Introduction
Osteosarcoma is the most common primary bone
malignancy in adolescents and young adults (1,2). With
the introduction of neoadjuvant and adjuvant chemotherapy, the
5-year survival rate of patients with osteosarcoma has increased
from 20% in the 1970s to a range of 60–80% (3–5).
However, this improvement has since reached a plateau, where the
5-year survival rate of patients has not further improved over the
past 10 years. Lung metastasis is one of the main factors
restricting the improvement of patient survival, rendering it the
main cause of mortality in patients with osteosarcoma (6,7).
Timely, accurate and comprehensive identification of
pulmonary nodules in patients with osteosarcoma is vital for
maximizing long-term survival of patients with osteosarcoma
(3,4). Chest CT is the preferred imaging
method for evaluating the presence of pulmonary metastasis from
primary osteosarcoma (3–5,7).
However, manual assessment of pulmonary nodules in patients with
osteosarcoma is time-consuming and laborious because of the large
number of chest CT scans and the long reading time required by the
diagnostic physician.
In recent years, the application of artificial
intelligence-assisted diagnostic technology in the medical field
has been on the rise. One study showed that average accuracy rate
by Neural Network Algorithm is 93.5% in a total of 1,000 eyes
fundus images from diabetic patients in which 298 eyes were
diagnosed as DR by two ophthalmologists (8). A Deep learning (DL) algorithm achieved
a voxel-wise area under the curve of 0.94 and a subject-level
accuracy of 92% for endovascular treatment eligibility (9). Another study showed that an algorithm
using a convolutional neural network (CNN) was able to
fully-automatically extract on a mean 92% of clinically relevant
coronary artery segments (10).
Therefore, the aim of the present study was to assess the efficacy
of the artificial intelligence diagnostic DCNN model for evaluating
pulmonary nodules in adolescent and young adult patients with
osteosarcoma. In addition, the present study aimed to compare its
clinical application value with another manual model.
Materials and methods
The present retrospective study was approved by the
Ethics Committee of Hangzhou Third People's Hospital (Hangzhou,
China) and the requirement for informed consent was waived. All
methods were performed in accordance with expert consensus on data
labeling and the quality control of pulmonary nodules was performed
in reference to the ‘Consensus on the rule and quality control of
pulmonary nodule annotation based on thoracic CT’ from 2018
(11). The inclusion criteria were
as follows: i) All patients were confirmed histologically with
osteosarcoma; ii) patients were 9–35 years old; iii) patients
underwent thin-slice CT examination, with a slice thickness of ≤3
mm; iv) the diameter range of all nodules was 2–150 mm; and v)
there was no obvious cavity in the thin-slice CT images. The
exclusion criteria were the following: i) Incomplete lung lobe
scan; ii) serious artifacts in the image; iii) missing layers or
faults in the image; and iv) the image did not conform to the DICOM
3.0 protocol (12). The images data
of chest CT screening diagnosed with osteosarcoma was
retrospectively collected from Hangzhou Third People's Hospital
(Hangzhou, China) between March 2011 and February 2022. All
examinations were performed as part of routine healthcare plans.
Disappearance of the small pulmonary nodules was observed for 3
months continuously, with the CT scanning images re-examined once a
month to confirm.
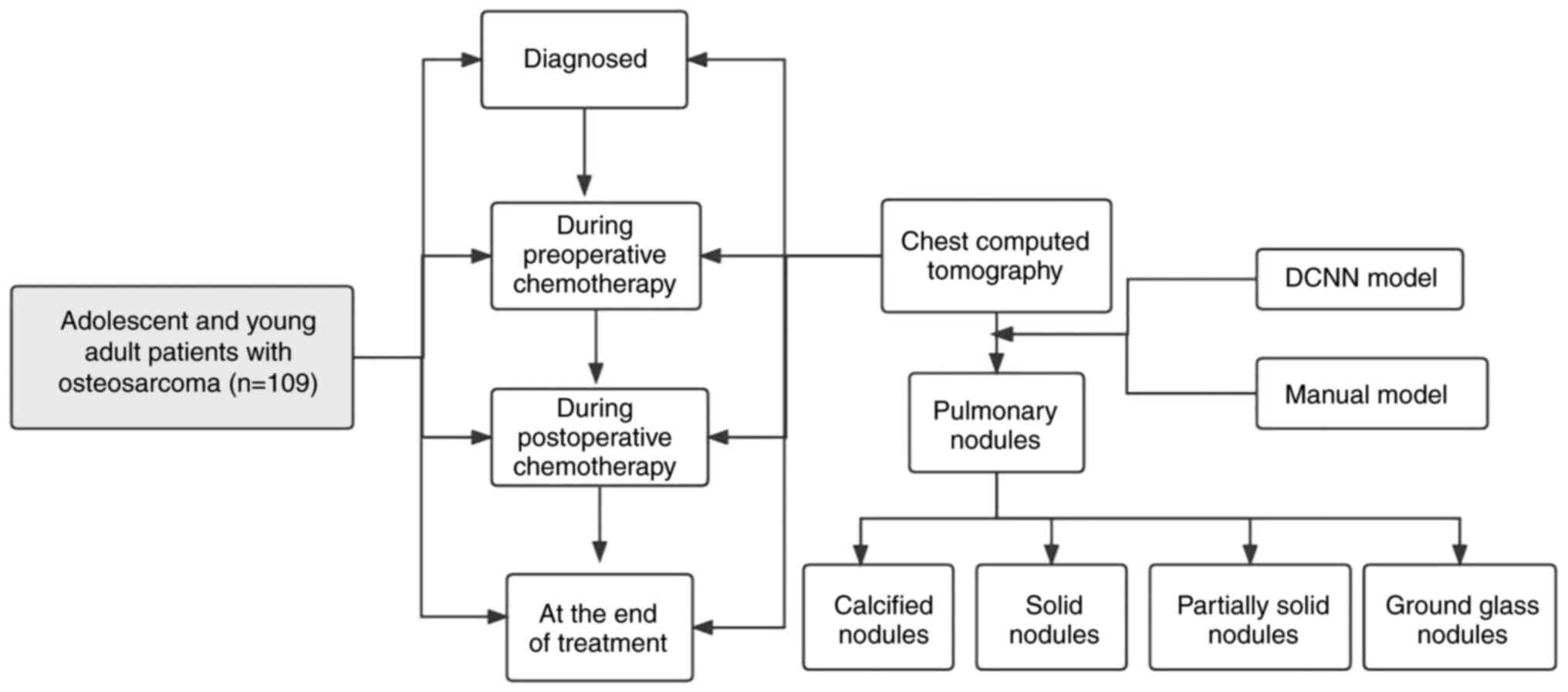
A total of 109 adolescent and young adult patients
with osteosarcoma underwent a total of 675 chest CT scans using
Somatom Definition Edge 64-slice spiral CT, GE Revolution 16-slice
spiral CT [General Electric Medical Investment (China) Co., Ltd.]
and uCT-510 16-slice CT (Shanghai United Imaging Healthcare Co.,
Ltd.). The scanning range was from the apex of the lung to the base
of the lung, including both chest walls and axilla. The lung window
was used for image analysis and the range of thickness of the
reconstructed lung window image was 1–3 mm, with the bone algorithm
used for reconstruction. The lung window was used for image
analysis, with a window width of 1,500 HU and a window level of
−400 HU. Chest CT scans were generally divided into the following
four stages: i) At the initial diagnosis of osteosarcoma; ii)
during preoperative chemotherapy; iii) within 1 year of the end of
treatment; and iv) 1 year after the end of treatment. The selection
process for patients included in the present study is shown in
Fig. 1.
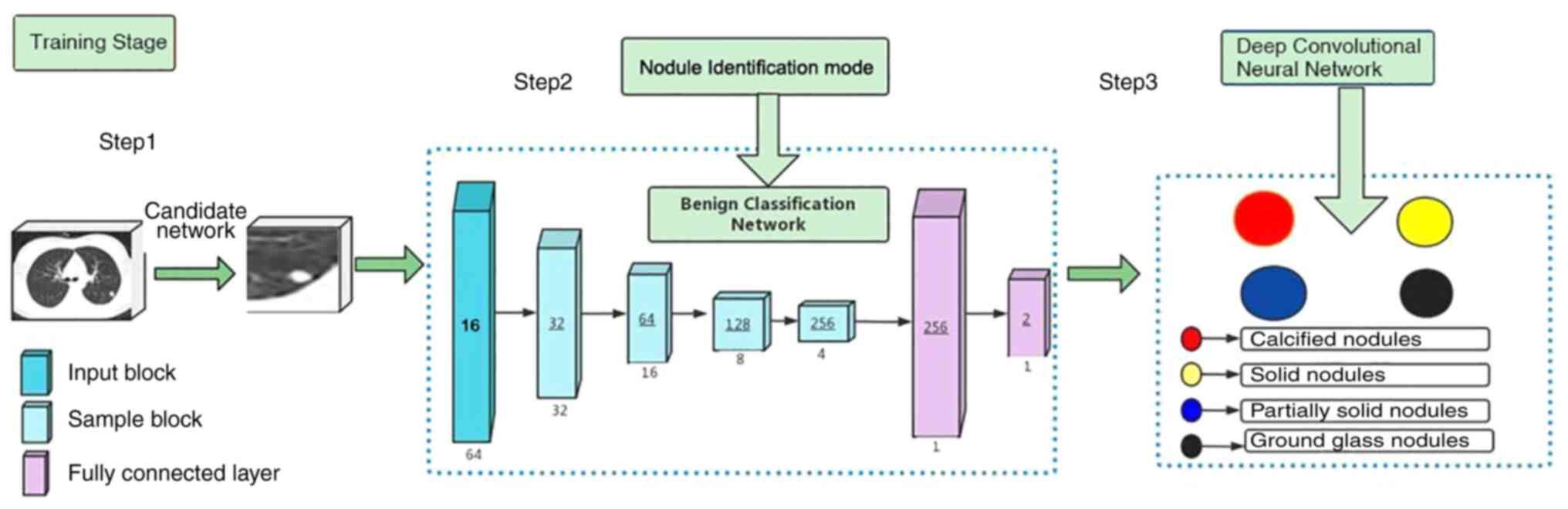
Pulmonary nodule CT image assistance software used
in the present study was developed by Shanghai United Imaging
Medical Co., Ltd., approved by the National Medical Products
Administration and the China Medical AI Certificate III was
obtained (certificate no. National Mechanical Note 20213210471).
The artificial intelligence software is based on the
three-dimensional convolutional neural network method (13). The simple workflow scheme of the
DCNN model development is presented in Fig. 2.
According to the random number table method,
(14) CT images were divided into
groups A and B, which included a total of 140,610 and 136,032
images, respectively. For group A, the DCNN model was used for
artificial intelligence-assisted image analysis. The distribution
of nodules in the lung was analyzed by artificial intelligence and
the nodules were automatically classified into ground glass
nodules, solid nodules, solid nodules and calcified nodules. The
short-and long-axis diameters of the pulmonary nodules were
automatically measured with the units in millimeters (mm). The
long-axis diameter was defined as the farthest two-point distance
in the maximum cross-sectional space within the nodule. The
short-axis diameter was defined as the longest distance
perpendicular to the long diameter within the nodule and the
reading time was recorded in seconds (sec). The length of total
reading time included the time from DCNN model identification time
to the completion of diagnostic report. For group B, The manual
mode was used, whereby simple manual reading was applied. The
junior physicians (XCZ, WTY and LLS) recorded the number, location
and size of nodules According to the maximum diameter of the
nodules, and to the China National Guideline of Classification,
Diagnosis and Treatment for Lung Nodules (2016 version) (15), the nodules were divided into four
categories: Ground glass nodules, solid nodules, partially solid
nodules and calcified nodules. The density of the ground glass
nodules was slightly higher than that of the surrounding lung
parenchyma, but the contours of blood vessels and bronchi within
the nodules were still visible. All solid nodules were soft tissue
density nodules with uniform density and concealed by blood vessels
and bronchi. Partially solid nodules were defined as nodules
containing both ground glass density and solid soft tissue density,
with uneven density. Calcified nodules were defined as round or
round (spherical or spherical) well-defined lesions of complete
calcium salt deposition within the lung parenchyma. By contrast,
The senior diagnosticians (YLN, SDJ and ZTW) issued the diagnostic
report based on the written report and personal opinion, the length
of total reading time includes the junior physicians reading time
to the senior diagnosticians was recorded. The thin-slice chest CT
images of patients with osteosarcoma were analyzed by two
diagnostic radiologists with the title of chief physician and
>10 years of work experience to determine the number and nature
of pulmonary nodules. If the judgment results were inconsistent, a
third imaging diagnostic physician with the title of associate
chief physician or higher rank and >15 years of work experience
would act as the arbiter. This procedure was used as the gold
standard for the diagnosis of pulmonary nodules in patients with
osteosarcoma.
The CT imaging features of the enrolled nodules were
labeled in the standard lung window. Each nodule was diagnosed and
labeled by two chest imaging diagnostic physicians with >10
years of work experience in a blinded manner. The decision was made
by a third imaging expert with the ranking of associate chief
physician or above with >15 years of experience before the final
summary of opinions was used as the gold standard for nodule
diagnosis and labeling. True-positive detection was defined as when
the positioning box of pulmonary nodules detected by the DCNN model
coincided with any of the positioning boxes in the gold standard.
Otherwise, the detection of a pulmonary nodule would be considered
a false-positive. The following four categories were defined: i)
True positive was predicted and observed; ii) true negative was
predicted and observed; iii) false negative was observed, but it
was predicted; and iv) false positive was observed, but it was
predicted. In the present study, the criteria for pulmonary
metastasis from the primary osteosarcoma were as follows: i)
Pathologically confirmed pulmonary metastasis of osteosarcoma; ii)
osteosarcoma pulmonary micrometastasis, whereby thin-slice chest CT
was routinely re-examined every 3 months at the beginning, followed
by once a month until dynamic changes of pulmonary small nodules
were observed; if the small nodules were stable for 12 months,
thin-slice chest CT was re-examined every 3 months; iii)
intrapulmonary micrometastasis was defined by the enlargement or
increase in the number of small nodules in the lung; and iv) ≥
three times of follow-up after diagnosis. The disappearance of
small pulmonary nodules was observed continuously for 3 months and
CT scan was re-examined once a month.
Statistical analysis
SPSS software (version 18.0; SPSS, Inc.) was used
for data analysis and processing, where the adoption rate of count
data was expressed in the form of n (%). Data comparison was
conducted using the χ2 test. The unpaired t-test was
used to analyze the difference in the reading time between the two
groups of samples. The DeLong test was used to compare the
diagnostic performance of the two models. Receiver operating
characteristic (ROC) curve analysis was applied to compare the
predictive performance of the two models. P<0.05 was considered
to indicate a statistically significant difference.
Results
A total of 109 patients, 65 males and 44 females,
with an age range of 9–34 years and median age of 16 years, were
included in the present study. All patients were confirmed
histologically with osteosarcoma.
Diagnostic performance
measurements
Table I presents the
evaluation performance of the DCNN and the manual models for
assessing pulmonary nodules in patients with osteosarcoma. In the
DCNN model group, 3,087 nodules were detected but 278 nodules were
missed compared with the determination using the gold standard,
which was performed by two diagnostic radiologists. In the manual
model group, 2,442 nodules were detected but 657 nodules were
missed. The unpaired t-test was used to analyze the difference in
the reading times between the DCNN and the manual model groups.
 | Table I.DCNN and manual model performance in a
cohort of 109 patients with osteosarcoma. |
Table I.
DCNN and manual model performance in a
cohort of 109 patients with osteosarcoma.
|
| Testing cohort |
|---|
|
|
|
|---|
| Performance | DCNN model | Manual model |
|---|
| True positives | 3,087 | 2,442 |
| True negatives | 343 | 355 |
| False positives | 257 | 246 |
| False negatives | 278 | 657 |
| Sensitivity | 0.923 | 0.908 |
| Specificity | 0.552 | 0.351 |
| Area under the
curve | 0.795 | 0.687 |
| 95% confidence
interval | (0.743-0.846) | (0.629-0.732) |
| Reading time
(sec) | 173.25±24.10 | 328.32±22.72 |
| P-value | <0.05 |
|
The reading time of the DCNN model was significantly
shorter compared with that of the manual model group [mean ±
standard deviation (SD); 173.25±24.10 vs. 328.32±22.72 sec;
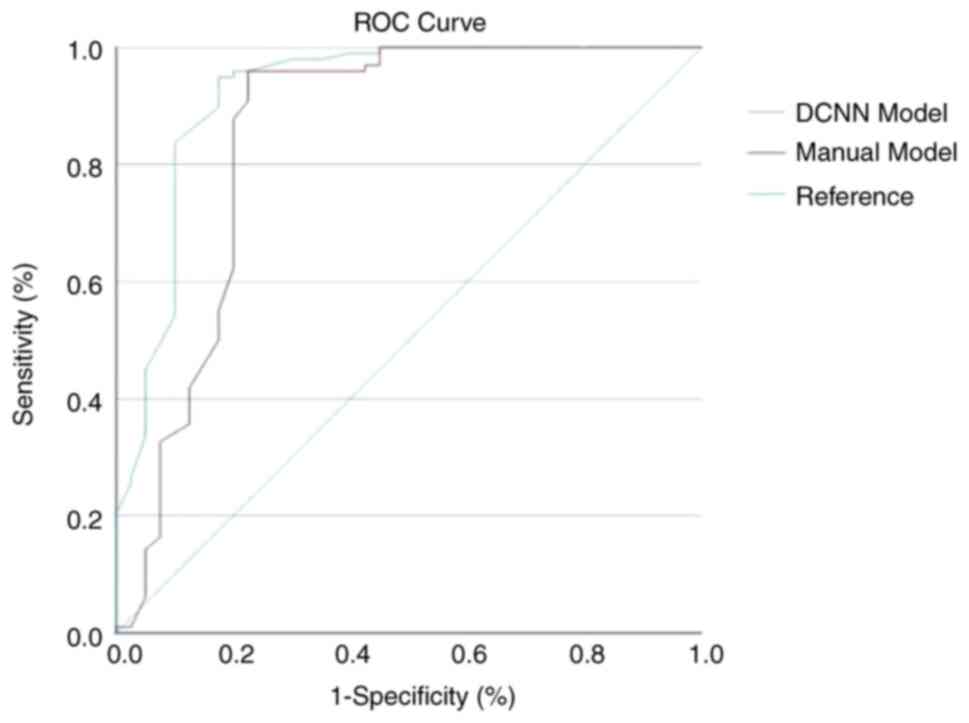
P<0.05; Table I]. The DeLong
test was used to compare the diagnostic performance of the two
models. ROC curve analysis was applied to compare the predictive
performance of the two models. The area under the ROC curve in the
DCNN model group was 0.795 (95% CI, 0.743-0.846), outperforming
that of the manual model (AUC, 0.687; 95% CI, 0.629-0.732;
P<0.05; Fig. 3). The ROC curve
analysis indicated that the DCNN-based model was superior compared
to the manual model. The χ2 test was used to compare the
sensitivity and specificity of the DCNN and manual models. The DCNN
model had significantly higher sensitivity and specificity compared
with the manual model (sensitivity, 0.923 vs. 0.908, respectively;
specificity, 0.552 vs. 0.351, respectively; P<0.05).
Nodules characteristics based on the
DCNN model
Table II indicates
that among the 3,087 nodules detected by the DCNN model, the number
of calcified nodules, solid nodules, partially solid nodules and
ground glass nodules was 744, 1,540, 206 and 597, respectively,
whereas the AUC was 0.766, 0.771, 0.761 and 0.796, respectively
(Fig. 4). Table II also presents the diameter,
classification and distribution of the pulmonary nodules in the
DCNN model.
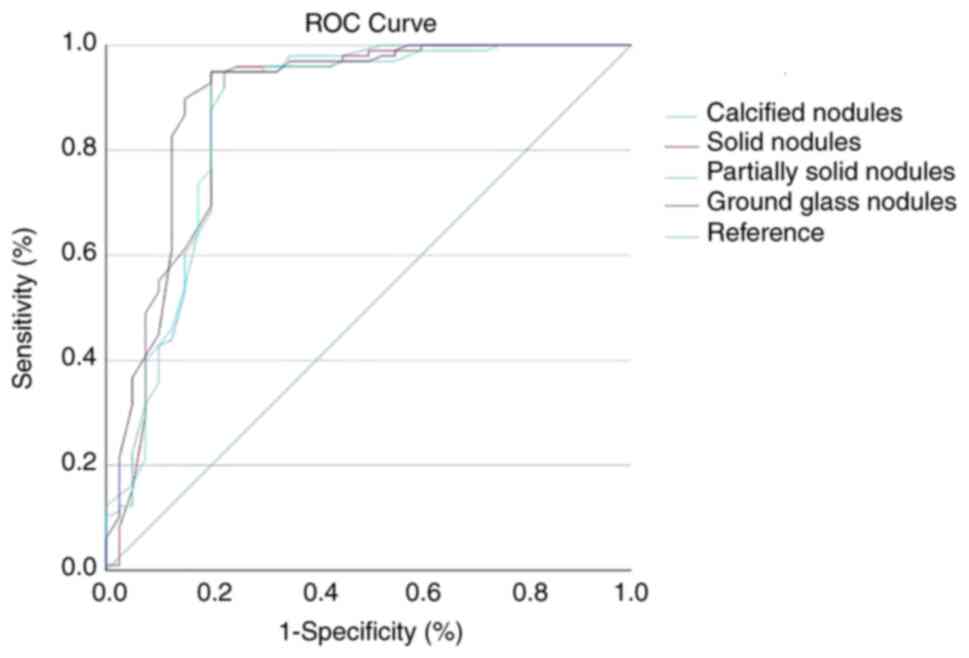 | Figure 4.ROC curves of the different types of
pulmonary nodules testing with the deep convolutional neural
network model (n=109). The number of calcified nodules, solid
nodules, partially solid nodules and ground glass nodules was 744,
1,540, 206 and 597 respectively as detected by the DCNN Model,
whereas the ROC was 0.766, 0.771, 0.761 and 0.796. ROC, receiver
operating characteristic; DCNN, deep convolutional neural
network. |
 | Table II.Indicators of pulmonary nodules
detected by the deep convolutional neural network. |
Table II.
Indicators of pulmonary nodules
detected by the deep convolutional neural network.
| Variable | Nodules ≤5 mm
(n=2,434) | Nodules >5 mm
(n=653) |
|---|
| Size, mm |
|
|
| 5 | 307 (12.6) | N/A |
| 4 | 577 (23.7) | N/A |
| 3 | 1,083 (44.5) | N/A |
| 2 | 467 (19.2) | N/A |
|
>20 | N/A | 60 (9.2) |
|
16-20 | N/A | 97 (14.8) |
|
11-15 | N/A | 161 (24.7) |
| 6-10 | N/A | 335 (51.3) |
| Calcified
nodules | 518 (21.3) | 226 (34.6) |
| Solid nodules | 1,287 (52.9) | 253 (38.7) |
| Partially solid
nodules | 135 (5.5) | 71 (10.9) |
| Ground glass
nodules | 494 (20.3) | 103 (15.8) |
| Right upper
lobe | 567 (23.3) | 101 (15.5) |
| Right middle
lobe | 195 (8.0) | 57 (8.7) |
| Right lower
lobe | 604 (24.8) | 185 (28.3) |
| Left upper
lobe | 528 (21.7) | 129 (19.8) |
| Left lower
lobe | 540 (22.2) | 181 (27.7) |
Dynamically evaluated by the DCNN
model
The DCNN model was used to dynamically evaluate and
analyze 675 chest CT images of 109 adolescent and young adult
patients with osteosarcoma. The DCNN model indicated that >50%
of the pulmonary nodules existed at the initial diagnosis (62/109;
58.0%), where cases with multiple pulmonary nodules tended to be
more common compared with those with only single pulmonary nodules
(68/109, 62.4% vs. 41/109, 37.6%; Table III). At diagnosis, during
preoperative chemotherapy, during postoperative chemotherapy and at
the end of all treatments, A total of 40 patients exhibited dynamic
changes in the pulmonary nodules; among them, the number of
pulmonary nodules increased in 17 patients, the diameter of
pulmonary nodules increased in 15 patients, the size and number of
pulmonary nodules did not change but the density of pulmonary
nodules increased in 5 patients, and the number of pulmonary
nodules decreased in 3 patients. Finally, 33 patients were
diagnosed with pulmonary metastases(Figs. 5, S1 and S2
and Table III). Among them, 16
cases occurred at the time of diagnosis of osteosarcoma, 8 cases of
pulmonary metastasis occurred during preoperative chemotherapy, 5
cases during postoperative chemotherapy and 4 cases occurred at the
end of all treatment. The DCNN model indicated that the diameter of
the majority of the pulmonary nodules was <5 mm (2,434/3,087,
78.8%). Based on density analysis, solid nodules accounted for 50%
of the total nodules, whereas calcified nodules and ground glass
nodules ranked in the second (21.3%) and third places (20.3%),
followed by part-solid nodules. Pulmonary nodules were randomly
distributed in either the left or right lobes (Table II).
 | Table III.Clinical data of 109 patients with
osteosarcoma and results of the dynamic monitoring of pulmonary
nodules using the deep convolutional neural network model. |
Table III.
Clinical data of 109 patients with
osteosarcoma and results of the dynamic monitoring of pulmonary
nodules using the deep convolutional neural network model.
| Parameter | Value |
|---|
| Age, years | 16 (9–34) |
| Sex |
|
|
Male | 69 (63.3) |
|
Female | 40 (36.7) |
| Site |
|
|
Limb | 102 (93.6) |
|
Non-limb | 7 (6.4) |
| Diagnosed |
|
|
Dynamic | 19 (30.6) |
| Dynamic (diagnosed
metastases) | 16 |
|
Stability | 43 (69.4) |
| During preoperative
chemotherapy |
|
|
Dynamic | 9 (39.1) |
|
Dynamic(diagnosed
metastases) | 8 |
|
Stability | 14 (60.9) |
| During
postoperative chemotherapy |
|
|
Dynamic | 7 (46.7) |
| Dynamic
(diagnosed metastases) | 5 |
|
Stability | 8 (53.3) |
| At the end of all
treatment |
|
|
Dynamic | 5 (55.6) |
| Dynamic
(diagnosed metastases) | 4 |
|
Stability | 4 (44.4) |
| Nodules (type not
defined) |
|
|
Single | 41 (37.6) |
|
Multiple | 68 (62.4) |
Discussion
The purpose of the present study was to evaluate the
ability of artificial intelligence-assisted technology to identify
pulmonary nodules in patients with osteosarcoma. Furthermore, the
present study aimed to monitor the dynamic changes in small nodules
of ≤5 mm in diameter in the lungs of patients with osteosarcoma
using such technology. Patients with osteosarcoma were found to
have a higher incidence of pulmonary small nodules (76.9%), where a
portion of the pulmonary nodules was diagnosed as lung metastases
with dynamic changes (13.4%; 16/119). During the regular follow-up
of patients with osteosarcoma, the number and density of pulmonary
small nodules may increase or decrease, disappear or remain stable.
The enlargement or increase of pulmonary small nodules, the
increase or decrease of nodule density and the decrease or
disappearance of pulmonary small nodules were defined as the
dynamic changes of pulmonary small nodules. The possibility of
pulmonary metastasis was considered, especially the enlargement or
increase of pulmonary small nodules and the increase of nodule
density were more likely to be pulmonary metastasis. In addition,
50% of the patients were shown to have small pulmonary metastatic
nodules at the time of diagnosis, whilst others were identified
during treatment and follow-up. Therefore, long-term dynamic
monitoring of small pulmonary nodules throughout the diagnostic and
treatment process is pivotal.
Deep learning algorithms are becoming increasingly
recognized as a promising technology for application in the medical
field (16,17). Zhang et al (18) previously applied a deep learning
algorithm for the detection of pulmonary nodules, and they found
that the trained sensitivity and specificity of the model was 84.4%
(95% CI, 80.5-88.3%) and 83.0% (95% CI, 79.5-86.5%), respectively.
Subgroup analysis of smaller nodules of <10 mm indicated
significant sensitivity and specificity, similar to those of larger
nodules with a size range of 10–30 mm. In another study, Nibali
et al (19) used DCNN for
detecting lung nodules, which yielded a sensitivity and specificity
of 91.7 and 88.6%, respectively. The present study found that
artificial intelligence algorithms may be used to automatically
monitor the size, number, location and density of pulmonary nodules
in patients with osteosarcoma. Therefore, using thin-slice chest CT
and artificial intelligence-assisted technology to dynamically
observe the nature of microscopic nodules in the lungs of patients
with osteosarcoma appears to be a viable method for improving the
diagnostic efficiency for radiologists.
The most commonly applied screening method for
osteosarcoma lung metastases is CT, which may improve the
sensitivity and specificity for the detection of lung
space-occupying lesions. CT scans are not complex and relatively
straightforward to perform, and they may be used to detect small
pulmonary nodules early to avoid other invasive examinations such
as thoracoscopy, bronchoscopy and CT-guided needle biopsy. Tsuchiya
et al (20) acknowledged
that CT scans may detect small pulmonary nodules <5 mm in
diameter, in addition to detecting pulmonary nodules with a
thickness of 10 mm. Since there can be omissions during CT
screening, fine scanning of small nodules should be performed
(21). Iwano et al (22) showed that a slice thickness of 1 mm
was more accurate for identifying benign and malignant solitary
pulmonary nodules. With the advent of fine tomographic CT, the
detection rate of sub-cm nodules is increasing. Brader et al
(23) reported that of the 117
nodules in patients with osteosarcoma tested, 80 (68%) were
pathologically malignant and 37 (32%) were benign. Specifically,
the assessors correctly classified 93–94% of the malignant nodules.
However, for benign lesions, the results were more inaccurate and
the assessors were only able to correctly classify 11–30% of them.
The majority of benign lesions were classified by the assessors to
be indeterminate (54–65%), but of a nodule size of 5 mm, and the
presence of calcification was found to be associated with an
increased likelihood of malignancy (P<0.05). Ghosh et al
(24) previously classified
osteosarcoma nodules into the following three categories: i) No
lung metastases; ii) indeterminate nodules; and iii) metastatic
nodules. The survival of patients with indeterminate pulmonary
nodules was significantly improved compared with that of patients
with metastatic disease but survival was similar to that of
patients without lung metastases, with metastatic nodules tending
to be >5 mm. However, the authors then argued that indeterminate
nodules remain a clinical and diagnostic challenge. In recent
years, artificial intelligence-assisted technology has been used
for thoroughly evaluating pulmonary nodules, yielding promising
evaluation results for judging the nature of uncertain pulmonary
nodules (17,25). In the present study, the application
of artificial intelligence-assisted technology for dynamic
monitoring indicated that 95 patients with osteosarcoma had
pulmonary small nodules (87.1%), while 40 patients with
osteosarcoma showed dynamic changes in the pulmonary nodules
(36.7%) and 33 (82.5%) were diagnosed with lung metastases during
the treatment period, comprising the time windows of diagnosis,
neoadjuvant chemotherapy, adjuvant chemotherapy and after all
treatments. Previous studies have found lung metastases at the
initial diagnosis of all high-grade III osteosarcoma to be ~20%
(26). In the present study, lung
metastases at the initial diagnosis of high-grade III osteosarcoma
were found to be ~50%. There may be several reasons behind this
finding. First, The detection rate of pulmonary small nodules based
on DCNN model was higher than that based on the manual model, which
caused a statistical difference. Previous studies were based on all
age groups. The present study focused on adolescents and young
adults (9–34 years old). Furthermore, artificial intelligence may
quickly identify nodules and shorten the reading time. However, the
sensitivity of artificial intelligence technology for pulmonary
nodules is high, but the accuracy still needs to be further
improved. However, in the current study, the identification of
small nodules was based on the evaluation of CT images rather than
on histological diagnosis, and diagnostic accuracy of pulmonary
nodules is difficult to evaluate by imaging evaluation. In
addition, it is likely that in addition to metastasis, microscopic
nodules in the lungs of patients with osteosarcoma may also present
with various conditions, such as inflammation, hemorrhage and lymph
node inflammation.
Determining the nature of small osteosarcoma
nodules, benign or metastatic, is a difficult challenge for medical
imaging. The artificial intelligence-based DCNN model was therefore
developed to evaluate pulmonary nodules. With the advent of
fine-slice CT, the detection of sub-cm nodules is increasing.
Artificial intelligence can quickly identify nodules and shorten
the reading time. In addition, during the follow-up re-examinations
of small nodules, artificial intelligence can accurately detect any
changes in nodules, such as size enlargement or reduction, and
increase or decrease in quantity. In general, the increase and/or
increase in pulmonary nodule size is associated with an increased
probability of nodules being metastases. Artificial intelligence
classified pulmonary nodules into four categories: Calcified
nodules, solid nodules, partially solid nodules and ground glass
nodules. The majority of osteosarcoma metastases were calcified
nodules and solid nodules. The increase in calcified nodules and
solid nodules indicates an increased probability of metastasis. A
limitation of this study is that small nodules metastasis of
osteosarcoma were diagnosed based on artificial intelligence
technology rather than histopathological diagnosis. Traditionally,
the gold standard for the diagnosis of pulmonary nodules as
osteosarcoma metastasis is not typically the judgement made by the
physician, but is instead possible following resection and
pathological analysis. The methods used to accurately identify the
micrometastatic nodules of osteosarcoma require further study by
medical imaging experts. However, with further data support or
improvement of imaging techniques, such as MRI/positron emission
tomography (PET) or PET/CT (27,28),
it is expected to be possible in the future to distinguish small
metastatic from benign nodules.
To conclude, artificial intelligence-assisted
diagnostic technology may be beneficial for the evaluation and
monitoring of pulmonary nodules in patients with osteosarcoma. For
patients with osteosarcoma, minute pulmonary nodules with a
diameter of <5 mm are frequently found. For patients with
osteosarcoma and with small nodules in the lungs, artificial
intelligence-assisted diagnosis technology may assist in evaluating
whether it is a lung metastasis to timely modify the chemotherapy
regimen or to determine the timing of surgery for lung metastases.
This may improve the long-term survival rate of patients. However,
further research remains necessary.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Hangzhou Science and
Technology Foundation (grant no. 2020123Y028 and the Natural
Science Foundation of Zhejiang Province (grant no.
LQ18H290001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLN, XCZ and HL confirm the authenticity of all the
raw data. YLN and HL conceived and designed the study, developed
the methodology used and drafted the original manuscript. XCZ
curated the data and carried out the formal analysis. XJS
visualized the data and participated in the investigation. YLN, YFX
and HL conducted deep learning modeling and statistical analysis
based on data. HL validated and revised the final manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study involving human participants was
reviewed and approved by the Medical Ethics Committee of Hangzhou
Third People's Hospital (Hangzhou, China). Written informed consent
for participation was not required for the current study in
accordance with the national legislation and institutional
requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Whelan J, Seddon B and Perisoglou M:
Management of osteosarcoma. Curr Treat Option Oncol. 7:444–455.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Picci P: Osteosarcoma (Osteogenic
sarcoma). Orphanet J Rare Dis. 2:1–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giger ML, Bae KT and Macmahon H:
Computerized detection of pulmonary nodules in computed tomography
images. Invest Radiol. 29:459–465. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaner EG, Chang AE, Doppman JL, Conkle
DM, Flye MW and Rosenberg SA: Comparison of computed and
conventional whole lung tomography in detecting pulmonary nodules:
A prospective radiologic-pathologic study. Am J Roentgenol.
131:51–54. 1978. View Article : Google Scholar
|
|
5
|
Obata H, Kuratsu S, Uchida A, Araki N,
Myoui A, Ueda T and Yoshikawa H: Analysis of organ selectivity in
the metastatic behavior of Dunn osteosarcoma. Clin Orthop Relat
Res. 398:212–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciccarese F, Bazzocchi A, Ciminari R,
Righi A, Rocca M, Rimondi E, Picci P, Reggiani MLB, Albisinni U,
Zompatori M and Vanel D: The many faces of pulmonary metastases of
osteosarcoma: Retrospective study on 283 lesions submitted to
surgery. Eur J Radiol. 84:2679–2685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakajima J, Murakawa T, Fukami T, Sano A,
Sugiura M and Takamoto S: Is finger palpation at operation
indispensable for pulmonary metastasectomy in colorectal cancer?
Ann Thorac Surg. 84:1680–1684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao K, Xu J and Zhao WQ: Artificial
intelligence on diabetic retinopathy diagnosis: An automatic
classification method based on grey level co-occurrence matrix and
naive Bayesian model. Int J Ophthalmol. 12:1158–1162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang K, Shou Q, Ma SJ, Liebeskind D, Qiao
XJ, Saver J, Salamon N, Kim H, Yu Y, Xie Y, et al: Deep learning
detection of penumbral tissue on arterial spin labeling in stroke.
Stroke. 51:489–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolterink JM, van Hamersvelt RW, Viergever
MA, Leiner T and Išgum I: Coronary artery centerline extraction in
cardiac CT angiography using a CNN-based orientation classifier.
Med Image Anal. 51:46–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Institutes for Food and Drug
Control, Cardio-thoracic Working Group, Chinese Society of
Radiology, . Chinese Medical Association: Expert consensus on the
rule and quality control of pulmonary nodule annotation based on
thoracic CT. Chin J Radiol. 53:9–15. 2019.(In Chinese).
|
|
12
|
Drnasin I, Grgić M and Gogić G: JavaScript
access to DICOM network and objects in web browser. J Digit
Imaging. 30:537–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Ahmad S, Fan J, Shen D and Yap
PT: Difficulty-aware hierarchical convolutional neural networks for
deformable registration of brain MR images. Med Image Anal.
67:1018172021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarmah BK and Chakrabarty D: Examination
of proper randomness of the numbers generated by rand corporation
(1955) random number table: t-Test.
2015.DOI:10.15680/IJIRSET.2015.0410007.
|
|
15
|
Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G,
Huang Y, Wang X, Wu N, Zhang G, Zheng X and Bu H: China national
guideline of classification, diagnosis and treatment for lung
nodules (2016 Version). Zhongguo Fei Ai Za Zhi. 19:793–798.
2016.(In Chinese). PubMed/NCBI
|
|
16
|
Ardila D, Kiraly AP, Bharadwaj S, Choi B,
Reicher JJ, Peng L, Tse D, Etemadi M, Ye W, Corrado G, et al:
End-to-end lung cancer screening with three-dimensional deep
learning on low-dose chest computed tomography. Nat Med.
25:954–961. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massion PP, Antic S, Ather S, Arteta C,
Brabec J, Chen H, Declerck J, Dufek D, Hickes W, Kadir T, et al:
Assessing the accuracy of a deep learning method to risk stratify
indeterminate pulmonary nodules. Am J Respir Crit Care Med.
202:241–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Sun X, Dang K, Li K, Guo XW,
Chang J, Yu ZQ, Huang FY, Wu YS, Liang Z, et al: Toward an expert
level of lung cancer detection and classification using a deep
convolutional neural network. Oncologist. 24:1159–1165. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nibali A, He Z and Wollersheim D:
Pulmonary nodule classification with deep residual networks. Int J
Comput Assist Radiol Surg. 12:1799–1808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuchiya H, Kanazawa Y, Abdel-Wanis ME,
Asada N, Abe S, Isu K, Sugita T and Tomita K: Effect of timing of
pulmonary metastases identification on prognosis of patients with
osteosarcoma: The Japanese musculoskeletal oncology group study. J
Clin Oncol. 20:3470–3477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Briccoli A, Rocca M, Salone MC, Fiore MD,
Vanel D, Balladelli A and Alberghini M: ‘Bubble-like’ lung
metastases in osteosarcoma patients. Eur J Radiol. 71:144–146.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwano S, Makino N, Ikeda M, Itoh S,
Tadokoro M, Satake H and Ishigaki T: Solitary pulmonary nodules:
Optimal slice thickness of high-resolution CT in differentiating
malignant from benign. Clin Imaging. 28:322–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brader P, Abramson SJ, Price AP, Ishill
NM, Emily ZC, Moskowitz CS, La Quaglia MP and Ginsberg MS: Do
characteristics of pulmonary nodules on computed tomography in
children with known osteosarcoma help distinguish whether the
nodules are malignant or benign? J Pediatr Surg. 46:729–735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh KM, Lee LH, Beckingsale TB, Gerrand
CH and Rankin KS: Indeterminate nodules in osteosarcoma: What's the
follow-up? Brit J Cancer. 118:634–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao Q, Gu Y, Wu J, Wang Z and Huang Y:
Abstract P6-02-19: Machine learning based analysis of CT radiomics
for the simultaneous indeterminate pulmonary nodules of breast
cancer. Cancer Res. 79:P6–02-19-P6-02-19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Guo X, Xu Y, Han X, Cai J, Wang X
and Wang G: Lung metastases at the initial diagnosis of high-grade
osteosarcoma: Prevalence, risk factors and prognostic factors. A
large population-based cohort study. Sao Paulo Med J. 137:423–429.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Susam S, Cinkooglu A, Ceylan KC, Gürsoy S,
Kömürcüoğlu BE, Mertoğlu A, Çırak AK, Tuksavul F, Gayaf M, Güldaval
F, et al: Diagnostic success of transthoracic needle biopsy and
PET-CT for 1 to 2 cm solid indeterminate pulmonary nodules. Clin
Respir J. 14:453–461. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grisanti F, Zulueta J, Rosales JJ, Morales
MI, Sancho L, Lozano MD, Mesa-Guzmán M and García-Velloso MJ:
Diagnostic accuracy of visual analysis versus dual time-point
imaging with 18F-FDG PET/CT for the characterization of
indeterminate pulmonary nodules with low uptake. Rev Esp Med Nucl
Imagen Mol (Engl Ed). 40:155–160. 2020.PubMed/NCBI
|