Introduction
Prostate cancer is the second most common malignancy
in men worldwide and the most commonly diagnosed cancer type in men
in developed countries (1). Its
incidence rate is the highest in Europe, America and Oceania and
the lowest in North Africa and Asia (1). Prostate cancer is the fifth leading
cause of cancer-associated mortalities in men worldwide, with the
highest mortality rates in the Caribbean, South Africa and Central
Africa (1). Furthermore, it is the
sixth most common malignancy in men in China, and its morbidity and
mortality rates have been increasing recently (2).
Transcription factor E-26 transformation-specific
(ETS)-related gene (ERG) is a member of the ETS family (3,4). ETS
transcription factors are essential for development and
differentiation and are involved in embryogenesis, angiogenesis,
hematopoiesis and neural development (5,6). ERG
is highly expressed in the embryonic mesoderm and endodermis and
plays a key role in the vascular system, urogenital tract and bone
development (7,8). To the best of our knowledge, Tomlins
et al (9) reported for the
first time in 2005 that in prostate cancer the transmembrane
protease serine 2 (TMPRSS2) gene is fused with ERG,
resulting in overexpression of the ERG protein. Subsequently,
studies have been conducted on the roles of the TMPRSS2-ERG
fusion and ERG protein expression in the pathogenesis, detection,
diagnosis and prognosis of prostate cancer (10–15).
Sedarsky et al (10)
reported the frequency of ERG expression in men with prostate
cancer from different ethnic groups worldwide. Salagierski and
Schalken (11) reported that the
TMPRSS2-ERG fusion can serve as a diagnostic indicator for
prostate cancer. Current research has mainly focused on the
detection and diagnostic value of ERG; however, its prognostic
value remains controversial.
Biochemical recurrence (BCR) is a marker of early
disease progression in patients after radical prostatectomy.
According to some reports, patients with positive expression of
fusion genes demonstrate a lower risk of BCR compared with patients
with negative expression (12,13).
However, other studies have shown no difference in disease
prognosis or BCR risk between patients with positive and negative
expression of fusion genes (14,15).
There are three main methods for detecting the
presence of fusion genes: Reverse transcription polymerase chain
reaction (PCR), fluorescence in situ hybridization and
immunohistochemistry (IHC); among these, IHC is the most popular
and convenient. The ERG protein is a routine immunohistochemical
marker used for prostate puncture and radical prostatectomy
specimens (15). Elucidating the
hitherto unknown prognostic value of ERG IHC could provide a
valuable reference for prostate cancer prognosis. Therefore, in the
present study, IHC was used to detect ERG protein in patients
undergoing radical prostatectomy. The relationships among ERG
protein levels, clinicopathological data and patient prognosis were
examined to further clarify the role of ERG IHC results in the
prognosis of prostate cancer.
Materials and methods
Clinical data
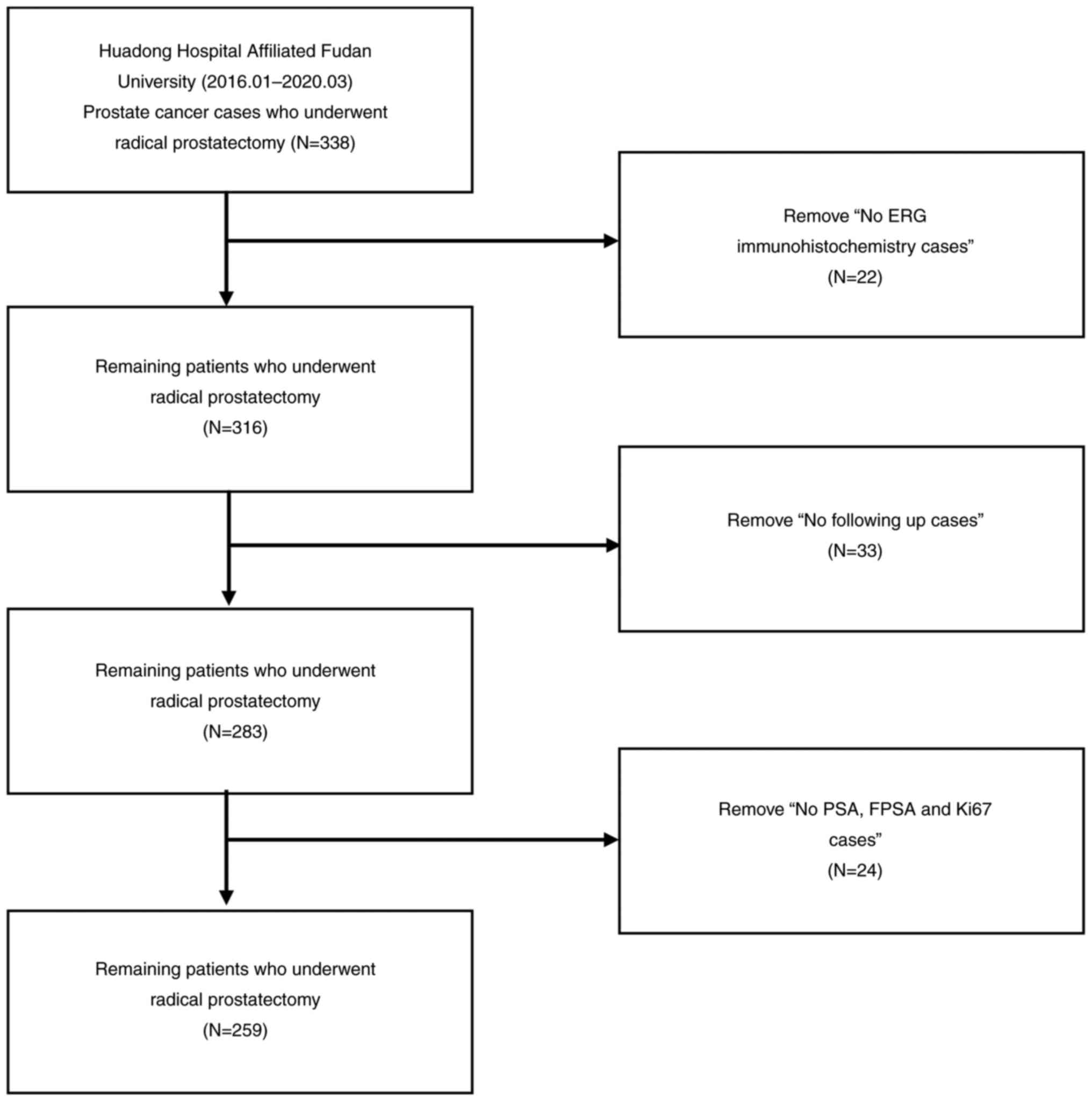
The present retrospective study included 338
patients with prostate cancer who underwent radical prostatectomy
at the Huadong Hospital, affiliated with Fudan University
(Shanghai, China), between January 2016 and March 2020. Patient
data were obtained through their medical records. Of the 338 cases,
22 were excluded because ERG, 33 were excluded because they were
not followed up, 24 were excluded because of missing PSA, free
prostate-specific antigen (FPSA) and Ki-67 data; finally, 259 cases
were analyzed in total (Fig. 1).
The patient ages ranged from 47 to 82 years, with a median age of
69 years. Paraffin-embedded sections of all surgical specimens were
prepared according to standard procedures and were reviewed
independently by two senior pathologists. These sections were
graded using the Gleason scoring system (16), and clinical staging was performed
according to the 2017 American Joint Committee on Cancer (AJCC)
tumor, node and metastasis (TNM) staging system (17) to determine the involvement of the
surgical margins in tumors and the proportion of tumors.
Simultaneously, the patients needed a monthly PSA review within 6
months after radical surgery and PSA and other related examinations
every 3 months. The primary follow-up endpoint was BCR (a PSA value
of >0.2 ng/ml for two consecutive measurements) and the
secondary endpoint was death.
Immunohistochemical reagents, methods
and judgment of results
A rabbit monoclonal antibody against human ERG (the
primary antibody) was purchased from Fuzhou Maixin Biotech Co.,
Ltd. (cat. no. RMA-0748). A peroxidase-labeled polymer conjugated
to goat anti-mouse and goat anti-rabbit immunoglobulins (secondary
antibody) were purchased from DAKO (EnVision two-step staining kit;
cat. no. GK500705; Agilent Technologies, Inc.). IHC was used to
detect the expression of ERG protein in prostate cancer cells. Both
lesions were stained in bilateral cases. The EnVision two-step
staining kit (DAKO; Agilent Technologies, Inc.) was used for IHC
analysis. The prostate cancer tissues were fixed using 10% neutral
buffered formalin for 24 h at room temperature. Paraffin-embedded
tissues were dissected at a thickness of 4 µm and dewaxed. Antigen
retrieval was performed with Tris-EDTA buffer (pH 9.0) for 20 min
in a microwave and then allowed to cool down at room temperature
for other 20 min. The sections were washed three times with
phosphate-buffered saline for 3 min each time. Endogenous
peroxidase was blocked with 0.3% hydrogen peroxide in
phosphate-buffered saline at room temperature for 15 min. The
sections were then incubated with primary antibodies (diluted at
1:200) for 60 min at room temperature, incubated with secondary
antibody (not diluted) for 45 min at room temperature, treated with
diaminobenzidine color, counterstained with hematoxylin at room
temperature for 4 min and tablet sealed. Each step was performed as
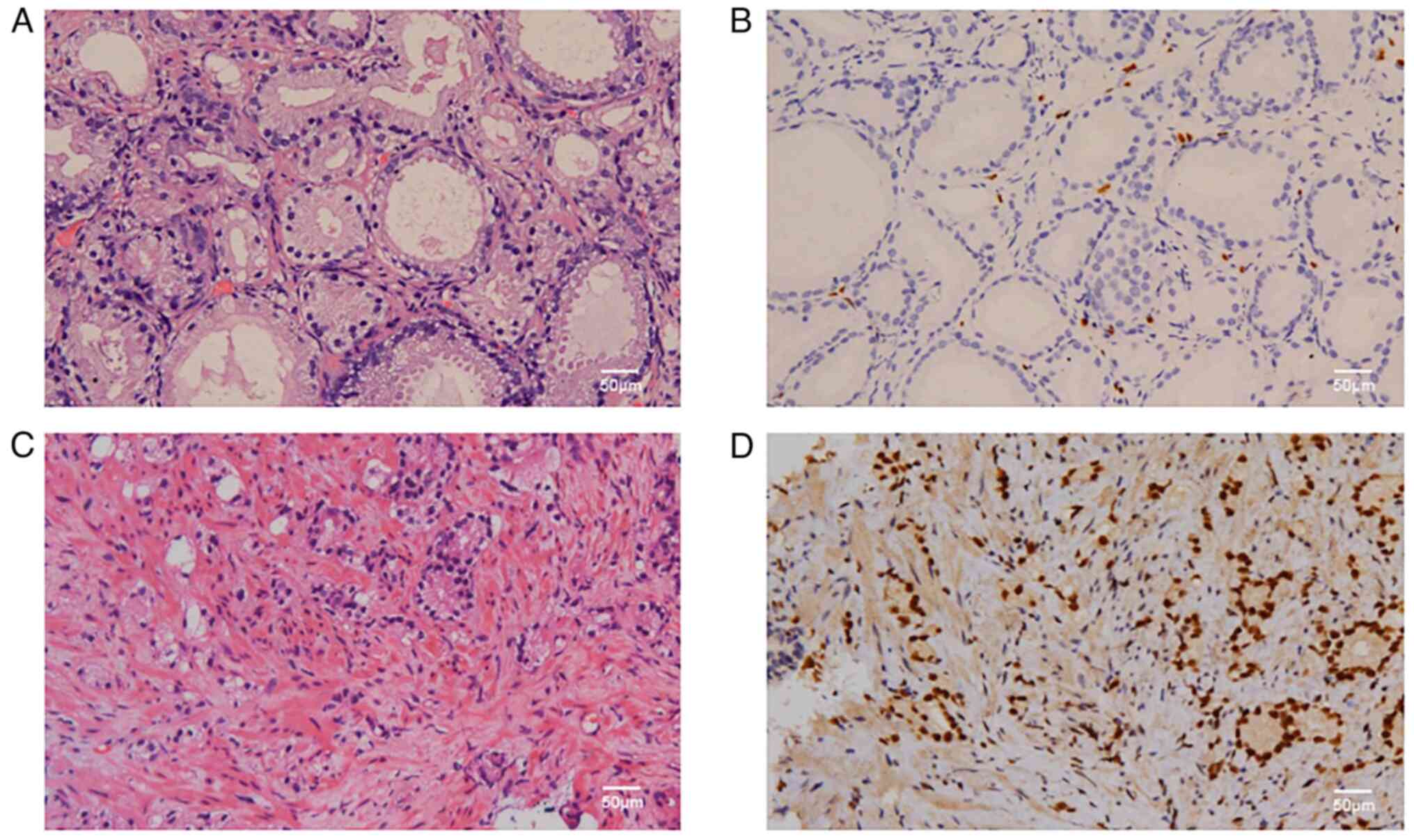
per the kit manufacturer's instructions (18). The results of ERG IHC were observed
by the light microscope (Olympus Corporation). Positive ERG
expression was indicated by medium- to strong-brown staining in the
nucleus (Fig. 2).
Statistical analyses
When the total sample size was >40, the lowest
expected count of the analyzed contingency table was >1 and the
expected count in <20% of the cells of the analyzed contingency
table was ≤5, the χ2 test was used to compare
categorical variables. When the expected count could not meet the
assumptions of using χ2 test, the Fisher's test was
used. Finally, the χ2 test was used to compare surgical
margins, tumor percentage and staining of Ki-67, FPSA or BCR
between ERG-positive and -negative cases. The Fisher's test was
used to compare the Gleason score, TNM stages, age and PSA group
between ERG-positive and -negative cases. An independent-samples
t-test was used to compare the biochemical failure-free survival
(BFFS) (the period of survival before BCR after radical
prostatectomy) and overall survival (OS) between the two groups of
patients. The Kaplan-Meier method was used to estimate BFFS and the
log-rank test was used to evaluate the distribution. Univariate and
multivariate Cox regression analyses were used to evaluate
prognostic factors; a=0.05 and P<0.05 was considered to indicate
a statistically significant difference. All data were analyzed
using SPSS 26.0 software (IBM Corp.) and R4.0.4 software (R
Development Core Team; http://www.R-project.org).
Results
Clinicopathological features in
patients undergoing radical prostatectomy
Among the specimens from 259 patients, 43 (16.6%)
were ERG-positive and 216 (83.4%) were ERG-negative. The patient
ages ranged from 47 to 82 years, with a median age of 69 years, and
30 patients (11.6%) were aged ≤60 years. The PSA levels ranged from
0.003 to 187.400 ng/ml before treatment, and the median PSA level
was 9.58 ng/ml. Before treatment, the FPSA levels ranged from
0.0059 to 30.6100 ng/ml, and the median FPSA level was 1.13 ng/ml.
In total, 18 patients (6.9%), 162 patients (62.5%), 29 patients
(11.2%), 49 patients (18.9%) and 1 patient (0.4%) had Gleason
scores of 6, 7, 8, 9 and 10, respectively. According to the 2017
AJCC TNM staging system, there were 7 cases of T1 (2.7%), 130 cases
of T2 (50.2%), 115 cases of T3 (44.4%), 7 cases of T4 (2.7%), 237
cases of N0 (91.5%), 12 cases of N1 (4.6%) and 10 cases of Nx
(3.9%) stages. There were 255 cases of M0 (98.5%) and 4 cases of M1
(1.5%) stages. The minimum tumor proportion of the radical
resection specimens was 1.0%, the maximum was 95% and the median
was 30%. Patients were followed up until December 2020; the longest
follow-up time was 60 months, the shortest was 10 months and the
median was 30 months. During the follow-up, BCR occurred in 48
patients (18.5%), four patients died (1.5%) and five were lost to
follow-up after BCR. The mean BFFS was 28.6 months.
Comparison of clinicopathological
features between patients with positive and negative ERG
IHC results
Patients were classified according to age as ≤60 and
>60 years old; tumor percentage as ≤25, 25–50, 50–75 and
>75%; Ki-67-positive staining as ≤5 and >5%; PSA-positive
staining as ≤10, 10–20, 20–100 and >100 ng/ml; and FPSA-positive
staining as ≤1, 1–4 and >4 ng/ml. Patients with ERG-positive or
-negative prostate surgical specimens were compared for the Gleason
scores, TNM stages, surgical margins, ages, tumor percentages,
Ki-67, PSA, FPSA, BCR, BFFS and OS. Analysis using the independent
sample t-test, χ2 test and Fisher's test revealed no
significant differences in the abovementioned indicators, except
for BCR, between the two groups of patients (P>0.05; Table I); there was a significant
difference in the distribution of BCR (P=0.017) between the groups
(Table I).
 | Table I.Comparison of clinical patient
characteristics with ERG protein expression. |
Table I.
Comparison of clinical patient
characteristics with ERG protein expression.
| Variables | Group | Overall | ERG-negative | ERG-positive | P-value |
|---|
| N |
| 259 | 216 | 43 |
|
| ERG (%) | Negative | 216 (83.4) | 216 (100.0) | 0 (0.0) |
<0.001a |
|
| Positive | 43 (16.6) | 0 (0.0) | 43 (100.0) |
|
| GS (%) | 6 | 18 (6.9) | 13 (6.0) | 5 (11.6) | 0.375 |
|
| 7 | 162 (62.5) | 133 (61.6) | 29 (67.4) |
|
|
| 8 | 29 (11.2) | 27 (12.5) | 2 (4.7) |
|
|
| 9 | 49 (18.9) | 42 (19.4) | 7 (16.3) |
|
|
| 10 | 1 (0.4) | 1 (0.5) | 0 (0.0) |
|
| T (%) | T1 | 7 (2.7) | 6 (2.8) | 1 (2.3) | 0.807 |
|
| T2 | 130 (50.2) | 109 (50.5) | 21 (48.8) |
|
|
| T3 | 115 (44.4) | 96 (44.4) | 19 (44.2) |
|
|
| T4 | 7 (2.7) | 5 (2.3) | 2 (4.7) |
|
| N (%) | N0 | 237 (91.5) | 198 (91.7) | 39 (90.7) | 0.899 |
|
| N1 | 12 (4.6) | 10 (4.6) | 2 (4.7) |
|
|
| Nx | 10 (3.9) | 8 (3.7) | 2 (4.7) |
|
| M (%) | M0 | 255 (98.5) | 212 (98.1) | 43 (100.0) | 1.000 |
|
| M1 | 4 (1.5) | 4 (1.9) | 0 (0.0) |
|
| Margins (%) | Negative | 183 (70.7) | 153 (70.8) | 30 (69.8) | 1.000 |
|
| Positive | 76 (29.3) | 63 (29.2) | 13 (30.2) |
|
| Age (%) | ≤60 years | 30 (11.6) | 22 (10.2) | 8 (18.6) | 0.122 |
|
| >60 years | 229 (88.4) | 194 (89.8) | 35 (81.4) |
|
| Tumor percent
(%) | ≤25% | 124 (47.9) | 101 (46.8) | 23 (53.5) | 0.446 |
|
| 25–50% | 67 (25.9) | 60 (27.8) | 7 (16.3) |
|
|
| 50–75% | 28 (10.8) | 22 (10.2) | 6 (14.0) |
|
|
| >75% | 40 (15.4) | 33 (15.3) | 7 (16.3) |
|
| Ki-67 group
(%) | ≤5% | 219 (84.6) | 184 (85.2) | 35 (81.4) | 0.691 |
|
| >5% | 40 (15.4) | 32 (14.8) | 8 (18.6) |
|
| PSA group (%) | ≤10 ng/ml | 134 (51.7) | 113 (52.3) | 21 (48.8) | 0.240 |
|
| 10–20 ng/ml | 77 (29.7) | 67 (31.0) | 10 (23.3) |
|
|
| 20–100 ng/ml | 45 (17.4) | 34 (15.7) | 11 (25.6) |
|
|
| >100 ng/ml | 3 (1.2) | 2 (0.9) | 1 (2.3) |
|
| FPSA group (%) | ≤1 ng/ml | 115 (44.4) | 100 (46.3) | 15 (34.9) | 0.232 |
|
| 1–4 ng/ml | 126 (48.6) | 103 (47.7) | 23 (53.5) |
|
|
| >4 ng/ml | 18 (6.9) | 13 (6.0) | 5 (11.6) |
|
| BCR (%) | 0 | 211 (81.5) | 182 (84.3) | 29 (67.4) | 0.017a |
|
| 1 | 48 (18.5) | 34 (15.7) | 14 (32.6) |
|
| BFFS, months [mean
(SD)] |
| 28.6 (15.1) | 29.0 (15.2) | 26.7 (14.6) | 0.378 |
| OS, months [mean
(SD)] |
| 32.6 (13.5) | 32.4 (14.1) | 33.6 (9.9) | 0.592 |
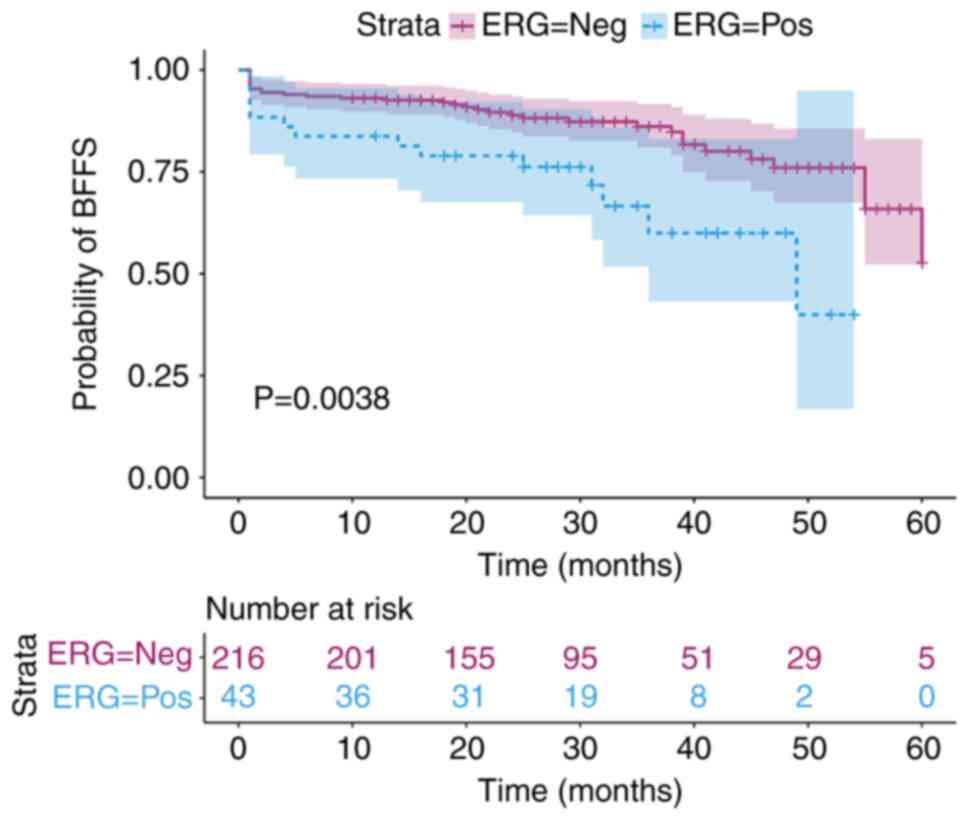
BFFS evaluation in patients undergoing
radical prostatectomy
BFFS in patients who showed positive and negative
results for ERG was estimated using the Kaplan-Meier method, and a
BFFS curve was generated; the difference in BFFS curves between
these two groups of patients was significant (P=0.0038; Fig. 3) and the distribution was tested
using the log-rank test. Patients with ERG-positive status had a
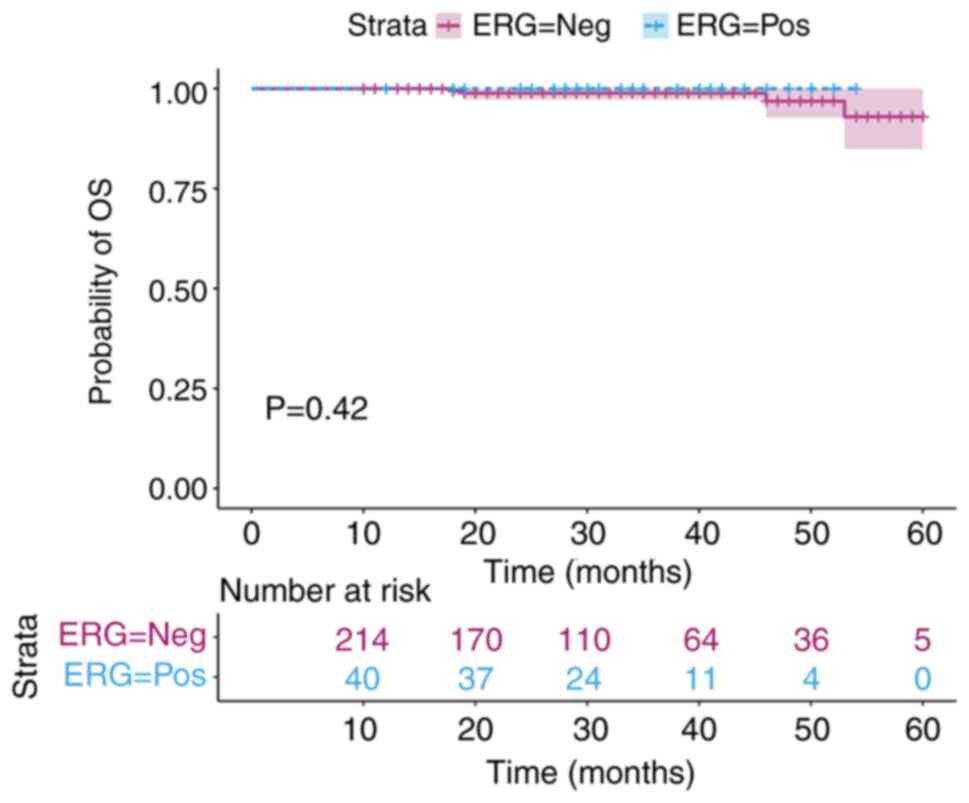
worse BFFS compared with those with ERG-negative status. In
addition, OS was estimated using the Kaplan-Meier method. There was
no significant difference in the OS curves between these two groups
of patients (Fig. 4).
BFFS was estimated in patients with different
Gleason scores, positive and negative surgical margins, tumor
proportions, Ki-67 scores and FPSA values using the Kaplan-Meier
method. BFFS curves were generated, and the distribution was tested
using the log-rank test. The difference in Gleason scores among the
BFFS curves was significant (P=0.0021; Fig. 5A). The lower the Gleason score, the
higher the BFFS and the lower the susceptibility to BCR. The
difference in tumor proportions among the BFFS curves was
significant (P=0.008; Fig. 5B). The
BFFS in patients with tumor proportions between 50 and 70% was
lower compared with that of in patients with other tumor
proportions; these patients were more prone to BCR. The difference
in BFFS curves between patients with positive and negative surgical
margins was significant (P=0.03; Fig.
5C). The BFFS in patients with negative surgical margins was
higher compared with that in patients with positive surgical
margins, and patients with positive surgical margins were more
prone to BCR. The differences in BFFS curves among the Ki-67 groups
were significant (P=0.013; Fig.
5D). The BFFS of Ki-67 ≤5% cases was higher compared with that
of Ki-67>5% cases, and patients with Ki-67>5% were more prone
to BCR. The differences in BFFS curves among the FPSA groups were
statistically significant (P=0.032; Fig. 5E). The BFFS in FPSA >4 ng/ml
cases was lower compared with that in other cases. The difference
in BFFS curves among the N stages groups was significant (P=0.0052;
Fig. 5F). The BFFS of N1 stage
cases was lower compared with that of cases with other N
stages.
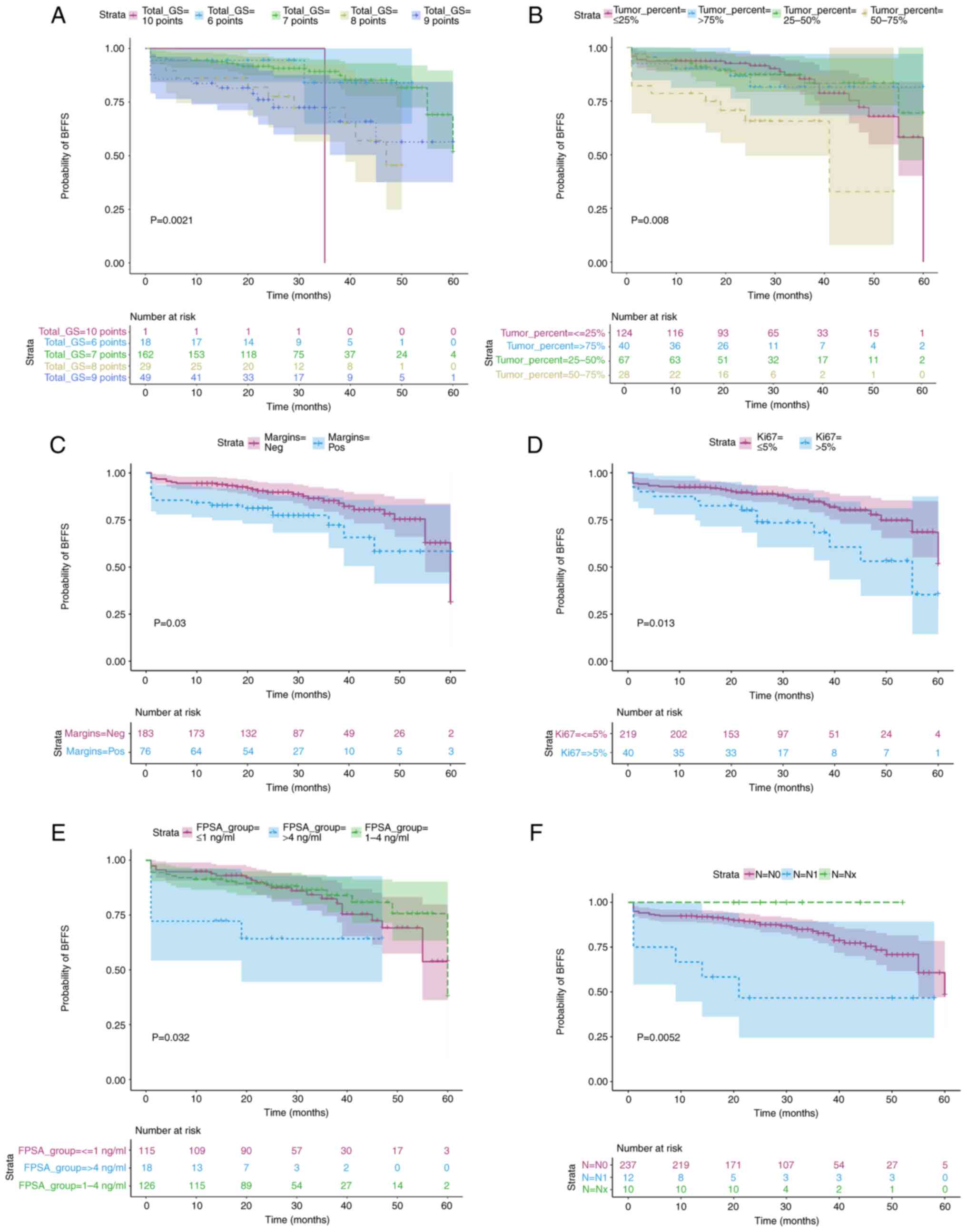 | Figure 5.BFFS curves for GS, tumor
proportions, surgical margins, FPSA, Ki-67 and N stages after
radical prostatectomy. The shaded area corresponds to the 95%
confidence interval; the table below is the risk exposure table.
(A) BFFS curves for Gleason scores, P=0.0021. (B) BFFS curves for
tumor proportions, P=0.008. (C) BFFS curves for surgical margins,
P=0.03. (D) BFFS curves for Ki-67, P=0.013. (E) BFFS curves for
FPSA, P=0.032. (F) BFFS curves for N stages, P=0.0052. ERG,
ETS-related gene; FPSA, free prostate-specific antigen; IHC,
immunohistochemistry; BFFS, biochemical failure-free survival; GS,
Gleason Score; N, node. |
Univariate and multivariate Cox
regression analyses in patients undergoing radical
prostatectomy
Univariate Cox regression analysis was performed for
ERG IHC, PSA, FPSA, age, Gleason score, surgical margins, tumor
percentage, Ki-67 and TNM stages in patients undergoing radical
prostatectomy. Multivariate Cox regression analysis was performed
based on results of the univariate Cox regression analysis. In the
univariate Cox regression analysis, positive IHC staining of ERG
[hazard ratio (HR), 2.48; 95% confidence interval (CI), 1.32-4.66;
P=0.005], FPSA >4 ng/ml (HR, 2.84; 95% CI, 1.14-7.05; P=0.025),
positive surgical margin (HR, 1.91; 95% CI, 1.06-3.43; P=0.030),
tumor proportion of 50–75% (HR, 3.06; 95% CI, 1.43-6.53; P=0.004),
Ki-67 scores >5% (HR, 2.16; 95% CI, 1.16-4.02; P=0.016) and N1
stage (HR, 3.39; 95% CI, 1.43-8.03; P=0.006) were risk factors for
patients undergoing radical prostatectomy (Table II). In the multivariate Cox
regression analysis, results of positive IHC staining of ERG were
observed (HR, 4.08; 95% CI, 2.03-8.17; P=0.000074). Gleason scores
of 8 (HR, 5.23; 95% CI, 1.01-27.15; P=0.049) and 10 (HR, 18.45; 95%
CI, 1.58-216.20; P=0.020) were independent prognostic factors for
these patients (Table II).
 | Table II.Univariate and multivariate Cox
regression analyses. |
Table II.
Univariate and multivariate Cox
regression analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| ERG |
| 0.009a |
|
|
| Negative | Ref |
| Ref |
|
| Positive | 2.48
(1.32-4.66) | 0.005a | 4.08
(2.03-8.17) |
0.000074a |
| PSA group,
ng/ml |
| 0.335 |
|
|
|
≤10 | Ref |
|
|
|
|
10-20 | 1.48
(0.78-2.84) | 0.232 |
|
|
|
20-100 | 1.59
(0.74-3.40) | 0.231 |
|
|
|
>100 | 5.18
(0.68-39.20) | 0.111 |
|
|
| FPSA group,
ng/ml |
| 0.074 |
|
|
| ≤1 | Ref |
|
|
|
|
1-4 | 0.85
(0.46-1.57) | 0.607 |
|
|
|
>4 | 2.84
(1.14-7.05) | 0.025a |
|
|
| Age group,
years |
| 0.699 |
|
|
|
≤60 | Ref |
|
|
|
|
>60 | 1.20
(0.47-3.03) | 0.705 |
|
|
| Total GS |
| 0.009a |
|
|
| 6 | Ref |
| Ref |
|
| 7 | 1.05
(0.25-4.52) | 0.944 | 1.40
(0.31-6.26) | 0.661 |
| 8 | 3.13
(0.69-14.30) | 0.141 | 5.23
(1.01-27.15) | 0.049a |
| 9 | 2.80
(0.63-12.30) | 0.175 | 2.83
(0.51-15.57) | 0.232 |
| 10 | 8.56
(0.77-95.00) | 0.081 | 18.45
(1.58-216.20) | 0.020a |
| Margins |
| 0.035a |
|
|
|
Negative | Ref |
| Ref |
|
|
Positive | 1.91
(1.06-3.43) | 0.030a | 1.56
(0.78-3.11) | 0.204 |
| Tumor percent,
% |
| 0.032a |
|
|
|
≤25 | Ref |
| Ref |
|
|
25-50 | 0.81
(0.38-1.71) | 0.581 | 0.74
(0.34-1.62) | 0.450 |
|
50-75 | 3.06
(1.43-6.53) | 0.004a | 1.65
(0.69-3.94) | 0.262 |
|
>75 | 0.96
(0.39-2.39) | 0.931 | 0.59
(0.21-1.67) | 0.317 |
| Ki-67, % |
| 0.023a |
|
|
| ≤5 | Ref |
| Ref |
|
|
>5 | 2.16
(1.16-4.02) | 0.016a | 1.50
(0.74-3.06) | 0.262 |
| T |
| 0.052 |
|
|
| T1 | Ref |
|
|
|
| T2 | 20000000
(0-Inf) | 0.997 |
|
|
| T3 | 36100000
(0-Inf) | 0.996 |
|
|
| T4 | 40500000
(0-Inf) | 0.996 |
|
|
| N |
| 0.010 a |
|
|
| N0 | Ref |
| Ref |
|
| N1 | 3.39
(1.43-8.03) | 0.006a | 2.18
(0.80-5.96) | 0.129 |
| Nx |
3.34×10−8 (0-Inf) | 0.997 |
4.86×10−8 (0-Inf) | 0.995 |
| M |
| 0.464 |
|
|
| M0 | Ref |
|
|
|
| M1 | 2.32
(0.32-17.10) | 0.408 |
|
|
Discussion
Several studies have investigated the prognostic
role of ERG IHC in prostate cancer worldwide; however, the findings
have been inconsistent (12–15).
In the present study, patients with ERG IHC-positive status had a
higher BCR and worse BFFS compared with patients with ERG
IHC-negative status after radical prostatectomy. According to the
multivariate Cox regression analysis, the ERG-positive status was
an independent prognostic factor for patients undergoing radical
prostatectomy. Overall, the reason for the differences between the
results of the current study and those of previous studies may be
that the specimens used in the present study were all derived from
radical prostatectomy cases and all patients enrolled were from
China. Prostate cancer has multiple foci and the biopsy specimens
cannot represent the whole cancer. Furthermore, there exist
differences in gene mutations between patients with prostate cancer
of different races/ethnicities. Nevertheless, the current findings
confirmed the prognostic value of ERG IHC in prostate cancer.
Therefore, more aggressive treatment strategies should be adopted
for patients with positive ERG IHC results, and comprehensive
perioperative treatment should be administered to patients
undergoing radical prostatectomy.
In the current study, there were no differences in
the Gleason score, TNM stages, surgical margins, age, tumor
percentage, Ki-67, PSA and FPSA between patients with positive and
negative ERG IHC. The Kaplan-Meier method and Cox regression
analysis were used to estimate the prognosis in patients using ERG
IHC, PSA, FPSA, age, Gleason score, surgical margins, tumor
percentage, Ki-67 and TNM stages; results confirmed that ERG IHC,
Gleason score, tumor proportion, surgical margins and Ki-67 were
among the factors affecting prostate cancer prognosis. Among these,
positive ERG IHC status and Gleason scores of 8 and 10 were
independent prognostic factors for prostate cancer.
The TMPRSS2-ERG fusion gene has been studied
widely and is a common molecular occurrence in high-grade
intraepithelial neoplasia of the prostate as well as prostate
cancer (19). It induces
intraepithelial neoplasia in normal prostate cells in transgenic
mice but does not transform into invasive carcinoma; when
accompanied by phosphatase and tensin homolog loss, aggressive
cancer may develop (20).
Therefore, fusion genes may play important roles in prostate cancer
development and have significant clinical value for the diagnosis
and prognosis of prostate cancer. The TMPRSS2-ERG fusion
leads to ERG protein overexpression. One study reports that
ERG silencing leads to cell cycle arrest in prostate cancer
cells (21); this is consistent
with the report that lowering ERG protein expression reduces the
proliferation and migration of prostate cancer cells (22). Both studies suggest that ERG
proteins play an important role in prostate cancer. The functions
of ERG among prostate cancer-related genes are attracting
attention worldwide, with increasing studies on this topic. ERG IHC
has become a common detection tool used for prostate biopsy and
radical prostatectomy specimens (23). The positivity rate of ERG IHC in the
present study was 16.6%, slightly lower compared with the average
level of 20% in Asia and notably lower compared with the average of
50% in Europe and America (24);
these differences may be caused by differences in
races/ethnicities. The genomics of prostate cancer in the
population in Asia differs from that in the population in Europe
and America, such as the presence of TMPRSS2-ERG, BRCA2 and
FOXA1 (24).
The fusion of TMPRSS2 and ERG results
from long-term exposure to androgen, increased androgen receptor
activity and inhibition of the protein PIWIL1, which prevents DNA
double-strand breaks (25). Thus,
the TMPRSS2-ERG fusion gene is a unique molecular marker for
prostate cancer and this finding is of great significance for
prostate cancer diagnosis. Nguyen et al (26) proposed that the urine-based
detection of the TMPRSS2-ERG fusion gene can be used as a
marker for prostate cancer diagnosis with good specificity and
sensitivity, providing a new non-invasive test for diagnosis of
prostate cancer. Lin et al (27) previously reported that urine
TMPRSS2-ERG levels after digital rectal examination are
associated with higher tumor volumes and Gleason scores in
subsequent prostate biopsies.
High-grade intraepithelial prostate tumors
containing the TMPRSS2-ERG fusion gene are easily
transformed into prostate cancer (28), revealing the prognostic value of the
TMPRSS2-ERG fusion gene in high-grade intraepithelial
neoplasia of the prostate. Active surveillance of patients with
high-grade intraepithelial neoplasia of the prostate and positive
IHC staining of ERG in prostate biopsy pathology is therefore
necessary.
The present study has several limitations. First,
this was a single-center study conducted at the Huadong Hospital,
affiliated with Fudan University. The majority of the participants
were Asian and the sample size was small, which could have caused
selection bias and systematic errors in the study. Performing
multi-center studies and expanding the sample size and other sample
data would help strengthen the credibility of the results. Most of
the samples included are ERG negative. The difference between the
number of ERG-positive samples and the number of ERG-negative
samples is unavoidable, because the average positivity rate of ERG
IHC is 20% in Asia. Second, this was a retrospective study, having
several limitations when compared with a prospective study, and
there may be interfering factors that lack credibility and have not
been considered. Third, the IHC method used in this study was
qualitative, and its application in the detection of ERG expression
has certain limitations. In future, prostate cancer specimens could
be divided according to the proportion of tumor cells stained by
ERG IHC (low, intermediate and high). Quantitative methods such as
western blotting and qPCR might also yield more convincing
results.
As precision medicine has become mainstream,
molecular detection has become a common clinical approach (29). For instance, breast cancer can be
classified into subtypes based on the estrogen receptor and human
epidermal growth factor receptor 2 (HER2) (30). Estrogen receptors and HER2 can
predict tumor progression and help in deciding optimal breast
cancer treatments (30). Similarly,
the TMPRSS2-ERG fusion gene is commonly and uniquely found
in prostate cancer cases; however, whether it can act as a
potential indicator for prostate cancer typing requires further
study.
The present study identified differences in BCR
between patients with positive and negative ERG IHC results.
Patients with ERG IHC-positive status had a worse prognosis and
were more prone to BCR compared with those with ERG IHC-negative
status. ERG IHC positivity is thus an independent risk factor for
predicting postoperative BCR in prostate cancer. ERG IHC is
expected to become a prognostic indicator of prostate cancer, and
its clinical application has been further improved. In conclusion,
the present study revealed that patients with positive ERG IHC
status were prone to BCR after radical prostatectomy and that
positive ERG expression was an independent prognostic risk factor
for prostate cancer.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS, YZ, JT and RW designed and investigated the
trial. YZ, JT and RW analyzed and interpreted the data and wrote
the manuscript. HX, ZC, LX and HD collected and analyzed the data.
YZ, JT, RW, HX, ZC, LX and HD reviewed and revised the manuscript.
ZS, YZ, JT and RW confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study, which involved human participants, was
reviewed and approved by the Ethics Committee of the Huadong
Hospital, affiliated with Fudan University (approval no. 20220050).
The study was conducted in accordance with the Declaration of
Helsinki (as revised in 2013). Since this study was a retrospective
cohort study, an informed consent waiver was applied.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
BCR
|
biochemical recurrence
|
|
BFFS
|
biochemical failure-free survival
|
|
CI
|
confidence interval
|
|
ERG
|
ETS-related gene
|
|
ETS
|
E-26 transformation-specific
|
|
FPSA
|
free prostate-specific antigen
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
IHC
|
immunohistochemistry
|
|
TMPRSS2
|
transmembrane protease serine 2
|
|
TNM
|
tumor, node and metastasis
|
|
OS
|
overall survival
|
|
PCR
|
polymerase chain reaction
|
|
PSA
|
prostate-specific antigen
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leprince D, Gegonne A, Coll J, de Taisne
C, Schneeberger A, Lagrou C and Stehelin D: A putative second
cell-derived oncogene of the avian leukaemia retrovirus E26.
Nature. 306:395–397. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nunn MF, Seeburg PH, Moscovici C and
Duesberg PH: Tripartite structure of the avian erythroblastosis
virus E26 transforming gene. Nature. 306:391–395. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sperone A, Dryden NH, Birdsey GM, Madden
L, Johns M, Evans PC, Mason JC, Haskard DO, Boyle JJ, Paleolog EM
and Randi AM: The transcription factor Erg inhibits vascular
inflammation by repressing NF-kappaB activation and proinflammatory
gene expression in endothelial cells. Arterioscler Thromb Vasc
Biol. 31:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan L, Le Bras A, Sacharidou A, Itagaki
K, Zhan Y, Kondo M, Carman CV, Davis GE, Aird WC and Oettgen P:
ETS-related gene (ERG) controls endothelial cell permeability
via transcriptional regulation of the claudin 5 (CLDN5)
gene. J Biol Chem. 287:6582–6591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birdsey GM, Dryden NH, Amsellem V,
Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC and Randi AM:
Transcription factor Erg regulates angiogenesis and endothelial
apoptosis through VE-cadherin. Blood. 111:3498–3506. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vijayaraj P, Le Bras A, Mitchell N, Kondo
M, Juliao S, Wasserman M, Beeler D, Spokes K, Aird WC, Baldwin HS
and Oettgen P: Erg is a crucial regulator of
endocardial-mesenchymal transformation during cardiac valve
morphogenesis. Development. 139:3973–3985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomlins SA, Rhodes DR, Perner S,
Dhannasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. Science. 310:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sedarsky J, Degon M, Srivastava S and Dobi
A: Ethnicity and ERG frequency in prostate cancer. Nat Rev Urol.
15:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salagierski M and Schalken JA: PCA3 and
TMPRSS2-ERG: Promising biomarkers in prostate cancer diagnosis.
Cancers (Basel). 2:1432–1440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saramäki OR, Harjula AE, Martikainen PM,
Vessella RL, Tammela TL and Visakorpi T: TMPRSS2:ERG fusion
identifies a subgroup of prostate cancers with a favorable
prognosis. Clin Cancer Res. 14:3395–3400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brady L, Carlsson J, Baird AM, Casey O,
Vlajnic T, Murchan P, Cormican D, Costigan D, Gray S, Sheils O, et
al: Correlation of integrated ERG/PTEN assessment with biochemical
recurrence in prostate cancer. Cancer Treat Res Commun.
29:1004512021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berg KD, Vainer B, Thomsen FB, Røder MA,
Gerds TA, Toft BG, Brasso K and Iversen P: ERG protein expression
in diagnostic specimens is associated with increased risk of
progression during active surveillance for prostate cancer. Eur
Urol. 66:851–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong DP, Chen R, Zhang CL, Zhang W, Xiao
GA, Wang FB, Ta N, Gao X and Sun YH: Prevalence and clinical
application of TMPRSS2-ERG fusion in Asian prostate cancer
patients: A large-sample study in Chinese people and a systematic
review. Asian J Androl. 22:200–207. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the Eighth Edition of the
tumor-node-metastasis staging classification for urologic cancers.
Eur Urol. 73:560–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gębska E, Sikora-Żydek A, Michalski M,
Reichman-Warmusz E, Kurek J, Dudek D, Skowron W, Jarząb J and
Wojnicz R: Tissue hemostasis is shifted toward thrombogenesis in
the psoriatic plaques. Pathol Res Pract. 213:1125–1129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perner S, Mosquera JM, Demichelis F, Hofer
MD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, De
Marzo AM and Rubin MA: TMPRSS2-ERG fusion prostate cancer: An early
molecular event associated with invasion. Am J Surg Pathol.
31:882–888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Chi P, Rockowitz S, Iaquinta PJ,
Shamu T, Shukla S, Gao D, Sirota I, Carver BS, Wongvipat J, et al:
ETS factors reprogram the androgen receptor cistrome and prime
prostate tumorigenesis in response to PTEN loss. Nat Med.
19:1023–1029. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Wang Y, Zhang J, Hu Q, Zhi F,
Zhang S, Mao D, Zhang Y and Liang H: Significance of the
TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep.
16:5450–5458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei Y, Peng J, He S, Huang H, Lin L, Zhu
Q, Ye L, Li T, Zhang X, Gao Y and Zheng X: miR-223-5p targeting ERG
inhibits prostate cancer cell proliferation and migration. J
Cancer. 11:4453–4463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah RB: Clinical applications of novel
ERG immunohistochemistry in prostate cancer diagnosis and
management. Adv Anat Pathol. 20:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland
SJ, Zheng Y and Ye D: Epidemiology and genomics of prostate cancer
in Asian men. Nat Rev Urol. 18:282–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bastus NC, Boyd LK, Mao X, Stankiewicz E,
Kudahetti SC, Oliver RT, Berney DM and Lu YJ: Androgen-induced
TMPRSS2:ERG fusion in nonmalignant prostate epithelial cells.
Cancer Res. 70:9544–9548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen PN, Violette P, Chan S, Tanguay S,
Kassouf W, Aprikian A and Chen JZ: A panel of TMPRSS2:ERG fusion
transcript markers for urine-based prostate cancer detection with
high specificity and sensitivity. Eur Urol. 59:407–414. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin DW, Newcomb LF, Brown EC, Brooks JD,
Carroll PR, Feng Z, Gleave ME, Lance RS, Sanda MG, Thompson IM, et
al: Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort:
Results from a baseline analysis in the canary Prostate Active
Surveillance Study. Clin Cancer Res. 19:2442–2450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park K, Dalton JT, Narayanan R, Barbieri
CE, Hancock ML, Bostwick DG, Steiner MS and Rubin MA: TMPRSS2:ERG
gene fusion predicts subsequent detection of prostate cancer in
patients with high-grade prostatic intraepithelial neoplasia. J
Clin Oncol. 32:206–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sisodiya SM: Precision medicine and
therapies of the future. Epilepsia. 62 (Suppl 2):S90–S105. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|