Introduction
To date, lung cancer is the tumor type that is most
likely to be diagnosed, and it is also responsible for the largest
proportion of cancer-associated deaths worldwide. In one single
year (2020), ~200 million new cases of lung cancer and 180 million
associated deaths were reported (1). Among all the new cases of lung cancer,
non-small cell lung cancer (NSCLC) cases comprise the largest
percentage (~85%). In the early stages, this disease may present
with no symptoms, and so the majority of patients are not diagnosed
until they have progressed to the advanced stages. The standard
method of care for these patients is concurrent chemoradiotherapy,
and when applying this method, the local control rate is typically
observed to be in the order of 50–60% (2,3).
The effectiveness of radiation therapy is determined
by the absorbed dose in the planning target volume (PTV) and the
organ at risk (OAR). In an ideal situation, the higher the dose
that is received by the PTV, and the lower the dose received by the
OAR, the better. A previously published study demonstrated that a
higher radiation dose intensity (>40 Gy) was associated with
improved survival time for patients with metastatic disease
(4). However, there is a defined
limit for the dose of radiation that the OARs can receive, and
these limits restrict the doses that can be applied for the PTV;
therefore, it is of great importance to find an appropriate
radiotherapy technique that will increase the dose associated with
the PTV, while decreasing the dose for the OAR.
The effectiveness of radiation therapy has gradually
improved, as the technology has shifted from two-dimensional to
three-dimensional (3D) accurate radiation therapy. 3D conformal
radiation therapy (3D-CRT) and intensity-modulated radiotherapy
(IMRT) now represent the two most accurate radiotherapy techniques
(5,6). Numerous studies have been published
that have evaluated the advantages of using IMRT over 3D-CRT, in
particular with respect to: i) Improving the homogeneity index (HI)
and conformity index (CI) of the PTV; and ii) decreasing the
absorbed dose in OARs (7–10). However, the disadvantages of using
IMRT should be borne in mind; its increased treatment time may
contribute to uncertainties in the prescribed treatment position,
which may result in dose changes in PTV. For example, a ‘low-dose
bath’ was shown to increase the risk of lethal pneumonitis
(11). In 2008, a new form of IMRT
was proposed by Otto (12), termed
volumetric modulated arc radiotherapy (VMAT). The biggest
difference from fixed-field IMRT is that, with VMAT, it is possible
to coordinate the dose rate, multileaf collimator movement and
gantry rotation at the same time. Apart from these advantages, VMAT
has also been shown to achieve an improved dose distribution with
decreasing treatment time in numerous types of cancer (e.g., in
prostate, locally advanced lung carcinoma and various head and neck
cancer applications) (13–17). The hybrid-IMRT (h-IMRT) technique,
which is discussed in the present study, comprises a combination of
3D-CRT and IMRT, and a previous study has suggested that this
technique can improve the plan quality when an appropriate ratio
between the use of 3D-CRT and IMRT is set; in the study, the
conventional component consisted of a nominal fraction dose of 1.8
Gy; the IMRT component consisted of a nominal 0.2 Gy per fraction,
and in this ratio, compared to 3D-CRT, using the h-IMRT technique
can improve the PTV coverage and avoid hot spots (18).
Although all four methods are able to achieve the
necessary objectives for PTV and OAR, when it comes to considering
which method is the most optimal technique for NSCLC, no unanimous
consensus has been reached. Therefore, the present study performed
upon a dosimetric comparison of the four techniques, aiming to
determine the following: i) Whether adding 20% IMRT to the 3D-CRT
base plan (3D-CRT:IMRT ratio, 4:1) leads to any improvement in the
plan quality; and ii) to identify the optimal technique for
patients with NSCLC (and the subgroup patients), through evaluating
the dose parameters [CI, HI, dose histogram volume (DVH) of the
OARs and the treatment monitor units (MUs)] of these four
techniques, so as to make the best of current radiation
resources.
Patients and methods
Patients' general characteristics
Between January 2017 and October 2021, 40 patients
with histologically or cytologically confirmed NSCLC who were
scheduled for radiotherapy were selected at our radiotherapy center
(Radiotherapy Center of The First Affiliated Hospital of Yangtze
University, Jingzhou, China). The inclusion criteria were as
follows: i) Non-small-cell lung cancer confirmed by histology or
cytology; ii) existence of measurable lesions according to the
Response Evaluation Criteria in Solid Tumors (19); iii) the patients were either
inoperable or did not wish to have surgery; iv) patients were in
stage III based on the TNM classification (20). Exclusion criteria were as follows:
i) Lung carcinoid or small cell lung cancer; ii) patients with any
distant metastasis. The scheme of therapy selected for them was
radical radiotherapy or combined chemoradiotherapy.
Computed tomography (CT)
simulation
All patients were fixed in place with an integrated
carbon fiber board and thermoplastic phantom or vacuum air cushion.
Both of the patient's hands were placed on the arm-fixing device in
a headfirst supine position. After intravenous injection of the
contrast enhancer (100 ml iodopanol injection), all patients were
scanned in free breathing mode using Philips Brilliance 16
simulation CT (Philips Medical Systems, Inc.). No special measures
were taken to control the target movement caused by respiratory
movement. The scanning range for each patient included the whole
neck, chest and upper abdomen, and the layer thickness was 5 mm.
The simulated positioning CT images obtained were subsequently
transmitted to the treatment planning system used for planning
design.
Target and contouring of the OARs
Experienced doctors who had positions at Deputy
Director level or higher in the hospital outlined the target and
OARs. The doctors outlined the tumor target volume in a Varian
Eclipse™ 15.6 planning system (Varian Medical Systems), according
to the requirements of the International Commission Radiological
Units 83 report (21). The gross
tumor volume (GTV) was defined as the primary tumor and clinical
positive lymph nodes. Clinical positive lymph nodes themselves were
defined as lymph nodes with a diameter ≥1 cm on the CT scan, or
lymph nodes observed to have high metabolic activity on positron
emission-CT. The clinical target volume (CTV) generally refers to a
1.5-cm margin in the head-foot direction and a 1-cm margin in other
directions on the basis of the GTV. Considering the patient's
respiratory movement during treatment and allowing for the
positioning errors and other factors, 0.8 cm was used on the basis
of the CTV to form a PTV. The OARs in the present study included
the left lung, right lung, esophagus, heart and spinal cord.
Requirement of target volume and the
constraints of OARs
A margin of 0.5 cm on the spinal cord formed the
planning OAR volume (PRV) for the spinal cord. The dose limits for
the bilateral lungs were as follows: Mean lung dose (MLD) <20
Gy, percentage of lung volume receiving a dose >20 Gy
(V20) <30%, or V20<35%,
V30<20% and V5<60% (22). Regarding the other OARs, the spinal
cord limits were as follows: Spinal cord PRV maximal dose
(Dmax) <45 Gy (23).
The esophageal limits were as follows: Mean esophagus dose (MED)
<34 Gy, V45<40% and Dmax<110%
prescription dose (PD). The cardiac dose limits were as follows:
Dmax<55 Gy, Dmax<105% PD when
overlapping with the target, V40<30% and mean heart
dose (MHD) <26 Gy (24,25). All patients were administered a PD
of 60 Gy in the PTV with 2 Gy/fraction. A total of 95% of the PTV
volume would receive at least 95% of the PD. The minimum dose in
the target volume was no less than 90% of the PD, and no more than
115% of the PD. ‘Vx’ here refers to the percentage of X
(Gy) dose received by the tissue in the total volume of the
tissue.
Planning design
The selection of the radiation field angle in a
radiotherapy plan generally follows the following principles: i)
Visually, the distance from the radiation source to the PTV is the
closest, and the properties of the tissue through which the
radiation passes are similar; ii) the incoming ray passes through
the stable tissue of the human body (avoiding loose tissue, such as
fat tissue with high mobility, so as to prevent positioning errors
during the implementation of the plan); iii) avoiding direct
irradiation of OARs close to the PTV; and iv) avoiding passing
through substances with large atomic number (such as metal buckles
or prosthetic devices). If the CI or HI of the radiotherapy plan is
reduced under the above conditions, or the clinical dose
requirements cannot be well met, the angle of the radiation field
should be adjusted to meet the clinical requirements. In order to
avoid skin toxicity, it is best to try to ensure that there is no
crossing over between each field, and that the proportion of the
radiation field that directly passes through the OARs is as small
as possible.
All four radiotherapy plans utilized in the present
study were designed by senior radiotherapy physicists utilizing the
Eclipse™ Treatment Planning System (Varian Medical Systems) with 6
MV of energy. Concerning the specific arrangement of the radiation
fields, the first plan design, i.e., the 3D-CRT radiotherapy plan
design, usually used 3–5 radiation fields, and the angle of the
radiation field was close to the tumor location. Following the
above principles, it was possible to improve the CI and HI of the
target volume by adjusting the weight of the radiation field while
avoiding hot spots and cold spots. Concerning the second plan, the
IMRT radiotherapy plan design used five radiation fields, and
repeated optimization was performed through appropriate
optimization conditions, so as to obtain a radiotherapy plan
suitable for clinical use. The third plan included in this study
was the hybrid plan of 3D-CRT and IMRT, in which the proportion of
3D-CRT was 80%, and therefore, the proportion of IMRT was 20%.
After the 3D-CRT radiotherapy plan had been formulated, it was
regarded as the base dose plan. On this basis, the IMRT field was
arranged and optimized. Generally, in the hybrid field plan, the
IMRT plan has three fields. Finally, the fourth plan was the VMAT
plan with a single full arc or double half arc. The choice of arc
depended on the location of the tumor. The angle of the collimator
was fixed at 20 or 340°, and the optimization conditions were
consistent with those of the second plan (i.e., the IMRT
radiotherapy plan). By repeating the optimization process, it was
possible to finally obtain a radiotherapy plan that met all the
patients' clinical needs.
Plan evaluation
The PTV and OAR parameter values were obtained from
the DVH. The parameters of V98, V2 and
V50 were selected, as the HI values could be calculated
according to these indexes, and the accompanying formula was
HI=(D2%-D98%)/D50% (26); the lower the value for HI, the
better. Subsequently, the CI values were calculated. The
calculation formula was as follows:
CI=(VROI,PD)2/(VROI ×
Vbody,PD) (27), where
VROI,PD is the volume of PTV covered by the PD,
VROI is the volume of PTV and Vbody,PD is the
total volume covered by the PD. The higher the value of CI (i.e.,
the closer it is to 1), the better. The evaluation parameters of
OARs were as follows: Total lung mean dose (Dmean),
V5, V13, V20 and V30;
ipsilateral lung Dmean, V5, V13,
V20 and V30; contralateral lung
Dmean, V5, V13, V20 and
V30; spinal cord Dmax; heart
Dmean, V30, V40 and
V45; and esophagus Dmean, Dmax and
V50.
Each radiotherapy plan recorded the final treatment
MUs and execution time. The execution time refers to the time from
the moment when the patient's position was set up until the
completion of the treatment. In the first three treatment methods,
the execution time was calculated according to the following
calculation method: Gantry rotation time (5.8°/sec) plus treatment
MU time (total MU/10) plus wedge changing time (60 sec for each
replacement). According to the actual evaluation, the treatment
time according to the VMAT plan was ~120 sec, and so all VMAT plans
were fixed for 120 sec and then compared with other radiotherapy
methods.
Statistical analysis
SPSS version 25 statistical software (IBM Corp.) was
used for the statistical analysis, and all data are expressed as
the mean ± standard deviation. One-way ANOVA was used to compare
the four radiotherapy plans. When statistical significance had been
reached, Tukey's Honestly Significant Difference method was used
for post hoc analysis and pairwise comparisons. All P-values
displayed are bidirectional. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients' clinical data
Table I shows the
basic characteristics of the 40 enrolled patients. The median age
of the patients was 63 years (range, 40–81 years). The median value
of the PTV was 315.6 cm3 (range: 114.2-429.1
cm3). Fig. 1 shows the
DVH diagram of a representative patient with stage III NSCLC when
using the 3D-CRT, IMRT, h-IMRT and VMAT technologies. Fig. 2 shows the isodose range of 5, 20,
30, 57 and 60 Gy for the same patient using the 3D-CRT, IMRT,
h-IMRT and VMAT technologies in cross-section, coronal plane and
sagittal plane, respectively.
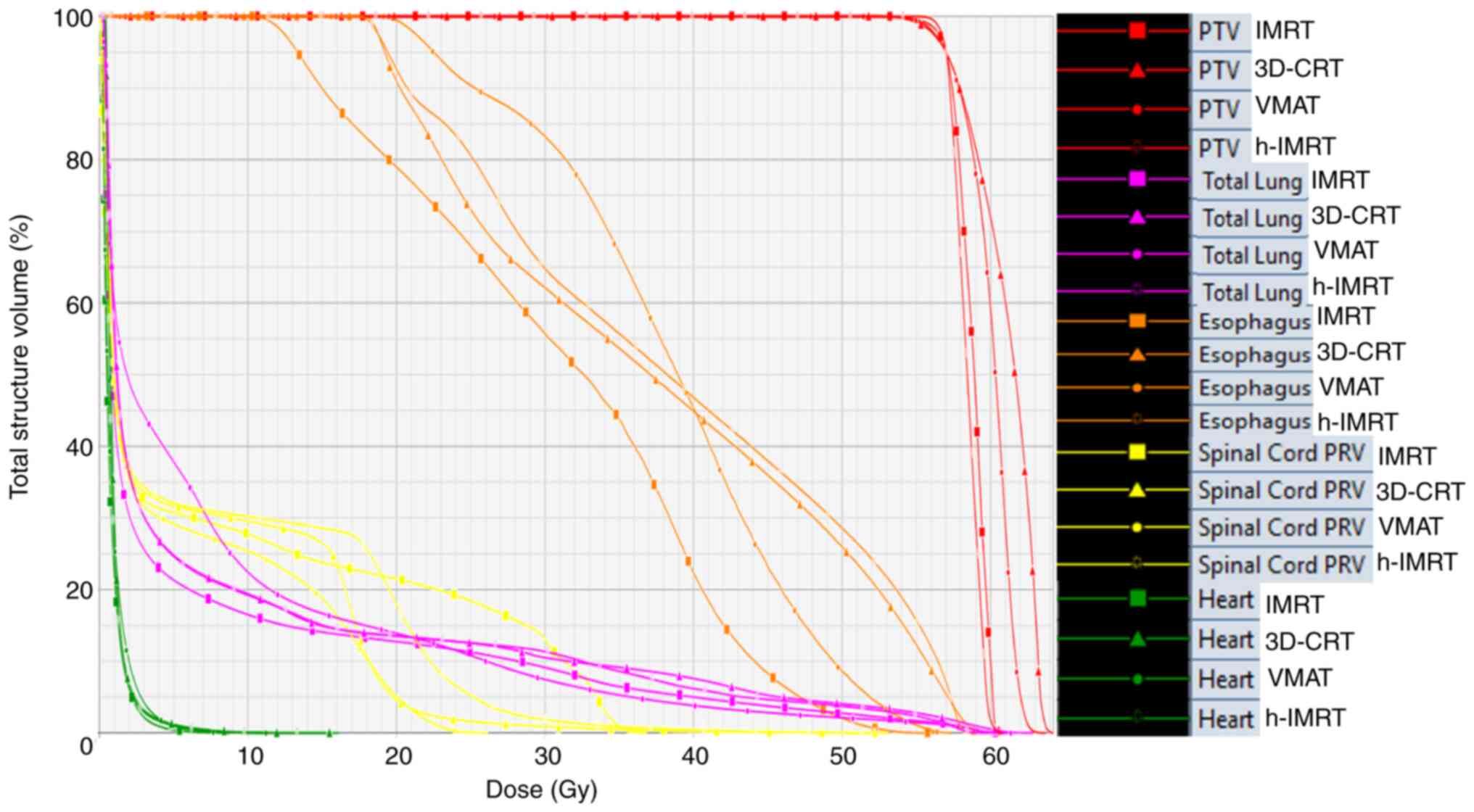 | Figure 1.Dose volume histogram comparison for
the target coverage and certain organs at risk, including total
normal lung, heart, esophagus and spinal cord, in 3D-CRT
(triangles), IMRT (squares), h-IMRT (hollow dots) and VMAT (solid
dots). The prescription dose was 60 Gy in 30 fractions. 3D-CRT,
three-dimensional conformal radiation therapy; IMRT,
intensity-modulated radiation therapy; h-IMRT, hybrid IMRT; VMAT,
volumetric-modulated arc therapy; PTV, planning target volume; PRV,
planning organ at risk volume; A, 3D-CRT; B, IMRT; C, h-IMRT; D,
VMAT. |
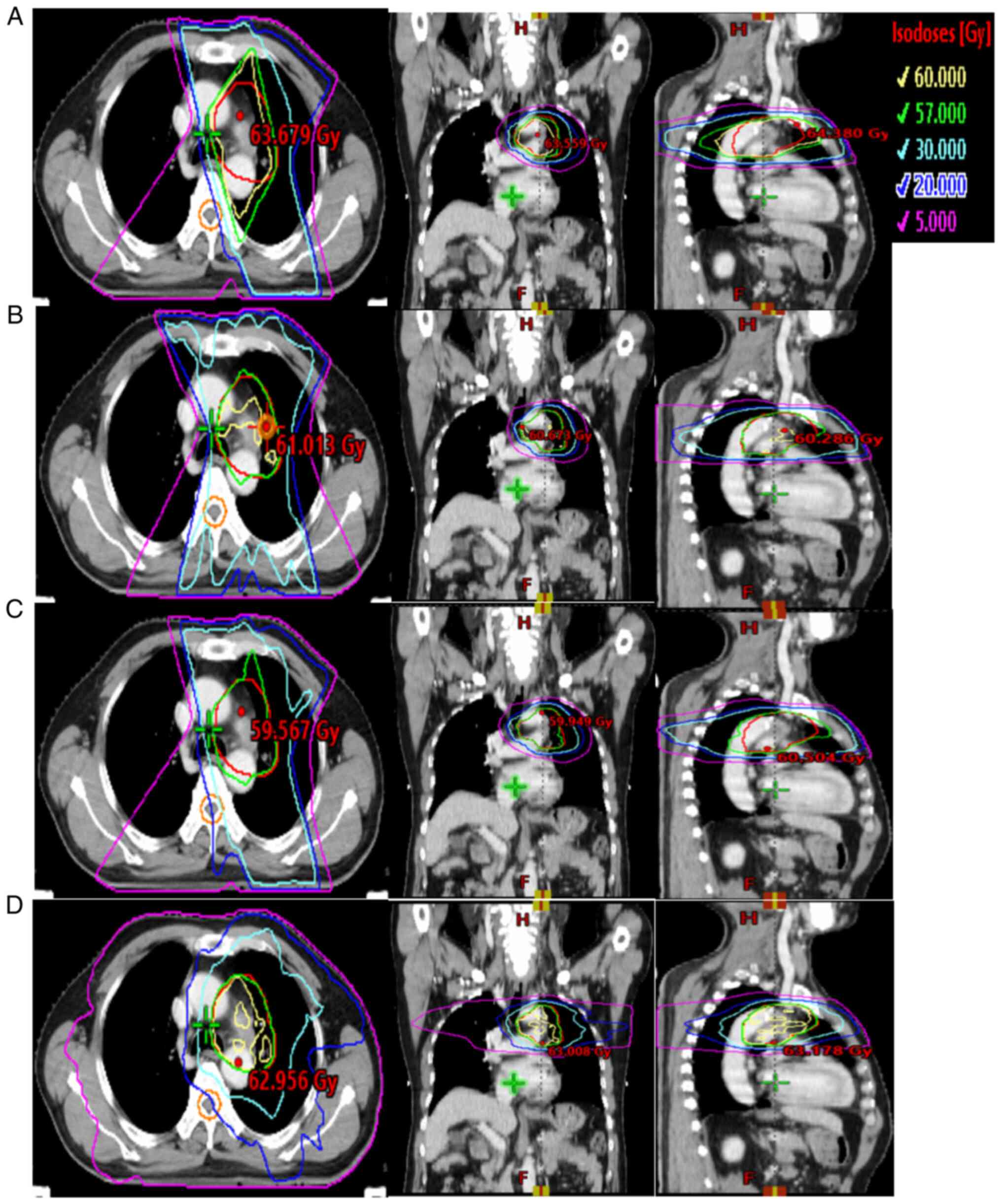 | Figure 2.Typical isodose distributions for the
four plans for a patient in the same computed tomography slice. (A)
Three-dimensional conformal radiation therapy, (B)
intensity-modulated radiation therapy, (C) hybrid
intensity-modulated radiation therapy and (D) volumetric-modulated
arc therapy. The red, blue, cyan, green and yellow lines represent
the dose curves of 5, 20, 30, 57 and 60 Gy (i.e., the prescription
doses), respectively. |
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Sex |
|
|
Male | 37 (92.5) |
|
Female | 3 (7.5) |
| Age, years | 63 (40–81) |
| Histology |
|
|
SCC | 28 (70.0) |
| AC | 12 (30.0) |
| Tumor location |
|
| LL | 10 (25.0) |
| RL | 30 (75.0) |
| T stage |
|
| T1 | 0 (0.0) |
| T2 | 8 (20.0) |
| T3 | 8 (20.0) |
| T4 | 24 (60.0) |
| N stage |
|
| N0 | 4 (10.0) |
| N1 | 7 (17.5) |
| N2 | 25 (62.5) |
| N3 | 4 (10.0) |
| TNM
stagea |
|
|
IIIA | 15 (37.5) |
|
IIIB | 25 (62.5) |
| Tumor size,
cm3 | 315.6
(114.2-429.1) |
| Lung volume,
cm3 | 3150.8
(1552.0-4797.0) |
Comparison of the four radiotherapy
plans in all patients
In terms of the average dose of PTV, the 3D-CRT
radiotherapy plan had the highest value (P<0.05); those of the
h-IMRT and VMAT techniques were similar (P>0.05), with IMRT
ranking last (P<0.05) (Table
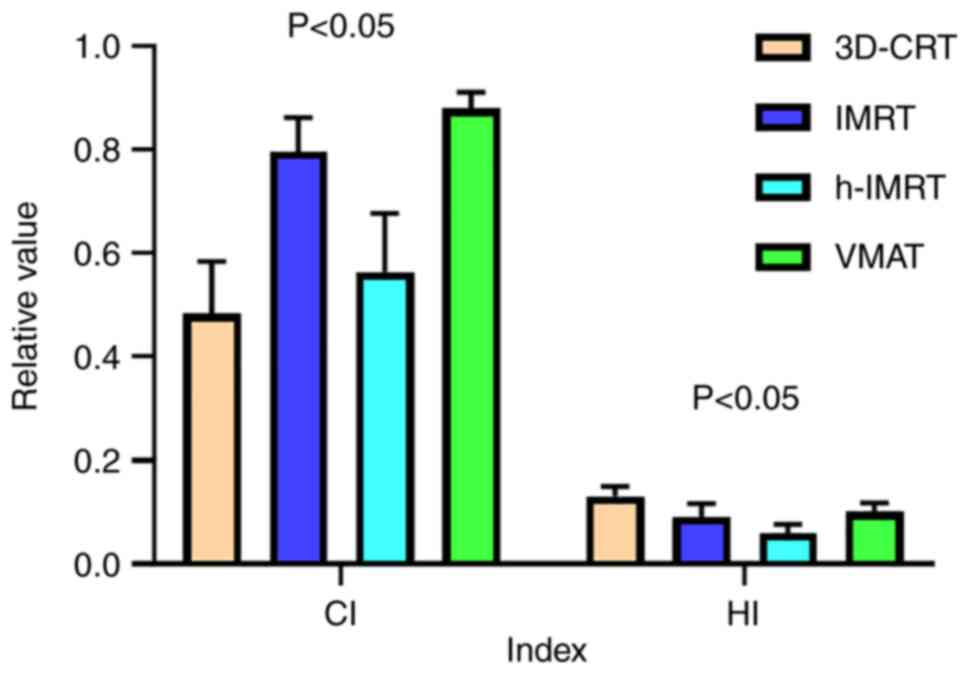
II). However, in terms of the CI, VMAT was found to have the
highest value, followed by IMRT, h-IMRT and finally 3D-CRT
(Fig. 3). Significant differences
in the CI value were identified among the four techniques
(P<0.05), as shown in Tables II
and III. With respect to the HI,
h-IMRT was the technique found to have the lowest value, and the HI
increased sequentially in the order IMRT, VMAT and 3D-CRT (which
had the highest HI value) (Fig. 3).
Similarly, as shown in Tables II
and III, significant differences
in the HI value were identified among the four techniques
(P<0.05).
 | Table II.Dose-volume histogram parameters for
PTV and OARs according to the radiotherapy techniques applied for
all the patients with non-small cell lung cancer. |
Table II.
Dose-volume histogram parameters for
PTV and OARs according to the radiotherapy techniques applied for
all the patients with non-small cell lung cancer.
| Dosimetric
parameter | 3D-CRT | IMRT | h-IMRT | VMAT | P-value |
|---|
| PTV |
|
|
|
|
|
| CI | 0.480±0.099 | 0.805±0.057 | 0.565±0.113 | 0.876±0.030 | <0.001 |
| HI | 0.130±0.020 | 0.085±0.025 | 0.058±0.016 | 0.093±0.019 | <0.001 |
|
D98, Gy | 55.73±0.33 | 55.94±0.69 | 57.38±0.85 | 55.99±0.27 | <0.001 |
|
D2, Gy | 63.65±1.17 | 61.00±1.15 | 60.82±0.78 | 61.56±1.00 | <0.001 |
|
Dmean, Gy | 60.65±0.82 | 59.16±0.74 | 59.50±0.62 | 59.54±0.65 | <0.001 |
| OAR |
|
|
|
|
|
| Normal
lung |
|
|
|
|
|
|
V5,
% | 36.13±9.35 | 35.10±9.23 | 36.69±9.27 | 51.64±10.78 | <0.001 |
|
V13,
% | 25.82±7.57 | 25.33±6.98 | 25.89±7.45 | 27.70±6.99 | 0.346 |
|
V20,
% | 21.21±7.30 | 20.97±6.04 | 21.09±7.28 | 20.27±6.02 | 0.887 |
|
V30,
% | 17.59±6.53 | 14.57±5.17 | 17.70±6.45 | 12.82±4.75 | <0.001 |
|
MLD, Gy | 12.38±3.75 | 10.77±2.94 | 14.14±14.29 | 11.81±2.92 | 0.142 |
|
Heart |
|
|
|
|
|
|
V30,
% | 17.45±14.89 | 14.31±12.31 | 17.67±14.72 | 9.88±11.52 | 0.009 |
|
V40,
% | 14.65±12.97 | 7.35±7.30 | 13.57±11.82 | 4.90±5.32 | <0.001 |
|
V45,
% | 11.37±9.97 | 5.24±5.74 | 9.78±9.33 | 3.50±3.87 | <0.001 |
|
MHD, Gy | 13.57±8.61 | 10.59±7.09 | 12.74±8.61 | 10.23±6.57 | 0.072 |
|
Esophagus |
|
|
|
|
|
|
V50,
% | 16.62±23.81 | 8.56±16.05 | 15.93±23.08 | 11.71±18.20 | 0.143 |
|
Dmax,
Gy | 45.25±19.90 | 42.43±20.45 | 44.62±19.36 | 47.35±15.05 | 0.600 |
|
MED, Gy | 21.70±15.14 | 19.28±13.19 | 22.35±14.60 | 26.64±10.68 | 0.042 |
| Spinal
cord |
|
|
|
|
|
|
Dmax,
Gy | 38.85±12.90 | 36.02±9.51 | 38.63±11.95 | 32.79±9.97 | 0.017 |
 | Table III.Comparison of the P-values of the
dose-volume histogram parameters for the PTV according to the four
radiotherapy techniques for all the patients with non-small cell
lung cancer. |
Table III.
Comparison of the P-values of the
dose-volume histogram parameters for the PTV according to the four
radiotherapy techniques for all the patients with non-small cell
lung cancer.
|
| P-values of
subgroup comparisonsa |
|---|
|
|
|
|---|
| Dosimetric
parameters | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|---|
| PTV |
|
|
|
|
|
|
| CI | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HI | <0.001 | <0.001 | <0.001 | <0.001 | 0.161 | <0.001 |
|
D98, Gy | 0.249 | <0.001 | 0.048 | <0.001 | 0.967 | <0.001 |
|
D2, Gy | <0.001 | <0.001 | <0.001 | 0.804 | 0.025 | 0.001 |
|
Dmean, Gy | <0.001 | <0.001 | <0.001 | 0.061 | 0.030 | 0.993 |
| OAR |
|
|
|
|
|
|
| Normal
lung |
|
|
|
|
|
|
|
V5,
% | 0.945 | 0.990 | <0.001 | 0.827 | <0.001 | <0.001 |
|
V13,
% | 0.985 | >0.999 | 0.536 | 0.977 | 0.326 | 0.567 |
|
V20,
% | 0.998 | >0.999 | 0.885 | >0.999 | 0.949 | 0.920 |
|
V30,
% | 0.036 | >0.999 | <0.001 | 0.027 | 0.394 | 0.015 |
|
MLD, Gy | 0.694 | 0.631 | 0.980 | 0.480 | 0.895 | 0.391 |
|
Heart |
|
|
|
|
|
|
|
V30,
% | 0.618 | >0.999 | 0.020 | 0.564 | 0.321 | 0.015 |
|
V40,
% | 0.001 | 0.941 | <0.001 | 0.007 | 0.572 | <0.001 |
|
V45,
% | <0.001 | 0.702 | <0.001 | 0.012 | 0.638 | <0.001 |
|
MHD, Gy | 0.194 | 0.946 | 0.045 | 0.475 | 0.995 | 0.337 |
|
Esophagus |
|
|
|
|
|
|
|
V50,
% | 0.178 | 0.998 | 0.602 | 0.247 | 0.856 | 0.710 |
|
Dmax,
Gy | 0.864 | 0.998 | 0.939 | 0.930 | 0.528 | 0.876 |
|
MED, Gy | 0.789 | 0.994 | 0.231 | 0.639 | 0.026 | 0.355 |
| Spinal
cord |
|
|
|
|
|
|
|
Dmax,
Gy | 0.556 | >0.999 | 0.027 | 0.620 | 0.438 | 0.036 |
In terms of the OARs, total lung V5 in
VMAT was statistically significant compared with the other three
radiotherapy plans (P<0.05). For total lung V30, IMRT
and VMAT were found to be better as techniques than the use of
3D-CRT and h-IMRT (P<0.05), while in terms of the mean dose to
the total lung, no significant differences existed in these four
techniques (P>0.05). In the comparisons of cardiac dose, the
V30 value decreased in VMAT, and this decrease was shown
to be statistically significant compared with 3D-CRT and IMRT
(P<0.05). By contrast, with heart V40 and
V45, IMRT and VMAT were found to have lower values
compared with 3D-CRT and h-IMRT (P<0.05); the difference between
the two pairs was not found to be statistically significant
(P>0.05). In terms of the mean dose, VMAT and IMRT had lower
values compared with h-IMRT and 3D-CRT, although these differences
did not reach the level of statistical significance (P>0.05).
The difference between the two pairs was also not found to be
statistically significant (P>0.05). In terms of esophageal
radiotherapy, no statistically significant differences were found
with regard to Dmax and V50, although VMAT
was identified as the technique with higher average dose than IMRT
(P<0.05). In the spinal cord, Dmax was found to have
the lowest value with the VMAT technology (P<0.05); these
findings are summarized in Tables
II and III.
There were, however, significant differences
identified in terms of the MUs and treatment times (P<0.05).
IMRT was found to have the highest value in terms of the MU
(P<0.05), followed sequentially (in decreasing order) by h-IMRT,
VMAT and 3D-CRT (the technique with the lowest MU) (P<0.05). In
terms of the treatment time, VMAT was the technique with the
shortest time (P<0.05), followed by IMRT, 3D-CRT and h-IMRT
(with the longest treatment time) (P<0.05). These data are
summarized in Table IV.
 | Table IV.Treatment time and MU comparison
according to the radiotherapy techniques in all the patients with
non-small cell lung cancer. |
Table IV.
Treatment time and MU comparison
according to the radiotherapy techniques in all the patients with
non-small cell lung cancer.
|
|
|
| P-values of
MUs/treatment time comparisonsa |
|---|
|
|
|
|
|
|---|
| Technology | MUs | Treatment time,
sec | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|---|
| 3D-CRT | 367.62±47.73 | 216.56±4.77 |
<0.001/<0.001 |
<0.001/<0.001 |
0.002/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
| IMRT | 658.76±163.39 | 201.12±16.34 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
| h-IMRT | 533.36±51.95 | 258.92±5.19 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
| VMAT | 433.19±59.31 | 120.00±0.00 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
<0.001/<0.001 |
Comparison of the radiotherapy plans
in their respective subgroups
The radiotherapy plans were subsequently divided
into three subgroups, according to the type, volume and location of
the primary tumors. When the tumor was located on the left, the
total lung V5 was still observed to be the highest when
using VMAT as the technique (with the lowest V30 value
and the highest CI value) (P<0.05), whereas the advantage of
using h-IMRT as the technique was seen most clearly in terms of the
HI value (P<0.05). VMAT also was advantageous from the
perspective of the V40 and V45 parameters in
the heart. When the tumor was located on the right side, the MLD
was higher for the 3D-CRT and h-IMRT techniques, although not
statistically significant (P>0.05), while the lowest
V30 value was identified for VMAT (P<0.05). In terms
of cardiac dose, the V30 value was also found to be
advantageous with VMAT (P<0.05), as shown in Table V.
 | Table V.Organs-at-risk dose parameters
according to the radiotherapy techniques in the left lung and right
lung of patients with non-small cell lung cancer. |
Table V.
Organs-at-risk dose parameters
according to the radiotherapy techniques in the left lung and right
lung of patients with non-small cell lung cancer.
| A, Left |
|---|
|
|---|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|---|
|
|
|
|
|
|
|
|---|
| Dosimetric
parameter | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|---|
| PTV |
|
|
|
|
|
|
|
|
|
|
| CI | 0.499±0.104 | 0.797±0.060 | 0.585±0.112 | 0.869±0.022 | <0.001 | 0.020 | <0.001 | <0.001 | 0.045 | <0.001 |
| HI | 0.129±0.015 | 0.090±0.027 | 0.055±0.016 | 0.092±0.019 | <0.001 | <0.001 | <0.001 | <0.001 | 0.993 | <0.001 |
| Lung |
|
|
|
|
|
|
|
|
|
|
|
V5, % | 32.25±7.60 | 30.05±7.85 | 33.15±7.82 | 47.62±10.44 | 0.875 | 0.990 | <0.001 | 0.712 | <0.001 | <0.001 |
|
V30, % | 15.60±4.99 | 13.31±4.37 | 15.74±4.89 | 11.73±4.63 | 0.496 | >0.999 | 0.043 | 0.446 | 0.763 | 0.043 |
| Heart |
|
|
|
|
|
|
|
|
|
|
|
V40, % | 14.88±16.26 | 8.89±10.19 | 14.21±15.57 | 5.15±6.08 | 0.286 | 0.999 | 0.048 | 0.618 | 0.827 | 0.126 |
|
V45, % | 11.58±13.23 | 6.78±8.42 | 11.36±12.84 | 3.64±4.08 | 0.254 | 0.950 | 0.038 | 0.501 | 0.579 | 0.084 |
|
| B,
Right |
|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|
|
|
|
|
|
|
| Dosimetric
parameter | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|
| Lung |
|
|
|
|
|
|
|
|
|
|
| MLD,
Gy | 12.98±3.89 | 11.34±2.90 | 15.56±17.02 | 12.22±2.87 | 0.859 | 0.605 | 0.983 | 0.183 | 0.975 | 0.379 |
|
V30, % | 18.50±7.00 | 15.15±5.45 | 18.61±6.92 | 13.32±4.78 | 0.048 | >0.999 | 0.002 | 0.038 | 0.572 | 0.002 |
| Heart |
|
|
|
|
|
|
|
|
|
|
|
V30, % | 17.09±12.97 | 13.64±10.44 | 17.36±12.62 | 10.09±12.43 | 0.614 | 0.554 | 0.048 | 0.554 | 0.592 | 0.040 |
In central lung cancer, for the spinal cord, VMAT
was the technique associated with the lowest Dmax value
(P<0.05). No significant differences in Dmax were
identified in the esophagus (P>0.05), although VMAT was the
technique that had the highest mean dose (P<0.05). In peripheral
lung cancer, no significant differences were identified comparing
between VMAT and IMRT in terms of CI (P>0.05), although this
pair of techniques was better compared with the other two
radiotherapy methods (P<0.05). VMAT and IMRT showed great
advantages in terms of the cardiac dose, as shown in Table VI. When the tumor volume was large,
the value for lung V5 was highest for VMAT (P<0.05),
although the V13 value was found not to be statistically
significant (P>0.05). In terms of the cardiac dose, for
V40, VMAT and IMRT were shown to be more effective as
techniques compared with the others (P<0.05). When the tumor
volume was small, the lung V30 was found to be the
lowest with VMAT (P<0.05), although no statistically significant
differences were identified in terms of the MLD (P>0.05). For
the spinal cord, the Dmax values for the VMAT technique
were lower compared with those for 3D-CRT and h-IMRT, (P<0.05),
as shown in Table VII.
 | Table VI.Dose-volume histogram parameters for
PTV according to radiotherapy techniques in patients with centrally
located and peripherally located non-small cell lung cancer. |
Table VI.
Dose-volume histogram parameters for
PTV according to radiotherapy techniques in patients with centrally
located and peripherally located non-small cell lung cancer.
| A, Central |
|---|
|
|---|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|---|
|
|
|
|
|
|
|
|---|
| Dosimetric
parameter | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|---|
| Spinal cord |
|
|
|
|
|
|
|
|
|
|
|
Dmax, Gy | 42.12±9.69 | 38.34±6.74 | 41.96±8.83 | 34.93±8.72 | 0.201 | >0.999 | 0.001 | 0.237 | 0.288 | 0.002 |
| Esophagus |
|
|
|
|
|
|
|
|
|
|
|
Dmax, Gy | 51.89±13.86 | 49.00±16.52 | 51.09±13.57 | 52.35±11.50 | 0.792 | 0.994 | 0.999 | 0.909 | 0.706 | 0.977 |
| MED,
Gy | 24.88±14.51 | 22.18±13.10 | 24.75±14.16 | 29.21±10.31 | 0.794 | >0.999 | 0.455 | 0.818 | 0.038 | 0.428 |
|
| B,
Peripheral |
|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|
|
|
|
|
|
|
| Dosimetric
parameter | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|
| PTV |
|
|
|
|
|
|
|
|
|
|
| CI | 0.508±0.108 | 0.814±0.050 | 0.597±0.133 | 0.873±0.025 | <0.001 | 0.048 | <0.001 | <0.001 | 0.324 | <0.001 |
| Heart |
|
|
|
|
|
|
|
|
|
|
|
V30, % | 16.85±13.99 | 9.55±8.55 | 16.86±13.68 | 6.21±6.35 | 0.217 | >0.999 | 0.042 | 0.315 | 0.858 | 0.042 |
|
V40, % | 14.24±11.85 | 4.47±4.91 | 12.31±9.32 | 3.14±3.42 | 0.013 | 0.922 | 0.004 | 0.048 | 0.972 | 0.021 |
 | Table VII.Organs at risk dose parameters
according to radiotherapy techniques in patients with non-small
cell lung cancer of smaller and larger PTV. |
Table VII.
Organs at risk dose parameters
according to radiotherapy techniques in patients with non-small
cell lung cancer of smaller and larger PTV.
| A, PTV ≥315.6
cm3 |
|---|
|
|---|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|---|
|
|
|
|
|
|
|
|---|
| Dosimetric
parameters | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|---|
| Lung |
|
|
|
|
|
|
|
|
|
|
|
V5, % | 38.56±9.71 | 38.13±10.23 | 38.92±9.54 | 55.43±10.83 | 0.999 | 0.999 | <0.001 | 0.999 | <0.001 | <0.001 |
|
V13, % | 27.95±7.34 | 27.37±6.98 | 27.87±7.19 | 29.99±6.38 | 0.990 | >0.999 | 0.706 | 0.993 | 0.515 | 0.681 |
| Heart |
|
|
|
|
|
|
|
|
|
|
|
V40, % | 15.65±14.33 | 9.04±8.65 | 14.69±13.50 | 6.76±6.62 | 0.048 | 0.989 | 0.020 | 0.257 | 0.879 | 0.048 |
|
| B, PTV <315.6
cm3 |
|
|
|
|
|
|
| P-values of
subgroup comparisonsa |
|
|
|
|
|
|
|
| Dosimetric
parameters | 3D-CRT | IMRT | h-IMRT | VMAT | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 |
|
| Lung |
|
|
|
|
|
|
|
|
|
|
|
V30, % | 15.25±6.47 | 12.56±5.10 | 15.47±6.60 | 10.69±4.38 | 0.315 | 0.999 | 0.021 | 0.247 | 0.627 | 0.014 |
| MLD,
Gy | 12.51±7.61 | 9.98±6.07 | 11.87±7.36 | 9.20±5.53 | 0.168 | 0.727 | 0.072 | 0.302 | 0.670 | 0.146 |
| Heart |
|
|
|
|
|
|
|
|
|
|
|
V30, % | 16.85±13.33 | 12.38±10.21 | 16.91±13.20 | 6.23±5.42 | 0.446 | >0.999 | 0.003 | 0.454 | 0.089 | 0.003 |
| Spinal cord |
|
|
|
|
|
|
|
|
|
|
|
Dmax, Gy | 38.51±12.56 | 35.48±9.30 | 38.28±11.72 | 31.34±9.95 | 0.741 | >0.999 | 0.042 | 0.783 | 0.511 | 0.049 |
Discussion
The present study compared the dosimetric
characteristics and treatment efficiencies of four radiotherapy
techniques in patients with stage III NSCLC (and its subgroups). To
date, and to the best of our knowledge, this study is the first to
have examined the application of 3D-CRT, IMRT, h-IMRT and VMAT in
stage III NSCLC and its subgroups. Based on the findings of the
study, it was clear that all four techniques were capable of
meeting their clinical objectives, although each technique had its
own specific characteristics. The main findings to be derived from
the dosimetric comparisons among these four radiotherapy techniques
were as follows: i) Compared with 3D-CRT alone, adding 20% IMRT to
the 3D-CRT base plan led to an improvement in both CI and HI, with
increased MUs and treatment time as a tradeoff; ii) compared with
3D-CRT and h-IMRT, additional OAR sparing was possible with IMRT
and VMAT; and iii) VMAT is comparable with IMRT in numerous
respects, although it possessed an improved conformal coverage,
with lower MUs and a shorter treatment time.
Guillemin et al (28) found that, in NSCLC radiotherapy
treatment, compared with 3D-CRT as a technique, IMRT could improve
the coverage of PTV without increasing the dose to OARs, and when
the numbers of patients with dysphagia were counted following
radiotherapy, that in the IMRT arm was significantly decreased. The
present results were found to be similar to this previous study, as
in the present study, compared with those for 3D-CRT, the CI and HI
values in the PTV of IMRT were improved, and the lung
V30, heart V40 and MHD parameters were
significantly decreased. This led to the conclusion that, at our
radiotherapy center, IMRT was superior to 3D-CRT as a technique in
terms of decreasing the risk of developing dysphagia. Peng et
al (29) conducted a clinical
trial in 3,872 patients with stage III NSCLC to compare their
survival outcomes when using 3D-CRT, IMRT and VMAT, and it was
concluded that: i) Survival is not compromised in patients using
IMRT or VMAT; and ii) given their dosimetric advantages (e.g., in
improving the conformality of high-dose regions), IMRT and VMAT
would be recommended for treating patients with stage III NSCLC.
According to these findings, the present study attempted to
identify the optimal technique for performing optimal dosimetrics
in patients with stage III NSCLC at our radiotherapy center.
Jang et al (30) conducted dosimetric comparisons
between 3D-CRT and IMRT in 31 lung tumors, and found that lung dose
differences between these two techniques were mainly associated
with the size of the PTV, rather than location-associated
parameters. In the present study, all the patients were divided
into 3 subgroups according to the size of the PTV, the location of
the tumors and the tumor type. Livingston et al (31) found that, among 15 patients with
larger PTVs (mean PTV size, 501 cm3) compared with
3D-CRT, V20 and V5 were reduced by IMRT to
3.3 and 6.4%, respectively. The MLD could be reduced by 1.4 Gy,
whereas in 15 patients with smaller PTV (mean PTV size, 168
cm3), the differences in the dosimetric parameters were
not found to be significant. In the present study, in the group
with a larger PTV, it was found that when comparing IMRT with
3D-CRT, a decrease of only 1.7% was observed for lung
V20 with IMRT, while V5 only decreased by
1.1%. The MLD was reduced by 1.77 Gy, which was different from the
results identified in previous studies. The main reason for this
difference may be the different definitions of larger and smaller
tumors; for example, Livingston et al (31) set 501 cm3 for the cutoff,
whereas the cutoff was set to 310.5 cm3 in the present
study. In a study by Xu et al (7), all the patients were divided into
several groups according to the patients' characteristics. The
study found that VMAT had improved CI and HI values compared with
IMRT when the tumor was located in the center; however, when the
tumor was peripherally located, no statistically significant
differences in the OARs (lung, heart, spinal cord and esophagus)
were observed among the three techniques, although VMAT was still
slightly better than IMRT in terms of the CI and HI. In the present
study, in central lung cancer, VMAT was shown to be better than
IMRT in terms of the CI, but it was not as good as IMRT with
respect to HI. In peripheral lung cancer, VMAT was also better than
IMRT in terms of CI, but no statistically significant differences
were identified between the two techniques in terms of HI. The
reason for these differences, when comparing the present study with
that by Xu et al (7), may be
that the proportions of patients with peripheral lung cancer and
central lung cancer were different. Among the patients enrolled in
the present study, there were 8 patients with peripheral lung
cancer and 32 with central lung cancer, whereas in the study by Xu
et al (7), the total number
of patients enrolled was only 30, and the proportions with
peripheral lung cancer or central lung cancer were unknown. Li
et al (13), when studying
the application of VMAT and IMRT in peripheral lung cancer and
central lung cancer, respectively, found that different types of
patients required different radiotherapy techniques. In peripheral
lung cancer, the V5 value was found to be lower in
half-arc VMAT compared with that in IMRT, and the V30 in
IMRT was lower compared with that in VMAT. In central lung cancer
with a PTV that did not include the mediastinum, increased values
for CI and HI were observed with single-arc VMAT compared with the
values with IMRT, and V30 and V5 were found
to be lower with VMAT compared with those with IMRT. In central
lung cancer with PTV including the mediastinum, the CI and HI
parameters were improved when using two-half-arc VMAT, but when
using double-half-arc VMAT, the V30 and V5
values were higher compared with those when using IMRT. The results
from the present study showed that there were no significant
differences in the V30 value comparing between the two
radiotherapy techniques (VMAT and IMRT) whether central or
peripheral lung cancer was under consideration, whereas VMAT was
always found to be better than IMRT in terms of the V5
value. The study by Li et al (13) did classify central lung cancer
again, i.e., i) a PTV that did not include the mediastinum; ii) a
PTV that included the mediastinum, which may provide the reason for
the different results.
In the RTOG 0617 trial, albeit with some caveats,
the survival benefits were offset by the toxicities associated with
the chemoradiation. In the trial, a dose of 60 Gy was found to be
superior to 74 Gy in terms of the survival rate (32). Cardiac injury, pneumonia,
esophagitis and myelitis caused by radiation therapy may be the
most important causes underlying this phenomenon.
Radiation pneumonia is an important factor that
threatens the prognosis of patients with stage III NSCLC. Patients
with severe radiation pneumonia are not usually responsive to
strict antibacterial treatment, respiratory support treatment or
high-dose corticosteroid treatment. Pneumonia-associated deaths may
provide the main reason for the poor efficacy of radiotherapy in
patients with stage III lung cancer (33). In conventional fractionated
radiotherapy, dosimetric parameters have been used to predict the
probability of pneumonia to a certain extent. In order to limit the
incidence of pneumonia, a number of researchers have put forward
their own views on the lung dose limit for radiotherapy (34–36).
Grambozov et al (37) found
that radiotherapy for stage III NSCLC led to a decline in pulmonary
function (PF). The study concluded that patients with a total lung
V20 <21% were at a low risk of PF decrease after
high-dose irradiation treatment.
Based on the above studies, the parameters of lung
V5, V13, V20, V30 and
MLD were selected as the criteria for evaluating the lung dose in
the present study. Zhang et al (8) found that the lung V5 and
V10 values obtained using VMAT were significantly higher
compared with those found when using IMRT as the technique. In the
present study, lung V5 was found to be the highest with
VMAT, especially for patients with a central tumor type and larger
PTV (53.59±10.51 and 55.43±10.8, respectively); however, in
peripherally located cancer, this parameter was small (46.06±9.88).
Although V5 was still larger with VMAT than that with
the other techniques, its value remained within an acceptable
range. Therefore, the actual probability of radiation pneumonia
caused by use of the VMAT technique in peripheral lung cancer may
be lower than that associated with central tumor type and larger
PTV. The advantage of using VMAT over the other three techniques is
focused on the medium and high (V30) dose values
associated with the total lung and ipsilateral lung, although it
must be considered whether it is reasonable to reduce the medium-
and high-dose areas in the lung at the expense of increasing the
low-dose area in the lung. This question requires further research
and discussion. In certain study, it has been shown that the use of
IMRT as the technique also leads to an increase in the low-dose
area (38). However, in the present
study, IMRT, as a technique, was not shown to differ significantly
from 3D-CRT or h-IMRT in terms of the total lung V5. The
possible reason for this finding was that the IMRT technology in
the present study used the 5-field technique. The more radiation
fields that are used, the larger the irradiation area will be. In
this way, the low-dose area will inevitably increase; moreover, the
present study also aimed to avoid lung tissue while selecting the
field angle. At the same time, it was found that, for the total
patients, the MLD for h-IMRT was higher compared with that for the
other techniques, although not statistically significant, this
suggests that the selection of h-IMRT should be made with certain
caution in these patients.
In the process of considering the most appropriate
radiotherapy treatment for patients with NSCLC, the heart is an
organ that requires special attention. Due to the short survival
time of patients with stage III lung cancer and the late occurrence
of radiation cardiotoxicity, there is a scarcity of data, and few
published studies are available on the cardiotoxicity of
radiotherapy in such patients. A previous study by Atkins et
al (39) did find that the
mortality of patients increased with an increase of the mean
cardiac dose above 10 Gy in patients without statin, but no
association between patient mortality and an increase of the mean
cardiac dose above 10 Gy was identified in patients who were taking
statin. The mean cardiac dose of patients in the present study was
>10 Gy in the whole group and in all subgroups, and the mean
dose was the highest when using 3D-CRT for the total patients, but
lower when using the VMAT and IMRT techniques. Further analysis of
each subgroup found that this trend also existed in the subgroup
with central lung cancer. Therefore, in terms of the mean cardiac
dose, either the VMAT or the IMRT technique appears to be the
better option for selection for patients with poor cardiac
function. Moreover, in the smaller PTV subgroup, the use of VMAT
had a greater potential for reducing the parameter of cardiac
V30, suggesting that it may be advantageous to use VMAT
in the subgroup with a smaller PTV for the purpose of protecting
the heart. Therefore, we consider that if patients have poor
cardiac function (such as myocardial infarction and ischemic heart
disease), it would be advisable to use VMAT instead of 3D-CRT and
h-IMRT, especially for patients with a smaller PTV.
Treatment-associated esophagitis is also a common
disease in radiotherapy for lung cancer. Grade 3 and higher
radiation-induced esophagitis will have a negative effect on the
patients' long-term survival (40).
In order to predict the probability of radiation esophagitis, the
three parameters of esophageal V50, Dmax and
Dmean were selected as the prediction reference values
in the present study. Compared with the other techniques, IMRT was
associated with a significant reduction in the esophageal
V50 value. This trend existed in the subgroup with large
PTV volume, although no statistically significant differences were
identified in the other subgroups. The dose parameter of
Dmean was found to be the highest with the VMAT
technique. This trend was also reflected in the subgroups where the
tumor was the central type, and for the larger PTV. Therefore, when
considering the treatment strategy for esophageal injury, using
IMRT would be the preferred option.
In determining which radiotherapy option would be
most suitable for patients with lung cancer, the probability of
spinal cord-associated side effects occurring is small; however,
spinal cord injuries caused by radiotherapy can be serious. Since
the spinal cord is a serial organ, if any part of the spinal cord
is irradiated beyond the dose limit, the function of the whole
spinal cord will be lost, thereby leading to paralysis (41). On this basis, in the present study,
only the parameter Dmax was selected in order to
evaluate the spinal cord dose. None of the four radiotherapy
techniques exceeded the maximum dose limit of the spinal cord.
Therefore, at least from this analysis, it was possible to conclude
that the four radiotherapy techniques were essentially safe to use.
On the basis of meeting the spinal cord dose limits, it was found
that the VMAT technology was capable of sustaining a reduced spinal
cord dose compared with the other techniques for the total
patients; looking at the subgroup analysis more specifically, this
trend was also found to exist in patients with the tumor located on
the right, in those with centrally located lung cancer and in
patients with a smaller PTV.
A previous study (42) reported that a significant reduction
in the number of MUs helps to minimize the systemic integrated
dose, thereby reducing the risk of radiation-induced carcinogenesis
and secondary cancer, especially in patients with long-term
survival times. In the present study, it was found that IMRT was
the technique that was associated with the highest number of MUs,
whereas 3D-CRT had the lowest number. Therefore, it is preferable
to select a plan featuring a lower number of MUs during
radiotherapy for younger patients. However, if other aspects can
meet the dose limit, then a plan with fewer MUs would be preferred
in order to reduce the probability of a secondary tumor. Treatment
time is also an index that is of concern for patients and medical
practitioners. Reducing the treatment time would not only improve
the patients' comfort and satisfaction, but it would also reduce
the positioning error in treatment. In the present study, it was
calculated that the treatment time using the VMAT technique was the
shortest, whereas the treatment time using h-IMRT was the longest.
Therefore, we consider that, if the patient's physical condition is
not good and they cannot tolerate being treated over a long period
of time, then VMAT, with its relatively short treatment time and
fewer MUs, should be preferred as the treatment option.
However, the present study did have certain
limitations. Firstly, this study was only a retrospective study.
Secondly, our patient population was relatively small, which may
have resulted in an inability to determine efficacies with a high
degree of confidence. Finally, in the present study, the planning
strategies, optimization algorithms and field angles would all have
affected the final dose parameter results. In the process of
implementing the aforementioned radiation therapy techniques, if
auxiliary equipment could be integrated into the procedures, then
the resultant values for the radiation therapy parameters may be
improved. For example, during the implementation of chest radiation
therapy, deep inspiration breath hold (DIBH) has been shown to have
dosimetric advantages in terms of reducing excessive lung exposure
and lung risk factors. This method can also reduce cardiac
exposure, although, at the same time, it incurs additional costs
and is difficult to implement for the patients (43,44).
Due to the lack of such equipment at our radiation therapy center,
this technology could not be used. Future studies should ideally
analyze the application of DIBH in these four radiotherapy
techniques.
In conclusion, the present study showed that,
compared with use of 3D-CRT alone, adding 20% IMRT to the 3D-CRT
base plan can improve the quality of the plan. Compared with 3D-CRT
and h-IMRT, using IMRT and VMAT as the treatment options provided
better dose coverage and sparing of OARs; moreover, for patients in
whom the lung V5 can be kept low enough, VMAT is a good
alternative, offering more possibilities for sparing of other OARs
and decreasing the treatment time and MUs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and ZC were responsible for the design and
conception of the study. CL, HL, WS, YH and JL collected patient
data, analyzed and interpreted the data and collaborated in the
discussion. CL prepared the manuscript and ZC revised it critically
for important intellectual content. ZC supervised the study. All
authors have read and approved the final manuscript. CL, ZC and HL
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Yangtze University (Jingzhou, China) approved the study
(approval no. KY202018). Since this is a retrospective analysis,
written consent for participation was not required. All the data
relating to the patients was anonymized to protect their privacy.
All the methods used were in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conibear J; AstraZeneca UK Limited, :
Rationale for concurrent chemoradiotherapy for patients with stage
III non-small-cell lung cancer. Br J Cancer. 123 (Suppl 1):S10–S17.
2020. View Article : Google Scholar
|
|
3
|
Punnett G, Fenemore J, Blackhall F and
Yorke J: Support and information needs for patients with non-small
cell lung cancer receiving concurrent chemo-radiotherapy treatment
with curative intent: Findings from a qualitative study. Eur J
Oncol Nurs. 64:1023252023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kao J, Farrugia MK, Frontario S, Zucker A,
Copel E, Loscalzo J, Sangal A, Darakchiev B, Singh A and Missios S:
Association of radiation dose intensity with overall survival in
patients with distant metastases. Cancer Med. 10:7934–7942. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alterio D, Gugliandolo SG, Augugliaro M,
Marvaso G, Gandini S, Bellerba F, Russell-Edu SW, Simone ID,
Cinquini M, Starzyńska A, et al: IMRT versus 2D/3D conformal RT in
oropharyngeal cancer: A review of the literature and meta-analysis.
Oral Dis. 27:1644–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faye MD and Alfieri J: Advances in
radiation oncology for the treatment of cervical cancer. Curr
Oncol. 29:928–944. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Deng W, Yang S, Li P, Kong Y, Tian
Y, Liao Z and Chen M: Dosimetric comparison of the helical
tomotherapy, volumetric-modulated arc therapy and fixed-field
intensity-modulated radiotherapy for stage IIB-IIIB non-small cell
lung cancer. Sci Rep. 7:148632017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Han A, Fu Z, Xu S and Zhang Z:
The dosimetric comparisons of CRT, IMRT, ARC, CRT+IMRT, and CRT+ARC
of postoperative radiotherapy in IIIA-N2 stage non-small-cell lung
cancer patients. Biomed Res Int. 2019:89892412019.PubMed/NCBI
|
|
9
|
Shrimali R, Chakraborty S, Bhattacharyya
T, Mallick I, Achari RB, Prasath S, Arun B, Mahata A, Shree MV,
Vishnupriya E and Chatterjee S: Development and validation of a
decision support tool to select IMRT as radiotherapy treatment
planning modality for patients with locoregionally advanced
non-small cell lung cancers (NSCLC). Br J Radiol. 92:201804312019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Truntzer P, Antoni D, Santelmo N,
Schumacher C, Falcoz PE, Quoix E, Massard G and Noël G: Superior
sulcus non-small cell lung carcinoma: A comparison of IMRT and
3D-RT dosimetry. Rep Pract Oncol Radiother. 21:427–434. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khalil AA, Hoffmann L, Moeller DS, Farr KP
and Knap MM: New dose constraint reduces radiation-induced fatal
pneumonitis in locally advanced non-small cell lung cancer patients
treated with intensity-modulated radiotherapy. Acta Oncol.
54:1343–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otto K: Volumetric modulated arc therapy:
IMRT in a single gantry arc. Med Phys. 35:310–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Wang J, Tan L, Hui B, Ma X, Yan Y,
Xue C, Shi X, Drokow EK and Ren J: Dosimetric comparison between
IMRT and VMAT in irradiation for peripheral and central lung
cancer. Oncol Lett. 15:3735–3745. 2018.PubMed/NCBI
|
|
14
|
Pokhrel D, Sanford L, Halfman M and Molloy
J: Potential reduction of lung dose via VMAT with jaw tracking in
the treatment of single-isocenter/two-lesion lung SBRT. J Appl Clin
Med Phys. 20:55–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iqbal MS, Richmond N, Ogilvie A, Pilling
K, Willis N, Byrne J, Walker C and West N: Dosimetric evaluation of
VMAT for palliative radiotherapy for non-small cell lung carcinoma.
Br J Radiol. 91:201801462018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osborn J: Is VMAT beneficial for patients
undergoing radiotherapy to the head and neck? Radiography (Lond).
23:73–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunte SO, Clark CH, Zyuzikov N and Nisbet
A: Volumetric modulated arc therapy (VMAT): A review of clinical
outcomes-what is the clinical evidence for the most effective
implementation? Br J Radiol. 95:202012892022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verbakel WF, van Reij E, Ladenius-Lischer
I, Cuijpers JP, Slotman BJ and Senan S: Clinical application of a
novel hybrid intensity-modulated radiotherapy technique for stage
III lung cancer and dosimetric comparison with four other
techniques. Int J Radiat Oncol Biol Phys. 83:e297–e303. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hodapp N: The ICRU Report 83: Prescribing,
recording and reporting photon-beam intensity-modulated radiation
therapy (IMRT). Strahlenther Onkol. 188:97–99. 2012.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haslett K, Bayman N, Franks K, Groom N,
Harden SV, Harris C, Hanna G, Harrow S, Hatton M, McCloskey P, et
al: Isotoxic intensity modulated radiation therapy in stage III
non-small cell lung cancer: A feasibility study. Int J Radiat Oncol
Biol Phys. 109:1341–1348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma C, Tian Z, Wang R, Feng Z, Jiang F, Hu
Q, Yang F, Shi A and Wu H: A prediction model for dosimetric-based
lung adaptive radiotherapy. Med Phys. 49:6319–6333. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JP, Dewalt J, Feldman A, Adil K,
Movsas B and Chetty IJ: Feasibility of radical cardiac-sparing,
treatment planning strategies for patients with locally advanced,
non-small cell lung cancer. J Appl Clin Med Phys. 23:e137842022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKenzie E, Zhang S, Zakariaee R, Guthier
CV, Hakimian B, Mirhadi A, Kamrava M, Padda SK, Lewis JH, Nikolova
A, et al: Left anterior descending coronary artery radiation dose
association with all-cause mortality in NRG oncology trial RTOG
0617. Int J Radiat Oncol Biol Phys. 115:1138–1143. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kataria T, Sharma K, Subramani V,
Karrthick KP and Bisht SS: Homogeneity Index: An objective tool for
assessment of conformal radiation treatments. J Med Phys.
37:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paddick I: A simple scoring ratio to index
the conformity of radiosurgical treatment plans. Technical note. J
Neurosurg. 93 (Suppl 3):S219–S222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guillemin F, Berger L, Lapeyre M and
Bellière-Calandry A: Dosimetric and toxicity comparison of IMRT and
3D-CRT of non-small cell lung cancer. Cancer Radiother. 25:747–754.
2021.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng J, Pond G, Donovan E, Ellis PM and
Swaminath A: A comparison of radiation techniques in patients
treated with concurrent chemoradiation for stage III non-small cell
lung cancer. Int J Radiat Oncol Biol Phys. 106:985–992. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang SS, Shin Y, Park SY, Huh GJ and Yang
YJ: Impact of tumor size and location on lung dose difference
between stereotactic body radiation therapy techniques for
non-small cell lung cancer. Thorac Cancer. 12:3310–3318. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livingston GC, Last AJ, Shakespeare TP,
Dwyer PM, Westhuyzen J, McKay MJ, Connors L, Leader S and Greenham
S: Toxicity and dosimetric analysis of non-small cell lung cancer
patients undergoing radiotherapy with 4DCT and image-guided
intensity modulated radiotherapy: A regional centre's experience. J
Med Radiat Sci. 63:170–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bradley JD, Hu C, Komaki RR, Masters GA,
Blumenschein GR, Schild SE, Bogart JA, Forster KM, Magliocco AM,
Kavadi VS, et al: Long-term results of NRG oncology RTOG 0617:
Standard- Versus high-dose chemoradiotherapy with or without
cetuximab for unresectable stage III non-small-cell lung cancer. J
Clin Oncol. 38:706–714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bradley JD, Paulus R, Komaki RR, Masters
G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco
A, et al: Standard-dose versus high-dose conformal radiotherapy
with concurrent and consolidation carboplatin plus paclitaxel with
or without cetuximab for patients with stage IIIA or IIIB
non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boonyawan K, Gomez DR, Komaki R, Xu Y,
Nantavithya C, Allen PK, Mohan R and Liao Z: Clinical and
dosimetric factors predicting grade ≥2 radiation pneumonitis after
postoperative radiotherapy for patients with non-small cell lung
carcinoma. Int J Radiat Oncol Biol Phys. 101:919–926. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tonison JJ, Fischer SG, Viehrig M, Welz S,
Boeke S, Zwirner K, Klumpp B, Braun LH, Zips D and Gani C:
Radiation pneumonitis after intensity-modulated radiotherapy for
esophageal cancer: Institutional data and a systematic review. Sci
Rep. 9:22552019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lewis GD, Agrusa JE, Teh BS, Gramatges MM,
Kothari V, Allen CE and Paulino AC: Radiation pneumonitis in
pediatric Hodgkin lymphoma patients receiving radiation therapy to
the chest. Pract Radiat Oncol. 8:e364–e368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grambozov B, Wolf F, Kaiser J, Wass R,
Fastner G, Gaisberger C, Rettenbacher L, Studnicka M, Pirich C,
Sedlmayer F and Zehentmayr F: Pulmonary function decreases
moderately after accelerated high-dose irradiation for stage III
non-small cell lung cancer. Thorac Cancer. 11:369–378. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang JY: Intensity-modulated
radiotherapy, not 3 dimensional conformal, is the preferred
technique for treating locally advanced lung cancer. Semin Radiat
Oncol. 25:110–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Atkins KM, Bitterman DS, Chaunzwa TL,
Williams CL, Rahman R, Kozono DE, Baldini EH, Aerts HJW, Tamarappoo
BK, Hoffmann U, et al: Statin use, heart radiation dose, and
survival in locally advanced lung cancer. Pract Radiat Oncol.
11:e459–e467. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Łazar-Poniatowska M, Kamińska J, Konopa K,
Dziadziuszko R and Jassem J: Contralateral esophageal sparing
technique in definitive radiotherapy for non-small cell lung
cancer: Dosimetric parameters and normal tissue complication
probability modeling. Rep Pract Oncol Radiother. 27:933–942. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schultheiss TE, Kun LE, Ang KK and
Stephens LC: Radiation response of the central nervous system. Int
J Radiat Oncol Biol Phys. 31:1093–1112. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sakthivel V, Mani GK, Mani S and Boopathy
R: Radiation-induced second cancer risk from external beam photon
radiotherapy for head and neck cancer: Impact on in-field and
out-of-field organs. Asian Pac J Cancer Prev. 18:1897–1903.
2017.PubMed/NCBI
|
|
43
|
Fjellanger K, Rossi L, Heijmen BJM,
Pettersen HES, Sandvik IM, Breedveld S, Sulen TH and Hysing LB:
Patient selection, inter-fraction plan robustness and reduction of
toxicity risk with deep inspiration breath hold in
intensity-modulated radiotherapy of locally advanced non-small cell
lung cancer. Front Oncol. 12:9661342022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guberina M, Santiago A, Pöttgen C,
Indenkämpen F, Lübcke W, Qamhiyeh S, Gauler T, Hoffmann C, Guberina
N and Stuschke M: Respiration-controlled radiotherapy in lung
cancer: Systematic evaluation of the optimal application practice.
Clin Transl Radiat Oncol. 40:1006282023. View Article : Google Scholar : PubMed/NCBI
|