Introduction
In China, gastric cancer (GC) is one of the most
common malignant tumors of the digestive system. As the fifth most
common cancer and third most deadly cancer, >1,000,000 new cases
and ~783,000 deaths from GC were reported worldwide in 2018
(1). Gastric cancer onset is
insidious, progresses rapidly, and is associated with poor
treatment efficacy. Although patients with GC can undergo radical
treatment through surgical resection in the early stages, the
postoperative recurrence rate is as high as 60%, and the long-term
prognosis of patients remains unsatisfactory. The postoperative
recurrence of GC has become the primary cause of death in GC
patients, which is related to the tumor size, degree of
differentiation, pathological type, and TNM stage (2,3). In
addition, several studies have confirmed that vessel invasion (VI)
is an independent risk factor for the recurrence and metastasis of
locally advanced GC (4–8). Therefore, accurate preoperative
evaluation of VI in locally advanced GC is of great clinical
significance. The evaluation of VI traditionally requires
postoperative pathological examination, which delays the
formulation of a preoperative individualized treatment plan
(9). Therefore, exploring methods
of noninvasive assessment of VI prior to surgery to determine the
best treatment strategy for patients with locally advanced GC is
particularly important. Radiomics is based on medical imaging
images to obtain more objective, quantitative, deeper, and visually
complex tumor features. With the continuous development and
improvement of radiomics, the diagnostic ability of artificial
intelligence (AI)-based methods has become equal to or even better
than that of human experts in several different types of cancer. As
a result of this success, AI approaches are currently helping solve
more complex clinical decision-making challenges, such as tumor
identification and prediction, the study of the tumor
microenvironment, assessment of the response to different treatment
modalities, identification of treatment-related changes, and
imaging representations of genotypic features associated with
prognosis (10–12).
In the present study, radiomics analysis was
performed based on computed tomography (CT) images of the portal
venous phase of locally advanced GC, combined with the diagnostic
model established by AI. A variety of machine-learning algorithms
were used for comparison, aiming to explore the value of
machine-learning models based on the radiomics characteristics of
portal vein phase CT in predicting VI of locally advanced GC before
surgery.
Materials and methods
Patients
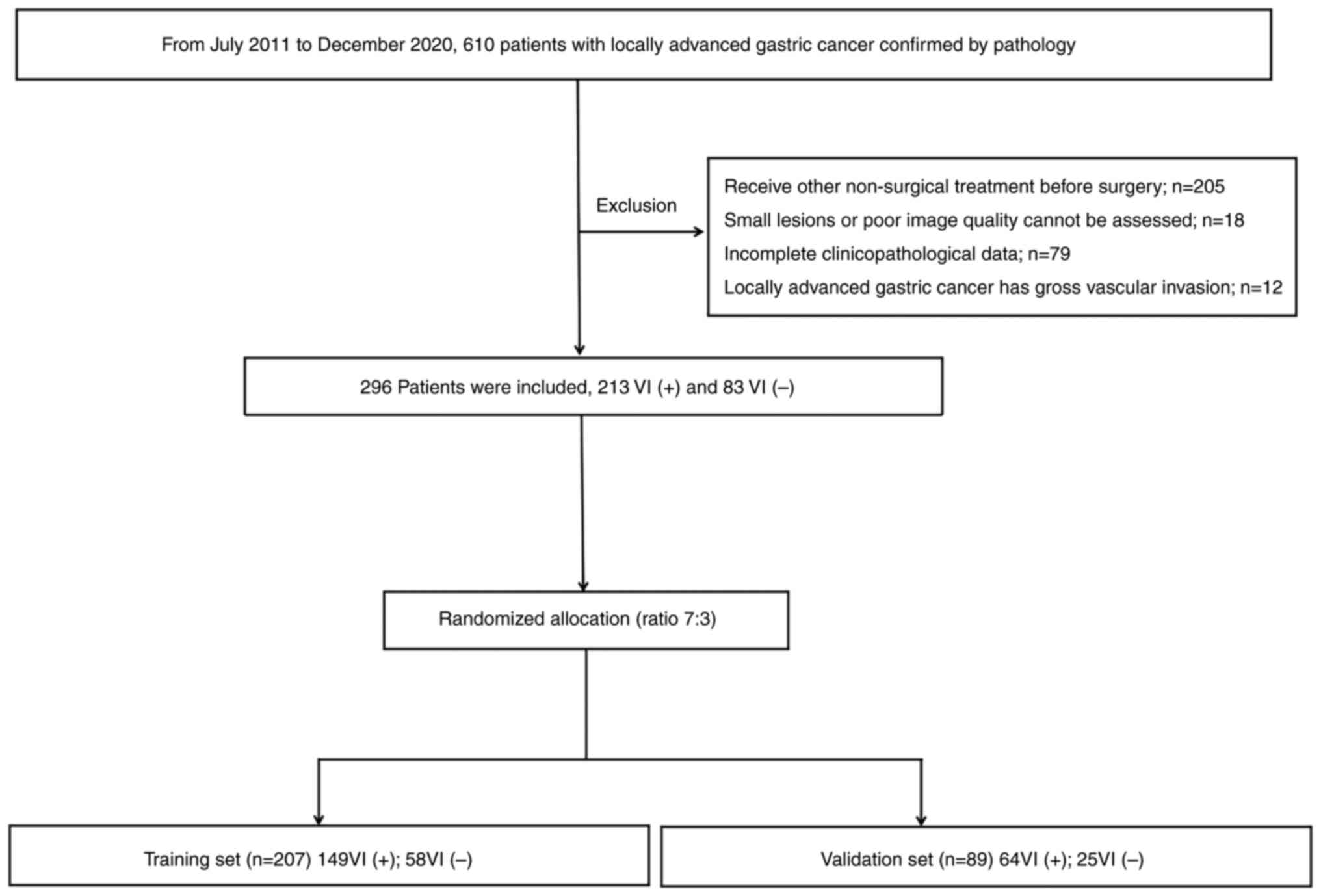
Between July 2011 and December 2020, information was
retrospectively collected from 296 patients (214 men and 82 women)
with locally advanced GC who were admitted to our hospital and
whose diagnoses were confirmed through pathological examination.
Their ages varied from 28 to 86 years, with the median age of 60.75
years. The inclusion criteria were as follows: Patients underwent
radical surgical resection, locally advanced GC was pathologically
confirmed with VI results, and abdominal contrast-enhanced CT
(CECT) examination was performed ≤2 weeks before surgery. The
exclusion criteria were as follows: Received radiotherapy,
chemotherapy, radiochemotherapy, or other nonsurgical treatments
before surgery; lesions too small or images where the quality was
too poor to be evaluated; incomplete clinicopathological data; and
macroscopic VI by the tumor. The pathological evaluation of VI was
based on the presence of tumor cell infiltration in the lymphatic
vessels and arteriovenous vessels of the peritumor under the
microscope (9). Based on
postoperative pathological results, 213 patients were confirmed as
VI+, and 83 patients were VI-. Patients were divided into training
(n=207) and validation sets (n=89) using a stratified sampling
method according to the ratio of 7:3. Among them, 149 VI+ and 58
VI- cases were assigned to the training set, while 64 VI+ and 25
VI- cases were assigned to the validation set. The flowchart of
case screening is presented in Fig.
1. Clinical data of patients in terms of sex, age, location,
TNM stage, degree of differentiation, Lauren's classification,
carcinoembryonic antigen levels, and CA125 levels were collected.
The study was approved by the Institutional Review Board of The
First Hospital of Zhengzhou University (Zhengzhou, China; approval
no. 2021-KY-1070-002).
CT image acquisition protocol
A Discovery CT 750HD scanner was used to perform the
CECT examination. The primary imaging parameters were as follows:
Tube voltage, 100 kVp; tube current, selection automatic mA; pitch,
1.375; detector collimation, 0.625 mm; rotation time, 0.5-0.6 sec;
and reconstruction slice thickness, 5 and 1.25 mm. Contrast medium
(370 mg I/ml) was injected through the cubital vein with a
high-pressure syringe at a flow rate of 3.5 ml/sec and a dose of
1.5 ml/kg body mass. Arterial phase and venous phase scans were
performed at 25 and 60 sec after the injection of the contrast
agent.
Radiomic analysis
The portal venous phase thin slice images (1.25 mm)
of locally advanced GC were introduced into Pyradiomics (version
3.0, http://pyradiomics.readthedocs.io) for imaging feature
extraction and analysis.
Tumor segmentation
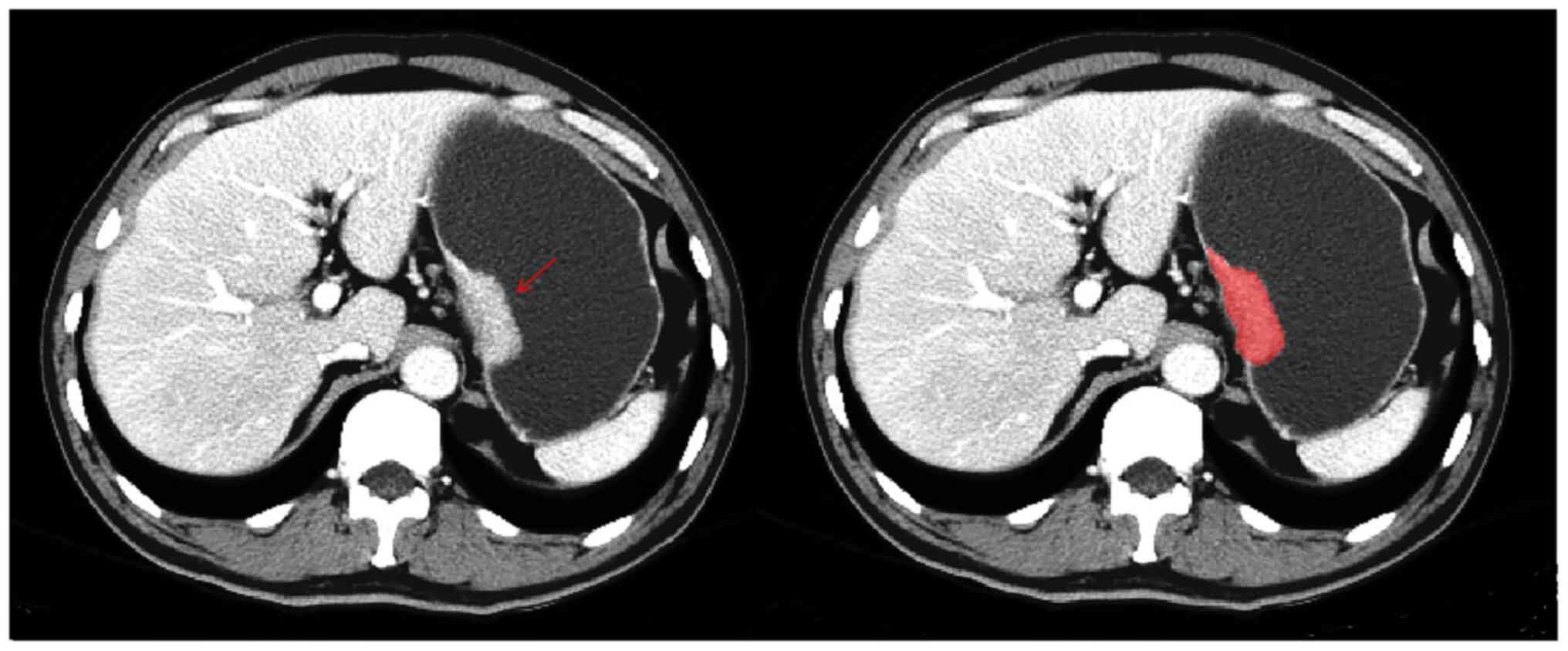
The tumor region of interest (ROI) was manually
delineated based on the portal venous phase CT images. The images
were exported in Digital Imaging and Communications in Medicine
format. Two abdominal imaging radiologists with 5 years of
diagnostic experience (doctor A) and 13 years of diagnostic
experience (doctor B), respectively, were asked to select the
largest slice of the tumor and determine the contour (Fig. 2). If an agreement could not be
reached, the results were judged by another radiologist with 36
years (doctor C) experience in abdominal diagnosis. CT images of 50
cases were randomly selected, and the ROIs were delineated by
doctors A and B every 2 weeks, respectively, and radiomics features
were extracted. An intraclass correlation coefficient (ICC)
>0.75 indicated good consistency.
Radiomics feature extraction and
screening
In total, 864 quantitative features of locally
advanced GC were extracted by Pyradiomics based on portal venous
phase images, including first-order features, shape features, gray
co-occurrence matrix features, gray run matrix features, gray
region size matrix features, adjacent gray difference matrix
features, and gray dependence matrix features. The Mann-Whitney U
test and Student's t-test were used for feature selection. After
filtering out low variance, the minimum absolute contraction and
selection algorithm was used to further screen the optimal feature
subset with low co-linearity. Meanwhile, linear fusion was adapted
to calculate the score of the radiomics signature for every patient
(Rad-score=constant + coefficient × imaging features).
Construction of predictive model
Four machine-learning algorithms, extreme gradient
boosting (XGBoost), logical regression, Gaussian naive Bayes (GNB),
and support vector machine (SVM) were used to select the optimal
feature subset. To evaluate the prediction ability of the model,
the receiver operating characteristic (ROC) and calibration curves
were used to test the prediction efficiency and fitness of the
model. The calibration curve was modified by 1,000
self-weightlifting resampling and then evaluated by the
Hosmer-Lemeshow test. The accuracy, sensitivity, and specificity
were calculated based on the optimal threshold value in the
training set.
Statistical analysis
R version 3.4.3 (http://www.r-project.org) (13) was used for statistical analysis
through R Studio (version 1.1.383) (14). Quantitative data were compared using
an independent samples Student's t-test if data were normally
distributed or otherwise a Mann-Whitney U test, and qualitative
data were analyzed using a χ2 test. The pROC package was
used to draw the ROC curve, and the rms package was applied to draw
the calibration curve. The glmnet package was used to realize the
least absolute shrinkage and selection operator (LASSO) regression
with a five-fold cross-validation method to screen radiomics
features. The XGBoost, logical regression, SVM, and GNB algorithms
were performed using the packages xgboost, glm, kernlab, and e1071,
respectively.
Results
Clinicopathological features
Statistically significant differences were found in
tumor location, TNM stage, differentiation degree, Lauren's
classification, CA125, and CA199 levels between VI+ and VI-
patients with locally advanced GC (all P<0.05, Table I), but no statistically significant
differences in sex, age, and CEA were observed (P>0.05, Table I). Multivariate logistic regression
model analysis showed that the degree of differentiation, Lauren's
classification, and CA199 levels were independent risk factors for
predicting VI in locally advanced GC (Table II).
 | Table I.Comparison of the clinicopathological
factors between the VI+ and VI- set of patients with locally
advanced gastric cancer. |
Table I.
Comparison of the clinicopathological
factors between the VI+ and VI- set of patients with locally
advanced gastric cancer.
| Factor | VI+ (n=213) | VI- (n=83) | χ2/t | P-value |
|---|
| Sex, n |
|
| −1.341 | 0.084 |
| Male | 148 | 66 |
|
|
|
Female | 65 | 17 |
|
|
| Age, years | 63 (54, 69) | 61 (54, 67) | −0.646 | 0.518 |
| Tumor location,
n |
|
| −3.386 | <0.001 |
|
Cardia | 98 | 56 |
|
|
| Gastric
body | 30 | 13 |
|
|
| Gastric
antrum | 85 | 14 |
|
|
| TNM, n |
|
| −5.833 | <0.001 |
| I | 9 | 20 |
|
|
| II | 37 | 32 |
|
|
|
III/IV | 167 | 31 |
|
|
| Differentiation,
n |
|
| −7.65 | <0.001 |
|
Middle | 50 | 67 |
|
|
| Low | 163 | 16 |
|
|
| Lauren, n |
|
| −1.896 | 0.042 |
|
Intestinal | 75 | 41 |
|
|
|
Diffuse | 46 | 14 |
|
|
|
Mixed | 92 | 28 |
|
|
| CEA,
ng/mla | 2.24 (1.43,
4.06) | 2.77 (1.68,
4.70) | 1.633 | 0.103 |
| CA125,
kU/la | 8.40 (6.14,
11.97) | 10.60 (7.31,
15.77) | 2.562 | 0.010 |
| CA199,
kU/la | 9.95 (4.97,
23.24) | 14.60 (7.19,
27.20) | 2.092 | 0.036 |
 | Table II.Multivariate logistic regression
model analysis. |
Table II.
Multivariate logistic regression
model analysis.
| Factor | P-value | Odds ratio | 95% Confidence
interval |
|---|
|
Differentiation | 0.003 | 13.651 | 7.265-25.650 |
| Lauren | 0.042 | 1.349 | 1.011-1.799 |
| CA199 | 0.044 | 1.796 | 1.406-2.186 |
Radiomics feature extraction
repeatability assessment
The extracted radiomics features were based on the
ROIs delineated by the doctors two times, which showed good
intragroup consistency, and with ICC values >0.75. Radiomics
features based on ROIs outlined by the two doctors also exhibited
good intergroup consistency.
Radiomic feature screening
A total of 864 radiomics features were extracted
based on the ROI from the CT images in the portal venous phase.
After filtering out low variance features, 236 radiomics features
were obtained. Next, 18 optimal radiomics features were finally
selected by the LASSO algorithm, including two original features
(original-gldm-Dependence Non-Uniformity Normalized,
original-glrlm-ShortRunEmphasis) and 16 wavelet features
(wavelet-LLLglszm Zone Entropy, wavelet-HLHgldm Dependence Non
Uniformity Normalized, wavelet-LLLngtdm Contrast, wavelet-LLLglrlm
Short Run Low Gray Level Emphasis, wavelet-LLHglszm Small Area Low
Gray Level Emphasis, wavelet-LHHglszm Zone Variance, wavelet-LHL
first order Median, wavelet-LLLglcm Imc1, wavelet-LHHglrlm Gray
Level Variance, wavelet-LHHglrlm Gray Level Non Uniformity
Normalized, wavelet-LLH first order Total Energy, wavelet-HHLgldm
Small Dependence Low Gray Level Emphasis, wavelet-HLHglszm Low Gray
Level Zone Emphasis, wavelet-HLHglszm High Gray Level Zone
Emphasis, wavelet-HHHngtdm Contrast, and wavelet-LLLglszm High Gray
Level Zone Emphasis). In the training set, the Rad-score value
[presented as median (lower quartile, upper quartile)] of VI+
patients [0.763 (0.681, 0.831)] was significantly higher than that
of VI- patients [0.672 (0.616, 0.753)]. In the validation set, the
Rad-score value of VI+ patients [0.746 (0.668, 0.800)] was higher
than that of VI- patients [0.721 (0.625, 0.794)], and the
difference between these two groups was statistically
significant.
Value of CT radiomics model in
predicting VI in advanced GC
The machine-learning model was constructed by
combining the 18 best image radiomics features screened using
venous CT images of locally advanced GC and the three independent
risk factors of GC VI in clinical features. The diagnostic efficacy
and ROC curve of different machine-learning models for predicting
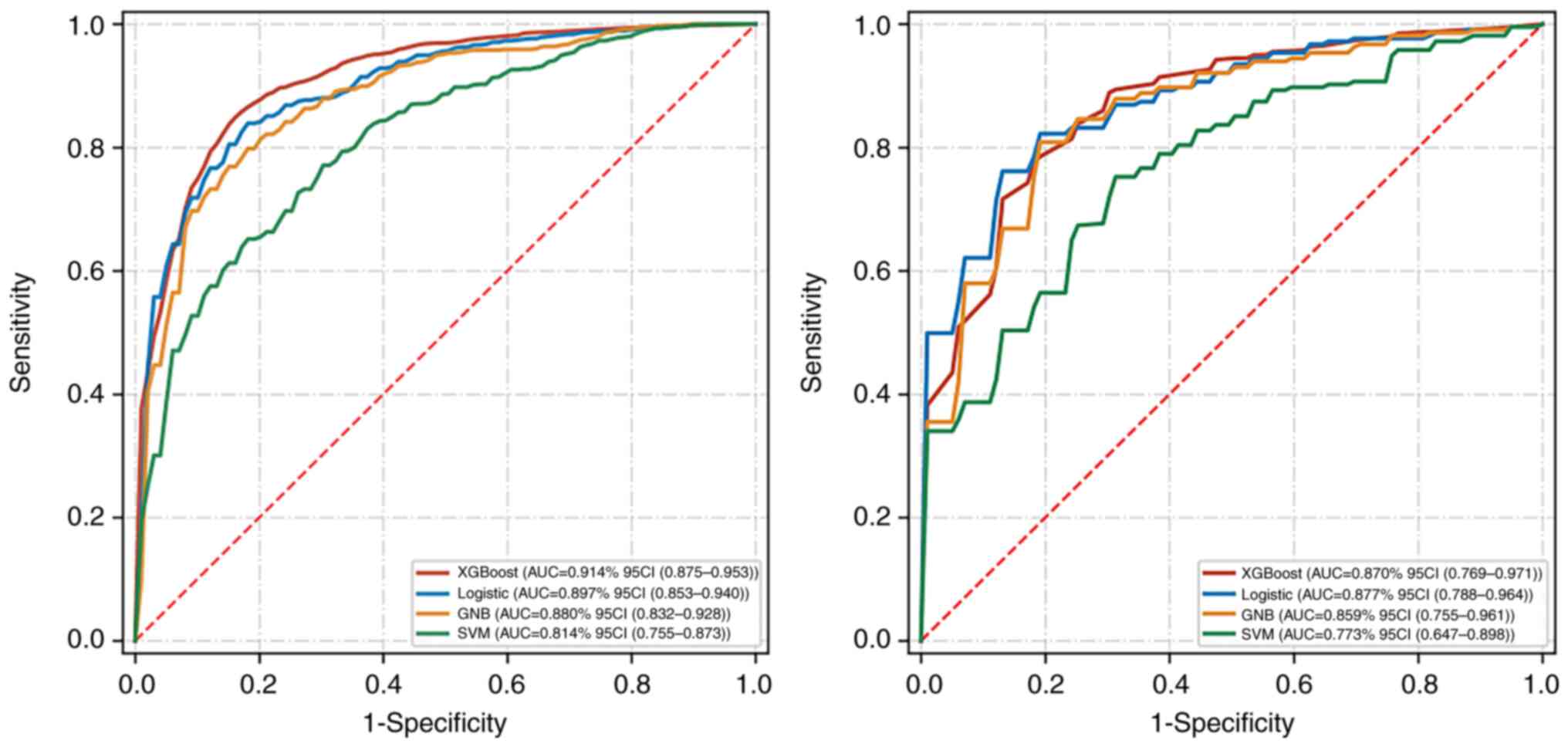
VI of locally advanced GC are shown in Table III and Fig. 3. The AUC values of prediction models
based on XGBoost, logical regression, GNB, and SVM in training sets
were 0.914, 0.897, 0.880, and 0.814, respectively. The AUC values
of prediction models in verification sets were 0.870, 0.877, 0.859,
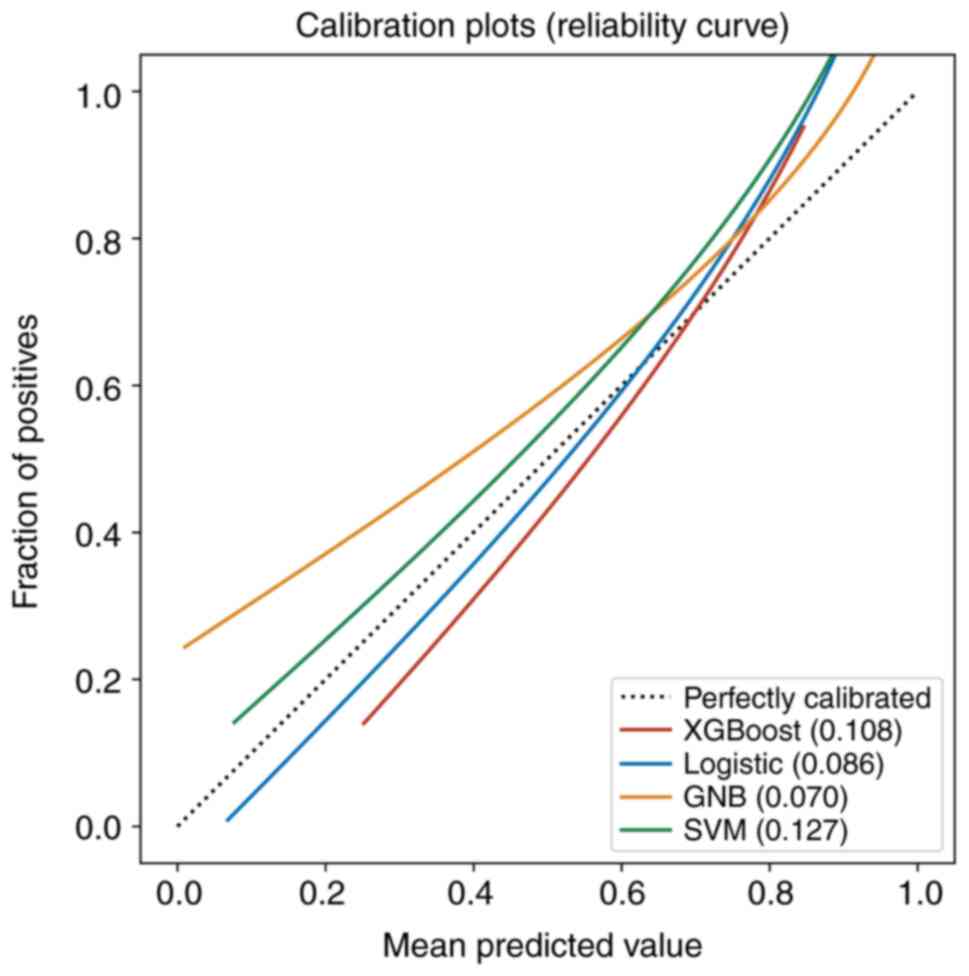
and 0.773, respectively. Calibration curves of the validation set
showed that the predicted results of the four machine-learning
models corresponded with the pathological results (Fig. 4). The results of the DeLong test
indicated no significant difference in AUC value between the
XGBoost and logical regression models either in the training set or
in the validation set. Among the four machine-learning models, the
logical regression model showed the highest AUC value of 0.877 (95%
confidence interval: 0.788-0.964) in the validation set. The
accuracy and F1 scores of the logical regression model were 77.7
and 84.0%, respectively (Table
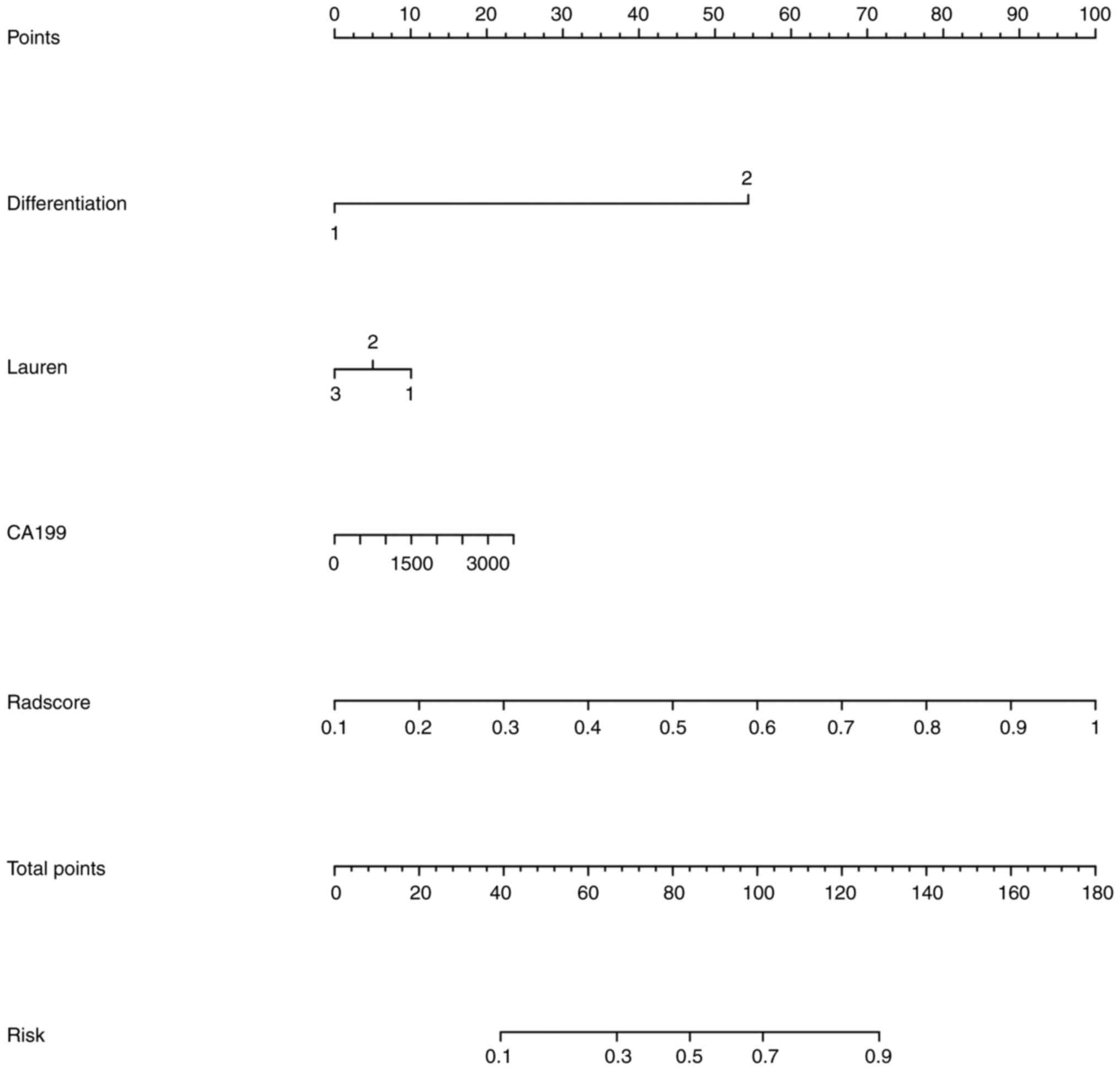
III). Finally, the nomogram is shown in Fig. 5.
 | Table III.Summary of the performance of the
different models in the training and test sets. |
Table III.
Summary of the performance of the
different models in the training and test sets.
|
| Training set | Validation set |
|---|
|
|
|
|
|---|
| Model | AUC (95% CI) | Accuracy (95%
CI) | F1 (95% CI) | AUC (95% CI) | Accuracy (95%
CI) | F1 (95% CI) |
|---|
| XGBoost | 0.914 | 0.816 | 0.885 | 0.870 | 0.787 | 0.876 |
|
| (0.875-0.953) | (0.774-0.857) | (0.860-0.910) | (0.769-0.971) | (0.751-0.823) | (0.852-0.900) |
| Logistic | 0.897 | 0.823 | 0.871 | 0.877 | 0.777 | 0.840 |
|
| (0.853-0.940) | (0.801-0.846) | (0.852-0.891) | (0.788-0.964) | (0.691-0.863) | (0.751-0.930) |
| GNB | 0.880 | 0.799 | 0.850 | 0.859 | 0.770 | 0.876 |
|
| (0.832-0.928) | (0.775-0.823) | (0.828-0.873) | (0.755-0.961) | (0.717-0.823) | (0.818-0.935) |
| SVM | 0.814 | 0.707 | 0.763 | 0.773 | 0.662 | 0.795 |
|
| (0.755-0.873) | (0.659-0.755) | (0.704-0.821) | (0.647-0.898) | (0.550-0.774) | (0.750-0.841) |
Discussion
In this study, 18 radiomics features screened using
venous CT images of locally advanced GC were used to construct
image omics labels, and three clinical features screened by
multivariate logistic regression were incorporated into the model
for training and testing, indicating the rationality of this study
in selecting the optimal feature subset. At the same time, XGBoost,
logistic, GNB, and SVM models were constructed in this study. Their
AUC values and accuracy, sensitivity, and specificity in the test
set were good, indicating that the four machine-learning models had
high predictive efficiency, suggesting the feasibility of feature
selection and model training in this study. In the test set, the
best efficacy and highest accuracy in terms of the AUC value of the
differentiation index was shown by the logistic model, followed by
the XGBoost, GNB, and SVM models. However, no statistical
significance in the AUC value difference between the four models
was found. The AUC of the XGBoost model in the training set was
0.914, but decreased to 0.870 in the test set, showing an obvious
overfitting phenomenon. The AUCs of the logistic model in the
training and test sets were 0.897 and 0.877, respectively, which
were relatively stable with no overfitting phenomenon and continued
to have good fitting and prediction abilities. In this study, the
calibration curves of the four models were good, indicating that
the results predicted by the four models showed high consistency
with the pathological results. Based on these results, the logistic
model is the best model to predict the VI status of locally
advanced GC.
The primary reason for the poor prognosis of locally
advanced GC is postoperative recurrence and metastasis, which has
become the primary cause of death. VI is closely related to the
pathological characteristics of tumor growth and metastasis.
Previous studies have confirmed that the detection of VI is of
great clinical value in the diagnosis and treatment of GC. The VI
status of GC is generally confirmed based on the postoperative
pathological examination, and the obtained results cannot fully
reflect the heterogeneity of locally advanced GC. Therefore, some
limitations in the guiding of preoperative management of GC
patients exist.
A previous study on primary liver cancer found that
the nomogram constructed using the random forest method is a
potential biomarker for predicting microvascular invasion and
relapse-free survival in solitary hepatocellular carcinoma
(15). Similarly, in a study of
intrahepatic cholangiocarcinoma with mass formation (16), both CECT radiomics analysis and
radiomics factors could predict microvascular invasion, and the
nomogram could further improve the prediction efficiency. Previous
studies have reported that CT radiomics demonstrates good
performance in the pathological grading of gastric neuroendocrine
tumors, prognostic prediction of GC patients, and neoadjuvant
chemotherapy response in advanced GC patients (17–19).
Other studies have shown that the normalized iodine concentration
based on energy spectrum CT can reflect the angiogenesis of GC
(20,21), which is closely related to the
recurrence and prognosis of GC. Based on these studies, the
prediction of VI of advanced GC based on radiomics should be
feasible. To the best of our knowledge, reports on the preoperative
prediction of VI in GC are rare (5,7).
Therefore, a preliminary study on the VI of GC based on radiomics
and machine learning was performed, and these results may form the
basis of further research.
Radiomics has excellent clinical application value
in the grading and staging of GC, predicting prognosis, and
evaluating response (5). Portal
venous phase CT images of locally advanced GC effectively reflect
the biological behavior of the tumor, and VI can promote the growth
and metastasis of GC cells. Therefore, radiomics analysis based on
portal venous phase CT images is helpful for the prediction of VI
status in locally advanced GC. In this study, original features and
wavelet features based on high-pass and low-pass were screened from
portal venous phase CT images, reflecting tumor morphological
differences and image inhomogeneity. In a study on the prediction
of the VI in borderline pancreatic cancer patients after
neoadjuvant therapy, Ahmed et al (22) found that a circumferential interface
≥180 degrees, contour deformity ≥grade 3, and/or a contact length
of tumor >2 cm may be important predictors, and its performance
of prediction yielded AUCs of 0.85-0.88 and 0.92- −0.87 for
arterial and venous invasion, respectively. However, the sample
size was small, and the clinical indicators were not included. Yang
et al (23) developed a
nomogram model integrating the clinical and radiomics features to
predict the microvascular invasion of hepatocellular carcinoma,
which showed that the AUC values of the model in the training and
validation sets were 0.943 and 0.850, respectively, and the
predictive performance of the model was lower than that of the
prediction models constructed by XGBoost, logistic regression, and
GNB in this study. Li et al (24) explored the value of the nomogram
based on prothrombin induced by vitamin K absence-II,
α-fetoprotein, and tumor size for predicting microvascular invasion
in early hepatocellular carcinoma. The results showed that the
model can predict microvascular invasion in early hepatocellular
carcinoma. However, the study included only 43 patients, only
clinical features were included in the nomogram model, and the
validation set was lacking. Given this, CT-based radiomics has
distinct value in the prediction of preoperative VI. Nevertheless,
the determination of optimal features for evaluating preoperative
VI is still controversial, and accounting for specificity and
sensitivity using a single feature is difficult. Related research
on the preoperative prediction of VI in GC using machine-learning
models has not yet been reported.
Machine learning has important clinical value in the
preoperative prediction of VI in locally advanced GC. In this
study, the AUC values of the XGBoost, logistic regression, and GNB
models constructed based on CT images in a portal venous phase were
higher than 0.80 for the prediction of the VI of locally advanced
GC in the validation set, indicating good diagnostic performance,
while the AUC of the SVM model in the validation set was relatively
low.
Currently, numerous reports have been published on
the use of machine-learning algorithms to predict tumor VI
(25–27). Using radiomics and clinical and CT
image features, Jiang et al (26) developed the XGBoost model and
three-dimensional convolutional neural network (3D-CNN) model to
predict microvascular invasion in hepatocellular carcinoma. The AUC
value of the 3D-CNN model was higher than that of the XGBoost model
in both the training and validation sets, which was 0.952 vs. 0.980
and 0.887 vs. 0.906, respectively. Moreover, its predictive
performance was higher than that of the prediction model in this
study. Although radiomics is an advanced technique for image
analysis, the extracted features are usually based on the manual
delineation of ROI and rely on personal experience; thus, it cannot
represent the most accurate decision (27). The most important advantage of the
3D-CNN model is the high efficiency in identifying microvascular
invasion, which can be done automatically with minimal work, time,
and materials. For the construction of the 3D-CNN model, only the
original image is required, and clinical data and radiological or
radiomics features are not required. In addition, Liu et al
(28) showed that the deep-learning
and SVM models based on arterial phase CT images and clinical
features can predict microvascular invasion in hepatocellular
carcinoma, and the deep-learning model exhibited better performance
with an AUC value of 0.845. The reason why the predictive
performance of the previous study was lower than that of the
present study may be due to the difference in sample sizes between
the two studies. With a smaller sample size, ‘falling into the
local optimal solution’ readily occurs, and achieving the global
optimal parameter value is thus difficult.
This study had several limitations. This study
preliminarily discussed the value of predicting VI in advanced
gastric cancer based on preoperative CT images, However, due to the
lack of data related to postoperative recurrence and prognosis,
further research is being conducted taking into account these
factors. The present study was a single-center study with a small
sample, and the stability and practicability of the models require
further confirmation. The machine-learning models in this study
were based only on internal data, and further external validation
is required. Finally, the models were only based on CT images in
the portal venous phase, and CT images in other phases should also
be compared.
In conclusion, machine-learning models based on
portal venous phase CT images can effectively predict the VI status
of locally advanced GC before surgery. The predictive performance
of the logistic regression model was relatively good and is
expected to be an advanced means for the preoperative noninvasive
prediction of VI status in locally advanced GC.
Acknowledgements
Not applicable.
Funding
The present study was funded by a grant from the National
Natural Science Foundation of China (grant no. 81701687).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZWH and JBG confirm the authenticity of all the raw
data. PL and RW are responsible for the delineation and
classification of the data. ZWH, ZLL, PL, and HL are responsible
for data collection. LLY and JBG are responsible for the
construction and statistical analysis of the machine learning
model. ZWH, ZLL, and PL are responsible drafting and editing the
manuscript. JBG gave final approval of the version to be published.
All authors listed have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research and
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University (approval no. 2021-KY-1070-002). Informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018:GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu WW, Chen QY, Zheng WZ, He QC, Huang
ZN, Xie JW, Wang JB, Lin JX, Lu J, Cao LL, et al: Postoperative
follow-up for gastric cancer needs to be individualized according
to age, tumour recurrence pattern, and recurrence time. Eur J Surg
Oncol. 48:1790–1798. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin Y, Xu Y, Li Y, Chen R and Cai W:
Integrative radiogenomics approach for risk assessment of
postoperative and adjuvant chemotherapy benefits for gastric cancer
patients. Front Oncol. 11:7552712021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Zhong L, Zhou X, Chen D and Li R:
Value of multiphase contrast-enhanced CT with three-dimensional
reconstruction in detecting depth of infiltration, lymph node
metastasis, and extramural vascular invasion of gastric cancer. J
Gastrointest Oncol. 12:1351–1362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng Y, Huang X, Liu J, Chen J, Bu Z, Wu
G, Xie W, Jeen F, Huang L, Tian C, et al: A novel nomogram for
individually predicting of vascular invasion in gastric cancer.
Technol Cancer Res Treat. 20:153303382110049242021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gresta LT, Rodrigues-Júnior IA, de Castro
LP, Cassali GD and Cabral MM: Assessment of vascular invasion in
gastric cancer: A comparative study. World J Gastroenterol.
19:3761–3769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Chu W, Li M, Xu P, Wang M, Peng M,
Wang K and Zhang L: Radiomics in gastric cancer: First clinical
investigation to predict lymph vascular invasion and survival
outcome using 18F-FDG PET/CT images. Front Oncol.
12:8360982022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang S, Zou X, Li J, Zhang A, Zhu L, Hu X
and Li C: The application value of ceMDCT in the diagnosis of
gastric cancer extramural vascular invasion and its influencing
factors. J Healthc Eng. 2022:42396002022.PubMed/NCBI
|
|
9
|
Rodríguez-Perálvarez M, Luong TV, Andreana
L, Meyer T, Dhillon AP and Burroughs AK: A systematic review of
microvascular invasion in hepatocellular carcinoma: Diagnostic and
prognostic variability. Ann Surg Oncol. 20:325–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKinney SM, Sieniek M, Godbole V, Godwin
J, Antropova N, Ashrafian H, Back T, Chesus M, Corrado GS, Darzi A,
et al: International evaluation of an AI system for breast cancer
screening. Nature. 577:89–94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehteshami Bejnordi B, Veta M, Johannes van
Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak
JAWM; the CAMELYON16 Consortium, ; Hermsen M, Manson QF, et al:
Diagnostic assessment of deep learning algorithms for detection of
lymph node metastases in women with breast cancer. JAMA.
318:2199–2210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bera K, Braman N, Gupta A, Velcheti V and
Madabhushi A: Predicting cancer outcomes with radiomics and
artificial intelligence in radiology. Nat Rev Clin Oncol.
19:132–146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
R Core Team, . R: A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: ISBN 3-900051-07-0. 2012, URL.
http://www.R-project.org/
|
|
14
|
RStudio Team, . RStudio: Integrated
Development for R. RStudio, Inc., Boston, MA. 2015.URL. http://www.rstudio.com/
|
|
15
|
Chong HH, Yang L, Sheng RF, Yu YL, Wu DJ,
Rao SX, Yang C and Zeng MS: Multi-scale and multi-parametric
radiomics of gadoxetate disodium-enhanced MRI predicts
microvascular invasion and outcome in patients with solitary
hepatocellular carcinoma ≤5 cm. Eur Radiol. 31:4824–4838. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang F, Wei S, Liu X, Liang X, Yang L and
Yan S: Radiomics analysis of contrast-enhanced CT for the
preoperative prediction of microvascular invasion in mass-forming
intrahepatic cholangiocarcinoma. Front Oncol. 11:7741172021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan X, Yang X, Hu S, Ge Y, Wu Q, Wang J
and Sun Z: Prediction of response to neoadjuvant chemotherapy in
advanced gastric cancer: A radiomics nomogram analysis based on CT
images and clinicopathological features. J Xray Sci Technol.
31:49–61. 2023.PubMed/NCBI
|
|
18
|
Wang R, Liang P, Yu J, Han YJ and Gao JB:
Diagnostic efficacy of a combined diagnostic model based on extreme
gradient boosting algorithm in differentiating the pathological
grading of gastric neuroendocrine neoplasms. Zhonghua Yi Xue Za
Zhi. 101:2717–2722. 2021.(In Chinese). PubMed/NCBI
|
|
19
|
Gao X, Ma T, Bai S, Liu Y, Zhang Y, Wu Y,
Li H and Ye Z: A CT-based radiomics signature for evaluating tumor
infiltrating Treg cells and outcome prediction of gastric cancer.
Ann Transl Med. 8:4692020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XH, Ren K, Liang P, Chai YR, Chen KS
and Gao JB: Spectral computed tomography in advanced gastric
cancer: Can iodine concentration non-invasively assess
angiogenesis? World J Gastroenterol. 23:1666–1675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang P, Ren XC, Gao JB, Chen KS and Xu X:
Iodine concentration in spectral CT: Assessment of prognostic
determinants in patients with gastric adenocarcinoma. AJR Am J
Roentgenol. 209:1033–1038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed SA, Mourad AF, Hassan RA, Ibrahim
MAE, Soliman A, Aboeleuon E, Elbadee OMA, Hetta HF and Jabir MA:
Preoperative CT staging of borderline pancreatic cancer patients
after neoadjuvant treatment: Accuracy in the prediction of vascular
invasion and resectability. Abdom Radiol (NY). 46:280–289. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Gu D, Wei J, Yang C, Rao S, Wang
W, Chen C, Ding Y, Tian J and Zeng M: A radiomics nomogram for
preoperative prediction of microvascular invasion in hepatocellular
carcinoma. Liver Cancer. 8:373–386. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Li T, Hu J and Liu J: A nomogram to
predict microvascular invasion in early hepatocellular carcinoma. J
Cancer Res Ther. 17:652–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Zhang HL, Liu QP, Sun SW, Zhang J,
Zhu FP, Yang G, Yan X, Zhang YD and Liu XS: Radiomic analysis of
contrast-enhanced CT predicts microvascular invasion and outcome in
hepatocellular carcinoma. J Hepatol. 70:1133–1144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang YQ, Cao SE, Cao S, Chen JN, Wang GY,
Shi WQ, Deng YN, Cheng N, Ma K, Zeng KN, et al: Preoperative
identification of microvascular invasion in hepatocellular
carcinoma by XGBoost and deep learning. J Cancer Res Clin Oncol.
147:821–833. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hosny A, Parmar C, Quackenbush J, Schwartz
LH and Aerts HJWL: Artificial intelligence in radiology. Nat Rev
Cancer. 18:500–510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu SC, Lai J, Huang JY, Cho CF, Lee PH,
Lu MH, Yeh CC, Yu J and Lin WC: Predicting microvascular invasion
in hepatocellular carcinoma: A deep learning model validated across
hospitals. Cancer Imaging. 21:562021. View Article : Google Scholar : PubMed/NCBI
|