Introduction
Meningiomas are a group of tumors of the central
nervous system (CNS) of meningothelial origin, and are the most
frequently histologically diagnosed of all CNS tumors, accounting
for ~36% of all CNS tumors (1). The
5th edition of the World Health Organization (WHO) classification
of CNS tumors describes 15 histological subtypes of meningiomas and
3 prognosis grades (2). The
majority of meningiomas are histologically benign (WHO Grade 1
2021) (1); 20–25% show atypical
features (WHO Grade 2 2021); and the morphological parameters of
1–6% of meningiomas are associated with a less favorable clinical
outcome and correspond to anaplastic tumor (WHO Grade 3 2021)
(3–5). The morphological grade system
describes the likelihood of recurrence. The frequency of recurrence
of benign meningiomas reaches a quarter (7–25%), atypical, just
over half (29–52%), and anaplastic (or malignant) meningiomas recur
with a frequency ranging from 50–94% of cases (6).
CNS metastases are a group of tumors with a source
of origin outside the CNS and a hematogenous route of spread; the
true occurrence rate of which has not been determined and is
probably underestimated (7). It is
hypothesized that ~30% of adult and 6–10% of pediatric cancer
patients suffer from a secondary brain tumor (8). Tumors and their molecular subtypes
differ in their propensity to metastasize to the CNS; the most
common source of brain metastases is lung cancer (most often
adenocarcinoma), followed by breast cancer, melanoma, renal cell
carcinoma, and colorectal cancer (9). Significant prognostic factors for
patients with intracranial metastases are age, Karnofsky index,
number of metastatic lesions, and severity of extracranial
involvement (10).
The vast majority of meningiomas have a meningeal
localization. Rarely, meningiomas may be intraventricular,
epidural, or even occur outside the CNS; >90% of meningiomas are
solitary (11); whereas
intracranial localization of intracranial metastases [solitary
intracranial dural metastases (IDM)] occurs in 8–9% of cases
(12). A total of ~50% of the
metastases are designated as single (located only in the CNS), and
even fewer as solitary (one metastatic lesion in the whole body)
(13).
It is usually safer to confidently assume a
diagnosis of meningioma, even with routine magnetic resonance
imaging (MRI). In the majority of cases, the task of distinguishing
metastases from other types of tumors is typically straightforward.
Both diagnoses are not uncommon in adult patients and, as a rule,
every clinician/radiologist is familiar with typical MRI findings.
Despite this, in the practice of an experienced physician, there is
occasionally a situation where the differential diagnosis between
these tumors is ambiguous, as intracranial meningiomas and IDM may
have similar imaging characteristics; namely, a cavity-less solid
structure, limited diffusion of water molecules, and the presence
of extensive peritumoral edema. The variety of sources of
metastases determines the variability of the cellular composition
and radiological manifestations of the tumor lesion, therefore, the
absence of characteristic of meningiomas (calcifications, ‘spoke
wheel’, enostotic spine, and ‘dural tail’) or metastasis (necrosis
cavity, hemorrhages, and large vessels) does not allow to reliably
exclude one option or the other (12,14).
In addition, a number of neoplastic lesions of the
meninges other than intracranial IDM such as mesenchymal tumors
[solitary fibrous tumors, hemangiopericytoma, smooth muscle tumors
(leiomyoma and leiomyosarcoma), epithelioid hemangioendothelioma
(EH), and peripheral primitive neuroectodermal tumor-Ewing sarcoma
(pPNET-ES)], intradural chordoma, leptomeningeal medulloblastoma,
melanocytic tumors, and hemangioblastoma may mimic meningioma
radiologically and even show the characteristic ‘dural tail’ of
this tumor (15,16). The clinical features of some of
these tumors in terms of age and sex distribution can be misleading
in making a diagnosis of meningioma. For example, EH is a rare
vascular soft tissue tumor with biological behavior intermediate
between malignant angiosarcoma and benign angioma. CNS involvement
is rare, with a total of 37 cases described in the literature.
Unlike soft tissue EH, intracranial EH affects young children.
Intracranial EH may present as an extra-axial meningeal lesion
showing a cystic appearance and contrast enhancement on MRI, which
may mimic a meningioma (15,17,18).
Or a tumor such as meningeal melanocytoma, which is a low-grade
solitary benign tumor that occurs at any age from 9 to 73 years,
but most often in the fifth decade with a slight female
predominance. On MRI, a melanocytoma appears as a
well-circumscribed, hyperintense extra-axial mass with homogeneous
contrast enhancement mimicking a meningioma. Therefore, although
MRI is an integral part of the diagnosis of CNS tumors,
histological examination of the tumor is of fundamental importance
for confirming or refuting the diagnosis of meningioma. However,
some of them also histologically resemble the various histotypes of
meningiomas (15,19).
Since at the stage of differential diagnosis, the
clinician/radiologist may not always have comprehensive data on the
anamnesis of the disease (moreover, in 10% of cases in patients
with brain metastases, the primary tumor was not detected at the
time of presentation) (9) and/or
previous studies, in the present study, it was attempted to
identify reliable criteria for distinguishing between intracranial
meningiomas and IDM with similar radiological presentations using
multiparametric MRI (mpMRI).
Materials and methods
Patients
The present study was approved by the Local Ethical
Committee of the Federal Center of Neurosurgery (Tyumen, Russian
Federation). Written informed consent for diagnostic analysis was
obtained from all patients. The total number of patients was 100
(50 patients with intracranial meningiomas and 50 patients with
IDM). The age of the patients ranged from 32–82 years and the
median was 58 years [Note: The value of age in the present study
can help in the differential diagnosis (see in Introduction), and
therefore only this parameter is indicated for patients in this
study, and other parameters may not affect performance].
Among the patients diagnosed with meningioma, the
most common histological subtype was meningothelial meningioma, WHO
Grade 1; 50% (n=25). The second most common subtype was mixed
meningioma, WHO Grade 1–24% (n=12). Atypical form (WHO Grade 2) was
diagnosed in 20% (n=10) of patients. In 4% (n=2) and 2% (n=1) of
cases, psammomatous and secretory forms of meningiomas were
identified (WHO Grade 2). The sex distribution of patients with
meningiomas was 76.5% (n=38) female and 23.5% (n=12) male [Note:
The value of sex in this study can help in the differential
diagnosis (see in Introduction), and therefore only this parameter
is indicated for patients in this study, and other parameters may
not affect performance].
The group of patients with IDM from primary foci of
different localization was 50 patients. Metastases with a primary
focus from the breast were diagnosed in 38% (n=19) of patients,
from the lungs in 34% (n=17), from the kidneys in 12% (n=6), from
the prostate in 10% (n=5), and the remaining metastases, 6% (n=3),
from the ovaries and large intestine. The sex of patients diagnosed
with IDM was 58% (n=29) female and 42% (n=21) male. Examples of
MRIs of patients with intracranial meningiomas and patients with
IDM are shown in Fig. 1, Fig. 2, Fig.
3, Fig. 4.
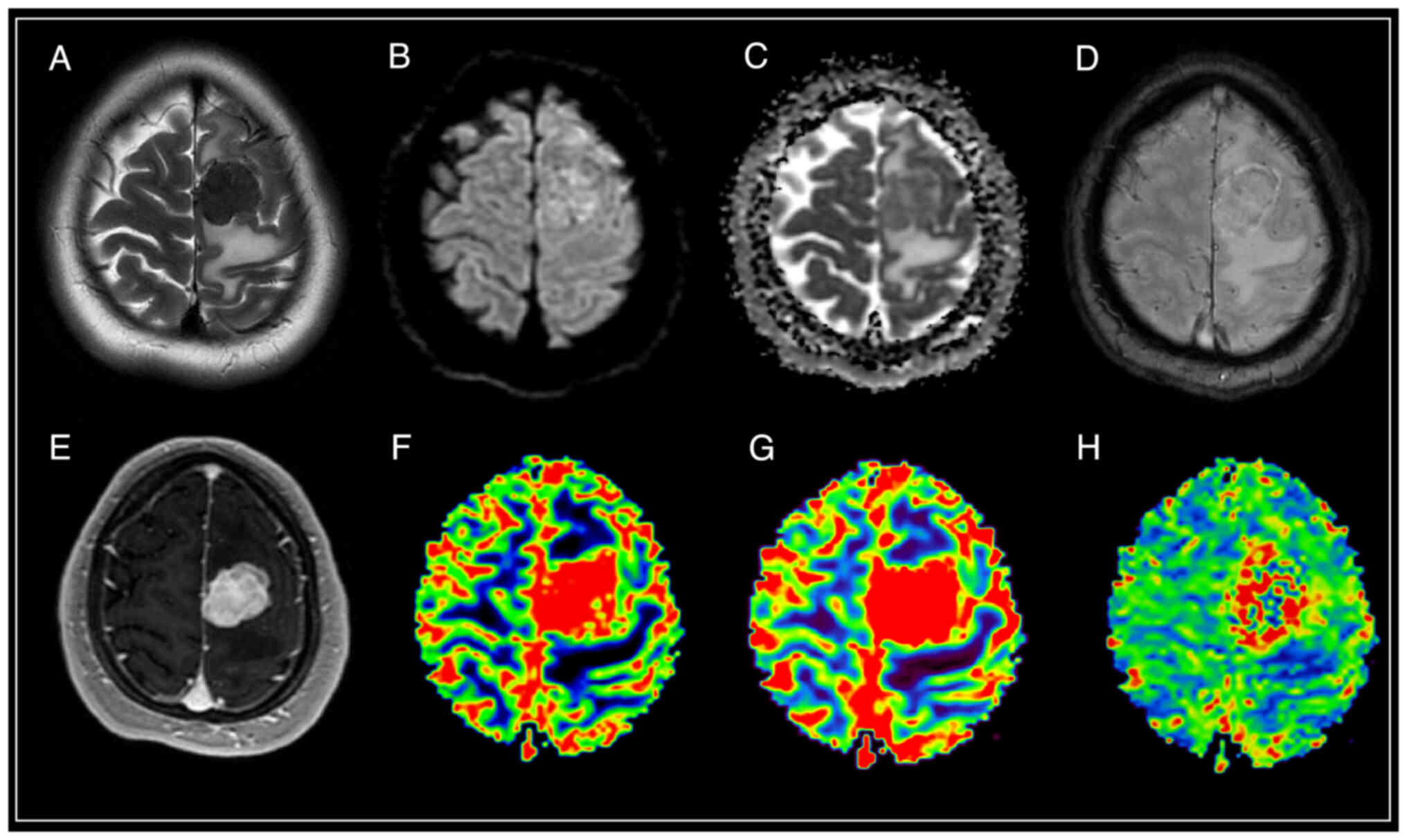 | Figure 1.Brain magnetic resonance imaging of a
patient with meningothelial meningioma (WHO Grade 1).
Supratentorially, in the left hemisphere of the frontal region,
against the background of moderate vasogenic edema, a clearly
demarcated extracerebral mass is visible, characterized by a
hypointense signal on T2-WI, intense and homogeneous accumulation
of a contrast agent, diffusion restriction with corresponding areas
of increased values of volumetric and velocity cerebral blood flow,
as well as prolongation of blood transit time. SWAN indicates the
presence of cortical draining veins in the formation structure. (A)
T2-WI; (B and C) DWI and ADC; (D) SWAN; (E) T1-WI with contrast;
(F) CBV; (G) CBF; and (H) MTT. SWAN, susceptibility-weighted
angiography; T1-WI, T1-weighted image; T2-WI, T2-weighted image;
DWI, diffusion-weighted imaging; ADC, apparent diffusion
coefficient; CBV, cerebral blood volume; CBF, cerebral blood flow;
MTT, mean transit time. |
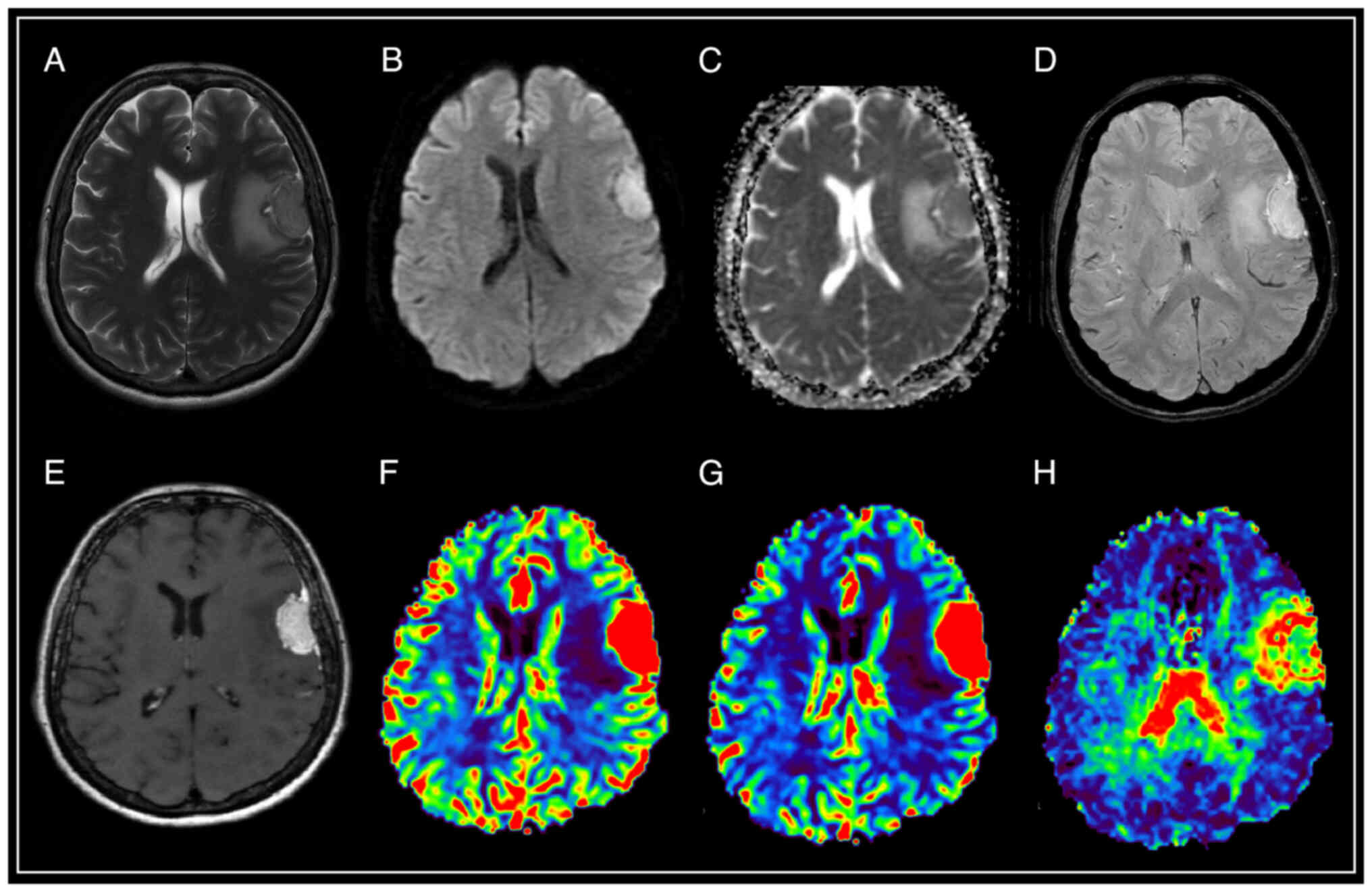
 | Figure 2.Brain magnetic resonance imaging with
solitary intracranial dural metastasis of lung adenocarcinoma. In
the parasagittal parts of the left frontal area, there is an
extracerebral mass with a base on the falciform process of the dura
mater, surrounded by a moderately pronounced zone of perifocal
edema, relatively homogeneously accumulating contrast throughout
the volume, with limited diffusion according to DWI and ADC, the
presence of artifacts from blood decay products in the tumor
structure, as well as high values of rCBV and rCBF, and lengthening
of MTT. (A) T2-WI; (B and C) DWI and ADC; (D) SWAN; (E) T1-WI with
contrast; (F) CBV; (G) CBF; and (H) MTT. SWAN,
susceptibility-weighted angiography; T1-WI, T1-weighted image;
T2-WI, T2-weighted image; DWI, diffusion-weighted imaging; ADC,
apparent diffusion coefficient; CBV, cerebral blood volume; CBF,
cerebral blood flow; r, relative; MTT, mean transit time. |
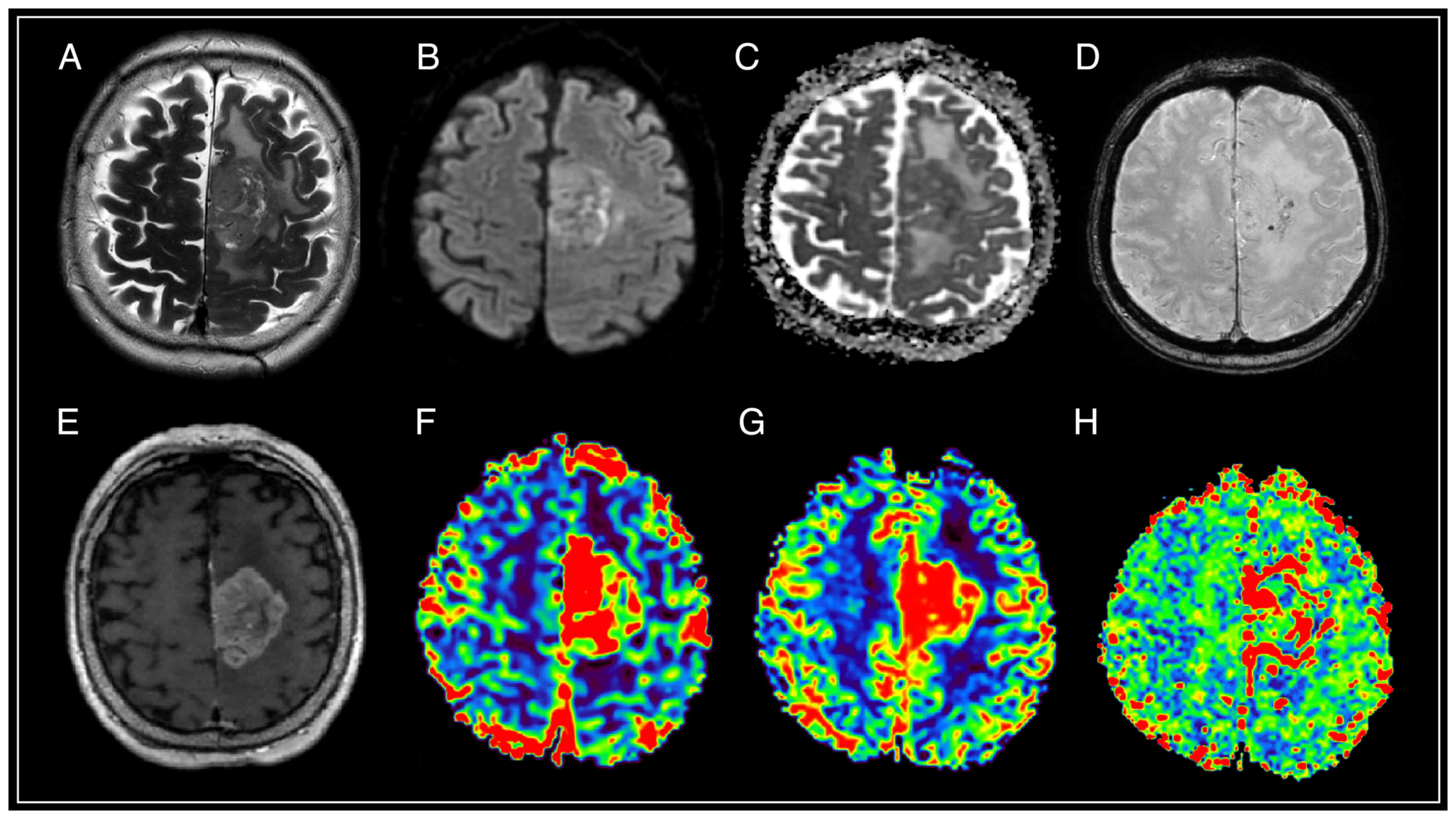
 | Figure 3.Brain magnetic resonance imaging of a
patient with atypical meningioma (WHO Grade 2). In the frontal
region of the left hemisphere, against the background of perifocal
edema, an extracerebral tumor is visible with an intense and
homogeneous accumulation of a contrast agent, the ‘dural tail’
phenomenon, diffusion limitation, an increase in volumetric and
velocity cerebral blood flow, and a prolongation of blood transit
time. The SWAN demonstrates the presence of peripheral draining
veins around the mass. (A) T2-WI; (B and C) DWI and ADC; (D) SWAN;
(E) T1-WI with contrast; (F) CBV; (G) CBF; and (H) MTT. SWAN,
susceptibility-weighted angiography; T1-WI, T1-weighted image;
T2-WI, T2-weighted image; DWI, diffusion-weighted imaging; ADC,
apparent diffusion coefficient; CBV, cerebral blood volume; CBF,
cerebral blood flow; MTT, mean transit time. |
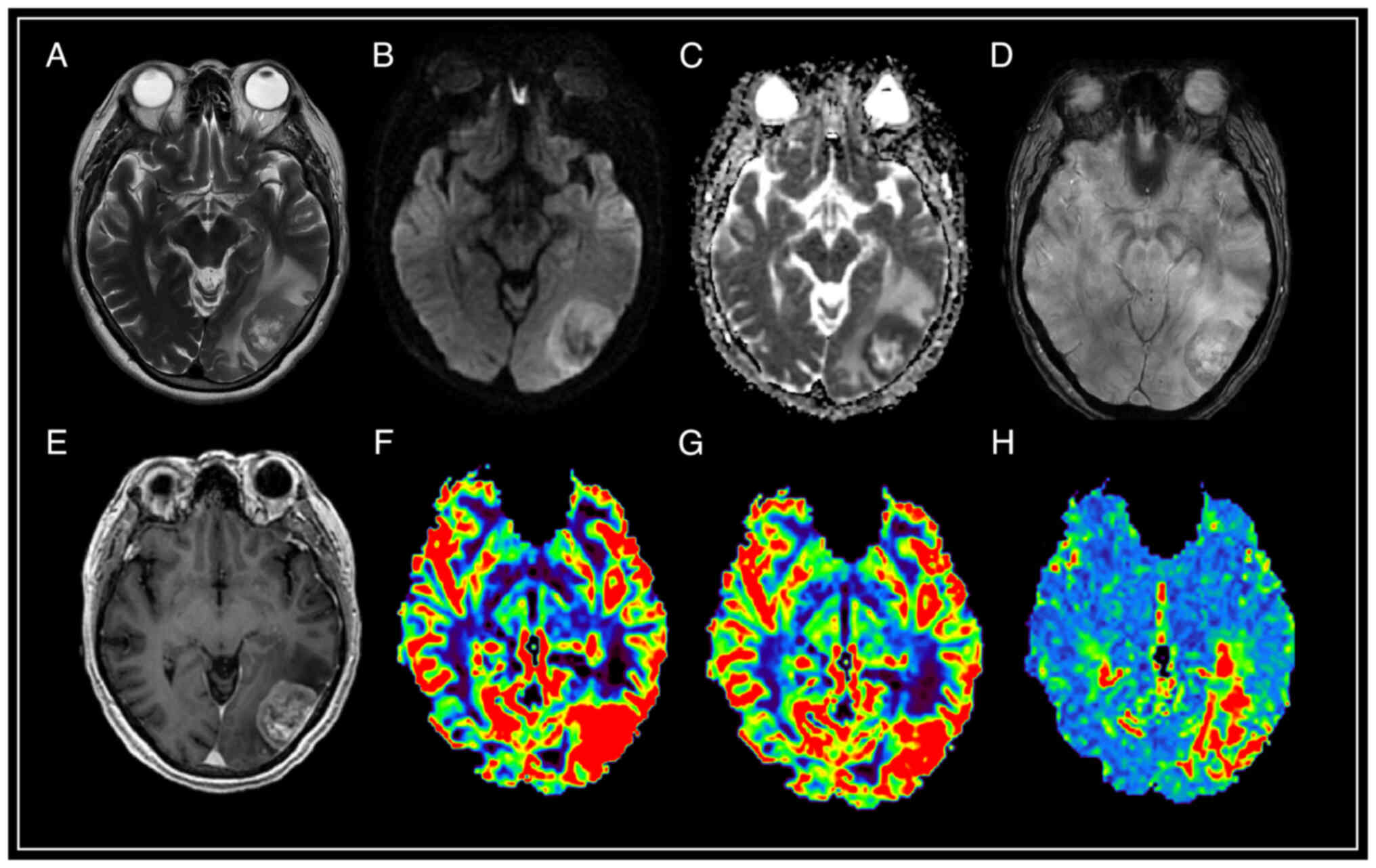 | Figure 4.Brain magnetic resonance imaging with
solitary intracranial dural metastasis of prostate adenocarcinoma.
In the occipital region of the left hemisphere, an extracerebral
formation is visible with heterogeneous contrast enhancement, and
restriction of diffusion, surrounded by a pronounced zone of edema.
SWAN indicates the presence of point artifacts of magnetic
susceptibility due to hemorrhages and intratumoral vascular shunts.
According to the results of MRI perfusion, high values of relative
cerebral blood volume (rCBV) and relative cerebral blood flow
(rCBF) in the tumor structure are determined. The mean transit time
(MTT) indicator is extended. (A) T2-WI; (B and C) DWI and ADC; (D)
SWAN; (E) T1-WI with contrast; (F) CBV; (G) CBF; and (H) MTT. SWAN,
susceptibility-weighted angiography; T1-WI, T1-weighted image;
T2-WI, T2-weighted image; DWI, diffusion-weighted imaging; ADC,
apparent diffusion coefficient; CBV, cerebral blood volume; CBV,
cerebral blood flow; r, relative; MTT, mean transit time. |
Execution protocol
MRI was performed using a General Electric 3T
Discovery W750 tomography with an 8-channel head coil (GE
Healthcare). Paramagnetic Clariscan (GE Healthcare) was used as a
contrast agent with a dose calculation of 0.2 ml/kg (0.1 mmol/kg).
The introduction of a contrast agent was performed in two stages: A
primary dose of 0.1 mmol/kg and an additional dose of 0.2 mmol/kg.
The contrast agent was injected into the cubital vein using an
automatic injector at an injection rate of 5 ml/sec.
The MRI study protocol included the following pulse
sequences: T1 GRE ‘BRAVO’, T1 SE CUBE, T2 SE, SWAN, DWI with ADC
mapping, PWI-DSC-T2* (dynamic susceptibility contrast DSC-T2*).
Image post-processing was performed on a GE
Advantage Window 4.5 graphics station (GE Healthcare). Blood flow
parameters were assessed using three perfusion maps: Cerebral blood
flow (CBF) in ml/100 g/min; cerebral blood volume (CBV) in ml/100
g; and mean transit time (MTT) in sec. The region of interest (ROI)
in the intact white matter of the semioval centers was used to
normalize blood flow parameters. The normalized blood flow
parameters were calculated as the ratio of the parameter values in
the area of interest to the intact brain substance, that is the
relative (r)CBF and rCBV. Given the similar imaging characteristics
of meningiomas with metastatic lesions, a detailed comparative
analysis of all tumors was performed using both routine and
specialized MRI sequences: Perfusion-weighted imaging (PWI),
diffusion-weighted imaging (DWI), apparent diffusion coefficient
(ADC) and susceptibility-weighted angiography (SWAN).
Data verification
The obtained data were verified using histological
and immunohistochemical (IHC) analysis as follows: i) Neutral
buffered formalin (10%) was used for fixation (24 h in room
temperature) and in frozen sections, cold acetone was used for 1
min; ii) Paraffin embedding (mostly 4 µm) and frozen sections
ranged between 4–6 µm in thickness. The IHC analysis included
routine staining with both Carazzi's hematoxylin and eosin alcohol
solution at room temperature (~24°C) for 15 min. The range of IHC
markers used were as follows: 7 ml Anti-EMA (E29) (cat. no.
Z2048MP; Thermo Fisher Scientific, Inc.), 500 µl Ki-67 (SP6) (cat.
no. PIMA514520; Invitrogen™; Thermo Fisher Scientific, Inc.), 7 ml
Cytokeratin Cocktail (AE1 & AE3) (cat. no. MBS370057;
MyBioSource, Inc.), and 100 µg/vial Anti-Vimentin (v9) (cat. no.
MA1102; Boster Biological Technology). Incubation Time/Temperature
of IHC markers: Anti-EMA (E29) 1–3 min/room temperature; Ki-67
(SP6) 30–60 min/room temperature; Cytokeratin Cocktail (AE1 &
AE3) 15 min/room temperature; and Anti-Vimentin (v9) 5–10 min/room
temperature. Cells were counted using an Aperio ImageScope
12.4.6-Pathology Slide Viewing Software using the nuclear,
cytoplasmic, and membrane staining module in the Aperio Image
Analysis Workstation.
CBV and CBF characteristics
CBV and CBF in high-grade intracranial meningiomas
and intracranial metastases show a high degree of heterogeneity.
Low and high CBV and CBF threshold values may be the result of
various pathological features. Therefore, to characterize the
tumor, it is necessary to stratify the CBV/CBF into low and high
regions. When choosing the thresholds, the following three facts
were taken into account: i) Normal brain tissue has intrinsic
differences depending on tissue type as CBV and CBF are greater in
gray matter than in white matter, and even higher in large vessels;
ii) some tumor vessels may originate from cerebral vessels; and
iii) several studies have that the distribution of CBV in the tumor
is more or less similar to that in normal tissue, although the
vasculature of intracranial meningiomas and IDM has morphological
and functional abnormalities (20–22).
Thus, instead of choosing arbitrary threshold values, the CBV and
CBF values in normal gray and white matter reported in published
studies to were used divide tumor perfusion into regions of high,
medium, and low levels (23,24).
The area of high CBF was defined as >120 ml/100 g/min.
Similarly, the lower region was defined as less than 50 ml/100
g/min but >10 ml/100 g/min. A lower threshold value of 10 ml/100
g/min was used to exclude voxels that could represent a surgical
cavity or necrosis. Similarly, CBV threshold values were divided
into three groups: High CBV, >4%; average CBV, 1.7–4%; and low
CBV, 0.2–1.7%.
Statistical analysis
Statistical processing of the obtained results was
performed using descriptive statistics and correlation analysis.
Sex, age, presence of dislocation of midline structures, bone
invasion, and severity of perifocal edema were compared for both
groups of patients using a Pearson's χ2 test or ANOVA as
appropriate. ADC, CBV, rCBV, CBF, rCBF, and MTT values were
compared for both groups of patients using a Mann-Whitney U test.
The optimal cut-off value, which can provide the sensitivity and
specificity needed to differentiate meningioma from dura
metastases, was determined by analysis of ROC curves. The area
under the ROC-curve values (AUC) was calculated for the CBV, rCBV,
CBF, and rCBF values. CBV, rCBV, CBF, and rCBF value parameters
were analyzed on the MRI software SPHERE® version 3.0.
Statistical analyses were performed using IBM SPSS version 24.0
software (IBM Corp.), and the graphs were generated using GraphPad
Prism version 8.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Neuroimaging data
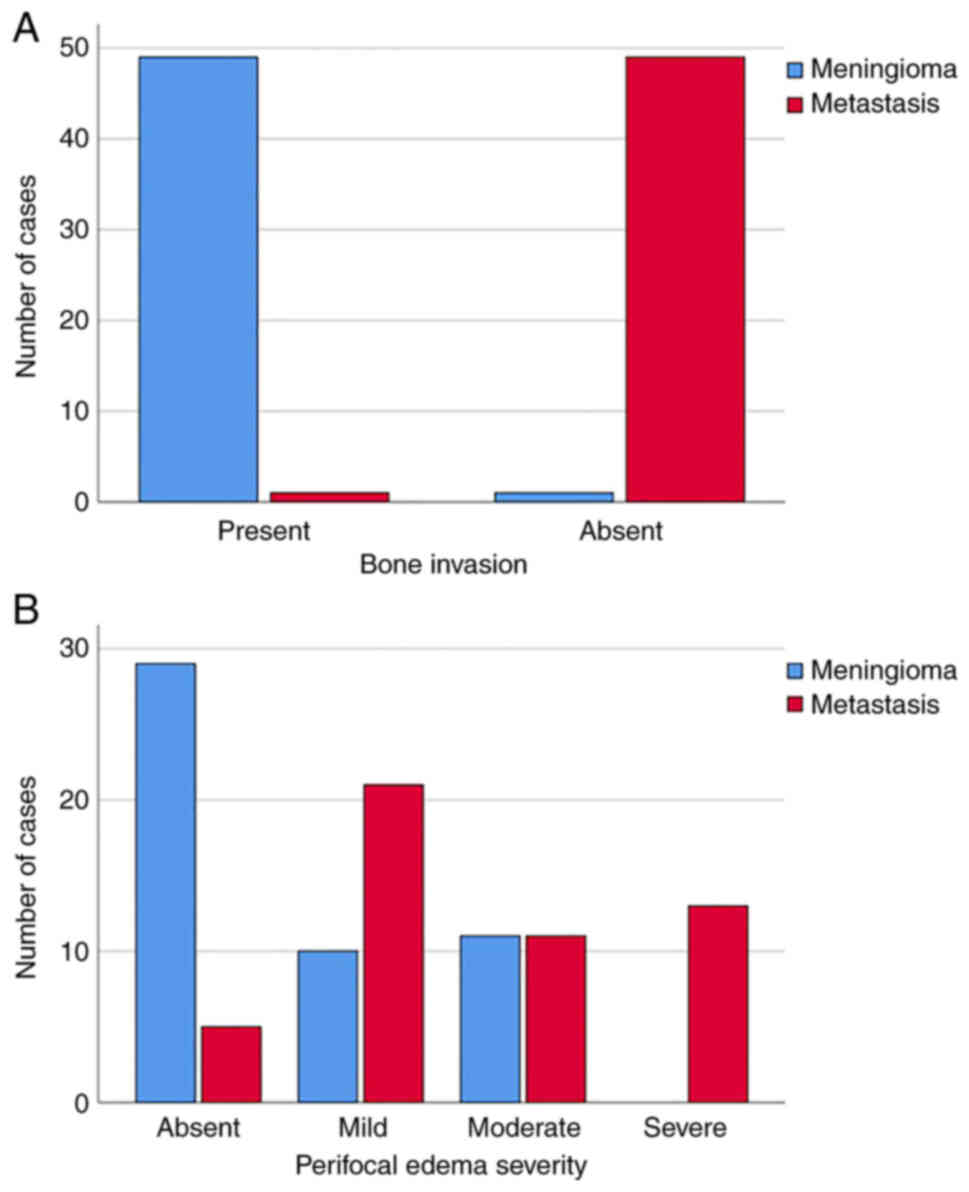
The results indicated that IDM affected bone less
frequently (P≤0.001) than intracranial meningiomas; bone invasion
by metastasis was observed in only 2% (n=1) of cases (Fig. 5A).
In addition, dislocation of the midbrain structures
in patients with meningiomas was observed in 12% of cases (n=6),
and in patients with IDM in 22% of cases (n=11). There were no
significant differences in dislocation between the different groups
of patients (P=0.169). In 60% of cases (n=30) there was no
perifocal edema in the meningioma patient group, whereas in 40% of
perifocal edema cases was detected; 20% (n=10) of mild severity and
20% (n=10) of moderate severity. Moreover, perifocal edema in the
group of patients with IDM was significantly more common (P≤0.001)
compared with patients with meningioma, where 48% of patients
(n=24) had moderate and severe edema, 42% (n=21) had mild edema,
and 10% (n=5) was absent (Fig.
5B).
In the group of intracranial meningiomas, the mean ±
SD ADC was 912.14×10−6±102.7×10−6
mm2/sec. The median CBV was 19.25 ml/100 g (CI,
18.08-28.96 ml/100 g) and the median increase in rCBV was 4.1-fold
(CI, 4.09-5.46). The median CBF was 155 ml/100 g/min (CI,
157.48-206.65 ml/100 g/min) and the median increase in rCBF was
3.85-fold (CI, 3.98-5.28). The median MTT was 11 sec (CI,
10.18-11.29 sec).
In the IDM group, the mean ± SD ADC was
867.67×10−6±138.6×10−6 mm2/sec.
The median CBV was 39.85 ml/100 g (CI, 36.50-46.83 ml/100 g) and
the median increase in rCBV was 7.15-fold (CI, 6.64-7.80). The
median CBF was 293 ml/100 g/min (CI, 261.65-306.12 ml/100 g/min)
and the median increase in rCBF was 6.7-fold (CI, 5.97-6.93). The
median MTT was 10.85 sec (CI, 10.15-10.86 sec).
According to the results of the comparative
analysis, a statistically significant difference in the values of
the CBV, rCBV, CBF, and rCBF indicators was revealed. In the IDM
group, perfusion values were significantly higher (P≤0.001). There
were no differences between ADC and MTT (P=0.071 and P=0.127,
respectively) in IDM and meningioma patient groups. The results of
the comparisons are shown in Table
I and Fig. 6.
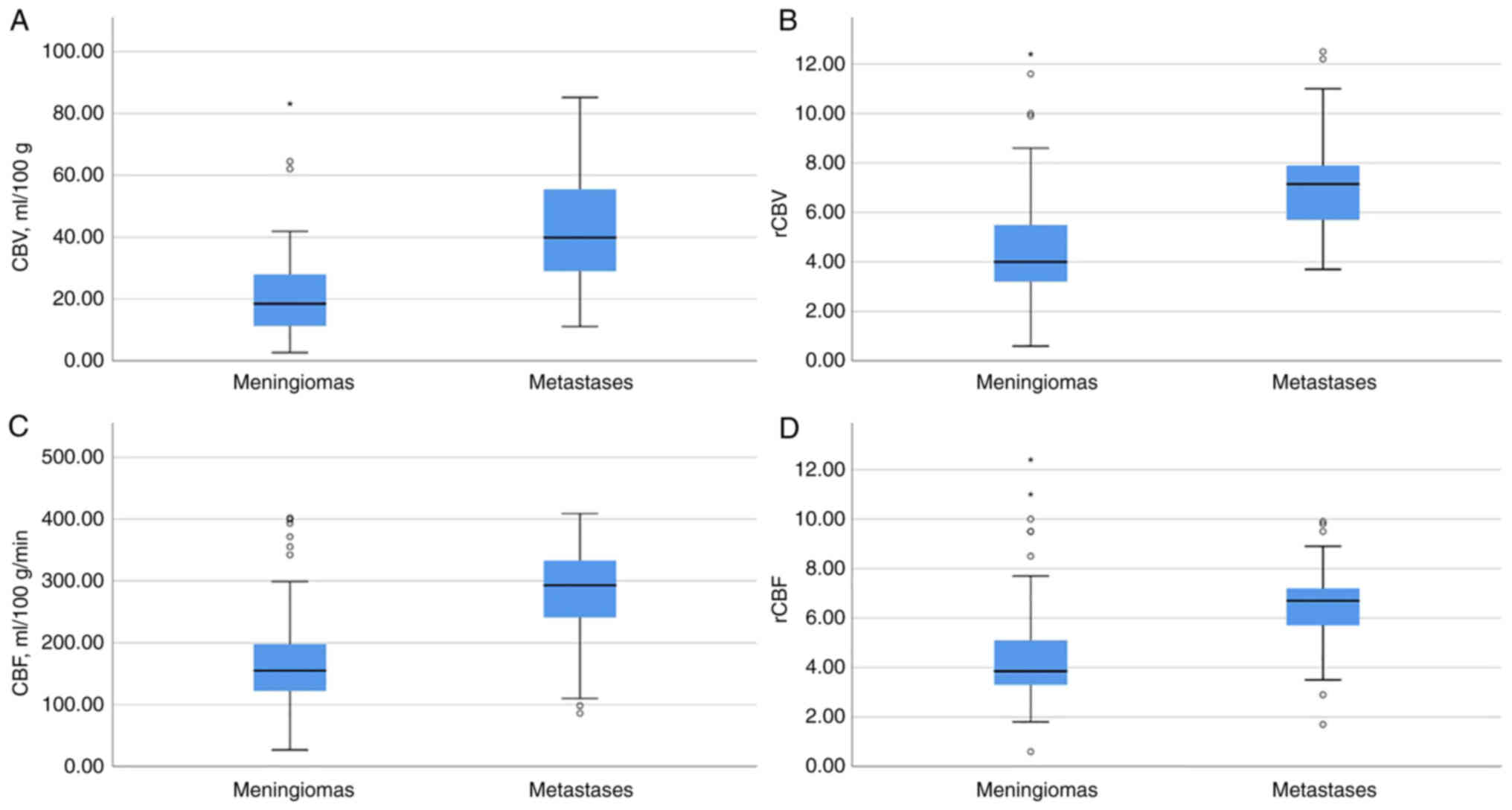 | Figure 6.Demonstration of evaluating the
results of the values of the CBV, rCBV, CBF, and rCBF. Graphs of
(A) CBV, (B) normalized rCBV, (C) CBF velocity, and (D) normalized
rCBF velocity for intracranial meningiomas and solitary IDM. (A)
The median CBV was significantly higher for IDM than for
intracranial meningiomas (P≤0.001). The Y-axis plots the values of
the CBV in ml/100 ml; (B) the median increase in rCBV was
significantly higher for IDM than for intracranial meningiomas
(P≤0.001). The Y-axis plots the ratio of rCBV in the ROI to the
normal white matter of the semioval center, representing the
normalized cerebral blood flow volume; (C) Median CBF was
significantly higher for IDM than for intracranial meningiomas
(P≤0.001). The Y-axis shows the values of CBF velocity in ml/100
g/min. (D) The median rCBF was significantly higher for IDM than
for intracranial meningiomas (P≤0.001). The Y-axis plots the ratio
of rCBF velocity in the ROI to the normal white matter of the
semioval center, representing the normalized rCBF velocity. ROI,
region of interest; IDM, intracranial dural metastasis; CBV,
cerebral blood volume; CBV, cerebral blood flow; r, relative. |
 | Table I.Average ADC, MTT, averaged absolute
BF and BV, and BFn and BVn in tumors based on the histological
affiliation. |
Table I.
Average ADC, MTT, averaged absolute
BF and BV, and BFn and BVn in tumors based on the histological
affiliation.
| Significative | Intracranial
meningioma | Intracranial dural
metastases | P-value |
|---|
| ADC,
×10−6 mm2/sec | 912.14 (SD:
±102,7) | 867.67 (SD:
±138,6) | 0.071 |
| BV, ml/100 g | 19.25 (CI:
18,08-28,96) | 39.85 (CI:
36,50-46,83) | <0.001 |
| BVn | 4.1 (CI:
4,09-5,46) | 7.15 (CI:
6,64-7,80) | <0.001 |
| BF, ml/100
g/min | 155.0 (CI:
157,48-206,65) | 293.0 (CI:
261,65-306,12) | <0.001 |
| BFn | 3.85 (CI:
3,98-5,28) | 6.7 (CI:
5,97-6,93) | <0.001 |
| MTT, sec | 11 (CI:
10,18-11,29) | 10.85 (CI:
10,15-10,86) | 0.127 |
Diagnostic value
Determination of perfusion threshold values (for
indicators with significant differences-CBV, rCBV, CBF, and rCBF)
for differentiating intracranial meningiomas and IDM was performed
by constructing ROC curves and calculating the optimal sensitivity
and specificity values (Fig. 7).
The threshold value for CBV was 28.25 ml/100 g; the sensitivity and
specificity were 76.5 and 78.0%, respectively (Fig. 7A). The threshold value for rCBV was
5.4; The sensitivity and specificity of the method are 74.5 and
82.0%, respectively (Fig. 7B). The
threshold value for CBF was 217.9 ml/100 g/min; the sensitivity and
specificity were 80.4 and 86.0%, respectively (Fig. 7C). The threshold value of the rCBF
was 5.6; the sensitivity and specificity were 82.4 and 76.0%,
respectively (Fig. 7D).
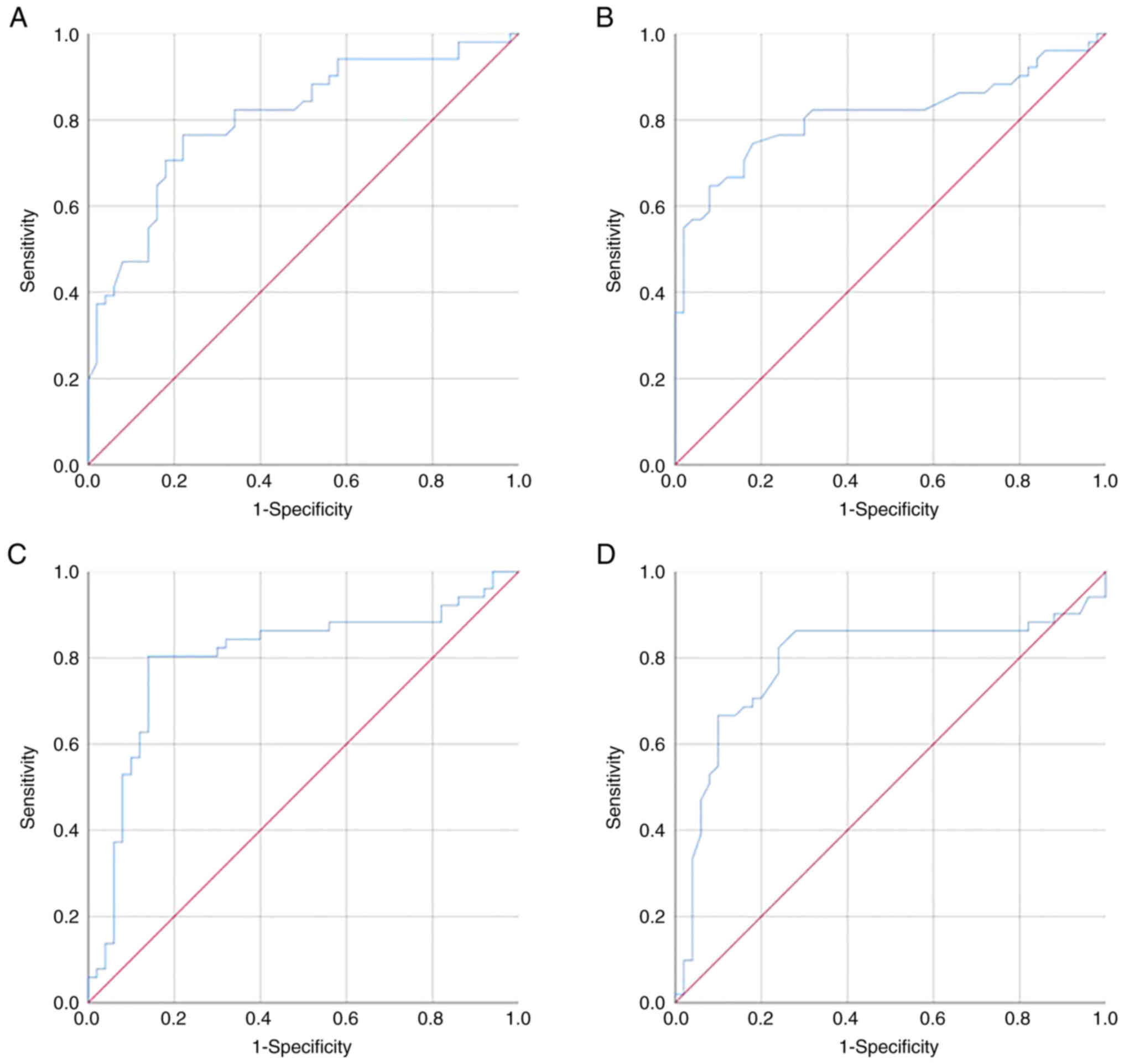 | Figure 7.The ROC curve and the AUC analysis.
(A) The AUC corresponding to CBV for differentiating intracranial
meningiomas and solitary IDM was 0.805±0.44 [95% CI, 0.719-0.890]
(P≤0.001) with sensitivity and specificity values of 76.5 and
78.0%, respectively; (B) The AUC corresponding to rCBV for
differentiating intracranial meningiomas and IDM was 0.811±0.46
[95% CI, 0.722-0.900] (P≤0.001) with sensitivity and specificity
values of 74.5 and 82.0%, respectively. (C) The AUC corresponding
to CBF for differentiating intracranial meningiomas and IDM was
0.8±0.48 [95% CI, 0.706-0.894] (P<0.001) with sensitivity and
specificity values of 80.4 and 86.0%, respectively. (D) The AUC
corresponding to rCBF for differentiating intracranial meningiomas
and IDM was 0.79±0.5 [95% CI, 0.692-0.888] (P≤0.001) with
sensitivity and specificity values of 82.4 and 76.0%, respectively.
If the values of CBV, rCBV, CBF, and rCBF were ≤ threshold value,
the patient was predicted to have an intracranial meningioma. The
threshold value of CBV was 28.25 ml/100 g, for rCBV it was 5.4, for
CBF it was 217.9 ml/100 g/min, and for rCBF was 5.6. ROC, receiver
operating characteristic; AUC, area under the curve; IDM,
intracranial dural metastasis; CBV, cerebral blood volume; CBV,
cerebral blood flow; r, relative. |
With the indicated threshold values of all the
listed perfusion indicators ≤ threshold, it was worth considering
an intracranial meningioma.
Histological and immunohistochemical
methods
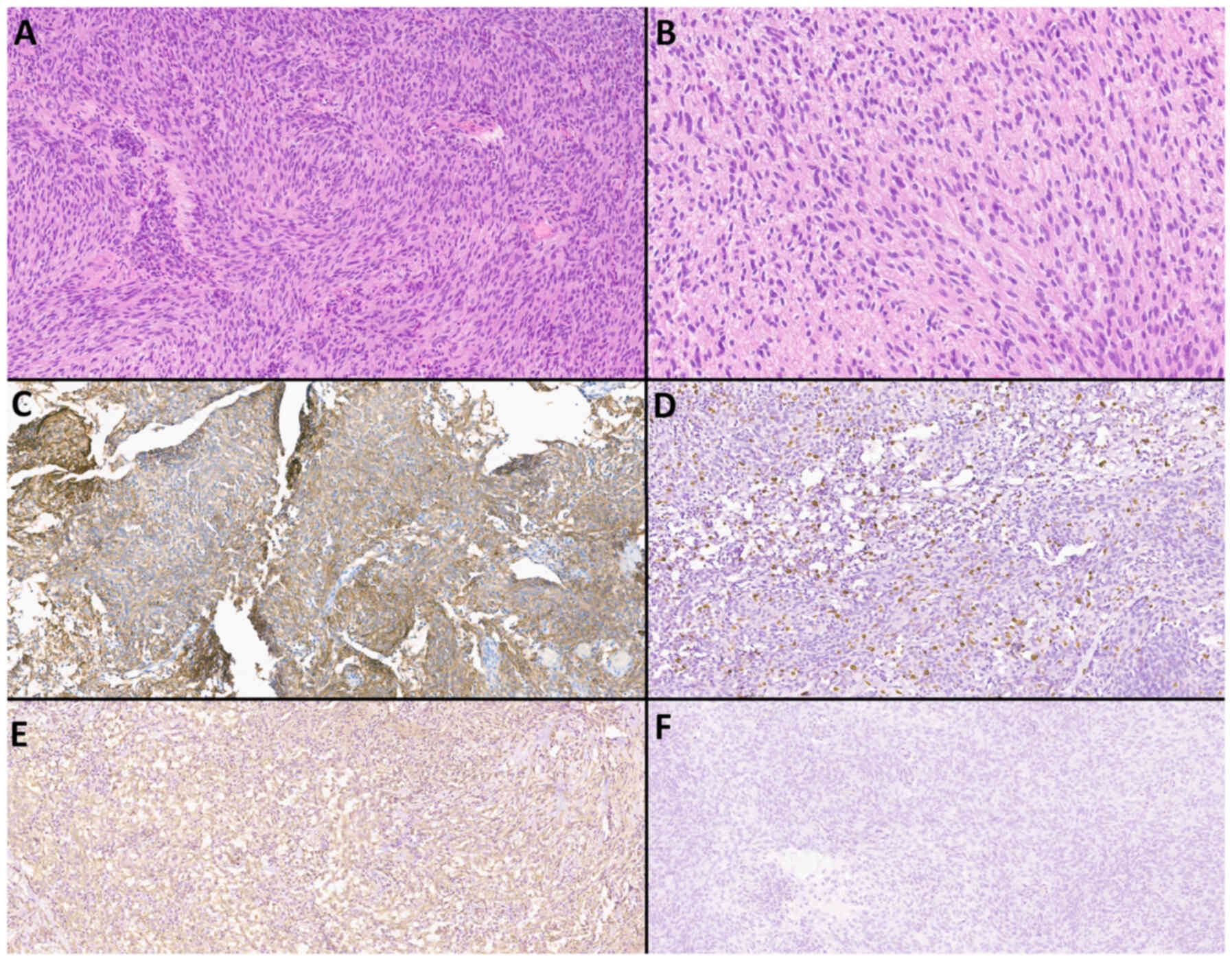
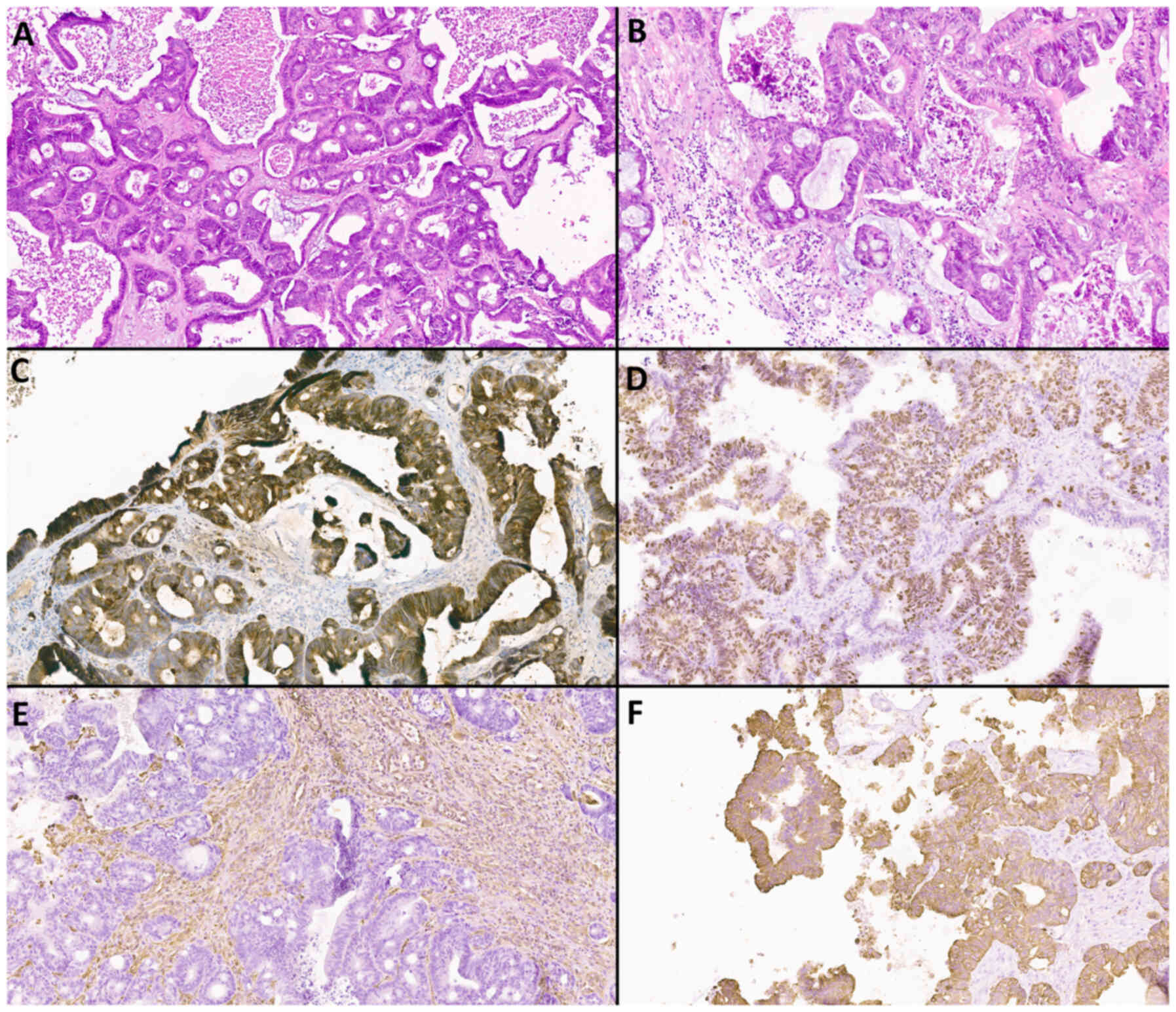
Examples of histological and immunohistochemical
studies of a patient with atypical meningioma (WHO Grade 2) and an
IDM patient with a primary focus on adenocarcinoma and clear cell
renal cell carcinoma are shown in Fig.
8, Fig. 9, Fig. 10, Fig.
11.
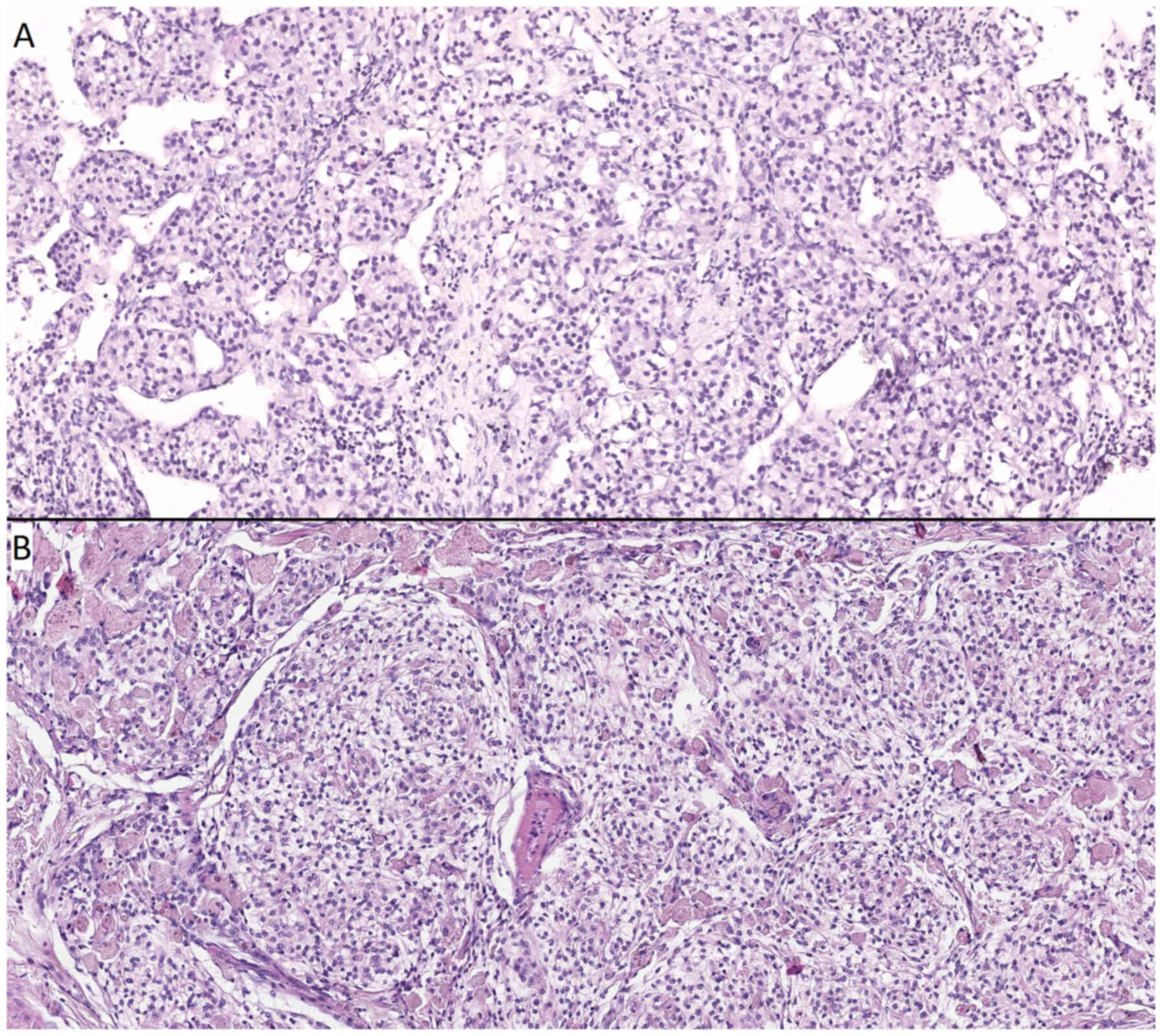
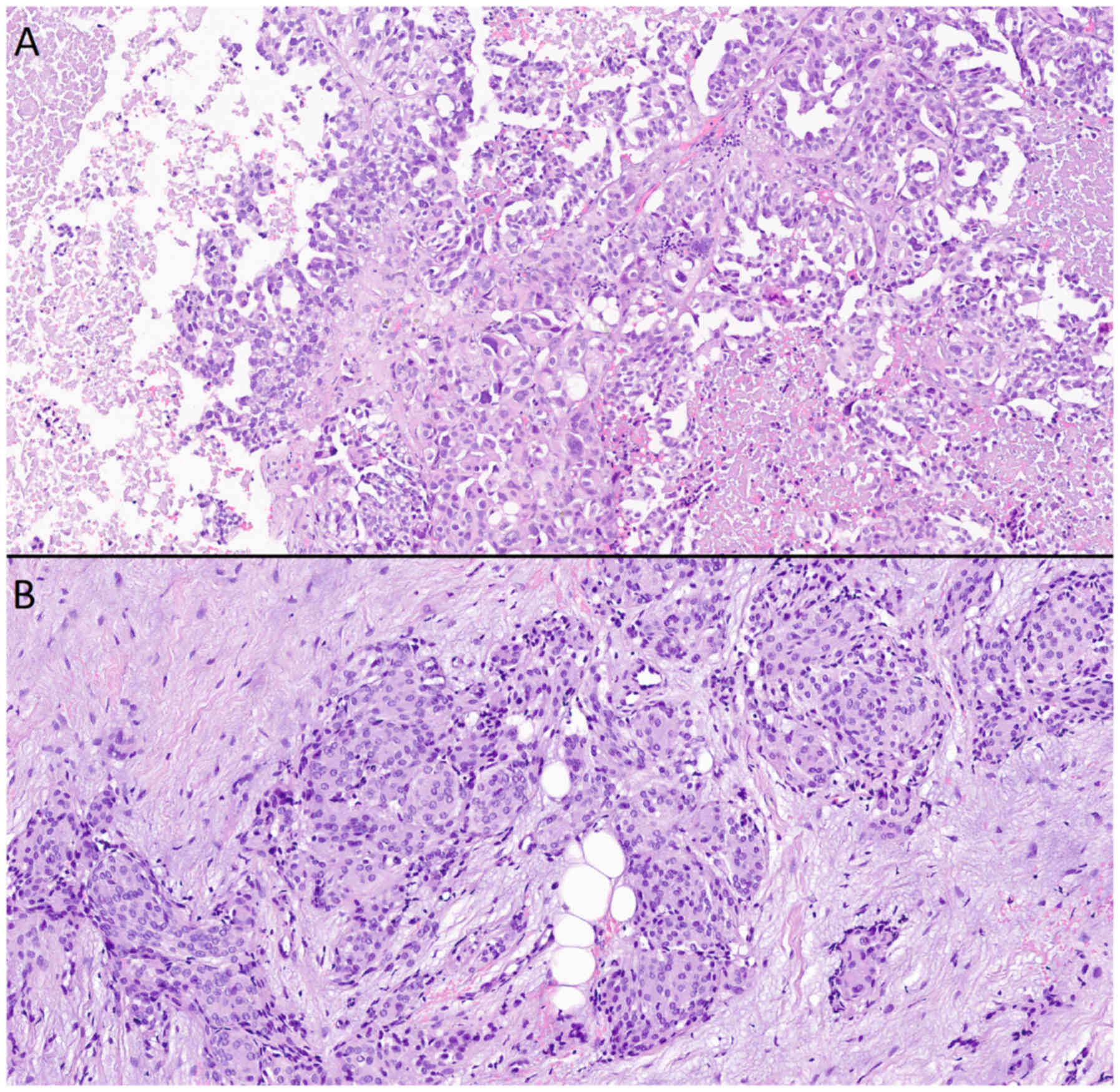 | Figure 11.Microscopic examination of
histological preparations from a patient with intracranial
meningioma and solitary intracranial dural metastasis (metastasis
of acinar adenocarcinoma of the prostate). (A) Metastasis of acinar
adenocarcinoma of the prostate, stained with hematoxylin and eosin,
(magnification, ×20). Cells of a large size with glandular
morphology, nuclei polymorphic in shape and size, with a cytoplasm
that appeared outlined, optically light, varied in volume, with
moderate lymphocytic infiltration, and continuous fields of
necrosis on the left and right. (B) Meningothelial meningioma,
stained with hematoxylin and eosin (magnification, ×20). Cells were
of a medium size, with a similar structure, and an arachnoid
endothelial appearance, forming typical microconcentric structures;
the nuclei were round-oval and monomorphic; the stroma was fibrous
and well expressed. |
Discussion
The most common meningeal tumor is meningioma, which
is regularly diagnosed through MRI scans as incidental findings.
Most meningiomas are histologically and biologically benign,
characterized by non-aggressive, very slow growth, and a low risk
of recurrence (WHO Grade 1). Small meningiomas are clinically
asymptomatic. At the same time, large tumors and tumors with
perifocal edema have a mass effect with the manifestation of a
variety of neurological symptoms, in particular cerebral
(headaches) and focal (paresis).
Data from the largest single-center study, which
included 1,000 cases from between 2004 and 2010, showed that 2% of
resected dural masses, initially regarded radiologically and
intraoperatively as meningiomas, were, in fact, mimic pathologies,
amongst which the largest number were metastases (25). The exact incidence of IDM is
difficult to estimate. Carcinomatous infiltration of the dura mater
is detected in patients with primary extraneural malignancies in
8–9% of cases and usually as a late manifestation (12). In ~20% of IDM cases, there are no
symptoms. Otherwise, the most common clinical manifestations are
symptoms of increased intracranial pressure, neurological deficits,
and seizures (12).
As a rule, the differential diagnosis of meningioma
and CNS metastases does not cause difficulties for a
neuromorphologist. In rare cases, at the initial stage of
microscopic examination of histological slides stained with
hematoxylin and eosin, a clear picture may not immediately emerge
(Figs. 10 and 11). On such occasions, the pathologist
should use IHC to determine the histogenesis of the tumor.
From a radiological point of view, a typical
meningioma and a typical metastasis appear notably different in
routine MRI examination. Benign meningiomas are close to spherical
or plaque-shaped, creeping along the dura matter configuration.
Often, meningiomas are delineated from the brain parenchyma by a
cerebrospinal fluid cleft, sometimes containing depressed
extracerebral vessels. Characteristic of meningiomas is the
presence of almost always vivid and homogeneous contrast
enhancement, accompanied by non-pathognomonic reactive thickening
of the dura in the form of a ‘dural tail’, visible in 60–72% of
cases (25). Additionally, ~25% of
meningiomas contain calcifications, the presence of which is
associated with a slow growth rate and a low degree of malignancy
(26). Hemorrhages are not
characteristic of meningiomas. In 20% of cases, the bone bearing
the base of the meningioma shows focal reactive hyperostosis in the
form of a spike (27). Since the
center of the meningioma is supplied with blood through the stalk
at the point of attachment to the dura matter from the branches of
the external carotid artery (for example, from the middle meningeal
artery), the supplying artery ‘radiates’ from one point to the
periphery of the tumor, appearing as ‘sun rays’ or a ‘spoked wheel’
on T2-WI and on postcontrast T1-WI. Meningiomas can grow into the
adjacent bone and even the scalp, into the lumen of the dural
sinus, or envelop nerves and arteries, typically causing stenosis
of the latter. Of note, >50% of meningiomas have peritumoral
vasogenic edema (28). On DWI,
meningiomas appear as tumors with high cellularity as they show a
high signal corresponding with low ADC values. It is hypothesized
that malignant meningiomas have lower ADC values; however, benign
meningiomas can also have similar diffusion index numbers, which
creates confusion (29).
Meningiomas are highly vascularized tumors. Surgical
resection of a meningioma carries a high risk of blood loss
(30). In PWI, meningiomas show
elevated rCBV values, which vary slightly based on the specific
histological subtype (31).
Intra-axial metastases, in the vast majority of
cases, are easily distinguishable from meningiomas according to
mpMRI, and have a number of typical imaging characteristics.
Metastases, as a rule, are typically located at the border of the
gray-white matter and in the border zones between the territories
of the arterial pools, accompanied by variably pronounced vasogenic
edema. For metastasis, a ring-shaped contrast enhancement pattern
is typical with a central area of necrosis and hemorrhages. Average
ADC values are within 919.4±200×10−6 mm2/sec.
Increased perfusion values along the periphery of the formation are
characteristic (32). According to
previous studies, IDM may look identical to intracranial
meningiomas, and any meningeal lesion is subject to a differential
diagnosis (33,34).
IDM typically presents as a focal nodular dural
thickening accompanied by perifocal vasogenic edema of variable
severity unrelated to the size of the lesion. Extensive edema has a
mass effect, compressing the brain parenchyma (35). In rare cases, swelling may be
absent. Post-contrast IDM series show intense accumulation of the
paramagnet. In half of the cases, the phenomenon of the ‘dural
tail’ occurs, sometimes hemorrhages are observed in the structure
of the tumor. A number of authors have hypothesized that IDM is
more likely to exhibit facilitated diffusion (12,16,36). A
small number of studies have found a correlation between low ADC
values and low metastatic differentiation, as well as increased
cellularity (37). However, it has
also been shown that there is no correlation between ADC values and
the histological nature of the metastasis (38); thus, this remains a contested
result. A summary and comparison of routine imaging findings are
shown the Table II.
 | Table II.Comparison of a meningioma, an
intra-axial metastasis, and routine MRI imaging findings of
IDM. |
Table II.
Comparison of a meningioma, an
intra-axial metastasis, and routine MRI imaging findings of
IDM.
|
| Lesion |
|---|
| Routine imaging
findings |
|
|---|
| Meningioma | Intra-axial
metastasis | Intracranial dural
metastasis |
|---|
| Morphology | Close to spherical
or plaque-shaped | Close to
spherical | Close to spherical
or focal dural thickening |
| CSF cleft sign | Characteristic | No CSF cleft
sign | Present |
| Contrast
enhancement characterization | Vivid and
homogeneous | Vivid with ring
pattern | Vivid and often
homogeneous |
| Dural tale
sign | Occurs in 60–72% of
cases | No dural tale
sign | Occurs in 50% of
cases |
| Calcifications | Occurs in 25% of
cases | Absent | Absent |
| Hemorrhages | Absent | Typical | Occurs but depends
on the source of tumor nature |
| Focal
hyperostosis | Occurs in 20% of
cases | Absent | Absent |
| Sun rays or spoked
wheel sign | Typical
feature | Absent | Absent |
| Extracerebral
vessels involvement | Arterial encasement
with lumen stenosis; dural venous sinuses invasion | No extracerebral
vessels involvement | Arterial encasement
without lumen stenosis; no dural venous sinuses invasion |
| Bone invasion | Occurs without bone
destruction | No bone
invasion | Occurs with bone
destruction |
| Vasogenic
edema | Occurs in 50% of
cases | Typical and depends
of tumor origin | Typical and depends
of tumor origin |
Perfusion of metastases varies depending on the
nature of the primary lesion, and can be either hypo- or
hypervascular. The majority of metastases, in particular those of
renal carcinoma, melanoma, and neuroendocrine carcinoma, are
hypervascular. According to Kremer et al (36). and Furtner et al (39), both of which had small sample sizes,
IDMs were less vascularized than intracranial meningiomas. The work
of Fink and Fink (40) and Bendini
et al (41) showed that rCBF
and rCBV values were similar to those of meningiomas on perfusion
maps. Lui et al (42), did
not reveal statistically significant differences in rCBV and MTT
values between the study groups based on the analysis of 12 cases
of intracranial meningiomas and 8 cases of IDM. A summary and
comparison of advanced imaging findings are presented in Table III.
 | Table III.Comparison of a meningioma, an
intra-axial metastasis, and advanced MRI imaging findings of
IDM. |
Table III.
Comparison of a meningioma, an
intra-axial metastasis, and advanced MRI imaging findings of
IDM.
|
| Lesion |
|---|
| Routine imaging
findings |
|
|---|
| Meningioma | Intra-axial
metastasis | IDM |
|---|
| Diffusion
restriction | Typical and
observed in entire mass | Varies and observed
thenon-necrotic mass periphery | Varies and depends
from the source tumor cellularity |
| CBV and CBF, rCBV
and rCBF values | Increased in entire
mass | Increased in
non-necrotic mass periphery | Vary and depend
from the source tumor nature |
CBV and the CBF also highlight the importance of
cerebral vascular autoregulation. The CBV threshold value of
angiomatous meningiomas is higher than that of meningothelial
meningiomas and higher than that of fibroblastic meningiomas
(36). A high CBV threshold value
with swelling around the lesion is indicative of anaplastic
meningioma (36). The CBV threshold
value observed in the metastatic peritumoral area was lower than
that observed in anaplastic meningioma due to tumor infiltration
around the lesion seen in anaplastic meningioma (36,43).
This CBV threshold value appears to be reliable in clinical
practice for distinguishing between two tumor masses even though
there is overlap. For instance, patients with high- or low-grade
gliomas with a high relative rCBV (>1.75) have a significantly
more rapid time to progression than patients who have high-grade
gliomas and low-grade gliomas with a low relative CBV (44,45).
In addition, CBV and rCBV have been shown to correlate with both
catheter angiography scores of tumor hypervascularization and
histopathological measurements of tumor neovascularization and
mitotic activity (46).
Intratumoral perfusion in intracranial meningiomas is increased
with a median rCBV of 8.9 due to the increased vascularization and
absence of the blood-brain barrier (BBB), while similar indicators
in various intracranial IDM are only slightly increased with a
median rCBV of 1.8 (24,47). However, hypervascular intracranial
IDM from primary tumors such as melanoma, renal cell carcinoma, and
Merkel cell carcinoma may present with an elevated rCBV
indistinguishable from that of meningiomas (37,39).
Hakyemez et al (48)
demonstrated that typical meningiomas had an rCBV value of SD
6.63±2.87, whereas in atypical meningiomas it was 12.25±4.63
(P<0.01). The CBF of a normally perfused area is >40 ml/100
g/min, the CBF of a zone of oligemia is between 20–40 ml/100 g/min,
the CBF of a zone of penumbra is between 10–20 ml/100 g/min, the
CBF of an ischemic zone is <10 ml/100 g/min (49). Lin et al (50) suggested that compared with solitary
metastases, CBF values in the peritumoral edema of glioblastoma may
be elevated by tumor cell angiogenesis. Based on this, it was
hypothesized that the difference in CBF values from an area of
edema close to an enhanced lesion to an area close to
normal-looking white matter in intracranial meningiomas and IDM
could be reflected as a gradient-the CBF gradient.
Despite histological and radiological differences,
there are situations where meningiomas and IDMs appear similar on
MRI, and the differential diagnosis between these tumors based on
imaging alone is ambiguous. In addition, the medical history is not
always exhaustive, and archival data may not be available, making
it impossible to assess growth dynamics. Preoperative
differentiation is required to determine the treatment approach:
Dynamic observation, surgical resection, additional examination to
search for the primary focus and staging, and the possibility of
using adjuvant therapy (39,41,42,51,52).
IDM, unlike parenchymal and leptomeningeal metastases, are
localized outside the BBB, thus remaining susceptible to systemic
chemotherapy (34). Therefore, the
search for reliable radiological markers to differentiate IDM is
essential in clinical practice. In such situations, the radiologist
should try to increase the specificity with additional instruments
and perform a multiparametric study, in particular DWI/ADC and PWI.
According to the results of the present study, there was no
statistically significant difference between the mean ADC and MTT
values for meningiomas and IDM. When analyzing perfusion parameters
(CBV, rCBV, CBF, and rCBF), differences were found, including
results that contrasted the results reported by Kremer et al
and Furtner et al namely, IDM perfusion rates were higher in
patients with meningiomas. In addition, the threshold value of the
CBF indicator was calculated, a value above this which makes it
possible to predict IDM with sensitivity and specificity in the
region of 90.0% (80.4 and 86.0%, respectively).
As it has been aforementioned, there are differences
in the degree of bone invasion and surrounding edema between
meningiomas and isolated intracranial IDM on MRI. Indeed, these
factors undoubtedly affect the prognosis and treatment options, and
again proving the importance of a correct diagnosis using one of
the methods such as neuroimaging. The use of high-dose
dexamethasone may produce symptomatic relief, even if there is no
evidence of cerebral edema on MRI. Immediate evacuation of a
subdural hematoma can be a life-saving procedure. Surgical
resection is the best treatment when the lesion is unique,
accessible, and circumscribed, particularly when the systemic
disease is controlled or not immediately life-threatening (53). However, even in the case of
progressive systemic cancer, some surgeons recommend resection when
the IDM causes severe symptoms. After total removal of the tumor,
the resected dura is replaced by an artificial dura (54). In some data, surgery was performed
in 83% of cases, alone or associated with another treatment. In our
experience, this figure largely overestimates the number of
patients eligible for surgery and is probably biased by the
publication of a higher number of surgical series. Radiation
therapy is indicated in patients who cannot be operated on because
the IDM is inaccessible or widespread or because life expectancy
due to the progression of the systemic diseases does not exceed a
few months. Systemic chemotherapy was used in very few cases but
could not be evaluated because of its almost constant association
with surgery and/or radiotherapy. Considering that this also
depends on the individuality of the case, depending also on the
experience of the surgeon, the equipment of the operating room and
the patient's systemic diseases (55,56).
In conclusion, diffusion-weighted images are not
reliable criteria for differentiating intracranial meningiomas from
IDM and should not influence the diagnosis suggested by imaging.
The meningeal lesion perfusion technique predicts metastasis with a
sensitivity and specificity close to 80–90% and deserves attention
in the diagnosis. Since IDM differs from meningiomas in the
severity of neoangiogenesis and, accordingly, in greater vascular
permeability, the technique for assessing vascular permeability
(wash-in parameter with dynamic contrast enhancement) may
potentially be used as an additional criterion for distinguishing
between dural lesions. In addition, this method has not been
brought to extensive practice, thus the purpose of this study was
to examine it. Since it was observed that this is possible, the
next step is multicenter prospective randomized trials on this
topic.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Bashkir State Medical
University Strategic Academic Leadership Program
(PRIORITY-2030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, OB and IG made substantial contributions to
conception and design and confirm the authenticity of all the raw
data RT and AS made substantial contributions to the acquisition of
data. XG, AB and TI made substantial contributions to the analysis
and interpretation of data. OB and IG were involved in drafting the
manuscript or revising it critically for important intellectual
content. HW gave final approval for the version published. All
authors read and approved the final manuscript and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by The approval was provided by the ethics
committee of Federal Center of Neurosurgery (Tyumen, Russian
Federation). Written informed consent to participate in this study
was provided by the participants' legal guardian/next of kin.
Written informed consent was obtained from the individual(s), and
minor(s)' legal guardian/next of kin, for the publication of any
potentially identifiable images or data included in this
article.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IDM
|
intracranial dural metastases
|
|
CNS
|
central nervous system
|
|
MRI
|
magnetic resonance imaging
|
|
ROC
|
receiver operating characteristic
curve
|
|
AUC
|
area under the curve analysis
|
|
mpMRI
|
multiparametric MRI
|
|
ADC
|
apparent diffusion coefficient
values
|
|
CBF
|
cerebral blood flow
|
|
WHO
|
World Health Organization
|
|
MTT
|
mean transit time
|
|
ROI
|
the region of interest
|
|
PWI
|
perfusion-weighted imaging
|
|
DWI
|
diffusion-weighted imaging
|
|
ADC
|
apparent diffusion coefficient
|
|
SWAN
|
susceptibility-weighted
angiography
|
|
IHC
|
immunohistochemical
|
|
SSTR2a
|
somatostatin receptor 2a
|
|
EH
|
epithelioid hemangioendothelioma
|
|
pPNET-ES
|
peripheral primitive neuroectodermal
tumor-Ewing sarcoma
|
References
|
1
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: Primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14 (Suppl 5):v1–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olar A, Wani KM, Sulman EP, Mansouri A,
Zadeh G and Wilson CD: Mitotic index is an independent predictor of
recurrence-free survival in meningioma. Brain Pathol. 25:266–275.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perry A, Scheithauer BW, Stafford SL,
Lohse CM and Wollan PC: ‘Malignancy’ in meningiomas: A
clinicopathologic study of 116 patients, with grading implications.
Cancer. 85:2046–2056. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sughrue ME, Sanai N, Shangari G, Parsa AT,
Berger MS and McDermott MW: Outcome and survival following primary
and repeat surgery for World Health Organization Grade III
meningiomas. J Neurosurg. 113:202–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marciscano AE, Stemmer-Rachamimov AO,
Niemierko A, Larvie M, Curry WT, Barker FG II, Martuza RL, McGuone
D, Oh KS, Loeffler JS and Shih HA: Benign meningiomas (WHO grade I)
with atypical histological features: Correlation of
histopathological features with clinical outcomes. J Neurosurg.
124:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fox BD, Cheung VJ, Patel AJ, Suki D and
Rao G: Epidemiology of metastatic brain tumors. Neurosurg Clin N
Am. 221–6. (v)2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valiente M, Ahluwalia MS, Boire А,
Brastianos PK, Goldberg SB, Lee EQ, Le Rhun E, Preusser M, Winkler
F and Soffietti R: The evolving landscape of brain metastasis.
Trends Cancer. 4:176–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Preusser M, Capper D, Ilhan-Mutlu A,
Berghoff AS, Birner P, Bartsch R, Marosi C, Zielinski C, Mehta MP,
Winkler F, et al: Brain metastases: Pathobiology and emerging
targeted therapies. Acta Neuropathol. 123:205–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna W and Byhardtl R: Recursive
partitioning analysis (RPA) of prognostic factors in three
Radiation therapy Oncology Group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ostrom QT, Gittleman H, Liao P,
Vecchione-Koval T, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and other central nervous
system tumors diagnosed in the United States in 2010–2014. Neuro
Oncol. 19 (Suppl_5):v1–v88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laigle-Donadey F, Taillibert S, Mokhtari
K, Hildebrand J and Delattre JY: Dural metastases. J Neurooncol.
75:57–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gavrilovic IT and Posner JB: Brain
metastases: Epidemiology and pathophysiology. J Neurooncol.
75:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galldiks N, Angenstein F, Werner JM, Bauer
EK, Gutsche R, Fink GR, Langen KJ and Lohmann P: Use of advanced
neuroimaging and artificial intelligence in meningiomas. Brain
Pathol. 32:e130152022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Starr CJ and Cha S: Meningioma mimics:
Five key imaging features to differentiate them from meningiomas.
Clin Radiol. 72:722–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lyndon D, Lansley JA, Evanson J and
Krfishnan AS: Dural masses: Meningiomas and their mimics. Insights
Imaging. 10:112019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenberg A and Agulnik M: Epithelioid
Hemangioendothelioma: Update on diagnosis and treatment. Curr Treat
Options Oncol. 19:192018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohan SM, Symss NP, Pande A, Chakravarthy
VM and Ramamurthi R: Intracranial epithelioid hemangioendothelioma.
Childs Nerv Syst. 24:863–868. 2008. View Article : Google Scholar
|
|
19
|
Gamoh S, Tsuno T, Akiyama H, Kotaki S,
Nakanishi T, Tsuji K, Yoshida H and Shimizutani K: Intracranial
meningeal melanocytoma diagnosed using an interdisciplinary
approach: A case report and review of the literature. J Med Case
Rep. 12:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barbier EL, Lamalle L and Décorps M:
Methodology of brain perfusion imaging. J Magn Reson Imaging.
13:496–520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durmo F, Lätt J, Rydelius A, Engelholm S,
Kinhult S, Askaner K, Englund E, Bengzon J, Nilsson M,
Björkman-Burtscher IM, et al: Brain tumor characterization using
multibiometric evaluation of MRI. Tomography. 4:14–25. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito A, Inoue T, Suzuki S, Ezura M,
Uenohara H and Tominaga T: Relationship between pathological
characteristics and radiological findings on perfusion MR imaging
of meningioma. Neurol Med Chir (Tokyo). 61:228–235. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamrazi B, Shiroishi MS and Liu CS:
Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am.
27:137–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kremer S, Grand S, Rémy C, Pasquier B,
Benabid AL, Bracard S and Le Bas JF: Contribution of dynamic
contrast MR imaging to the differentiation between dural metastasis
and meningioma. Neuroradiology. 46:642–648. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibuya M: Pathology and molecular
genetics of meningioma: Recent advances. Neurol Med Chir (Tokyo).
55:14–27. 2015. View Article : Google Scholar
|
|
27
|
Boulagnon-Rombi C, Fleury C, Fichel C,
Lefour S, Marchal Bressenot A and Gauchotte G: Immunohistochemical
approach to the differential diagnosis of meningiomas and their
mimics. J Neuropathol Exp Neurol. 76:2892017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace E: The dural tail sign. Radiology.
233:56–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng L, Liang P, Jiao J, Chen J and Lei T:
Will an asymptomatic meningioma grow or not grow? A meta-analysis.
J Neurol Surg A Cent Eur Neurosurg. 76:341–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Leary S, Adams WM, Parrish RW and
Mukonoweshuro W: Atypical imaging appearances of intracranial
meningiomas. Clin Radiol. 62:10–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim BW, Kim MS, Kim SW, Chang CH and Kim
OL: Peritumoral brain edema in meningiomas: Correlation of
radiologic and pathologic features. J Korean Neurosurg Soc.
49:26–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filippi CG, Edgar MA, Uluğ AM, Prowda JC,
Heier LA and Zimmerman RD: Appearance of meningiomas on
diffusion-weighted images: Correlating diffusion constants with
histopathologic findings. AJNR Am J Neuroradiol. 22:65–72.
2001.PubMed/NCBI
|
|
33
|
Nania A, Granata F, Vinci S, Pitrone A,
Barresi V, Morabito R, Settineri N, Tomasello F, Alafaci C and
Longo M: Necrosis score, surgical time, and transfused blood volume
in patients treated with preoperative embolization of intracranial
meningiomas. Analysis of a single-centre experience and a review of
literature. Clin Neuroradiol. 24:29–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zimny A and Sasiadek M: Contribution of
perfusion-weighted magnetic resonance imaging in the
differentiation of meningiomas and other extra-axial tumors: Case
reports and literature review. J Neurooncol. 103:777–783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Talybov R, Beylerli O, Mochalov V,
Prokopenko A, Ilyasova T, Trofimova T, Sufianov A and Guang Y:
Multiparametric MR imaging features of primary CNS lymphomas. Front
Surg. 9:8872492022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kremer S, Grand S, Remy C, Esteve F,
Lefournier V, Pasquier B, Hoffmann D, Benabid AL and Le Bas JF:
Cerebral blood volume mapping by MR imaging in the initial
evaluation of brain tumors. J Neuroradiol. 29:105–113.
2002.PubMed/NCBI
|
|
37
|
Nayak L, Abrey LE and Iwamoto FM:
Intracranial dural metastases. Cancer. 115:1947–1953. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seki S, Kamide T, Tamase A, Mori K,
Yanagimoto K and Nomura M: Intraparenchymal hemorrhage from dural
metastasis of breast cancer mimicking meningioma. Neuroradiol J.
29:179–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Furtner J, Oth I, Schöpf V, Nenning KH,
Asenbaum U, Wöhrer A, Woitek R, Widhalm G, Kiesel B, Berghoff AS,
et al: Noninvasive differentiation of meningiomas and dural
metastases using intratumoral vascularity obtained by arterial spin
labeling. Clin Neuroradiol. 30:599–605. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fink KR and Fink JR: Imaging of brain
metastases. Surg Neurol Int. 4:209–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bendini M, Marton E, Feletti A, Rossi S,
Curtolo S, Inches I, Ronzon M, Longatti P and Di Paola F: Primary
and metastatic intraaxial brain tumors: Prospective comparison of
multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy,
perfusion MRI, and histopathological findings in a group of 159
patients. Acta Neurochir (Wien). 153:403–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lui YW, Malhotra A, Farinhas JM, Dasari
SB, Weidenheim K, Freeman K and LaSala PA: Dynamic perfusion MRI
characteristics of Dural metastases and meningiomas: A pilot study
characterizing the first-pass wash-in phase beyond relative
cerebral blood volume. AJR Am J Roentgenol. 196:886–890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi R, Jiang T, Si L and Li M:
Correlations of magnetic resonance, perfusion-weighed imaging
parameters and microvessel density in meningioma. J BUON.
21:709–713. 2016.PubMed/NCBI
|
|
44
|
Pillai JJ and Zacá D: Clinical utility of
cerebrovascular reactivity mapping in patients with low grade
gliomas. World J Clin Oncol. 2:397–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagesh V, Chenevert TL, Tsien CI, Ross BD,
Lawrence TS, Junck L and Cao Y: Quantitative characterization of
hemodynamic properties and vasculature dysfunction of high-grade
gliomas. NMR Biomed. 20:566–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kremer S, Grand S, Remy C, Esteve F,
Lefournier V, Pasquier B, Hoffmann D, Benabid AL and Le Bas JF:
Cerebral blood volume mapping by MR imaging in the initial
evaluation of brain tumors. J Neuroradiol. 29:105–113.
2002.PubMed/NCBI
|
|
47
|
Toh CH, Wei KC, Chang CN, Peng YW, Ng SH,
Wong HF and Lin CP: Assessment of angiographic vascularity of
meningiomas with dynamic susceptibility contrast-enhanced
perfusion-weighted imaging and diffusion tensor imaging. AJNR Am J
Neuroradiol. 35:263–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hakyemez B, Erdogan C, Bolca N, Yildirim
N, Gokalp G and Parlak M: Evaluation of different cerebral mass
lesions by perfusion-weighted MR imaging. J Magn Reson Imaging.
24:817–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grand S, Tahon F, Attye A, Lefournier V,
Le Bas JF and Krainik A: Perfusion imaging in brain disease. Diagn
Interv Imaging. 94:1241–1257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin L, Xue Y, Duan Q, Sun B, Lin H, Huang
X and Chen X: The role of cerebral blood flow gradient in
peritumoral edema for differentiation of glioblastomas from
solitary metastatic lesions. Oncotarget. 7:69051–69059. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hayashida Y, Hirai T, Morishita S,
Kitajima M, Murakami R, Korogi Y, Makino K, Nakamura H, Ikushima I,
Yamura M, et al: Diffusion-weighted imaging of metastatic brain
tumors: Comparison with histologic type and tumor cellularity. AJNR
Am J Neuroradiol. 27:1419–1425. 2006.PubMed/NCBI
|
|
52
|
Duygulu G, Ovali GY, Calli C, Kitis O,
Yünten N, Akalin T and Islekel S: Intracerebral metastasis showing
restricted diffusion: Correlation with histopathologic findings.
Eur J Radiol. 74:117–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koenig MA: Cerebral edema and elevated
intracranial pressure. Continuum (Minneap Minn). 24:1588–1602.
2018.PubMed/NCBI
|
|
54
|
Esquenazi Y, Lo VP and Lee K: Critical
care management of cerebral edema in brain tumors. J Intensive Care
Med. 32:15–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McKay MJ: Brain metastases: Increasingly
precision medicine-a narrative review. Ann Transl Med. 9:16292021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Harrison RA, Nam JY, Weathers SP and
DeMonte F: Intracranial Dural, calvarial, and skull base
metastases. Handb Clin Neurol. 149:205–225. 2018. View Article : Google Scholar : PubMed/NCBI
|