Introduction
Breast cancer (BC), with the highest incidence rate
among types of female cancer, has been reported to be the second
leading cause of death, jeopardizing the health of female patients
(1). Triple-negative BC (TNBC)
refers to a subtype of BC defined by the negative expression of
estrogen and progesterone receptors, and the lack of amplification
of the human epidermal growth factor-2 (2). Patients with TNBC have poor prognosis
and a high risk of relapse and metastasis compared with patients
with other subtypes due to the aggressive nature of TNBC, coupled
with the lack of targeted therapies (3–5). Thus,
effective TNBC therapies should be developed, and the design of
novel therapeutic strategies for more effective disease management
in TNBC has become a hotspot of research (6).
Clustered regulatory interspaced short palindromic
repeats (CRISPR)/Cas9 system is emerging as a powerful tool for
precision medicine (7,8). The CRISPR/Cas9 system, originally
identified as an adaptive immune defense mechanism in bacteria,
comprises two major components: Cas9 protein, as a nuclease, and
the synthetic guide RNA (sgRNA). With the regulation of sgRNAs,
Cas9 is recruited to the target site where it cuts DNA, allowing
the removal/deletion and addition/insertion of DNA fragments. The
CRISPR/Cas9 system has been successfully and effectively applied as
a revolutionary, yet feasible, genome editing tool in rodents and
embryonic stem cells and zygotes, as well as plants and other small
experimental animals (9,10). Wang et al (11) adeptly utilized the CRISPR/Cas9
technology to generate mice with multiple concurrent gene
mutations, achieving remarkably high efficiency. In contrast to
traditional techniques which require 2–3 years to produce a mouse
carrying five gene mutations, this cutting-edge method accomplishes
the same feat in a mere 2–3 weeks, and notably circumvents the need
for embryonic stem cells.
Recent research has suggested that enhancer of zeste
homolog 2 (EZH2) serves as a key component of polycomb repressive
complex 2 (PRC2) (12). EZH2 serves
a role in chromosomal structure formation, cell cycle regulation,
senescence, cell differentiation and promotion of tumor growth and
metastasis in malignant tumor models (13–15).
Hussein et al (16) reported
a notable correlation between high EZH2 expression and TNBC
phenotype, suggesting its biological role in this aggressive
disease. EZH2, serving as a potential oncogene in a wide variety of
malignancies, has a marked role in promoting MMP activity and
extracellular matrix degradation, indicating the role of EZH2 in BC
metastasis (17). Accordingly,
EZH2, a novel biomarker, may serve as a novel target for treating
TNBC.
CRISPR/Cas9 system has been developed as a novel
gene editing tool, capable of achieving high gene-targeted
efficiency in different cell lines and small experimental animals,
demonstrating that it is a promising gene therapy (18,19).
To the best of our knowledge, however, the role of the CRISPR/Cas9
system in the editing of EZH2 in the treatment of BC remains
unclear.
Effective targeted therapies for TNBC are limited,
highlighting the urgent need for the development of novel
therapeutic strategies. The CRISPR/Cas9 system has emerged as a
powerful tool for gene editing and shows great promise in
addressing the challenges posed by TNBC. By leveraging the precise
and efficient gene editing capabilities of CRISPR/Cas9, we choose
EZH2 as a targeting gene in TNBC, thereby providing valuable
insight into the molecular mechanisms underlying TNBC
pathogenesis.
Materials and methods
Chemicals and reagents
Table SI lists the
chemicals and reagents used in the present study.
Cell culture
MDA-MB-231 (cat. no. TCHu227) and 293A (cat. no.
SCSP-5094) cell lines purchased from the Cell Bank of the Chinese
Academy of Sciences. MDA-MB-231 was cultured in L-15 medium and
293A was cultured in DMEM medium supplemented with 10% FBS (Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C with 5% CO2.
gRNA design
The gRNA of EZH2 was designed online (http://crispor.tefor.net/; version 5.01) and that of
green fluorescent protein (GFP) was designed as a control. EZH2 or
GFP-specific gRNAs were designed using the N20NGG pattern. The
N20NGG pattern allows for flexibility in selecting the nucleotides
within the 20-base target region of the gRNA, enabling
customization for specific target sequences. By utilizing this
pattern, it increases the chances of finding suitable target sites
within the desired gene or DNA sequence. The gRNA sequences and
primers were synthesized by Sangon Biotech Co., Ltd. The parental
vector, pENTRY-U6-hEF1a-Cas9 (cat. no. 111385; Addgene, Inc.), was
used to construct the CRISPR vectors.
pENTRY-U6-hEF1a-Cas9 plasmid was digested with
restriction endonuclease XmaI/PmeI and the digested
product was purified. sgRNA oligos (Table SII) targeting the EZH2 or GFP
genomic loci were annealed and ligated with linear
pENTRY-U6-hEF1a-Cas9 using the Gibson enzyme. The following
recombinant reaction procedure was used: A total of 0.5 µl
pENTRY-U6-hEF1a-Cas9 plasmid (XmaI/PmeI double
digestion), 2.0 µl sgRNA fragment and 2.5 µl Gibson enzyme were
incubated at 50°C for 1 h, transformed with E. coli and
coated with Luria-Bertani (kanamycin-resistant plates). Next,
monoclonal colonies were selected to extract plasmids for Sanger
sequencing. The sequence was termed
pENTRY-U6-EZH2-sgRNA-hEF1a-Cas9.
Adenovirus packaging
sgRNA expression vector
pENTRY-U6-EZH2-sgRNA-hEF1a-Cas9 and the adenoviral backbone vector
pAD-U6-EF1a-Cas9 were combined using Gateway technology to
construct an adenoviral vector. To initiate the recombination
process, 0.5 µl of the pENTRY-U6-EZH2-sgRNA-hEF1a-Cas9 plasmid and
0.5 µl of the pAD vector were mixed together. Then, 1 µl of the
Gateway™ LR Clonase™ II enzyme mix (cat. no. 11791020; Thermo
Fisher Scientific, Inc.), which contains the necessary
recombinases, was added to the plasmid mixture. The plasmid
mixture, along with the enzyme mix, was incubated for 1 h at 25°C.
During this incubation period, the recombinases facilitated the
recombination of the pENTRY-U6-EZH2-sgRNA-hEF1a-Cas9 plasmid with
the pAD vector. This recombination process involved specific DNA
sequences within the plasmids, allowing for the insertion of the
sgRNA expression cassette into the adenoviral backbone vector.
GFP and EZH2 adenoviral vectors were transformed,
monoclonal colonies were selected by kanamycin and plasmid was
extracted and then identified with the Xba1 restriction
enzyme; the recombinant gRNA vectors were termed EZH2-pAD-Cas9 and
GFP-pAD-Cas9, respectively. The recombinant plasmid was linearized
with PacI enzyme, and the linearized pAD vector was
transfected into 293A cells using Lipofectamine® 3000
(Cat. No. L3000015; Thermo Fisher Scientific, Inc.). The virus was
collected when cells were lesioned. Viral particles were purified
by cesium chloride gradient centrifugation (8,000 × g, 5 min),
which yielded EZH2 and GFP adenoviruses. The viral titer was
determined based on the endpoint dilution method (20). The formula was used under the
following conditions: A−, the negative control, did not
have CPE and proliferation inhibition, while B−, the
minimum dilution of the crude virus extracts, resulted in CPE.
Transduction of CRISPR adenovirus
MDA-MB-231 cells were seeded in six-well plates at a
density of 1.5×106 cells/well 1 day prior to
transduction and grown to 70% confluence. MDA-MB-231 cells were
transfected with EZH2 adenovirus or a GFP adenovirus. According to
the cell MOI and virus titer, the corresponding virus volume was
added, as determined using the following formula: Virus volume=(MOI
× number of cells)/virus titer (MOI of MDA-MB-231: 10). At 48 h
post-transduction at 37°C by puromycin (2.00 µg/ml), MDA-MB-231
cells were harvested, and genomic DNA was extracted for PCR and T7
endonuclease I (T7EI) digestion. The T7EI editing assay was
performed as previously reported (21). With MDA-MB-231 cell genomic DNA as a
template and T7EI sense and antisense primers, a reaction mixture
was prepared containing 5 µl of PCR product (300 ng), 3 µl of
distilled water and 1 µl of 10×T7 buffer, resulting in a total
volume of 9 µl. An annealing program was performed with an initial
temperature of 95°C for 5 min, followed by a gradual decrease of
2°C per second until reaching 85°C, and then decrease at a rate of
0.5°C per second until reaching 16°C. After completion of the
annealing step, 1 µl of T7E1 enzyme was added to the annealed
product, the contents were thoroughly mixed and incubate at 37°C
for 20 min to allow for the digestion reaction to occur. Upon
completion of the reaction, 1.5 µl of 0.25 mol/l EDTA was added to
stop the digestion reaction.
Reverse transcription-quantitative PCR
(RT-qPCR)
At 48 h after EZH2 adenovirus infection, cells were
collected and total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse-transcribed into cDNA using SuperScript™ III First-Strand
Synthesis System (cat. no. 12574018; Thermo Fisher Scientific,
Inc.). Human GAPDH was used as an internal loading control for
qPCR. EZH2 was amplified using cDNA as a template to detect
expression of EZH2 mRNA in MDA-MB-231 cells following EZH2
adenovirus infection. qPCR was performed using reagents and
protocols from the Applied Biosystems SYBR Green Master Mix Kit
(cat. no. A46012; Thermo Fisher Scientific, Inc.). qPCRs were
performed using a Roter-Gene 300 thermal cycling instrument
(Corbett Life Science). The following thermocycling conditions were
used for qPCR: Initial denaturation at 95°C for 2 min, followed by
38 cycles of denaturation at 95°C for 15 sec, and annealing and
elongation at 60°C for 30 sec. The primers used to amplify EZH2 and
GAPDH are provided in Table SII.
The relative expression of EZH2 mRNA was calculated using the
2−∆∆Cq method (22).
Agarose gel electrophoresis
MDA-MB-231 cells were transfected with EZH2
adenovirus or a GFP adenovirus. At 48 h post-transduction,
MDA-MB-231 cells were harvested. Genomic DNA was extracted from
cells and EZH2 loci were analyzed using the T7EI assay. T7EI
recognizes and cleaves the mismatched DNA structure, resulting in a
short strand cleaved by T7EI. The DNA sample was resuspended in an
appropriate buffer and transferred to a PCR tube. The sample was
then heated to 68°C for 15 min, then 95°C for 10 min in a
thermocycler. The temperature was then reduced to 4°C. 2 µl of the
resuspended sample was used as a template for the PCR. The primers
used for this PCR were EZH2 sense primer and EZH2 antisense primer.
The PCR was then conducted with the following steps: Denaturation
at 95°C for 30 sec to separate the double-stranded DNA into single
strands. Annealing at 57°C for 30 sec for the primers to bind to
the DNA template. Extension at 72°C for 40 sec for the Taq
polymerase to replicate the DNA. These cycles were repeated 40
times. After the PCR was complete, the amplified DNA fragments were
run on a 2% agarose gel in 1X TBE buffer.
Western blot analysis
When MDA-MB-231 cells reached 70–80% confluence,
they were collected and lysed with RIPA (cat. no. P8340; Merck
KGaA) at 72 h after EZH2 adenovirus infection, and total protein
was extracted. Protein concentration was quantified with a BCA kit
and then detected by western blotting. A total of 50 µg
protein/lane was separated by 10% SDS-PAGE, transferred to PVDF
membranes (cat. no. 03010040001; Roche) and blocked with 5% skimmed
milk at 25°C for 1 h. Rabbit anti-human EZH2 polyclonal antibody
(cat. no. ab186006; 1:1,000 dilution; Abcam) and mouse anti-human
anti-GAPDH antibody (cat. no. D190090; 1:3,000 dilution; Sangon
Biotech) were added and membranes were incubated overnight at 4°C.
Subsequently, the PVDF membranes were washed three times with 20%
Tween-20 in tris-buffered saline (TBST) and incubated with
horseradish peroxidase-labeled goat anti-rabbit secondary antibody
or goat anti-mouse secondary antibody (1:5,000) for 2 h at 25°C.
Next, the membrane was washed three times with TBST. Protein bands
were visualized using ECL. Performing grayscale analysis of Western
Blot bands using ImageJ (version: 1.54D; National Institutes of
Health).
MTT assay
Prepare a working solution of MTT by dissolving MTT
powder in 0.01M PBS to the desired concentration (0.5 mg/ml).
MDA-MB-231 cells at the logarithmic proliferation phase were seeded
in 96-well plates (2,000/well). The adenovirus solution was added
when the cell density was 70–90%. The MTT assay was performed at
24, 48, 72 and 96 h following infection of MDA-MB-231 cells with
the adenovirus. The cells were assigned to three groups: EZH2-KO
group, Control group and Blank group. The optical density was
examined at 490 nm using a microplate reader. A cell proliferation
curve was plotted.
Transwell assay
MDA-MB-231 cells were divided into EZH2-KO group,
Control group and Blank group. A total of 500 µl/well L-15 medium
supplemented with 20% FBS was added into a 24-well plate. The
Transwell chamber was placed in a 24-well plate. The upper chamber
was inoculated with 200 µl (2×105) MDA-MB-231 suspension
supplemented in serum-free medium after 48 h of adenovirus
infection. Following 24 h cell culture at 37°C, the chamber was
removed and the cells in the upper chamber that did not pass
through the chamber membrane were removed with a cotton swab. The
cells were fixed with 4% paraformaldehyde for 10–15 min and stained
with 0.1% crystal violet solution at ambient temperature. After 30
min, samples were rinsed with water and then dried. A total of five
randomly selected fields of view were observed under a light
microscope (×100 magnification), and the number of cells passing
through the Transwell cell polycarbonate membrane was counted
manually. The experiment was performed in triplicate.
Would-healing assay
A total of 2×105 MDA-MB-231 cells from
the EZH2-KO Group, Control Group and Blank Group were placed in a
24-well plate. Then, a 200-microlitre sterile pipette tip was used
to draw a line across the central area of cell proliferation, after
cell debris was washed away with PBS, the cells were cultured with
5% FBS at 37 °C and captured at 0, 12, 24 and 48 h under a light
microscope (×200 magnification).
In vivo tumor biology
The animal experiments were approved by the Ethics
Review Committee of Guangxi University of Chinese Medicine.
NOD/SCID, 6-8-week-old mice, female, average weight of 25 g)
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd (Beijing, China). They were housed under a 12-h light/dark
cycle. In brief, 2×106 MDA-MB-231 cells from EZH2-KO,
control and blank groups in 0.2 ml PBS were inoculated
subcutaneously in the right flank each mouse (n=3 mice per group).
Tumor volume was evaluated with a slide caliper and determined by
the following formula: V=length ×
width2/2(mm3). These examinations were
conducted every 5 days, which ensured accurate tracking of
ulceration progression without causing undue disruption to the
typical behavior of animals or imposing unnecessary stress. At 25
days after inoculation, all mice were anaesthetized with
intramuscular injection of 50 mg/kg ketamine mixed with 5 mg/kg
xylazine (23). Mice were
euthanized by cervical dislocation and the tumor volume was
examined.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 9.5.1 software (Dotmatics). All quantitative data
from at least three independent experiments are expressed as the
mean ± SD. Statistical analysis of mRNA expression was performed
using one-way ANOVA with Bonferroni's post-hoc test to evaluate
differences between three groups. Statistical analysis of the
proliferation rate, migration ability and tumor volume were
performed using Kruskal-Wallis followed by Dunn's
multiple-comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
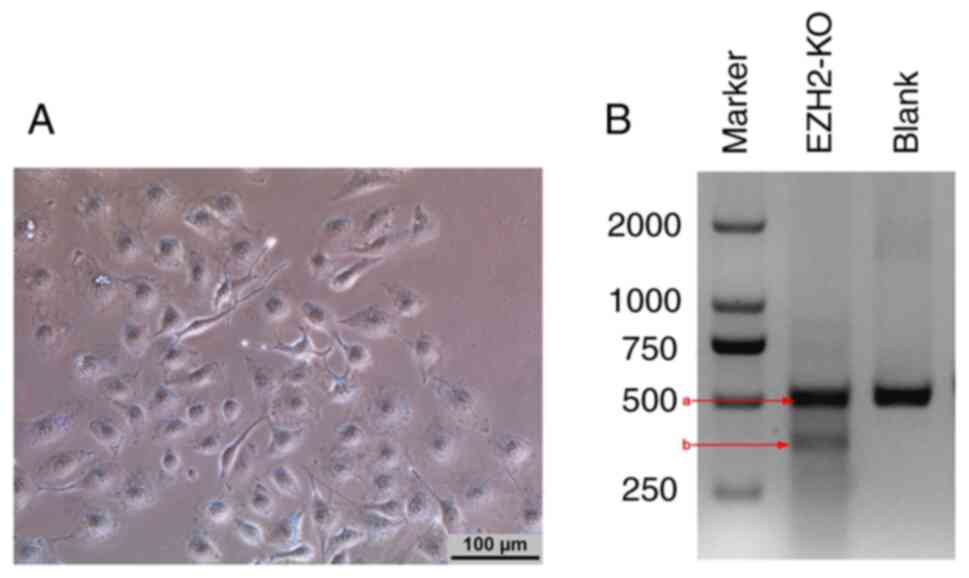
Virus preparation and confirmation of
in vitro CRISPR/Cas9 system knockout of EZH2 gene
The recombinant pAD plasmid linearized with
PacI was transfected into 293A cells for 7–9 days and CPE
was identified (Fig. 1A).
Before investigation of the in vitro and
in vivo EZH2-silencing effect of the CRISPR/Cas9 system to
generate EZH2 loss-of-function, the editing efficiency of
EZH2-sgRNA for EZH2 was tested in MDA-MB-231 cells. After
MDA-MB-231 cells were infected with the EZH2 adenovirus for 48 h,
genomic DNA was extracted from cells and EZH2 loci were analyzed
using the T7EI assay. T7EI recognizes and cleaves the mismatched
DNA structure, resulting in a short strand cleaved by T7EI
(Fig. 1B). Thus, two fragments were
generated by T7EI digestion (Fig.
1B).
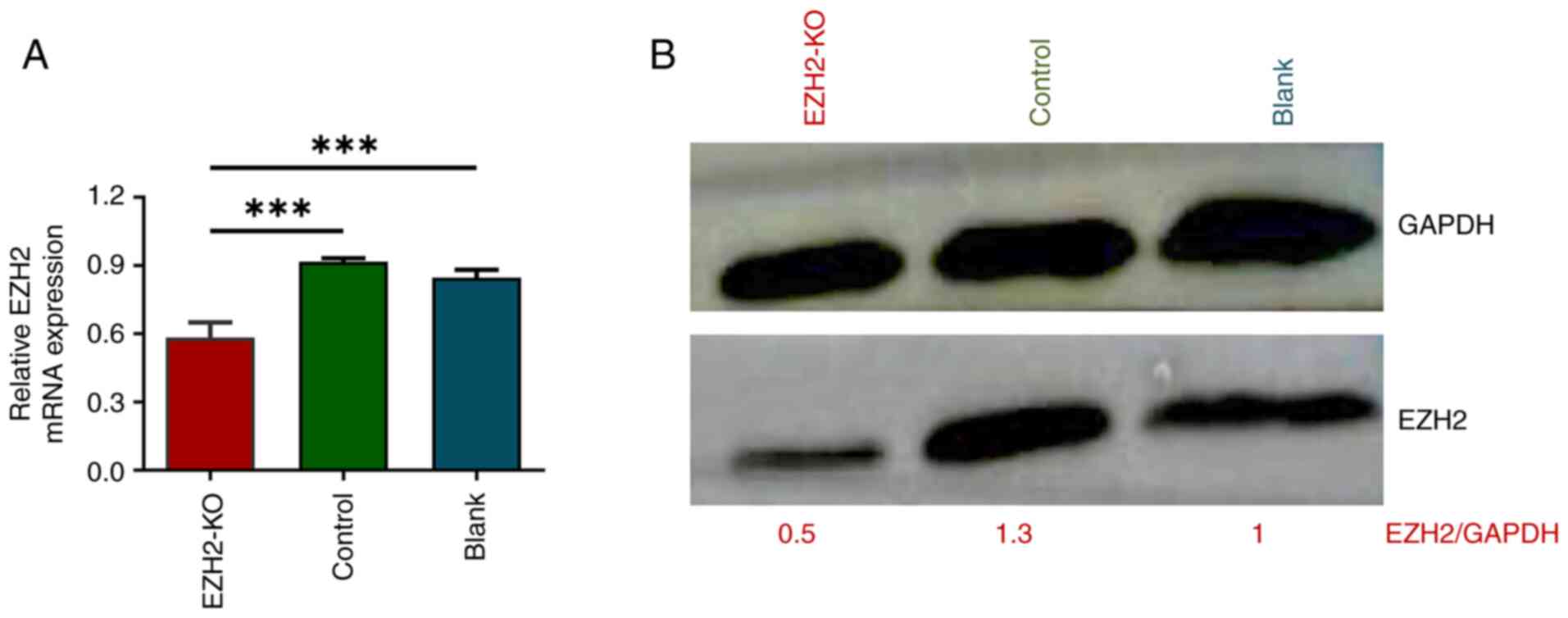
EZH2 expression is decreased at both
the mRNA and protein levels
Following adenovirus infection of MDA-MB-231 cells,
the mRNA expression of EZH2 in the EZH2-KO group was 0.58±0.06,
which was significantly lower than in the control group (0.92±0.02)
and in the blank group (0.846±0.035) (Fig. 2A).
Western blotting was performed 72 h after adenovirus
infection of MDA-MB-231 cells. EZH2 protein expression in
MDA-MB-231 cells infected with EZH2 adenovirus was downregulated
compared with that in control and blank groups (Fig. 2B). However, the expression of EZH2
in the control and blank groups was similar.
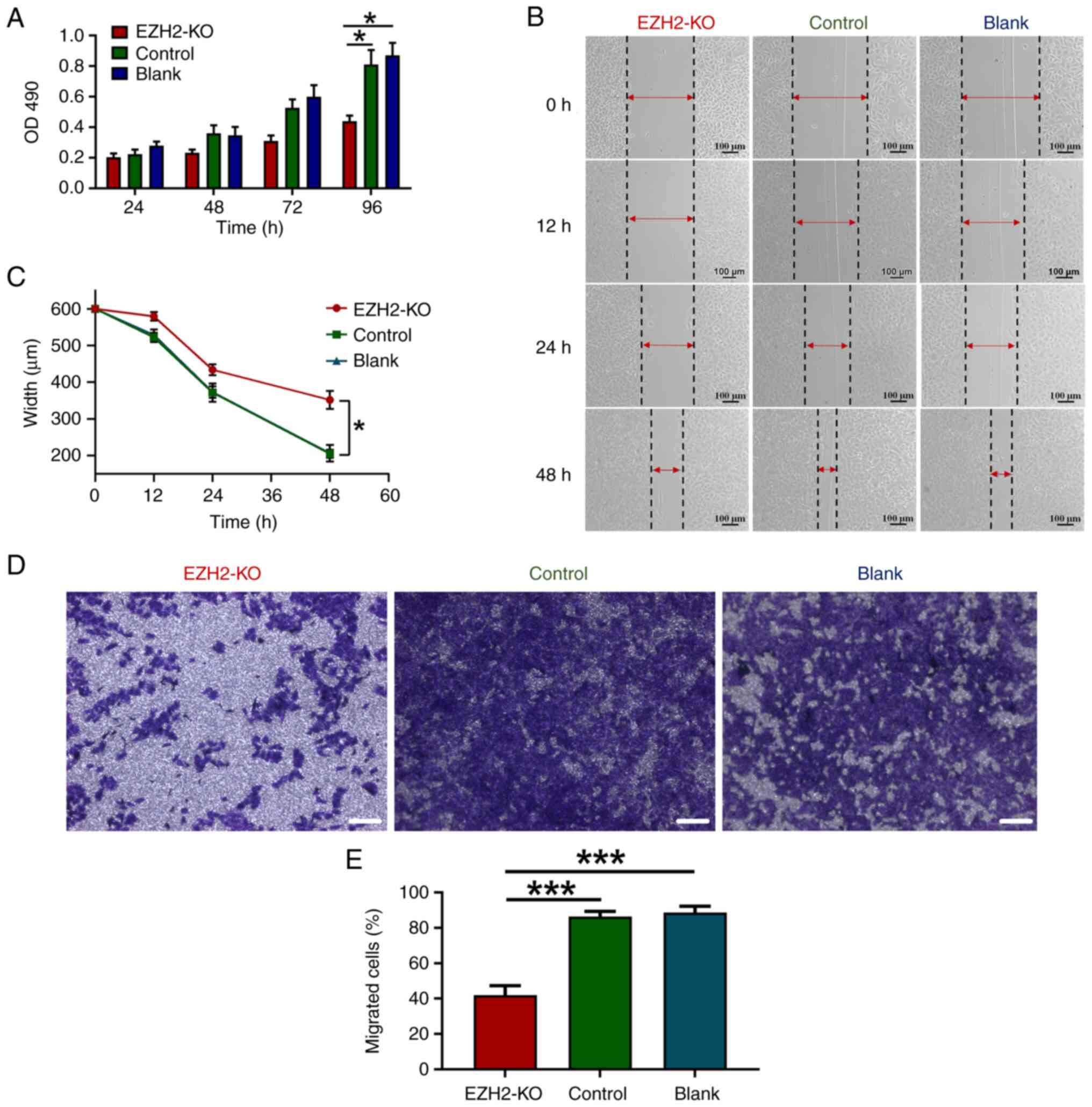
KO of EZH2 decreases proliferative and
migratory abilities of MDA-MD-231 cells
After MDA-MB-231 cells were infected with the
adenovirus for 24 h, the proliferation rate of the cells was
decreased. With the extension of the infection time, the
proliferation rate of MDA-MB-231 cells was significantly lower than
that of the control and blank groups (Fig. 3A). Compared with the control and
blank groups, the proliferation rate of MDA-MB-231 cells in the
EZH2-KO group declined after 48, 72 and 96 h, and the difference
was statistically significant at 96 h (P<0.05).
Wound healing assay suggested that the would-healing
area in EZH2-KO group was smaller compared with that in control and
blank groups (Fig. 3B and C).
Transwell assay showed the number of MDA-MB-231 having migrated
through the transwell, and the lower layer of the filter decreased
in EZH2-KO group (Fig. 3D and E).
these results suggest that inhibition of EZH2 expression decreased
the migration ability of MDA-MB-231 cells.
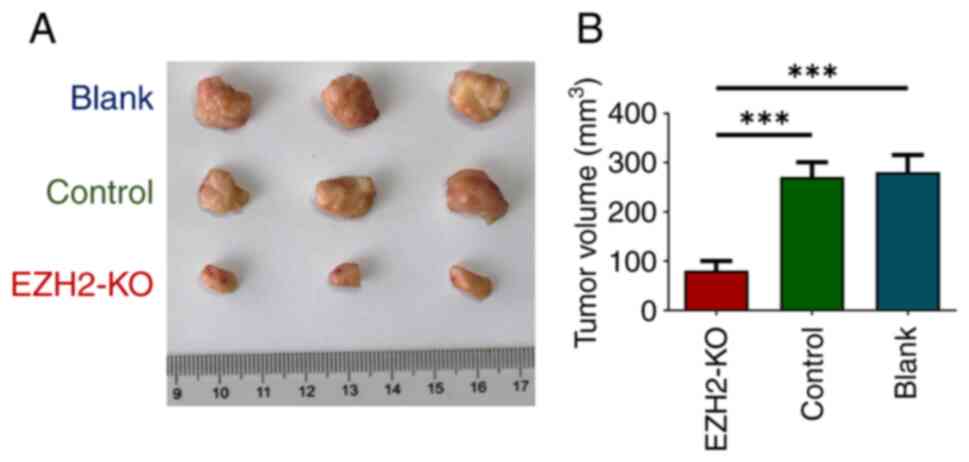
KO of EZH2 decreases tumor growth in
vivo
Smaller tumor size wase found in the EZH2-KO
group compared with the control and blank groups (Fig. 4A). The tumor volume in the EZH2-KO
group was smaller in than that in the control and blank groups
(Fig. 4B). The aforementioned
results suggest that KO of EZH2 suppressed tumor
proliferation in vivo.
Discussion
The development of TNBC, a common malignant tumor in
female patients, has been reported as a complex process of genetic
and epigenetic changes (24).
Epigenetics is a hereditary gene expression regulation mode that
involves DNA sequence changes, including histone modification, gene
imprinting and DNA methylation (25).
Introducing a foreign gene into cells of an organism
and achieving efficient expression or silencing of an endogenous
gene are the goals of gene therapy (26). With the advance of tumor therapy,
gene therapy has become a research topic in cancer treatment.
Notably, inhibition of oncogene expression has an inhibitory effect
on tumor growth due to the overexpression of certain dominant
oncogenes (27). Selecting optimal
gene therapy technology to inhibit oncogene expression is a key
step in gene therapy (28,29). At present, the CRISPR/Cas9
technology is widely used to establish cell and animal models
related to tumor genes (30). Thus,
the adenovirus system was used in the present study to mediate
CRISPR/Cas9-targeted KO of EZH2.
As a defense mechanism, prokaryotes developed an
adaptive immune system, termed CRISPR (31). The role of the short repeat
sequences remained unclear until 2005 when several groups described
similarities between the aforementioned sequences and phage DNA,
raising the hypothesis that the aforementioned sequences are part
of an adaptive immune system in bacteria (32,33).
In 2013, type II Cas9 protein from Streptococcus pyogenes
was first used for RNA-guided DNA cleavage in mammalian cells,
providing a basis for using CRISPR/Cas9 as a genome editing tool
(34). CRISPR/Cas9 technology is
applied to explore the role of specific genes in cancer
development, which may lead to the discovery of novel oncogenes or
tumor suppressor genes (30). Feng
et al (35) knocked down
CDK11 in osteosarcoma cell lines based on the CRISPR/Cas9 system;
cell viability, proliferation, migration and invasiveness were
significantly decreased following knockout, and CDK11 may serve as
a novel target for osteosarcoma therapy.
EZH2 is a key member of the PRC2 silencing complex
that exhibits methyltransferase activity and catalyzes histone H3
on lysine 27 trimethylation and heterochromatin formation, and
thereby mediates tumor suppressor gene expression silencing
(36). The overexpression of EZH2
has been identified in prostate and bladder cancer, melanoma and
TNBC (37–40). Moreover, extensive research has
suggested that EZH2 overexpression is associated with poor
prognosis in numerous types of cancer (41–43).
In the present study, the CRISPR/Cas9 gene editing
technology was used. mRNA and protein levels of EZH2 tended to
decrease after MDA-MB-231 cells were infected with the adenovirus.
CRISPR/Cas9 system targeting EZH2 was found to inhibit EZH2 mRNA
and protein expression in MDA-MB-231 cells. Functional assays
confirmed that knocking out EZH2 inhibited proliferation and
migration of MDA-MB-231 in vitro. Finally, the effect of
EZH2 KO in vivo was validated, knockout of EZH2 gene
expression inhibited tumor progression.
In the present study, a basis was provided for
clinical application of gene therapy for cancer using CRISPR/Cas9
adenovirus system. Monoclonal cells in which the EZH2 was knocked
out were selected using the in vitro dilution method
(17) and the functional role of
EZH2 in TNBC was examined. In future, the interaction of EZH2 with
certain signaling pathways, oncogenes or tumor suppressor genes
should be explored to gain more insight into the effects of EZH2 on
tumorigenesis and its role in the pathogenesis of tumors.
While the present study provides key insight into
the impact of gene therapy based on the CRISPR/Cas9 adenovirus
system, findings are based on experiments conducted in a single
cell line. Therefore, the extent to which these results can be
generalized to other cell lines or in vivo contexts remains
uncertain. Future studies should aim to validate and extend the
findings of the current study in different cell lines and more
complex biological systems.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the research grant of the
National Natural Science Foundation of Ningbo (grant no.
202003N4022), National Natural Science Foundation of China (grant
nos. NSFC81603575, NSFC81760551, NSFC81403382 and NSFC81760845),
Guangxi Natural Science Foundation (grant nos. 2014jjAA0334,
2016GXNSFBA380120, 2017GXNSFAA198045 and 2015GXNSFBA139128) and the
self-funded project of Guangxi Health Commission (grant no.
Z20210130).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QQM and LY conceived and designed the study and
methodology. QQM, PW, HL, XLF and XCG performed the experiments and
acquired the data. PW, HL, XLF and XCG analyzed and interpreted the
data. QQM, HL and LY wrote, reviewed and revised the manuscript. LY
provided study supervision. All authors have read and approved the
final manuscript. QQM and LY confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The Ethics Committee of The First Affiliated
Hospital of Guangxi University of Chinese Medicine approved the
present study. The animal experiments were approved by the Ethics
Committee of Guangxi University of Chinese Medicine (approval no.
20230214N1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ismail-Khan R and Bui MM: A review of
triple-negative breast cancer. Cancer Control. 17:173–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Darbeheshti F, Izadi P, Razavi ANE,
Yekaninejad MS and Tavakkoly BJ: Comparison of BRCA1 expression
between triple-negative and luminal breast tumors. Iran Biomed J.
22:210–214. 2018.PubMed/NCBI
|
|
4
|
Costa RLB and Gradishar WJ:
Triple-negative breast cancer: Current practice and future
directions. J Oncol Pract. 13:301–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu X, Baig A, Kasymjanova G, Kafi K,
Holcroft C, Mekouar H, Carbonneau A, Bahoric B, Sultanem K and
Muanza T: Pattern of local recurrence and distant metastasis in
breast cancer by molecular subtype. Cureus. 8:e9242016.PubMed/NCBI
|
|
6
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jennifer AD and Emmanuelle C: The new
frontier of genome engineering with CRISPR-Cas9. Science.
346:12580962014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurata M, Yamamoto K, Moriarity BS,
Kitagawa M and Largaespada DA: CRISPR/Cas9 library screening for
drug target discovery. J Hum Genet. 63:179–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Fan D, Adah D, Wu Z, Liu R, Yan Q,
Zhang Y, Du ZY, Wang D, Li Y, et al: CRISPR/Cas9-mediated hypoxia
inducible factor-1α knockout enhances the antitumor effect of
transarterial embolization in hepatocellular carcinoma. Oncol Rep.
40:2547–2557. 2018.PubMed/NCBI
|
|
10
|
Suemura S, Kodama T, Myojin Y, Yamada R,
Shigekawa M, Hikita H, Sakamori R, Tatsumi T and Takehara T: CRISPR
loss-of-function screen identifies the hippo signaling pathway as
the mediator of regorafenib efficacy in hepatocellular carcinoma.
Cancers (Basel). 11:13622019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Yang H, Shivalila CS, Dawlaty MM,
Cheng AW, Zhang F and Jaenisch R: One-step generation of mice
carrying mutations in multiple genes by CRISPR/Cas-mediated genome
engineering. Cell. 153:910–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bremer SCB, Bittner G, Elakad O, Dinter H,
Gaedcke J, König AO, Amanzada A, Ellenrieder V, Freiherr VHA,
Ströbel P and Bohnenberger H: Enhancer of zeste homolog 2
(EZH2) is a marker of high-grade neuroendocrine neoplasia in
gastroenteropancreatic and pulmonary tract and predicts poor
prognosis. Cancers (Basel). 14:28282022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Z, Wei B, Qiao A, Yang P, Chen W,
Zhen D and Qiu X: A novel EZH2/NXPH4/CDKN2A axis is involved in
regulating the proliferation and migration of non-small cell lung
cancer cells. Biosci Biotechnol Biochem. 86:340–350. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt A, Behrendt L, Eybe J, Warmann SW,
Schleicher S, Fuchs J and Schmid E: The effect of direct and
indirect EZH2 inhibition in rhabdomyosarcoma cell lines. Cancers
(Basel). 14:412021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alzrigat M, Jernberg-Wiklund H and Licht
JD: Targeting EZH2 in multiple myeloma-multifaceted anti-tumor
activity. Epigenomes. 2:162018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hussein YR, Sood AK, Bandyopadhyay S,
Albashiti B, Semaan A, Nahleh Z, Roh J, Han HD, Lopez-Berestein G
and Ali-Fehmi R: Clinical and biological relevance of enhancer of
zeste homolog 2 in triple-negative breast cancer. Hum Pathol.
43:1638–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chien YC, Liu LC, Ye HY, Wu JY and Yu YL:
EZH2 promotes migration and invasion of triple-negative breast
cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am J Cancer
Res. 8:422–434. 2018.PubMed/NCBI
|
|
18
|
Vukmirovic D, Seymour C and Mothersill C:
Deciphering and simulating models of radiation genotoxicity with
CRISPR/Cas9 systems. Mutat Res Rev Mutat Res. 783:1082982020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
BeltCappellino A, Majerciak V, Lobanov A,
Lack J, Cam M and Zheng ZM: CRISPR/Cas9-mediated knockout and in
situ inversion of the ORF57 gene from all copies of the kaposi's
sarcoma-associated herpesvirus genome in BCBL-1 cells. J Virol.
93:e00628–e00619. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed LJ and Muench H: A simple method of
estimating fifty per cent endpoint. Am J Epidemiol. 27:493–497.
1938. View Article : Google Scholar
|
|
21
|
Sentmanat MF, Peters ST, Florian CP,
Connelly JP and Pruett-Miller SM: A survey of validation strategies
for CRISPR-Cas9 editing. Sci Rep. 8:8882018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derakhshan F and Reisfilho JS:
Pathogenesis of triple-negative breast cancer. Ann Rev Pathol.
17:181–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanson MA and Godfrey KM: Genetics:
Epigenetic mechanisms underlying type 2 diabetes mellitus. Nat Rev
Endocrinol. 11:261–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang DY, Sun QC, Zou XJ, Song Y, Li WW,
Guo ZQ, Liu SS, Liu L and Wu DH: Long noncoding RNA UPK1A-AS1
indicates poor prognosis of hepatocellular carcinoma and promotes
cell proliferation through interaction with EZH2. J Exp Clin Cancer
Res. 39:2992020. View Article : Google Scholar
|
|
27
|
Guo B, Tan X and Cen H: EZH2 is a negative
prognostic biomarker associated with immunosuppression in
hepatocellular carcinoma. PLoS One. 15:e02421912020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun S, Gao J, Zhou S, Li Y, Wang Y, Jin L,
Li J, Liu B, Zhang B, Han S, et al: A novel circular RNA circ-LRIG3
facilitates the malignant progression of hepatocellular carcinoma
by modulating the EZH2/STAT3 signaling. J Exp Clin Cancer
Res. 39:2522020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Chen W, Ma W, Han C, Song K, Kwon
H and Wu T: EZH2 promotes cholangiocarcinoma development and
progression through histone methylation and microRNA-mediated
down-regulation of tumor suppressor genes. Am J Pathol.
192:1712–1724. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang SW, Gao C, Zheng YM, Yi L, Lu JC,
Huang XY, Cai JB, Zhang PF, Cui YH and Ke AW: Current applications
and future perspective of CRISPR/Cas9 gene editing in cancer. Mol
Cancer. 21:572022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishino Y, Shinagawa H, Makino K, Amemura M
and Nakata A: Nucleotide sequence of the iap gene, responsible for
alkaline phosphatase isozyme conversion in Escherichia coli, and
identification of the gene product. J Bacteriol. 169:5429–5433.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bolotin A, Quinquis B, Sorokin A and
Ehrlich SD: Clustered regularly interspaced short palindrome
repeats (CRISPRs) have spacers of extrachromosomal origin.
Microbiology (Reading). 151:2551–2561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pourcel C, Salvignol G and Vergnaud G:
CRISPR elements in Yersinia pestis acquire new repeats by
preferential uptake of bacteriophage DNA and provide additional
tools for evolutionary studies. Microbiology (Reading).
151:653–663. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA and Zhang F:
Multiplex genome engineering using CRISPR/Cas Systems. Science.
339:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng Y, Sassi S, Shen JK, Yang X, Gao Y,
Osaka E, Zhang J, Yang S, Yang C, Mankin HJ, et al: Targeting CDK11
in osteosarcoma cells using the CRISPR-Cas9 system. J Orthop Res.
33:199–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orlando V: Polycomb, epigenomes, and
control of cell identity. Cell. 112:599–606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morel KL, Sweeney CJ and Ellis L:
Targeting EZH2 and PI3K/mTOR for a novel combination therapeutic
strategy in aggressive variant prostate cancer. Mol Cancer Res.
18:B292020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Ma X, Wang Q and Kong Z: EZH2
targeting to improve the sensitivity of acquired radio-resistance
bladder cancer cells. Transl Oncol. 16:1013162022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tiffen J, Gallagher SJ, Filipp F,
Gunatilake D, Emran AA, Cullinane C, Dutton-Register K, Aoude L,
Hayward N, Chatterjee A, et al: EZH2 Cooperates with DNA
methylation to downregulate key tumor suppressors and IFN gene
signatures in melanoma. J Invest Dermatol. 140:2442–24544. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dale B, Anderson C, Park K, Kaniskan HÜ,
Ma A, Shen Y, Zhang C, Xie L, Chen X, Yu X and Jin J: Targeting
triple-negative breast cancer by a novel proteolysis targeting
chimera degrader of enhancer of zeste homolog 2. ACS Pharmacol
Transl Sci. 5:491–507. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RGAB, Otte AP, et al: The polycomb group protein EZH2 is
involved in progression of prostate cancer. Nature. 419:624–629.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takawa M, Masuda K, Kunizaki M, Daigo Y,
Takagi K, Iwai Y, Cho H, Toyokawa G, Yamane Y, Maejima K, et al:
Validation of the histone methyltransferase EZH2 as a
therapeutic target for various types of human cancer and as a
prognostic marker. Cancer Sci. 102:1298–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|