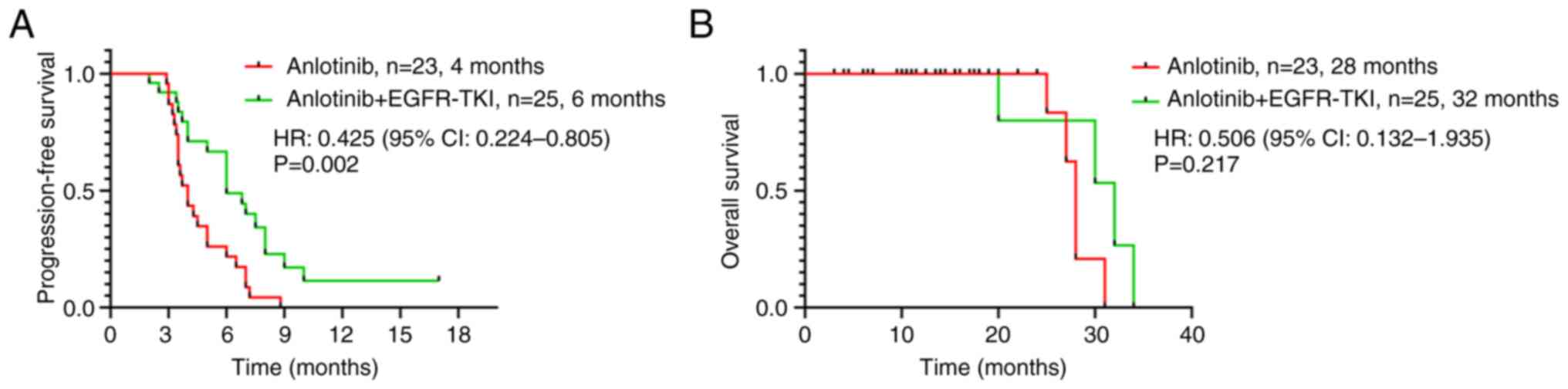
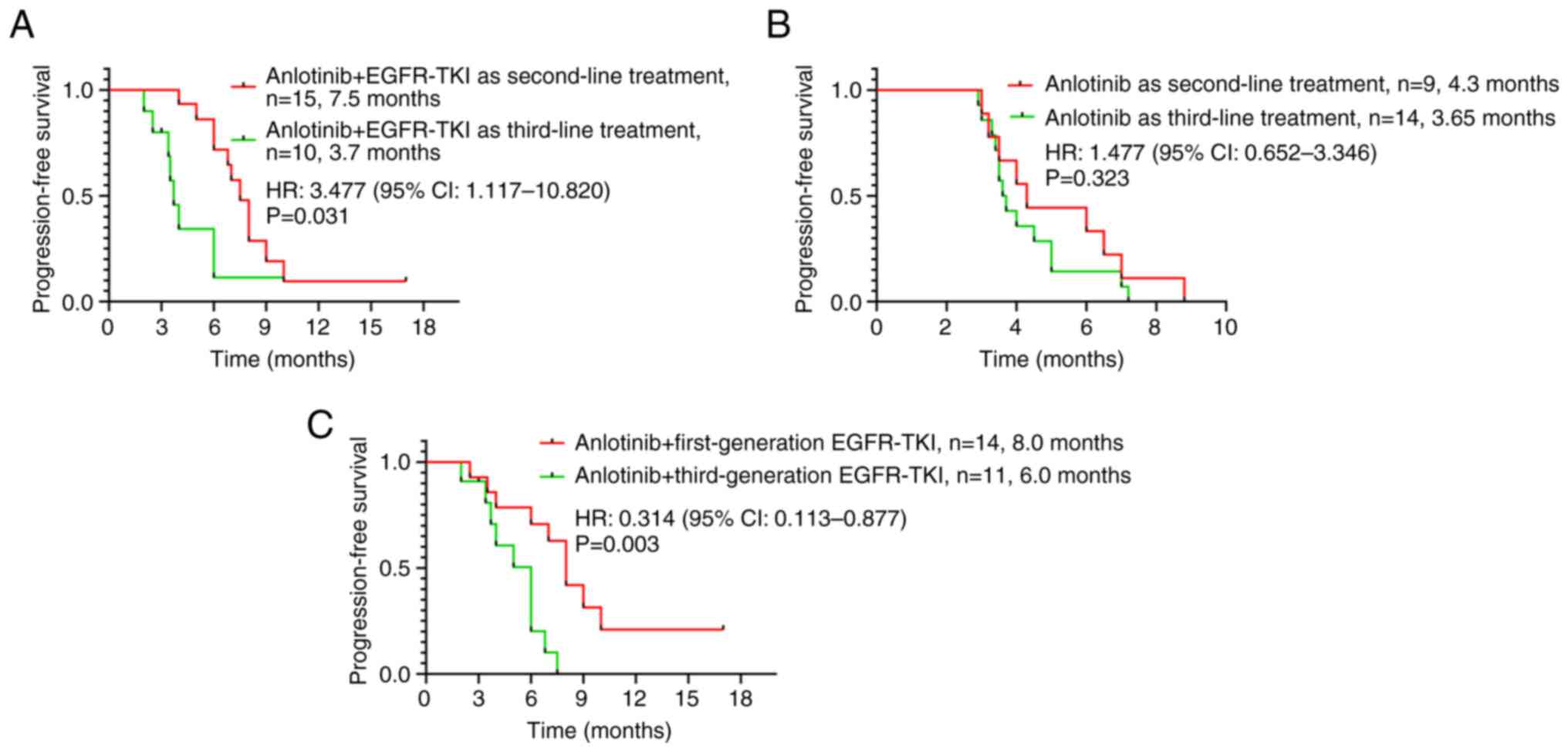
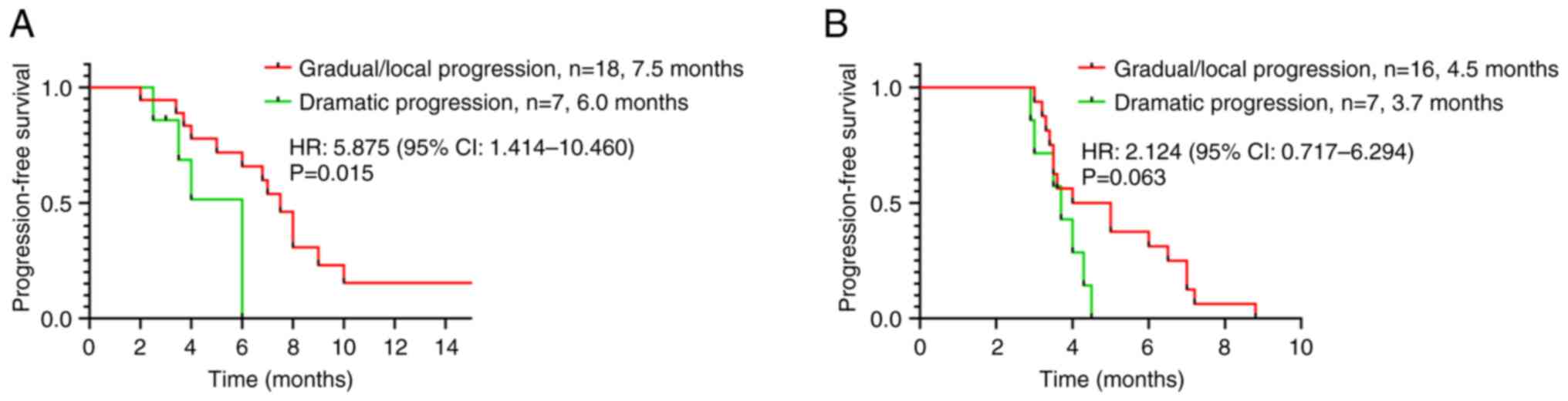
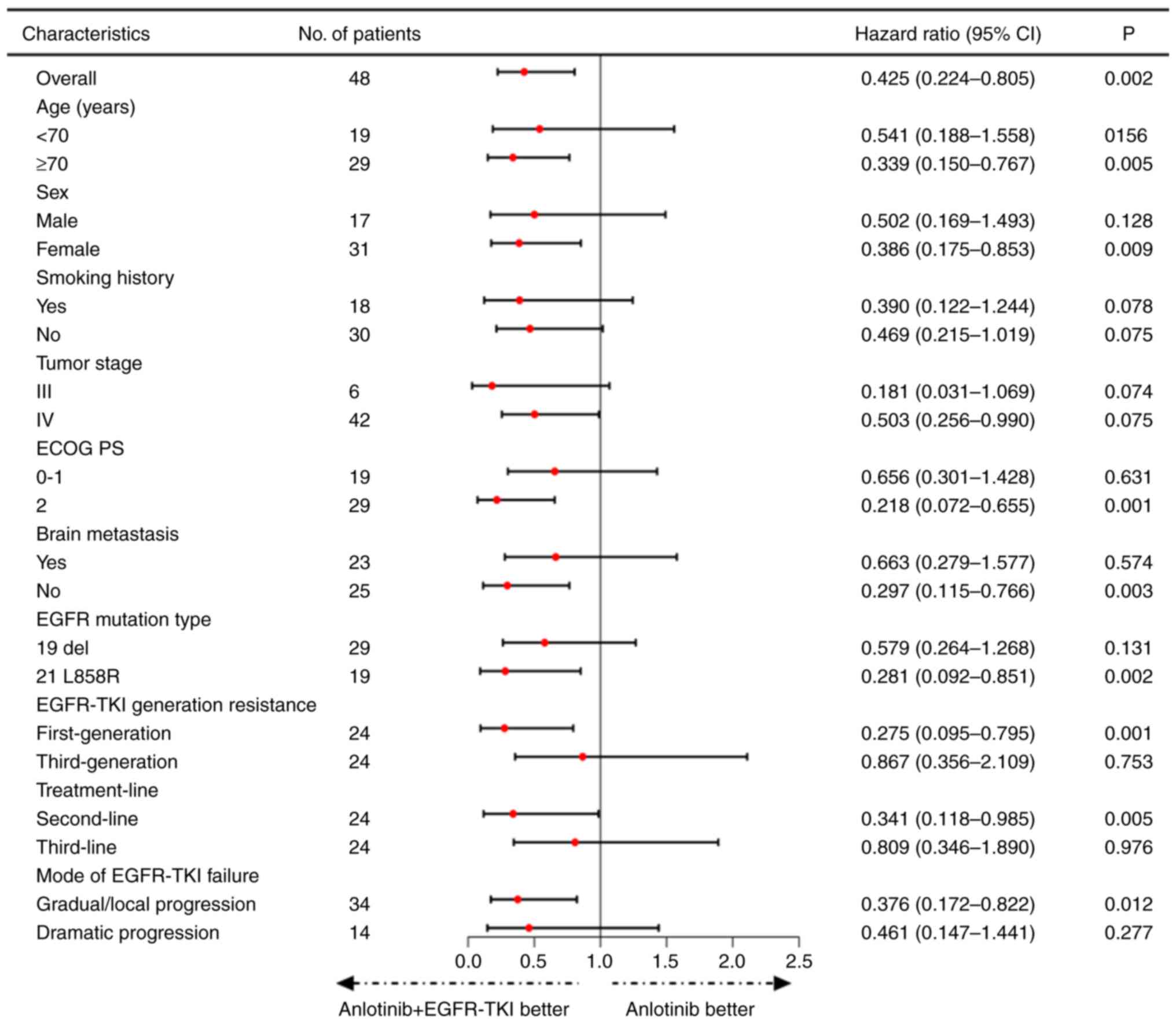
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JC, Sequist LV, Geater SL, Tsai CM,
Mok TS, Schuler M, Yamamoto N, Yu CJ, Ou SHI, Zhou C, et al:
Clinical activity of afatinib in patients with advanced
non-small-cell lung cancer harbouring uncommon EGFR mutations: A
combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung
6. Lancet Oncol. 16:830–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastaticnon-small-cell lung cancer With EGFR
exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Furmonertinib (AST2818) versus
gefitinib as first-line therapy for Chinese patients with locally
advanced or metastatic EGFR mutation-positive non-small-cell lung
cancer (FURLONG): A multicentre, double-blind, randomised phase 3
study. Lancet Respir Med. 10:1019–1028. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liam CK, Leow HR, How SH, Pang YK, Chua
KT, Lim BK, Lai NL, Kuan YC, Pailoor J and Rajadurai P: Epidermal
growth factor receptor mutations in non- small cell lung cancers in
a multiethnic malaysian patient population. Asian Pac J Cancer
Prev. 15:321–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camidge DR, Pao W and Sequist LV: Acquired
resistance to TKIs in solid tumours: Learning from lung cancer. Nat
Rev Clin Oncol. 11:473–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hung MS, Chen IC, Lin PY, Lung JH, Li YC,
Lin YC, Yang CT and Tsai YH: Epidermal growth factor receptor
mutation enhances expression of vascular endothelial growth factor
in lung cancer. Oncol Lett. 12:4598–4604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pore N, Jiang Z, Gupta A, Cerniglia G, Kao
GD and Maity A: EGFR tyrosine kinase inhibitors decrease VEGF
expression by both hypoxia-inducible factor (HIF)-1-independent and
HIF-1-dependent mechanisms. Cancer Res. 66:3197–3204. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang L, Yu Y, Li Y, Wang S, He J, Zhang R
and Sun YP: Upregulation of AREG, EGFR, and HER2 contributes to
increased VEGF expression in granulosa cells of patients with
OHSSdagger. Biol Reprod. 101:426–432. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osude C, Lin L, Patel M, Eckburg A, Berei
J, Kuckovic A, Dube N, Rastogi A, Gautam S, Smith TJ, et al:
Mediating EGFR-TKI resistance by VEGF/VEGFR autocrine pathway in
non-small cell lung cancer. Cells. 11:16942022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Syed YY: Correction to: Anlotinib: First
global approval. Drugs. 78:12872018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y
and Lou L: Preclinical characterization of anlotinib, a highly
potent and selective vascular endothelial growth factor receptor-2
inhibitor. Cancer Sci. 109:1207–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin B, Song X, Yang D, Bai D, Yao Y and Lu
N: Anlotinib inhibits angiogenesis via suppressing the activation
of VEGFR2, PDGFRbeta and FGFR1. Gene. 654:77–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou
Q, Su J, Wang Z, Xu CR, Huang YS, Wang BC, et al: Clinical modes of
EGFR tyrosine kinase inhibitor failure and subsequent management in
advanced non-small cell lung cancer. Lung Cancer. 79:33–39. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WSME, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu SG and Shih JY: Management of acquired
resistance to EGFR TKI-targeted therapy in advanced non-small cell
lung cancer. Mol Cancer. 17:382018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manzo A, Montanino A, Carillio G, Costanzo
R, Sandomenico C, Normanno N, Piccirillo MC, Daniele G, Perrone F,
Rocco G and Morabito A: Angiogenesis inhibitors in NSCLC. Int J Mol
Sci. 18:20212017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu HA, Schoenfeld AJ, Makhnin A, Kim R,
Rizvi H, Tsui D, Falcon C, Houck-Loomis B, Meng F, Yang JL, et al:
Effect of osimertinib and bevacizumab on progression-free survival
for patients with metastatic EGFR-mutant lung cancers: A phase 1/2
single-group open-label trial. JAMA Oncol. 6:1048–1054. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY,
Cui JW, Yang N, Song Y, Li XL, Lu S, et al: Bevacizumab plus
erlotinib in Chinese patients with untreated, EGFR-mutated,
advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study.
Cancer Cell. 39:1279–1291. e32021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hata A, Katakami N, Kaji R, Yokoyama T,
Kaneda T, Tamiya M, Inoue T, Kimura H, Yano Y, Tamura D, et al:
Afatinib plus bevacizumab combination after acquired resistance to
EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung
cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer.
124:3830–3838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li F, Zhu T, Cao B, Wang J and Liang L:
Apatinib enhances antitumour activity of EGFR-TKIs in non-small
cell lung cancer with EGFR-TKI resistance. Eur J Cancer.
84:184–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Cao H, Cui Y, Jin S, Gao W, Huang
C and Guo R: Concurrent use of anlotinib overcomes acquired
resistance to EGFR-TKI in patients with advanced EGFR-mutant
non-small cell lung cancer. Thorac Cancer. 12:2574–2584. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lian Z, Du W, Zhang Y, Fu Y, Liu T, Wang
A, Cai T, Zhu J, Zeng Y, Liu Z and Huang JA: Anlotinib can overcome
acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small
cell lung cancer without harboring EGFR T790M mutation. Thorac
Cancer. 11:1934–1943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li T, Qian Y, Zhang C, Uchino J, Provencio
M, Wang Y, Shi X, Zhang Y and Zhang X: Anlotinib combined with
gefitinib can significantly improve the proliferation of epidermal
growth factor receptor-mutant advanced non-small cell lung cancer
in vitro and in vivo. Transl Lung Cancer Res. 10:1873–1888. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Shao L, Xu Y, Song Z, Lou G, Zhang
Y and Chen M: Efficacy and safety of anlotinib with and without
EGFR-TKIs or immunotherapy in the treatment of elder patients with
non-small-cell lung cancer: A retrospective study. BMC Pulm Med.
22:1792022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chu T, Zhong R, Zhong H, Zhang B, Zhang W,
Shi C, Qian J, Zhang Y, Chang Q, Zhang X, et al: Phase 1b study of
sintilimab plus anlotinib as first-line therapy in patients with
advanced NSCLC. J Thorac Oncol. 16:643–652. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barron F, Cardona AF, Corrales L,
Ramirez-Tirado LA, Caballe-Perez E, Sanchez G, Flores-Estrada D,
Zatarain-Barrón ZL and Arrieta O; Latin American Consortium for the
Study of Lung Cancer (CLICaP), : Characteristics of progression to
tyrosine kinase inhibitors predict overall survival in patients
with advanced non-small cell lung cancer harboring an EGFR
mutation. J Thorac Dis. 10:2166–2178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Otsuka K, Hata A, Takeshita J, Okuda C,
Kaji R, Masago K, Fujita S and Katakami N: EGFR-TKI rechallenge
with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer
Chemother Pharmacol. 76:835–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui Q, Hu Y, Cui Q, Wu D, Mao Y, Ma D and
Liu H: Osimertinib rechallenge with bevacizumab vs. chemotherapy
plus bevacizumab in EGFR-mutant NSCLC patients with osimertinib
resistance. Front Pharmacol. 12:7467072021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wedding U, Honecker F, Bokemeyer C,
Pientka L and Hoffken K: Tolerance to chemotherapy in elderly
patients with cancer. Cancer Control. 14:44–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robinson L and Xavier NA: Managing older
patients with cancer. JAAPA. 33:31–34. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tan AC and Tan DSW: Targeted therapies for
lung cancer patients with oncogenic driver molecular alterations. J
Clin Oncol. 40:611–625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imyanitov EN, Iyevleva AG and Levchenko
EV: Molecular testing and targeted therapy for non-small cell lung
cancer: Current status and perspectives. Crit Rev Oncol Hematol.
157:1031942021. View Article : Google Scholar : PubMed/NCBI
|