Introduction
Liver cancer (LC) is a common malignancy worldwide
(1,2). The most recent data from the National
Cancer Centre in Beijing, China, show that LC is a common malignant
tumour, and the mortality (17.1 per 100,000) and morbidity (18.3
per 100,000) rates associated with LC in China are higher compared
with the global averages (mortality rate of 8 per 100,000 and
morbidity rate of 10.1 per 100,000) in 2018 (3–5).
Moreover, most patients with liver disease, such as hepatitis B or
C-associated viral infections, and patients who abuse alcohol,
which makes early diagnosis more difficult, are more likely to
develop LC compared with other individuals (6). The pathogenesis of LC is not clear,
and targeted treatments are lacking (7,8).
Therefore, it is important to identify specific tumour markers to
improve the treatment and prognosis of patients with LC.
Chromosomal instability is believed to be one of the
causes of LC (9). Timely activation
of the anaphase-promoting complex (APC) is considered to be an
important factor for maintaining accurate chromosome segregation
(10). APC is a polymeric protein
complex that is composed of multiple tetratricopeptide repeat
proteins: APC2, APC3, APC5, APC6, APC7, APC8 and APC11 (11). Previous studies have confirmed that
abnormal regulation of APC-related tetratricopeptide repeat
proteins may be involved in tumourigenesis (12–14),
including LC (15,16).
Cell division cycle 23 (CDC23), which is also known
as APC8, is an APC subunit that regulates mitosis by catalysing the
formation of ubiquitin conjugates (17,18).
Both the end of mitosis and the start of a new cell cycle depend on
the ubiquitin-mediated degradation of key cell cycle proteins
(19). CDC23 was identified in a
genetic screen using Saccharomyces cerevisiae, and it was
found that the products of CDC23 catalysis are required for
ubiquitination (20). A study on
papillary thyroid cancer (PTC) revealed that CDC23 was
overexpressed in PTC tissue compared with normal tissue and that
CDC23 exerts important biological effects on thyroid cancer cell
proliferation and cell cycle progression (21). In addition, a previous study
confirmed that inhibition of microRNA-204-3p by LINC00514 increased
CDC23 expression and further led to PTC progression (22). Moreover, knockdown of CDC23 by small
interfering RNA induced G2/M arrest in breast cancer
cells, although CDC23 has not been further studied in these cells
(23).
Notably, although numerous studies on APC-related
proteins have been carried out in cancer (12–14),
studies on the role of CDC23 in LC are still lacking. Therefore,
the main aim of the present study was to explore the role of CDC23
in LC using in vitro and in vivo experiments.
Materials and methods
Cell lines and culture
The cell lines used in the present study include the
normal human liver cell line THLE-2 and LC cell lines (HepG2,
Hep3B, Huh7, LM3), which were purchased from Shanghai Institutes
(Shanghai Biowing Applied Biotechnology, Co., Ltd.). These cell
lines were authenticated by short tandem repeat profiling. The
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% foetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). The cell culture media were supplemented
with 100 U/ml penicillin and streptomycin at 37°C in 5%
CO2.
Lentiviral infection
GV493 Lentivirus expressing short hairpin (sh)RNA
targeting CDC23 (sh-CDC23) and negative control (NC) lentivirus
(sh-NC) were designed and constructed by Shanghai Jikai Gene
Chemical Technology Co., Ltd. The sh-CDC23 target sequence was
5′-CCAGTGTTACATCAAATAT-3′, while the sh-NC target sequence was
5′-TTCTCCGAACGTGTCACGT-3′. HepG2 and LM3 cells were transduced with
these lentiviruses for 24 h (MOI=20). Subsequently, the medium was
replaced with fresh complete culture medium supplemented with 5
µg/ml puromycin for 5 days, and the cells were harvested for
subsequent experiments.
Western blotting
All cells and tissues were lysed in RIPA lysis
buffer (Applygen Technologies, Inc.) and centrifuged at 4°C for 30
min (300 × g) to obtain the lysates. The specific experimental
steps were described in our previous study (24). The protein concentration was
determined by the bicinchoninic acid (BCA) method. Proteins (50
µg/lane) were separated on 10% gels using SDS-PAGE and transferred
to PVDF membranes. The following primary antibodies were used:
Anti-CDC23 (cat. no. ab177148; 1:1,000), anti-E-cadherin (cat. no.
ab76055; 1:1,000), anti-N-cadherin (cat. no. ab18203; 1:1,000),
anti-vimentin (cat. no. ab8978; 1:1,000) (all from Abcam) and
anti-β-actin (cat. no. CL488-66009; 1:2,000; Proteintech Group,
Inc.). Antibodies were incubated with the PVDF membranes overnight
at 4°C. The Horseradish peroxidase-conjugated secondary antibodies
(1:8,000; cat. nos. sc-2357 and sc-2005; Santa Cruz Biotechnology,
Inc.) were incubated with the PVDF membranes at room temperature
for 1 h. The target protein was detected with a Pierce enhanced
chemiluminescence detection reagent (Thermo Fisher Scientific,
Inc.).
Cell proliferation assay
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8; Sigma-Adrich; Merck KGaA) assays according
to the manufacturer's instructions. For the CCK-8 assays,
1×104 HepG2 and LM3 cells/well were seeded in 96-well
plates. After 24, 48, 72 and 96 h, a total of 10 µl CCK-8 solution
was added to each well. The plates were incubated at 37°C for 2 h,
and the absorbance values at 450 nm were measured using a
microplate reader (Thermo Fisher Scientific, Inc.).
Colony formation assay
Cells in the logarithmic growth phase from the
sh-CDC23 group and sh-NC group were digested with EDTA + 0.25%
trypsin at 37°C for 3 min, and dissolved into single-cell
suspensions in DMEM culture media supplemented with 10% FBS. A
total of 2×102 cells were inoculated into in 6-well
plates, and the cells were cultured in 2 ml complete media. The
cells were placed in an incubator and cultured at 37°C with 5%
CO2 and saturated humidity for 14 days. Cell growth was
observed during culture, and the culture was stopped when
macroscopic colonies appeared in the culture dish. The supernatants
were discarded, and the cells were carefully washed twice with PBS.
The cells were fixed with 5 ml 100% methanol for 15 min at room
temperature and then stained using 1 ml 0.1% crystal violet for 15
min at room temperature. Each group was run in triplicate, and the
number of colonies formed (>50 cells) was manually
calculated.
Transwell assays
HepG2 and LM3 cells (2.0×105/ml) in DMEM
without serum were added to the upper chambers of a Transwell plate
(24-well, 8.0-µm pores) (BD Biosciences). DMEM supplemented with
10% FBS was added to the lower chambers. For the invasion assays, a
total of 50 µl Matrigel (BD Biosciences) was used to precoat the
membrane surface before addition of the cells at 37°C for 4 h.
After incubation at 37°C for 48 h, the non-invaded cells were
removed, and the invaded cells were stained with a 0.1% crystal
violet solution at 37°C for 10 min. The cells were observed under
an inverted fluorescence microscope.
Animal studies
A total of 10 male BALB/c nude mice (weight, 16–18
g; age, 4 weeks) were obtained from Hunan Slack Jingda Experimental
Animal Co., Ltd. [production permit no. SCXK (XIANG) 2013-0004].
The mice were randomly divided into two groups (sh-NC and sh-CDC23
groups; n=5 mice/group) and subcutaneously injected with
2×106 LM3 cells. The mice were monitored weekly
(25,26) and the tumour volume (formula: L ×
S2 × 0.5, where L and S represent the maximum and
minimum diameter of the tumour, respectively) was assessed. After 4
weeks, the mice were anesthetized with isoflurane (3–6%), followed
by cervical dislocation to ensure death. All details of the housing
conditions were described in our previous study (27). The present study was approved by the
Ethics Committee of The First Affiliated Hospital of Nanchang
University (Nanchang, China; approval no. 202112020). All
experiments were conducted in accordance with Nanchang University
and Canadian Council on Animal Care (CCAC) ethical guidelines. CCAC
guidelines were used to define humane endpoints, including a tumor
not exceeding 10% of the animal's body weight, a tumor location
that does not affect normal functions or cause pain, weight loss of
>20%, ulceration or infection of the growth site, metastases and
self-mutilation.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6 (GraphPad Software; Dotmatics) and SPSS 18 (SPSS, Inc.).
The data are presented as the mean ± standard deviation of three
independent experiments. Unpaired Student's t-test was used to
assess differences between two groups. One-way ANOVA followed by
Dunnett's multiple comparisons test was utilized to analyse the
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
CDC23 is highly expressed in LC cell
lines
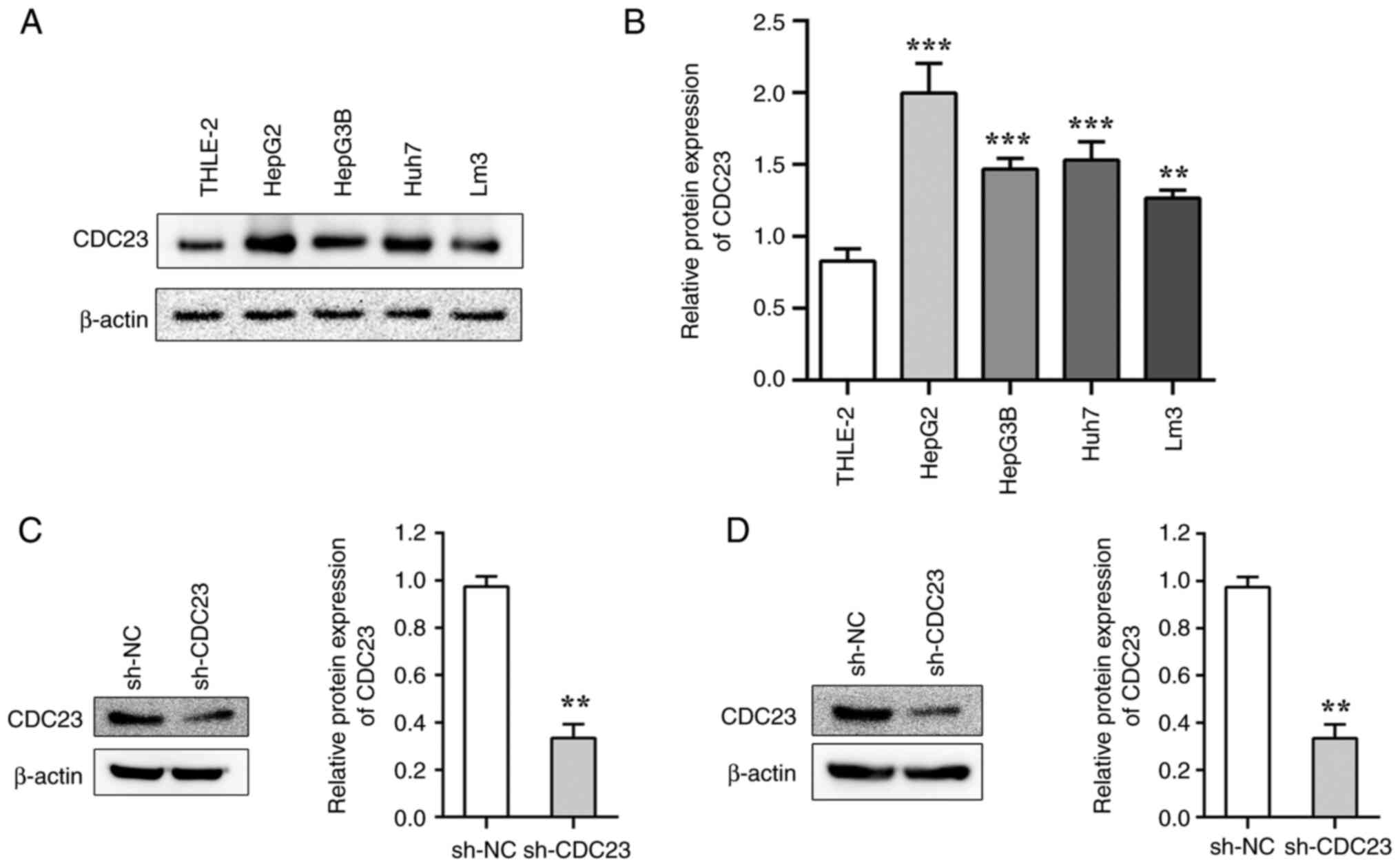
The results showed that the protein expression
levels of CDC23 were significantly higher in LC cell lines compared
with that in the normal liver cell line THLE-2 (Fig. 1A and B). Among the LC cell lines,
CDC23 was highly expressed in LM3 and HepG2. Therefore, the LM3 and
HepG2 cell lines were selected for further experiments. After
transduction with sh-CDC23, the protein expression levels of CDC23
were significantly decreased in LM3 and HepG2 cells compared with
the sh-NC group (Fig. 1C and
D).
Knockdown of CDC23 inhibits the
proliferation and colony formation of LM3 and HepG2 cells
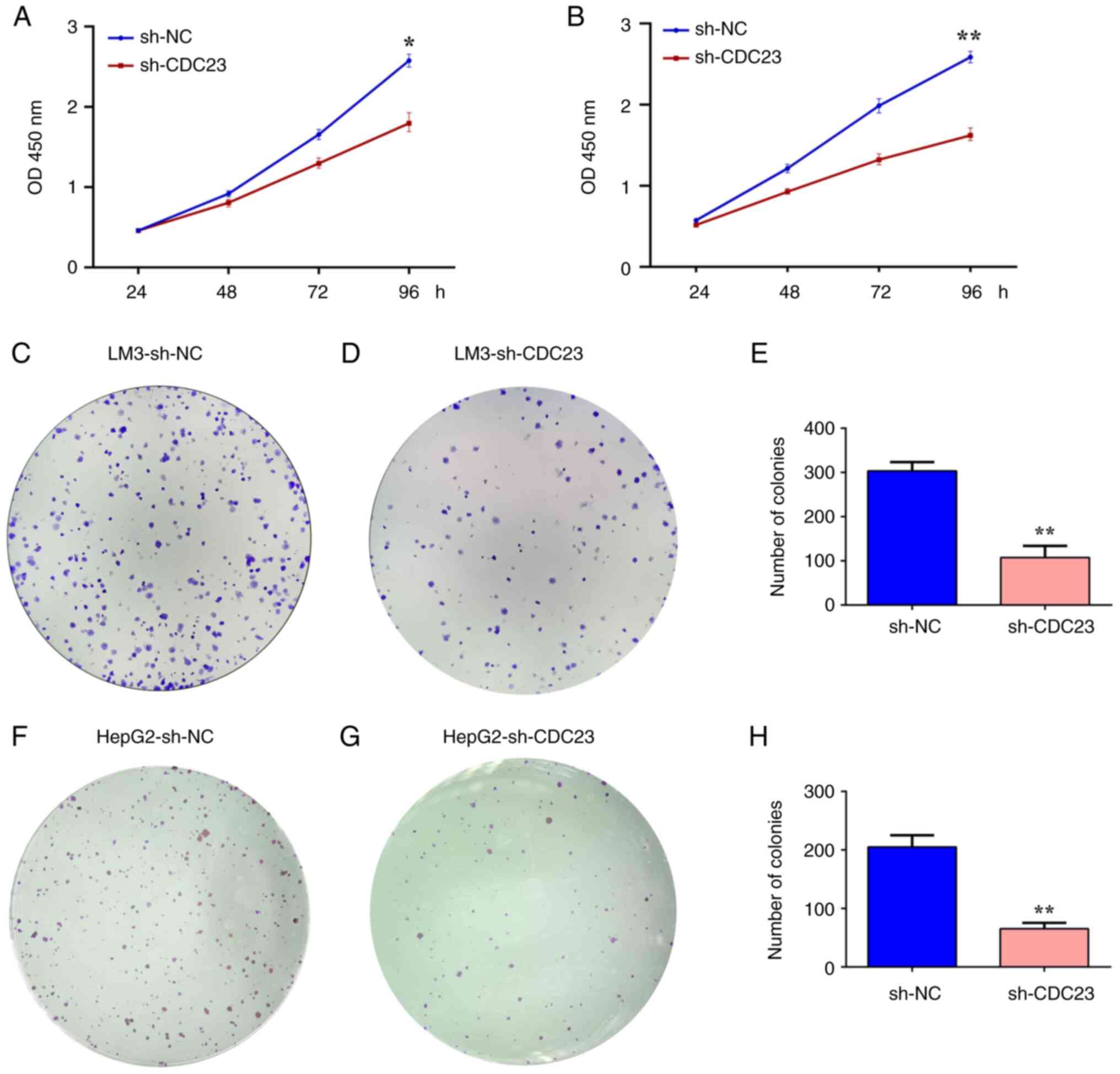
The experimental results of the present study showed
that CDC23 was highly expressed in LC cell lines, and we
hypothesized that CDC23 may promote the progression of LC. CCK-8
and colony formation assays were used to assess the effects of
CDC23 on the proliferation and colony formation ability of LC cell
lines. CCK-8 assays showed that knockdown of CDC23 significantly
suppressed the proliferation of LM3 and HepG2 cells (Fig. 2A and B). Colony formation
experiments also revealed that knockdown of CDC23 significantly
suppressed the clonogenic ability of LM3 and HepG2 cells (Fig. 2C-H).
CDC23 knockdown reduces the migratory
and invasive abilities of LC cells
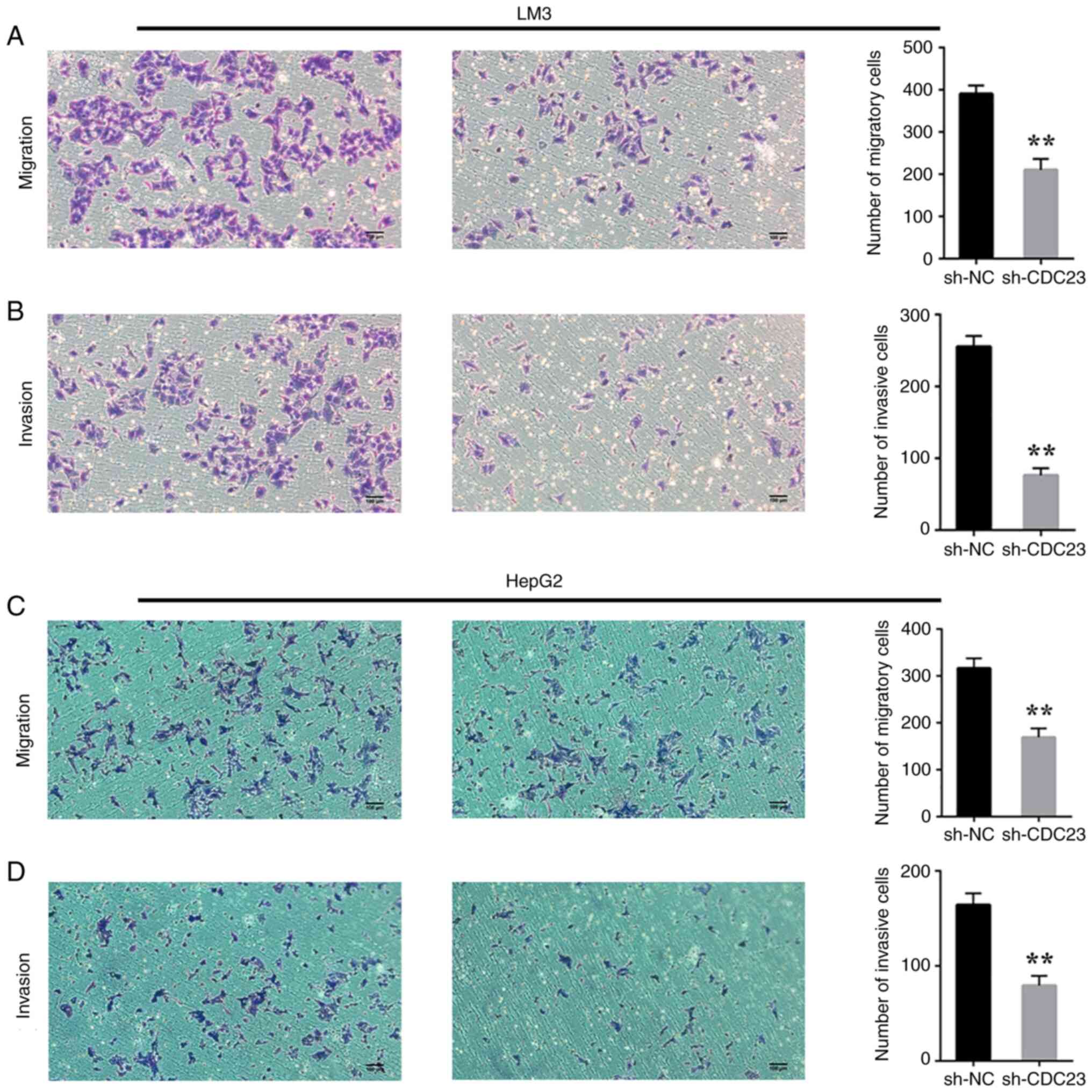
Considering the effect of CDC23 on the proliferation
and colony formation of LC cells, it was examined whether CDC23
also affects the invasion and migration of these cells. A Transwell
assay was carried out a to verify this hypothesis. As expected, the
assay results showed that knockdown of CDC23 significantly
inhibited the migratory and invasive abilities of LM3 (Fig. 3A and B) and HepG2 cells (Fig. 3C and D).
Effect of silencing CDC23 on the
expression of epithelial-mesenchymal transition (EMT)-related
molecules
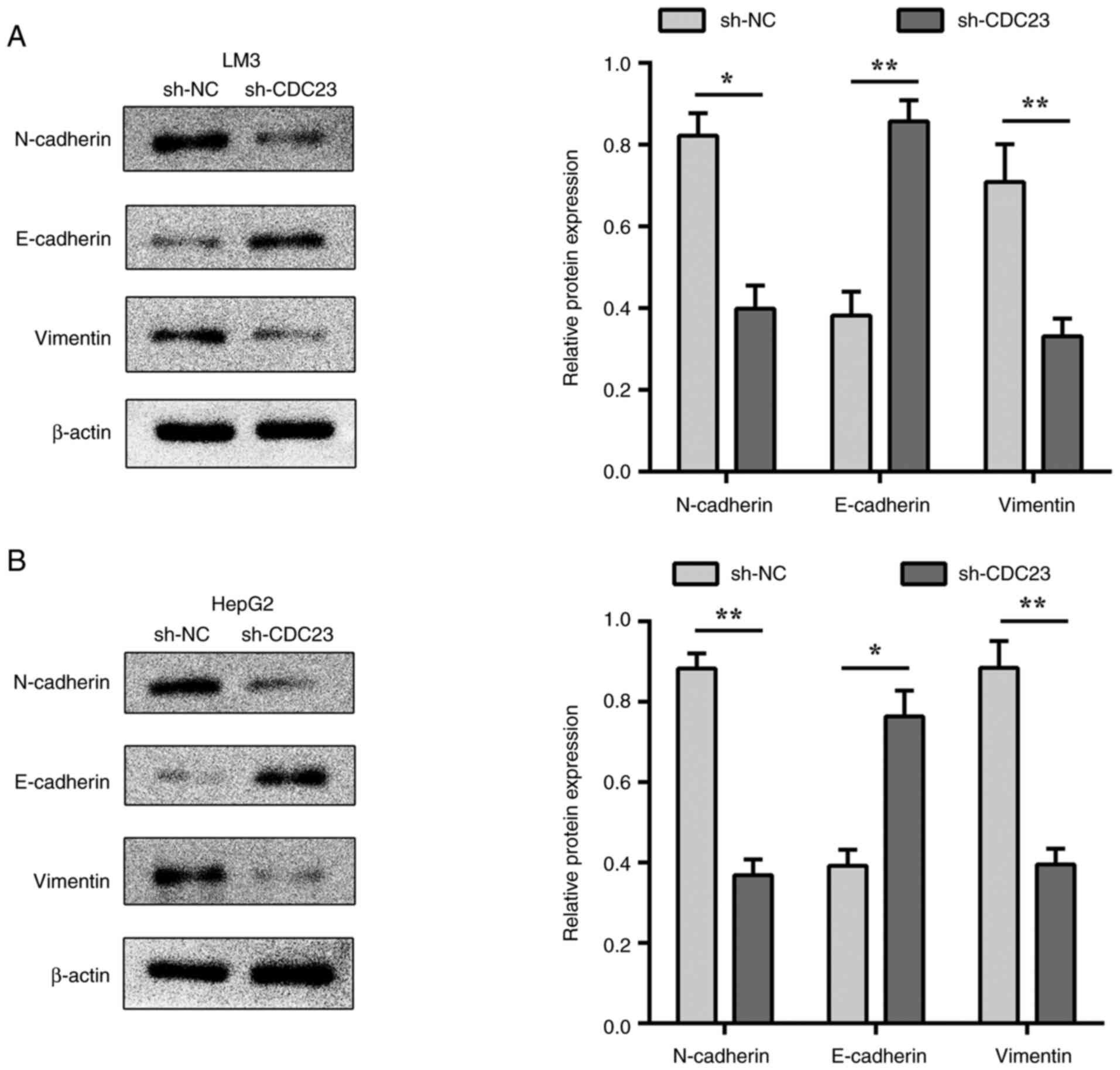
EMT plays a vital role in cancer cell metastasis
(28). N-cadherin, vimentin and
E-cadherin are key regulatory molecules of EMT (29). The results of the present study
indicated that the knockdown of CDC23 significantly increased
E-cadherin expression in LM3 and HepG2 cells (Fig. 4A and B), while the expression levels
of N-cadherin and vimentin were significantly decreased (Fig. 4). These results suggested that CDC23
may affect LC metastasis by regulating the EMT process in LC
cells.
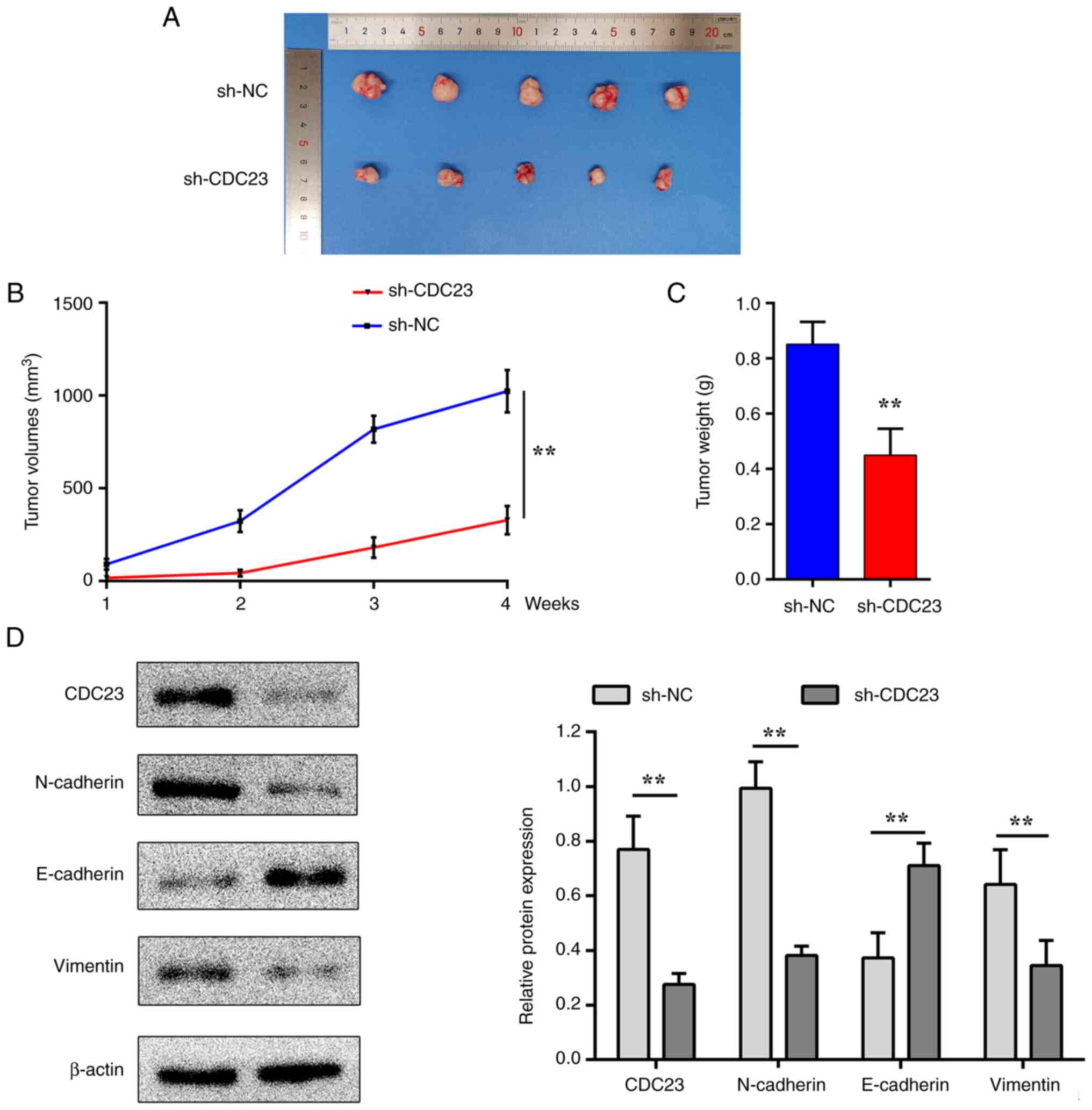
Knockdown of CDC23 inhibits tumour
growth in vivo
A tumour xenograft model was established to assess
the role of CDC23 in vivo. In vivo experiments were
further conducted to evaluate the effect of CDC23 knockdown on LM3
cells in mice (Fig. 5A). The
results indicated that the tumour volumes in the sh-CDC23 group
were significantly lower compared with those in the sh-NC group
(Fig. 5B). In addition, the tumour
weights were higher in the sh-NC group compared with the sh-CDC23
group (Fig. 5C). Western blot
assays also revealed that the protein expression level of
E-cadherin was increased in the sh-CDC23 group compared with sh-NC,
while those of N-cadherin and vimentin were decreased instead
(Fig. 5D). These results were
consistent with those from the in vitro experiments.
Discussion
The role of CDC23 in cancer has attracted the
attention of the scientific community (21,23).
Interestingly, a previous study assessed whether CDC23 is a tumour
suppressor gene by searching for CDC23 mutations in 5q-abnormal
myeloid leukaemia cells (30).
However, experimental results suggested that CDC23 may not be
involved in the progression of myeloid leukaemia characterized by
chromosome 5 abnormalities (30).
Thus, in the present study, it was experimentally
evaluated whether CDC23 suppresses or promotes LC.
To determine whether CDC23 is specifically expressed
in LC, the protein expression level of CDC23 was examined and found
to be significantly higher in LC cell lines compared with a normal
liver cell line. This finding may suggest that CDC23 may impact the
LC cell phenotype and functions. Migration and proliferation are
closely associated with the high mortality rate of cancer, and
these processes are also main obstacles for successfully curing
cancer (31–33). Therefore, whether CDC23 affects the
LC cell phenotype was investigated in the present study. The
experimental results indicated that the proliferation and migration
of the HepG2 and LM3 cell lines were significantly inhibited after
CDC23 gene knockdown. The results suggested that high CDC23
expression was closely associated with the proliferation and
migration of LC cells.
The metastatic capacity of tumour cells is closely
related to the high morbidity and mortality of cancer, with 50% of
1,438 patients with breast cancer developing metastasis within 10
years, and it is also a main hurdle in preventing cancer regression
(31). EMT is a key cellular
process required for embryonic development and its role in
initiating and promoting tumour cell metastasis and invasion, as
well as the underlying mechanism, have received increasing
attention (34,35). The metastasis of tumour cells
involves EMT, directed invasion of the surrounding tissue of tumour
cells, invasion of tumour cells into the blood circulation,
lymphatic circulation and vascular exudation (36). EMT is regulated by numerous
calcium-dependent cell adhesion molecules that regulate epithelial
properties, including E-cadherin, N-cadherin and vimentin (29,37).
These EMT markers have been confirmed to be associated with the
progression of various tumours, including breast cancer (38), laryngeal squamous cell carcinoma
(39) and pancreatic carcinoma
(40). However, the functions of
these three proteins (E-cadherin, N-cadherin and vimentin) on
tumour metastasis is not the same. For example, overexpression of
E-cadherin is involved in inhibiting tumour metastasis, whilst
overexpression of N-cadherin and vimentin is involved in promoting
tumour metastasis (38–40). Moreover, multiple studies have
confirmed that the functions of these three proteins in liver
cancer are similar to those aforementioned (41–43).
The experimental results of the present study
indicated that inhibition of CDC23 expression in LC cell lines
significantly reduced cancer cell invasion. Notably, it was
hypothesized that the mechanism by which CDC23 regulates
tumourigenesis involves tumour EMT and metastasis. In the present
study, the expression of EMT markers was investigated. The results
indicated that the expression of E-cadherin was significantly
increased while the expression levels of N-cadherin and vimentin
were significantly decreased after knockdown of CDC23 in HepG2 and
LM3 cells. The aforementioned results revealed that CDC23 may
regulate LC progression through EMT in vitro. However, how
CDC23 regulates EMT is not clearly established and the lack of
CDC23 overexpression experiments is a limitation of the present
study.
However, the biological environment is also
relevant, and whether these in vitro results can be verified
in vivo still requires further investigation. In vivo
experiments were performed to confirm the effect of the CDC23
knockdown that was observed in the in vitro experiments.
Nude mice were injected with cells that had been transduced with
sh-NC or sh-CDC23. After analysis, significant differences in the
tumour dimensions and weight between the two groups were observed.
In addition, western blot assays revealed that the protein
expression of E-cadherin was increased, while the expression levels
of CDC23, N-cadherin and vimentin were decreased in the sh-CDC23
group compared with sh-NC. These results indicated that CDC23
knockdown in LC cells inhibited tumour growth in vivo. Thus,
the in vitro results were consistent with the in vivo
results.
In conclusion, the present study demonstrated that
CDC23 is highly expressed in LC cell lines. In addition, CDC23
emerged as a regulator of the malignant biological behaviour of LC
cell lines through modification of the expression of EMT markers,
which may reveal a novel target for further studying LC growth and
metastasis.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82103165 to JX), The Education
Department of Jiangxi Province Science and Technology Research
Projects (grant no. GJJ160246 to JX) and Young Talent Cultivation
Project of the First Affiliated Hospital of Nanchang University
(grant no. YFYPY202007 to JX).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and JX designed the study. YZ, LL, CF and WH
analysed the data. YZ and LL contributed to performing the
experiments and wrote the manuscript. YL and JX confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal study was approved by the Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China; approval no. 202112020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng
J, Feletto E, Canfell K, Qu C and Chen W: Is it possible to halve
the incidence of liver cancer in China by 2050? Int J Cancer.
148:1051–1065. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu J and Wang H: Precision diagnosis and
treatment of liver cancer in China. Cancer Lett. 412:283–288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villanueva A: Hepatocellular Carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Lu Z and Zhao X: Tumorigenesis,
diagnosis, and therapeutic potential of exosomes in liver cancer. J
Hematol Oncol. 12:1332019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fulgenzi CAM, Talbot T, Murray SM,
Silletta M, Vincenzi B, Cortellini A and Pinato DJ: Immunotherapy
in hepatocellular carcinoma. Curr Treat Options Oncol. 22:872021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eferl R and Trauner M: Chromosomal
instability in HCC: A key function for checkpoint kinase 2. Gut.
67:204–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sudakin V, Chan GK and Yen TJ: Checkpoint
inhibition of the APC/C in HeLa cells is mediated by a complex of
BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 154:925–936. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vodermaier HC, Gieffers C, Maurer-Stroh S,
Eisenhaber F and Peters JM: TPR subunits of the anaphase-promoting
complex mediate binding to the activator protein CDH1. Curr Biol.
13:1459–1468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishida H, Miwa H, Tatsuta M, Masutani S,
Imamura H, Shimizu J, Ezumi K, Kato H, Kawasaki T, Furukawa H and
Kawakami H: Ki-67 and CEA expression as prognostic markers in
Dukes' C colorectal cancer. Cancer Lett. 207:109–115. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melloy PG: The anaphase-promoting complex:
A key mitotic regulator associated with somatic mutations occurring
in cancer. Genes Chromosomes Cancer. 59:189–202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
VanGenderen C, Harkness TAA and Arnason
TG: The role of anaphase promoting complex activation, inhibition
and substrates in cancer development and progression. Aging (Albany
NY). 12:15818–15855. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Gao JZ, Du JL, Huang ZX and Wei LX:
Increased CDC20 expression is associated with development and
progression of hepatocellular carcinoma. Int J Oncol. 45:1547–1555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Tang Q, Ni R, Huang X, Wang Y, Lu
C, Shen A, Wang Y, Li C, Yuan Q, et al: Early mitotic inhibitor-1,
an anaphase-promoting complex/cyclosome inhibitor, can control
tumor cell proliferation in hepatocellular carcinoma: Correlation
with Skp2 stability and degradation of p27(Kip1). Hum Pathol.
44:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prinz S, Hwang ES, Visintin R and Amon A:
The regulation of Cdc20 proteolysis reveals a role for APC
components Cdc23 and Cdc27 during S phase and early mitosis. Curr
Biol. 8:750–760. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hershko A: Mechanisms and regulation of
the degradation of cyclin B. Philos Trans R Soc Lond B Biol Sci.
354:1571–1575. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas C, Wetherall B, Levasseur MD,
Harris RJ, Kerridge ST, Higgins JMG, Davies OR and Madgwick S: A
prometaphase mechanism of securin destruction is essential for
meiotic progression in mouse oocytes. Nat Commun. 12:43222021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zachariae W and Nasmyth K: TPR proteins
required for anaphase progression mediate ubiquitination of mitotic
B-type cyclins in yeast. Mol Biol Cell. 7:791–801. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Rahbari R, He M and Kebebew E:
CDC23 regulates cancer cell phenotype and is overexpressed in
papillary thyroid cancer. Endocr Relat Cancer. 18:731–742. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Zhong W, Xu Y, Yu B and Liu H:
Silencing of lncRNA LINC00514 inhibits the malignant behaviors of
papillary thyroid cancer through miR-204-3p/CDC23 axis. Biochem
Biophys Res Commun. 508:1145–1148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Achari C, Winslow S, Ceder Y and Larsson
C: Expression of miR-34c induces G2/M cell cycle arrest in breast
cancer cells. BMC Cancer. 14:5382014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong J, Feng Z, Li Z, Zhong T, Yang Z, Tu
Y, Xiao T, Jie Z and Cao Y: Overexpression of TWA1 predicts poor
prognosis in patients with gastric cancer. Pathol Res Pract.
215:1525942019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhuang Y, Li X, Zhan P, Pi G and Wen G:
MMP11 promotes the proliferation and progression of breast cancer
through stabilizing Smad2 protein. Oncol Rep. 45:2021. View Article : Google Scholar
|
|
26
|
Rezaeian AH, Li CF, Wu CY, Zhang X,
Delacerda J, You MJ, Han F, Cai Z, Jeong YS, Jin G, et al: A
hypoxia-responsive TRAF6-ATM-H2AX signalling axis promotes HIF1α
activation, tumorigenesis and metastasis. Nat Cell Biol. 19:38–51.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Li Z, Fan X, Xiong J, Zhang G,
Luo X, Li K, Jie Z, Cao Y, Huang Z, et al: PRL-3 promotes gastric
cancer peritoneal metastasis via the PI3K/AKT signaling pathway
in vitro and in vivo. Oncol Lett. 15:9069–9074.
2018.PubMed/NCBI
|
|
28
|
Pan G, Liu Y, Shang L, Zhou F and Yang S:
EMT-associated microRNAs and their roles in cancer stemness and
drug resistance. Cancer Commun (Lond). 41:199–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:49472019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao N, Lai F, Fernald AA, Eisenbart JD,
Espinosa R, Wang PW and Le Beau MM: Human CDC23: cDNA cloning,
mapping to 5q31, genomic structure, and evaluation as a candidate
tumor suppressor gene in myeloid leukemias. Genomics. 53:184–190.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mierke CT: The matrix environmental and
cell mechanical properties regulate cell migration and contribute
to the invasive phenotype of cancer cells. Rep Prog Phys.
82:0646022019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Liu D, Feng Z, Mao J, Zhang C, Lu
Y, Li J, Zhang Q, Li Q and Li L: MicroRNA-138 modulates metastasis
and EMT in breast cancer cells by targeting vimentin. Biomed
Pharmacother. 77:135–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhandari A, Zheng C, Sindan N, Sindan N,
Quan R, Xia E, Thapa Y, Tamang D, Wang O, Ye X and Huang D: COPB2
is up-regulated in breast cancer and plays a vital role in the
metastasis via N-cadherin and Vimentin. J Cell Mol Med.
23:5235–5245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greco A, De Virgilio A, Rizzo MI, Pandolfi
F, Rosati D and de Vincentiis M: The prognostic role of E-cadherin
and β-catenin overexpression in laryngeal squamous cell carcinoma.
Laryngoscope. 126:E148–E155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L, Guo P, He Y, Chen Z, Chen L, Luo
Y, Qi L, Liu Y, Wu Q, Cui Y, et al: HCC-derived exosomes elicit HCC
progression and recurrence by epithelial-mesenchymal transition
through MAPK/ERK signalling pathway. Cell Death Dis. 9:5132018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|