ondansetron and cyclophosphamide during high-dose chemotherapy for
breast cancer. Cancer Chemother Pharmacol. 42:497–503. 1998.
View Article : Google Scholar : PubMed/NCBI
|