Introduction
Preoperative neoadjuvant long-course
chemoradiotherapy (LC-CRT) is the current standard of therapy for
locally advanced rectal cancer (LARC) with the lower edge of the
tumor <10-cm from the anal verge (1). Since neoadjuvant short-course
radiotherapy (SCRT) can achieve similar survival outcomes without
significantly increasing toxicities compared with LC-CRT it can be
used as an alternative option (2,3).
Numerous studies have confirmed the synergistic effects between
radiotherapy and immune checkpoint inhibitors (ICIs) (4,5).
Radiotherapy can increase exposure to tumor antigens, alter the
tumor microenvironment and promote the recruitment of inflammatory
factors (6–8), and ICIs can simultaneously strengthen
the ablility of the immune response of the patient against tumors.
Based on these theories and studies, the present study conducted a
phase 2 clinical trial of preoperative SCR followed by chemotherapy
and camrelizumab as a treatment regimen for LARC, which showed a
measurable increase in the pathological complete response (pCR)
rate (48.1%; 13/27) (9). A phase 3
randomized controlled clinical trial is underway and the current
results are concordant with the phase 2 results, showing a
significant increase in pCR rate compared with preoperative
neoadjuvant LC-CRT.
In rectal cancer, a number of patients may opt for
watchful waiting treatment due to the presence of special situation
of complete clinical remission (CCR). However, the dilemma is that
there is no uniform definition of CCR. Moreover, there is
controversy on watchful waiting as a treatment method (10). A prospective study has demonstrated
that imaging is not a sensitive predictor of pCR in patients with
rectal cancer. The accuracy of fluorodeoxyglucose positron emission
tomography and computed tomography (CT) in predicting pCR is only
54 and 19%, respectively (11).
This is also a hindrance when choosing treatment options (surgery
or observation) in clinical practice. Therefore, early and correct
identification of patients with pCR and avoiding them to have the
operation is one of the urgent clinical issues to be addressed.
Numerous studies have demonstrated that economical
and widely available hematological indicators are often used to
predict the prognosis of patients with cancer (12–14).
For example, in patients with rectal cancer treated with
neoadjuvant CRT, Huang et al (15) suggested that a higher neutrophil to
lymphocyte ratio (NLR) pretreatment is associated with poorer
prognosis, and that these patients not only have a higher risk of
recurrence, but also have worse overall survival (OS) and
disease-free survival (DFS). Even in patients with LARC treated
with SCRT, high levels of NLR are independently associated with
poor OS (16). Similarly, the
platelet to lymphocyte ratio (PLR) is negatively associated with
prognosis in patients with rectal cancer (17). In addition to these inflammatory
indicators, there are a number of peripheral blood indicators that
also reflect the prognosis of patients with cancer. For example,
elevated lactate dehydrogenase (LDH) at baseline serves as a poor
prognostic biomarker in advanced non-small cell lung cancer
(18). A meta-analysis of 76
studies indicated that LDH is a predictive biomarker of prognosis
in a variety of solid tumors (19).
In metastatic colorectal cancer, patients with high LDH also have a
poor prognosis (20). Notably, to
the best of our knowledge, studies of LDH in the neoadjuvant
treatment of LARC are rare.
The above findings suggest that baseline peripheral
blood indicators, including NLR, PLR and LDH, can be used as
predictors of prognosis in patients with cancer. Therefore, the aim
of the present study was to investigate whether these indicators
could predict the pathological response of patients with LARC
treated with SCR followed by chemotherapy and camrelizumab, while
also searching for hematological markers at baseline that may
potentially play a predictive role.
Patients and materials
Patients
Eligible patients at Cancer Center, Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China) were enrolled in the present
retrospective study between June, 2021 and October, 2022. The
percentages of females were 35.2, 31.0 and 31.4% and the mean age
were 56.91, 53.69 and 56.98 years in Arm A, Arm b and Arm C,
respectively. The study was approved by the Institutional Ethics
Committee of the Tongji Medical College, Huazhong University of
Science and Technology (approval no. 0271-21). The inclusion
criteria were as follows: i) Patients or their family members agree
to participate in the study and sign the informed consent form; ii)
aged 18–75 years, male or female; iii) histologically confirmed
T3-44 and/or N+ rectal adenocarcinoma (AJCC/UICC TNM staging (8th
Edition, 2017) (21); iv) inferior
margin ≤10 cm from the anal verge; v) it is expected to reach R0;
vi) Eastern Cooperative Oncology Group performance status score is
0–1; vii) can swallow pills normally; viii) untreated with
antitumor therapy for rectal cancer, including radiotherapy,
chemotherapy, surgery and traditional Chinese medicine; ix)
surgical treatment is planned after neoadjuvant treatment; x) there
was no operative contraindication; xi) laboratory tests were
required to meet the following requirements: White blood cells
≥4×109/l; absolute neutrophil count
≥1.5×109/l; platelet count ≥100×109/l;
hemoglobin ≥90 g/l; serum total bilirubin ≤1.5× upper limit of
normal (ULN); serum alanine aminotransferase and aspartate
aminotransferase ≤2.5× ULN; serum creatinine ≤1.5× ULN value or
creatinine clearance rate ≥50 ml/min; international normalized
ratio ≤1.5× ULN; activated partial thromboplastin time ≤1.5× ULN;
and xii) males or females with reproductive ability who are willing
to use contraception in the trial. The exclusion criteria were as
follows: i) Documented history of allergy to study drugs, including
any component of Camrelizumab, capecitabine, irinotecan,
oxaliplatin and other platinum drugs; ii) have received or are
receiving any of the following treatments: Any radiotherapy,
chemotherapy or other anti-tumor drugs for tumors; patients who
need to be treated with corticosteroid (dose equivalent to
prednisone of >10 mg/day) or other immunosuppressive agents
within 2 weeks prior to study drug administration; received live
attenuated vaccine within 4 weeks before the first use of the study
drug; underwent major surgery or severe trauma within 4 weeks
before the first use of the study drug; iii) any active autoimmune
disease or history of autoimmune disease; iv) have a history of
immunodeficiency, including HIV positive, or other acquired or
congenital immunodeficiency diseases, or have a history of organ
transplantation or allogeneic bone marrow transplantation; v) there
are clinical symptoms or diseases of heart that are not well
controlled; vi) severe infection (CTCAE >2) occurred within 4
weeks before the first use of the study drug; baseline chest
imaging revealed active pulmonary inflammation, signs and symptoms
of infection within 14 days prior to the first use of the study
drug or oral or patient is taking intravenous antibiotic therapy,
except for prophylactic use of antibiotics; vii) patients with
active pulmonary tuberculosis infection found by medical history or
CT examination, or with a history of active pulmonary tuberculosis
infection within one year before enrollment, or with a history of
active pulmonary tuberculosis infection >1 year ago but without
regular treatment; viii) the presence of active hepatitis B (HBV
DNA >2,000 IU/ml or 104 copies/ml) was positive for
hepatitis C (hepatitis C antibody) and HCV RNA was higher compared
with the lower limit of analytical method; ix) female subject who
is pregnant or breastfeeding; x) patients who are not suitable for
participation in clinical trials in the opinion of the
investigator. View inclusion criteria and exclusion criteria using
the following link: https://clinicaltrials.gov/ct2/show/NCT04928807?term=NCT04928807&draw=2&rank=1.
Eligible patients were divided into 3 cohorts
depending on the neoadjuvant treatment regimen: Arm A (SCRT plus 2
cycles of chemotherapy and camrelizumab); Arm B (SCRT plus 2 cycles
of chemotherapy); and Arm C (LC-CRT plus 2 cycles of chemotherapy).
The more detailed treatment regimen was presented in Fig. 1. Albumin, total cholesterol, LDH,
neutrophil, platelet and lymphocyte values in the peripheral blood
were collected before treatment. pCR was defined as postoperative
pathologic confirmation of no active cancer cells remaining in
either the primary site or the resected regional lymph nodes with a
ypT0N0M0 stage.
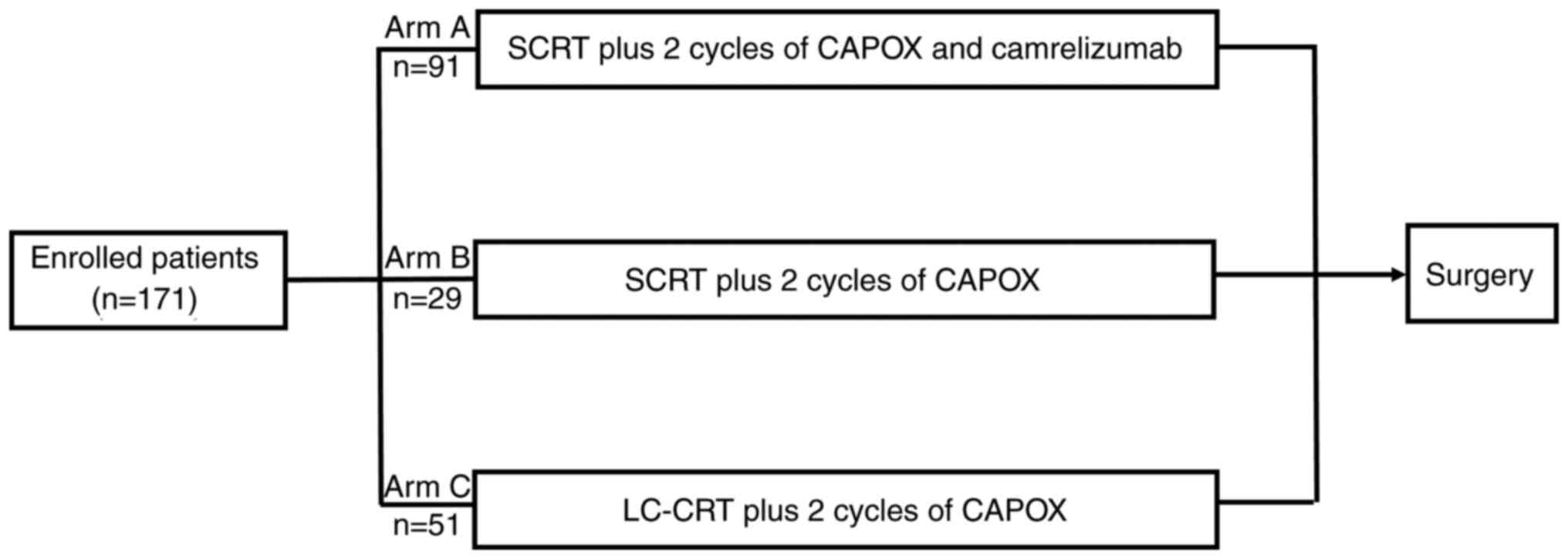 | Figure 1.Treatment regimen. CAPOX course,
oxaliplatin 130 mg/m2 intravenously, day 1; capecitabine
1,000 mg/m2 oral twice daily, days 1–14. Camrelizumab,
200 mg intravenous drip, day 1. LC-CRT course, 28×1.8 Gy on 5 days
per week, oral capecitabine (825 mg/m2, bid, days 1–5)
during radiotherapy. Surgery was performed according to total
mesorectal excision principles. CAPOX, capecitabine plus
oxaliplatin; SCRT, short-course radiotherapy; LC-CRT, long-course
chemoradiotherapy; SCRT, 5×5 Gy over 5 days. |
Statistical analysis
Continuous variables are described by mean ±
standard deviation (SD) or median and range (determined by whether
the data are normally distributed according to Shapiro-wilk test),
and categorical variables are presented as frequencies and
percentages. The non-parametric test, χ2 and Fisher's
exact tests were performed to compare the three groups. The
receiver operating characteristic (ROC) curve was used to determine
the optimal cut-off value to define the level of hematological
indicators and univariate and multivariate logistics regressions
were used to determine independent prognostic factors of pCR among
these indicators. P<0.05 was considered to indicate a
statistically significant difference. All data analysis was
performed in IBM SPSS 27.0 (IBM Corp.).
Results
Patient clinical features
The basic clinical characteristics of the three
cohorts of patients are presented in Table I. The mean ages of Arm A, Arm B and
Arm C were 56.91, 53.69 and 56.98 years old, respectively. The
percentages of males in Arm A, Arm B and Arm C were 64.8, 69.0 and
68.6%, respectively. The proportion of phase III in any cohort is
higher compared with the proportion of phase II (Arm A: 90.1% vs.
9.9%; Arm B: 75.9% vs. 24.1%; Arm C: 90.2% vs. 9.8%). Sex, age, T
stage, N stage, TNM stage and distance from primary tumor to anal
verge were generally well balanced among each group.
 | Table I.Baseline patient and tumor
characteristics in three cohorts. |
Table I.
Baseline patient and tumor
characteristics in three cohorts.
| Characteristic | Arm A (n=91) | Arm B (n=29) | Arm C (n=51) | P-value |
|---|
| Sex (%) |
|
|
| 0.863 |
|
Male | 59 (64.8) | 20 (69.0) | 35 (68.6) |
|
|
Female | 32 (35.2) | 9 (31.0) | 16 (31.4) |
|
| Age (mean ±
SD) | 56.91±0.962 | 53.69±11.720 | 56.98±8.620 | 0.243 |
| Clinical T stage
(%) |
|
|
| 0.440 |
|
2-3 | 69 (75.8) | 25 (76.2) | 38 (74.5) |
|
| 4 | 22 (24.1) | 4 (13.8) | 13 (25.5) |
|
| Clinical N stage
(%) |
|
|
| 0.161 |
|
0-1 | 48 (52.7) | 20 (69.0) | 24 (47.1) |
|
| 2 | 43 (47.3) | 9 (31.0) | 27 (52.9) |
|
| Tumor node
metastasis (%) |
|
|
| 0.102 |
| II | 9 (9.9) | 7 (24.1) | 5 (9.8) |
|
|
III | 82 (90.1) | 22 (75.9) | 46 (90.2) |
|
| MMR status (%) |
|
|
| 0.056 |
|
dMMR/unknown | 27 (29.7) | 10 (34.5) | 7 (13.7) |
|
|
pMMR | 64 (70.3) | 19 (65.5) | 44 (86.3) |
|
| Circumferential
resection margin (%) |
|
|
| <0.001 |
|
Positive | 48 (52.7) | 7 (24.1) | 30 (58.8) |
|
|
Negative | 27 (29.7) | 6 (20.7) | 17 (33.3) |
|
|
Unknown | 16 (17.6) | 16 (55.2) | 4 (7.8) |
|
| Extramural vascular
invasion (%) |
|
|
| <0.001 |
|
Positive | 40 (43.9) | 5 (17.2) | 24 (47.1) |
|
|
Negative | 29 (31.9) | 4 (13.8) | 19 (37.3) |
|
|
Unknown | 22 (24.2) | 20 (69.0) | 8 (15.7) |
|
| Distance from
primary tumor to anal verge (median + range, cm) | 5.0 (0.5-10.0) | 5.0 (2.0-10.0) | 6.0 (1.5-9.8) | 0.830 |
Efficacy
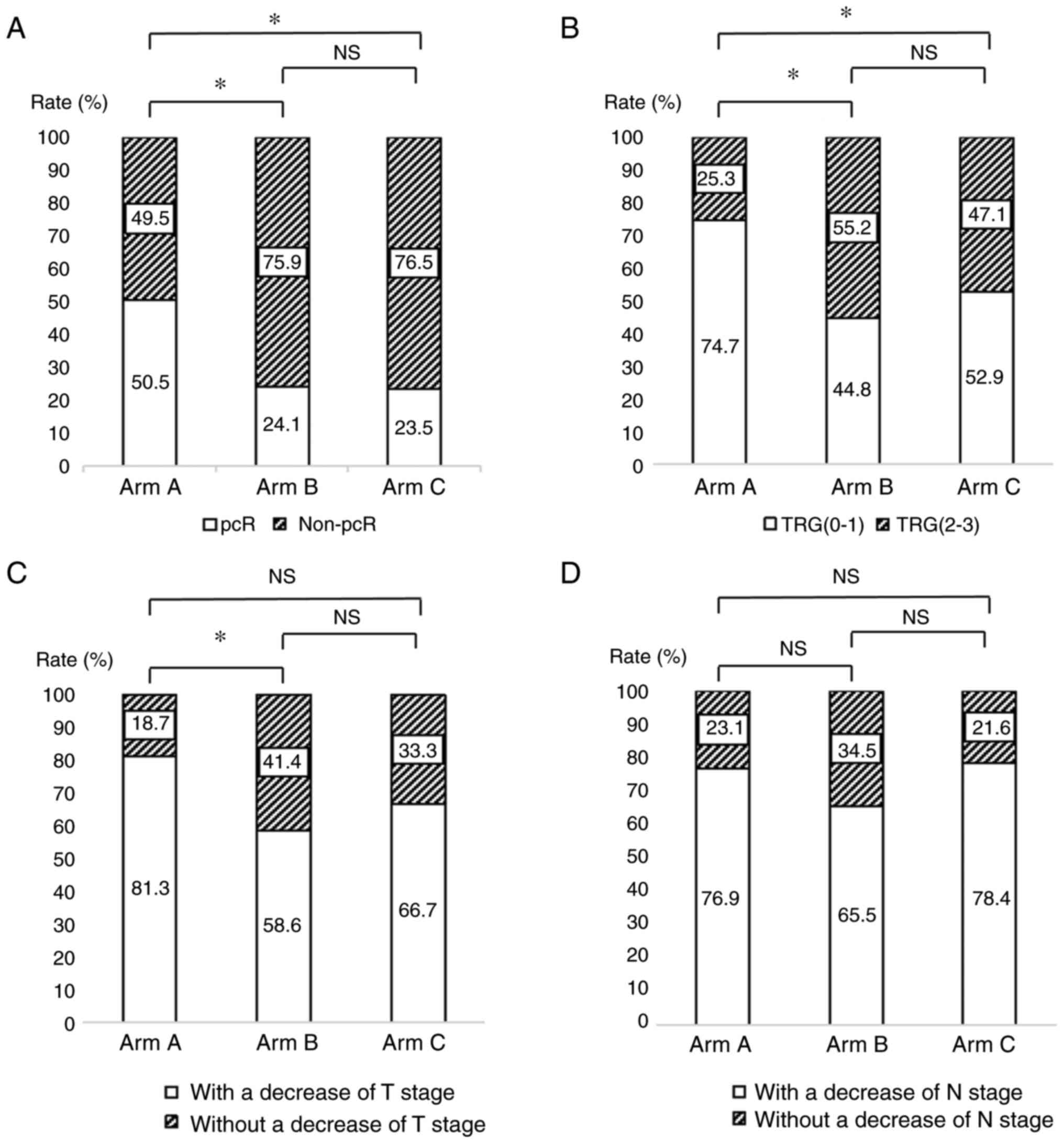
The pCR rates for Arm A, Arm B and Arm C were 50.5,
24.1 and 23.5%, respectively (Table
II). The pCR rate was different between Arm A and Arm B, and
between Arm A and Arm C, while there was no significant difference
between Arm B and Arm C (Fig. 2A).
The percentages of pT0 were 50.5 (Arm A), 24.1 (Arm B) and 23.5%
(Arm C) and pN0 were 80.2 (Arm A), 79.3 (Arm B) and 74.5% (Arm C)
in different groups, respectively (Table II). The percentage of TRG (the
criterion of TRG refers to the American Joint Committee on Cancer
8th edition) (21) of grade 0–1 was
higher in Arm A compared with that in the remaining two Arms (Arm
A: 76.9%, Arm B: 44.8%, Arm C:52.9%) (Table II and Fig. 2B). The proportions of Arm A, Arm B
and Arm C with a decrease of at least one level in T stage were
81.3, 58.6 and 66.7% and with a N downstaging at least one level
were 76.9, F65.5 and 78.4%, respectively (Table II). There was no difference in T
and N downstaging between either group, except that Arm A has a
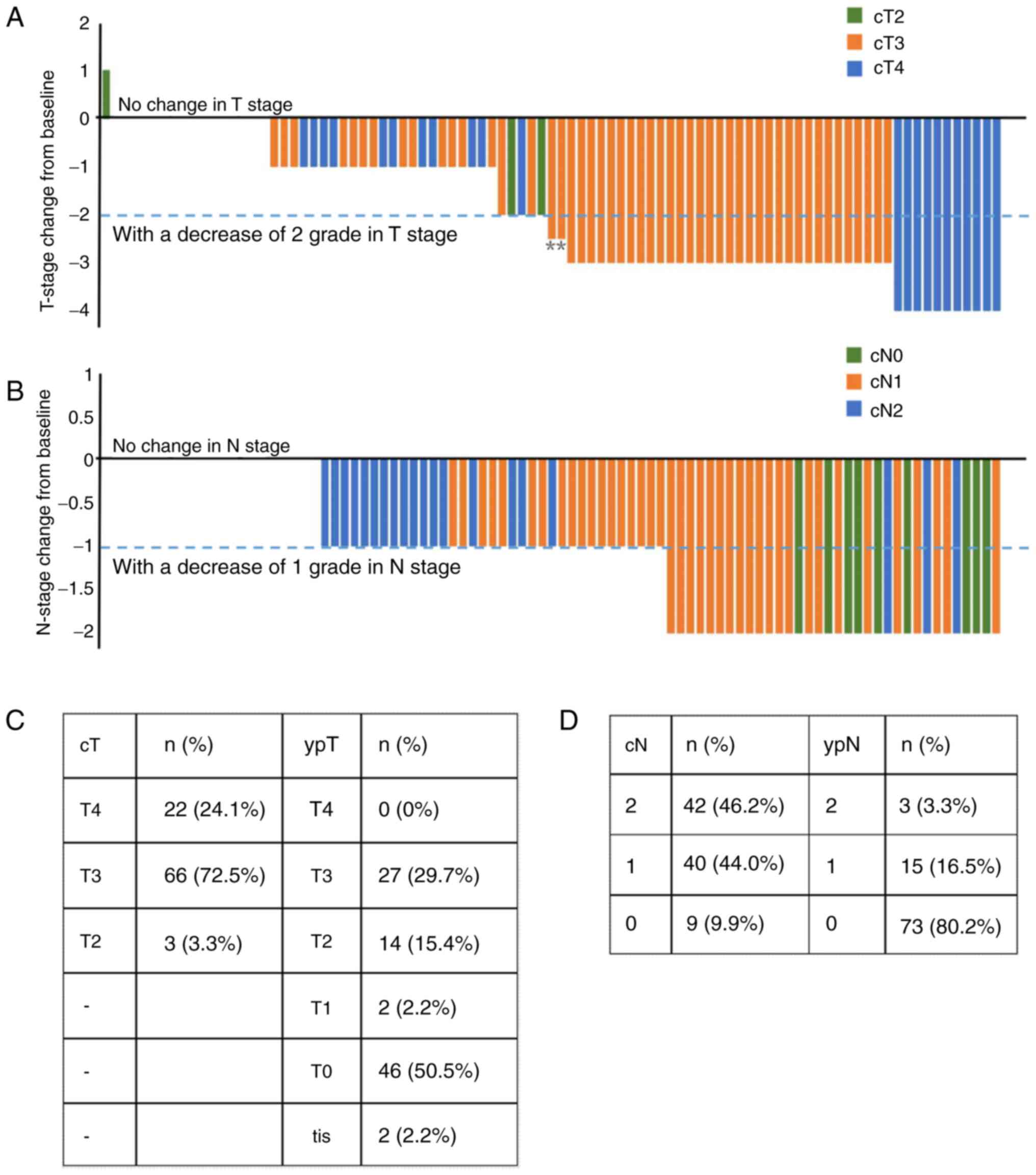
significant T-downstaging vs. Arm B (Fig. 2C and D). Fig. 3 presents the T stage and N stage of
Arm A patients at baseline and postoperative.
 | Table II.Postoperative pathological results in
Arm A, Arm B and Arm C. |
Table II.
Postoperative pathological results in
Arm A, Arm B and Arm C.
| Pathological
results | Arm A (n=91)
(%) | Arm B (n=29)
(%) | Arm C (n=51)
(%) |
|---|
| ypT |
|
|
|
|
tis | 2 (2.2) | 0 (0) | 0 (0) |
| 0 | 46 (50.5) | 7 (24.1) | 12 (23.5) |
| 1 | 2 (2.2) | 2 (6.9) | 2 (3.9) |
| 2 | 14 (15.4) | 5 (17.2) | 13 (25.5) |
| 3 | 27 (29.7) | 15 (51.7) | 23 (45.1) |
| 4 | 0 (0) | 0 (0) | 1 (2.0) |
| ypN |
|
|
|
| 0 | 73 (80.2) | 23 (79.3) | 38 (74.5) |
| 1 | 15 (16.5) | 5 (17.2) | 9 (17.6) |
| 2 | 3 (3.3) | 1 (3.4) | 4 (7.8) |
| ypTNM |
|
|
|
|
pCR | 46 (50.5) | 7 (24.1) | 12 (23.5) |
| 0 | 2 (2.2) | 0 (0) | 0 (0) |
| I | 9 (9.9) | 6 (20.7) | 10 (19.6) |
| II | 16 (17.6) | 10 (34.5) | 16 (31.4) |
|
III | 18 (19.8) | 6 (20.7) | 13 (25.5) |
| TRG |
|
|
|
| 0 | 46 (50.5) | 7 (24.1) | 12 (23.5) |
| 1 | 22 (24.2) | 6 (20.7) | 15 (29.4) |
| 2 | 19 (20.9) | 14 (48.3) | 22 (43.1) |
| 3 | 4 (4.4) | 2 (6.9) | 2 (3.9) |
Prognostic factors associated with pCR
in Arm A
In the ROC analysis for the albumin, total
cholesterol, LDH, neutrophil, platelet, lymphocyte, NLR and PLR
cutoff values, the areas under the curve were 0.525 (P=0.688),
0.594 (P=0.156), 0.502 (P=0.982), 0.581 (P=0.179), 0.541 (P=0.505),
0.507 (P=0.916), 0.549 (P=0.425) and 0.549 (P=0.422) for pCR,
respectively (data not shown). Finally, albumin >37.9 g/l, total
cholesterol >4.45 mmol/l, LDH >141 U/l, neutrophil
>2.62×109/l, platelet >244×109/l,
lymphocyte >1.44×109/l, NLR >3 and PLR >128
were classified into the high-level group. Binary logistic
regression analysis was performed for the following 10 criteria: i)
Sex; ii) age; iii) albumin; iv) total cholesterol; v) LDH; vi)
neutrophil; vii) platelet; viii) lymphocyte; ix) NLR; x) PLR.
Univariate logistic regression analysis was performed showing that
baseline total cholesterol >4.45 mmol/l, neutrophil
≤2.62×109/l and PLR >128 were significant prognostic
factors of pCR, and multivariate regression analysis showed that
baseline total cholesterol >4.45 mmol/l, neutrophil
≤2.62×109/l were selected as the independent factors for
pCR (Table III).
 | Table III.Univariate and multivariate logistics
regressions for predicting pCR. |
Table III.
Univariate and multivariate logistics
regressions for predicting pCR.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Albumin (g/l) |
|
|
|
|
|
>37.9 | 0.208 | 1.857
(0.708-4.868) |
|
|
|
≤37.9 |
| 1 |
|
|
| Total cholesterol
(mmol/l) |
|
|
|
|
|
>4.45 | 0.026 | 2.856
(1.134-7.190) | 0.016 | 3.456
(1.255-9.517) |
|
≤4.45 |
| 1 |
| 1 |
| LDH (U/l) |
|
|
|
|
|
≤141 | 0.217 | 0.465
(0.138-1.568) |
|
|
|
>141 |
| 1 |
|
|
| Neutrophil
(×109/l) |
|
|
|
|
|
≤2.62 | 0.012 | 3.810
(1.337-10.861) | 0.020 | 4.370
(1.258-15.180) |
|
>2.62 |
| 1 |
| 1 |
| Platelet
(×109/l) |
|
|
|
|
|
>244 | 0.090 | 2.175
(0.886-5.340) |
|
|
|
≤244 |
| 1 |
|
|
| Lymphocyte
(×109/l) |
|
|
|
|
|
>1.44 | 0.347 | 1.500
(0.644-3.492) |
|
|
|
≤1.44 |
| 1 |
|
|
| NLR |
|
|
|
|
| ≤3 | 0.999 | - |
|
|
|
>3 |
| - |
|
|
| PLR |
|
|
|
|
|
>128 | 0.047 | 2.390
(1.013-5.635) | 0.075 | 2.481
(0.913-6.744) |
|
≤128 |
| 1 |
| 1 |
Discussion
The present study validated that the regimen of SCRT
plus 2 cycles of chemotherapy and camrelizumab could significantly
increase the pCR rate in patients with LARC by two-fold (Arm A vs.
Arm C, 50.5% vs. 23.5%). Unfortunately, the mechanism of such a
high pCR rate with this regimen is not clarified. At the same time,
studies using hematological indicators to predict pCR in this
specific cohort have not been conducted. Therefore, the present
study performed this study on 91 patients who were enrolled in the
Arm A.
As aforementioned, NLR, LDH and PLR can be used as
predictors of prognosis in rectal cancer and are negatively
correlated with OS and PFS (15,22,23).
However, the results of a study that included 1,237 patients showed
that NLR and PLR not only did not predict the prognosis of patients
with locally advanced rectal cancer, but also did not predict the
pCR rate after neoadjuvant chemoradiotherapy (24). Instead, this is consistent with the
present findings, which showed that although PLR was associated
with pCR, it was not an independent predictor of pCR. These studies
with contradictory results indicates that the role of these
indicators in predicting prognosis needs to be explored in
depth.
However, notably, the present study revealed that
high total cholesterol and low neutrophils at baseline were
associated with higher pCR rates. As early as 1997, it has been
suggested that total serum cholesterol levels are positively
correlated with the development of colorectal cancer (25). Cholesterol can promote tumorigenesis
and progression by participating in the regulation of different
signaling pathways (26,27). Cholesterol plays an indispensable
role in maintaining the stability of cell membranes and in the
growth and differentiation of cells, which include but are not
specific to lymphocytes (28,29).
Therefore, we hypothesize that the explanation for the effective
and aggressive killing of tumor cells in the Arm A may also be that
cholesterol provided the material source needed for the lymphocytes
to function effectively. However, the specific mechanism needs to
be further explored before it can be clarified.
Neutrophils are often involved in the inflammatory
response in the body and can contribute to tumorigenesis,
progression and metastasis by secreting different factors (30,31).
Meng et al (32) have
demonstrated that high neutrophils indicate a worse DFS. Higher
neutrophil levels in tumor tissue are a risk factor for reduced
5-DFS in patients with colorectal cancer (33). Similarly, in squamous carcinoma of
the anal canal, the cohort of high neutrophils at baseline exhibit
poor local control rates (34).
Overall, high neutrophils are a detrimental presence for patients
with rectal cancer. The present results show that low neutrophils
at baseline are an independent influence in predicting high pCR,
corroborating that high neutrophil status may enhance tumor
resistance to therapy. To some extent, it can be considered that
the relationship between NLR and neutrophil levels is positively
correlated. Since immune checkpoint inhibitors eliminate tumor
cells by enhancing the cytotoxic effect of T cells, the number of
lymphocytes may not visually reflect the killing ability of
lymphocytes (35). It may explain
why lymphocytes and NLR are not associated with the pathological
response.
For patients with rectal cancer who receive
neoadjuvant therapy, if they achieve CCR, a treatment strategy of
observation can be adopted (10).
This can reduce the damage caused by the surgery itself including
psychological and physical damage to the patient (36,37).
However, the concordance between CCR rates and pCR rates varies and
is highly influenced by human consciousness. The result of a study
of 488 patients showed that only 25% of patients who achieved CCR
were pCR (38). This means that 75%
of these patients would be in a situation of delayed treatment if
they did not receive surgical treatment (38). By contrast, the rate of CCR (14.3%;
13/91) in the present study was lower compared with the pCR rate,
which would have led to patients receiving overtreatment. If pCR
can be accurately predicted through easy and cost-effective blood
indicators, then these indicators can provide some reference when
making treatment decisions for patients.
In conclusion, short-course radiotherapy followed by
chemotherapy and camrelizumab as a neoadjuvant treatment for
locally advanced rectal cancer could significantly improve the pCR
rate, and PLR, high cholesterol and low neutrophils at baseline
were favorable prognostic factors. Furthermore, high cholesterol
and low neutrophils at baseline were independently associated with
high pCR rates.
Acknowledgements
Not applicable.
Funding
Shanghai Cancer Prevention and Anti-Cancer Development
Foundation 2020 Discovery research funding (grant no. 2020HX025).
CSCO Tongshu Oncology Research Fund (grant no.
Y-tongshu2021/qn-0205). CSCO Xinda Oncology Immunotherapy Research
Fund (grant no. Y-XD202002-0168).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ, DY, TZ and ZL conceived and designed this study.
JY, MZ, LL participated in the data curation. FZ, DY, LZ and JW
analyzed the data. ZL provided the administrative support. FZ and
DY drafted the manuscript and all authors reviewed it. TZ and ZL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Ethics
Committee of the Tongji Medical College, Huazhong University of
Science and Technology (approval no. 0271-21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benson AB, Venook AP, Al-Hawary MM, Azad
N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: Rectal cancer, version 2.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:1139–1167. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bahadoer RR, Dijkstra EA, van Etten B,
Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID,
Beets-Tan RGH, Blomqvist LK, et al: Short-course radiotherapy
followed by chemotherapy before total mesorectal excision (TME)
versus preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:29–42.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, Michalski W, Bebenek M and Kryj M: Long-term results of a
randomized trial comparing preoperative short-course radiotherapy
with preoperative conventionally fractionated chemoradiation for
rectal cancer. Br J Surg. 93:1215–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagasaka M, Zaki M, Kim H, Raza SN, Yoo G,
Lin HS and Sukari A: PD1/PD-L1 inhibition as a potential
radiosensitizer in head and neck squamous cell carcinoma: A case
report. J Immunother Cancer. 4:832016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schoenhals JE, Seyedin SN, Tang C, Cortez
MA, Niknam S, Tsouko E, Chang JY, Hahn SM and Welsh JW: Preclinical
rationale and clinical considerations for radiotherapy plus
immunotherapy: Going beyond local control. Cancer J. 22:130–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng X, Feng R, Yang L, Xing L and Yu J:
The role of radiation oncology in immuno-oncology. Oncologist. 24
(Suppl 1):S42–S52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Deng W, Li N, Neri S, Sharma A,
Jiang W and Lin SH: Combining immunotherapy and radiotherapy for
cancer treatment: Current challenges and future directions. Front
Pharmacol. 9:1852018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez-Ruiz ME, Vitale I, Harrington
KJ, Melero I and Galluzzi L: Immunological impact of cell death
signaling driven by radiation on the tumor microenvironment. Nat
Immunol. 21:120–134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Z, Cai M, Zhang P, Li G, Liu T, Li X,
Cai K, Nie X, Wang J, Liu J, et al: Phase II, single-arm trial of
preoperative short-course radiotherapy followed by chemotherapy and
camrelizumab in locally advanced rectal cancer. J Immunother
Cancer. 9:e0035542021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Campos F, Martín-Martín M,
Fornell-Pérez R, García-Pérez JC, Die-Trill J, Fuentes-Mateos R,
López-Durán S, Domínguez-Rullán J, Ferreiro R, Riquelme-Oliveira A,
et al: Watch and wait approach in rectal cancer: Current
controversies and future directions. World J Gastroenterol.
26:4218–4239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guillem JG, Ruby JA, Leibold T, Akhurst
TJ, Yeung HW, Gollub MJ, Ginsberg MS, Shia J, Suriawinata AA,
Riedel ER, et al: Neither FDG-PET Nor CT can distinguish between a
pathological complete response and an incomplete response after
neoadjuvant chemoradiation in locally advanced rectal cancer: A
prospective study. Ann Surg. 258:289–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dayde D, Tanaka I, Jain R, Tai MC and
Taguchi A: Predictive and prognostic molecular biomarkers for
response to neoadjuvant chemoradiation in rectal cancer. Int J Mol
Sci. 18:5732017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto T, Kawada K, Hida K, Matsusue R,
Itatani Y, Mizuno R, Yamaguchi T, Ikai I and Sakai Y: Combination
of lymphocyte count and albumin concentration as a new prognostic
biomarker for rectal cancer. Sci Rep. 11:50272021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheong C, Shin JS and Suh KW: Prognostic
value of changes in serum carcinoembryonic antigen levels for
preoperative chemoradiotherapy response in locally advanced rectal
cancer. World J Gastroenterol. 26:7022–7035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CM, Huang MY, Tsai HL, Huang CW, Su
WC, Chang TK, Chen YC, Li CC and Wang JY: Pretreatment
neutrophil-to-lymphocyte ratio associated with tumor recurrence and
survival in patients achieving a pathological complete response
following neoadjuvant chemoradiotherapy for rectal cancer. Cancers
(Basel). 13:45892021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doi H, Yokoyama H, Beppu N, Fujiwara M,
Harui S, Kakuno A, Yanagi H, Hishikawa Y, Yamanaka N and Kamikonya
N: Neoadjuvant modified short-course radiotherapy followed by
delayed surgery for locally advanced rectal cancer. Cancers
(Basel). 13:41122021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ergen ŞA, Barlas C, Yıldırım C and Öksüz
D: Prognostic role of peripheral neutrophil-lymphocyte ratio (NLR)
and platelet-lymphocyte ratio (PLR) in patients with rectal cancer
undergoing neoadjuvant chemoradiotherapy. J Gastrointest Cancer.
53:151–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tjokrowidjaja A, Lord SJ, John T, Lewis
CR, Kok PS, Marschner IC and Lee CK: Pre- and on-treatment lactate
dehydrogenase as a prognostic and predictive biomarker in advanced
non-small cell lung cancer. Cancer. 128:1574–1583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrelli F, Cabiddu M, Coinu A, Borgonovo
K, Ghilardi M, Lonati V and Barni S: Prognostic role of lactate
dehydrogenase in solid tumors: A systematic review and
meta-analysis of 76 studies. Acta Oncol. 54:961–970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scartozzi M, Giampieri R, Maccaroni E, Del
Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi
L, Bittoni A and Cascinu S: Pre-treatment lactate dehydrogenase
levels as predictor of efficacy of first-line bevacizumab-based
therapy in metastatic colorectal cancer patients. Br J Cancer.
106:799–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. (8th edition).
Springer International Publishing: American Joint Commission on
Cancer; 2017, View Article : Google Scholar
|
|
22
|
Basile D, Garattini SK, Corvaja C, Montico
M, Cortiula F, Pelizzari G, Gerratana L, Audisio M, Lisanti C,
Fanotto V, et al: The MIMIC study: Prognostic role and cutoff
definition of monocyte-to-lymphocyte ratio and lactate
dehydrogenase levels in metastatic colorectal cancer. Oncologist.
25:661–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Partl R, Paal K, Stranz B, Hassler E,
Magyar M, Brunner TB and Langsenlehner T: The pre-treatment
platelet-to-lymphocyte ratio as a prognostic factor for
loco-regional control in locally advanced rectal cancer.
Diagnostics (Basel). 13:6792023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dudani S, Marginean H, Tang PA, Monzon JG,
Raissouni S, Asmis TR, Goodwin RA, Gotfrit J, Cheung WY and Vickers
MM: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as
predictive and prognostic markers in patients with locally advanced
rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer.
19:6642019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada K, Araki S, Tamura M, Sakai I,
Takahashi Y, Kashihara H and Kono S: Relation of serum total
cholesterol, serum triglycerides and fasting plasma glucose to
colorectal carcinoma in situ. Int J Epidemiol. 27:794–798. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oneyama C, Iino T, Saito K, Suzuki K,
Ogawa A and Okada M: Transforming potential of Src family kinases
is limited by the cholesterol-enriched membrane microdomain. Mol
Cell Biol. 29:6462–6472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian
W, Cui Y, Choi JC, Doh J, Han JK and Cho W: Cholesterol selectively
activates canonical Wnt signalling over non-canonical Wnt
signalling. Nat Commun. 5:43932014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blank N, Schiller M, Gabler C, Kalden JR
and Lorenz HM: Inhibition of sphingolipid synthesis impairs
cellular activation, cytokine production and proliferation in human
lymphocytes. Biochem Pharmacol. 71:126–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaguchi T and Ishimatu T: Effects of
cholesterol on membrane stability of human erythrocytes. Biol Pharm
Bull. 43:1604–1608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong S, Dong L and Cheng L: Neutrophils
in cancer carcinogenesis and metastasis. J Hematol Oncol.
14:1732021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan U, Chowdhury S, Billah MM, Islam KMD,
Thorlacius H and Rahman M: Neutrophil extracellular traps in
colorectal cancer progression and metastasis. Int J Mol Sci.
22:72602021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng X, Shi D, Zheng H, Xu Y, Li Q, Cai S
and Cai G: High preoperative peripheral blood neutrophil predicts
poor outcome in rectal cancer treated with neoadjunctive
chemoradiation therapy. Int J Clin Exp Pathol. 10:7718–7725.
2017.PubMed/NCBI
|
|
33
|
Rottmann BG, Patel N, Ahmed M, Deng Y,
Ciarleglio M, Vyas M, Jain D and Zhang X: Clinicopathological
significance of neutrophil-rich colorectal carcinoma. J Clin
Pathol. 76:34–39. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schernberg A, Huguet F, Moureau-Zabotto L,
Chargari C, Rivin Del Campo E, Schlienger M, Escande A, Touboul E
and Deutsch E: External validation of leukocytosis and neutrophilia
as a prognostic marker in anal carcinoma treated with definitive
chemoradiation. Radiother Oncol. 124:110–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint
signaling inhibition for cancer immunotherapy: mechanism,
combinations, and clinical outcome. Front Pharmacol. 8:5612017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paun BC, Cassie S, MacLean AR, Dixon E and
Buie WD: Postoperative complications following surgery for rectal
cancer. Ann Surg. 251:807–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mei F, Yang X, Na L and Yang L: Anal
preservation on the psychology and quality of life of low rectal
cancer. J Surg Oncol. 125:484–492. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hiotis SP, Weber SM, Cohen AM, Minsky BD,
Paty PB, Guillem JG, Wagman R, Saltz LB and Wong WD: Assessing the
predictive value of clinical complete response to neoadjuvant
therapy for rectal cancer: An analysis of 488 patients. J Am Coll
Surg. 194:131–136. 2002. View Article : Google Scholar : PubMed/NCBI
|