Introduction
Idiopathic CD4+ lymphocytopenia (ICL) is a rare
immunodeficiency disorder characterized by a decrease in CD4+ T
cells, an increased risk of opportunistic infections and no
evidence of infection with human immunodeficiency virus (HIV) types
1 and 2 or any other known immunodeficiencies or therapies that may
decrease T-cell numbers (1). In
addition to various opportunistic bacterial, viral, parasitic and
fungal infections, patients with ICL frequently experience
autoimmune diseases, including Sjögren's syndrome, systemic lupus
erythematosus and rheumatoid arthritis (1).
Similar to patients with HIV, patients with ICL
frequently experience acquired immunodeficiency syndrome-defining
cancers, such as Kaposi sarcoma, non-Hodgkin lymphoma and cervical
cancer (1). Due to the rarity of
cases, it remains unclear whether the incidence of other cancer
types in patients with ICL differs from those without ICL, and
various studies have described the incidence of malignancies that
coincide with ICL (1–4). According to a meta-analysis of
HIV-infected patients, HIV infection does not increase the
incidence of breast cancer (5),
which may be applied to ICL.
The present study reported on a rare case of early
breast cancer in a patient with ICL who experienced a severe
complication of neutropenia during adjuvant chemotherapy and was
forced to discontinue anticancer therapy.
Case report
In January 1994, a 41-year-old female patient had
been diagnosed with cytomegalovirus (CMV) retinitis. In August
2001, on initial presentation to the Center Hospital of the
National Center of Global Health and Medicine (Tokyo, Japan) at the
age of 49 years due to the progression of CMV retinitis, a CD4+
T-cell count of 112 and 102/mm3 (normal range,
500–1,500/mm3) was determined in two consecutive
peripheral blood counts. The patient had no abnormalities in B-cell
count, ranging around 150/mm3 (normal range,
100–600/mm3) in several tests, with normal IgM and IgG
production, and diminished IgA production of 68 mg/dl (normal
range, 110–410 mg/dl). The patient's natural killer cell activity
exhibited a deficiency, which was 5% (normal range, 18–40%). Bone
marrow aspiration and biopsy indicated no abnormalities. The
patient displayed no numerical or morphological abnormalities in
red blood cells, neutrophils, monocytes or platelets. The patient
tested negative for HIV antibodies, without any known other factors
that may decrease the CD4+ T cells, which led to the diagnosis of
ICL. Of note, the patient had no remarkable family history.
In June 2003, at the age of 50 years, the patient
was diagnosed with Mycobacterium avium complex lung disease
and was administered 400 mg/day of clarithromycin. In May 2017, at
the age of 65 years, the patient was diagnosed with type 1 diabetes
due to the occurrence of diabetic ketoacidosis, and
contrast-enhanced computed tomography during hospitalization
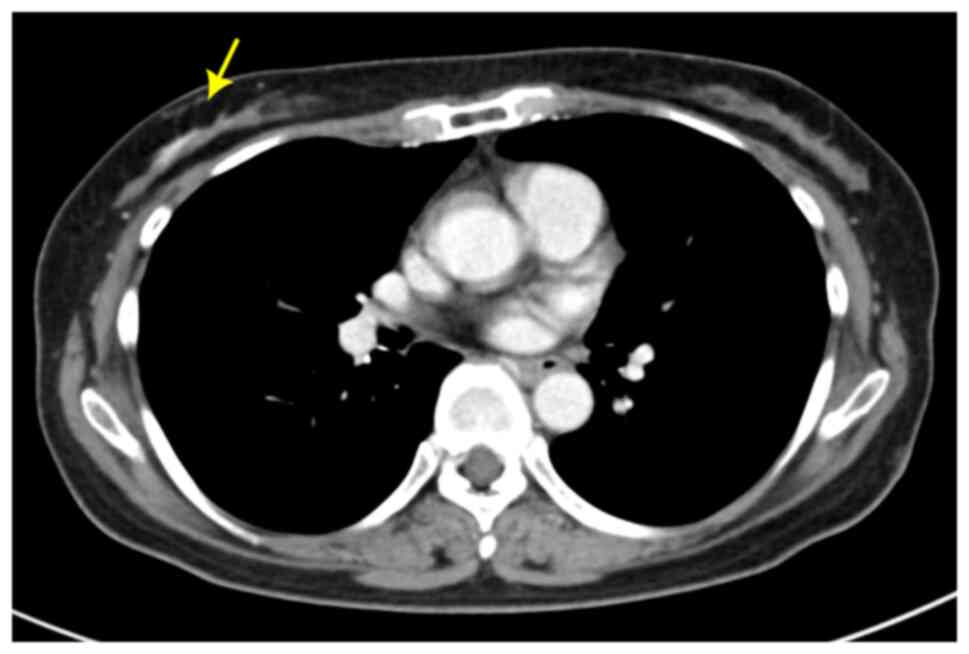
adventitiously revealed a contrast effect of the right breast
(Fig. 1). Ultrasonography was
performed, with no suspected malignancy, and the patient was
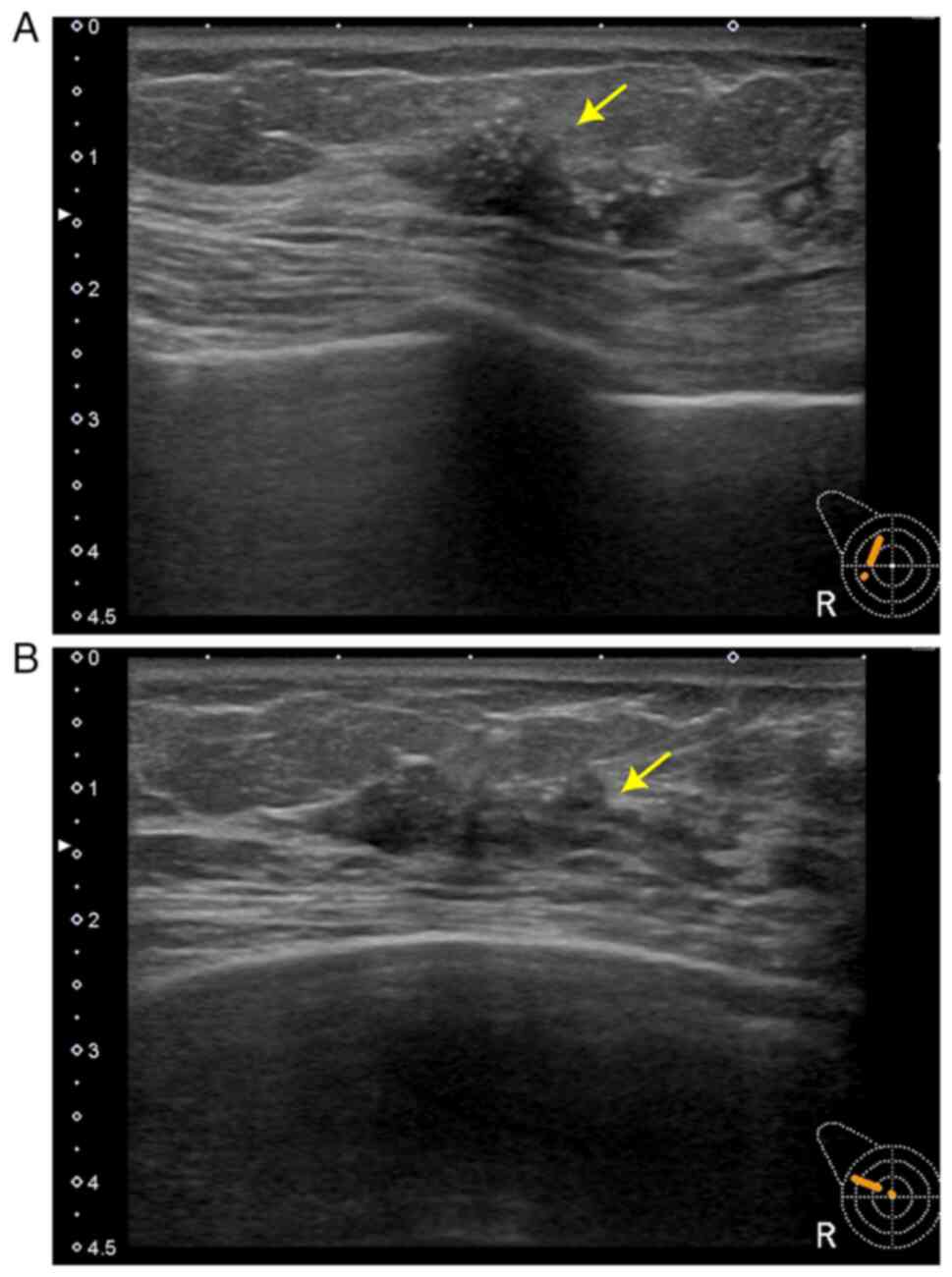
instructed to undergo a biyearly follow-up. After two years, in
September 2019, follow-up ultrasonography (Fig. 2A and B) and mammography revealed a
22×22×8 mm mass with calcification in the right breast and a core
needle biopsy was performed. Pathological examination revealed
invasive ductal carcinoma, estrogen receptor (ER)-, progesterone
receptor (PgR)+, human epidermal growth factor 2 (HER2) 3+ and a
Ki-67 index of 30–40%. Subsequently, a right breast resection and
sentinel lymph node biopsy were performed. Sentinel lymph nodes
were negative for breast cancer metastasis and axillary clearance
was not performed. The final pathological results demonstrated
invasive ductal carcinoma, ER-, PgR-, HER2 1+ and a Ki-67 index of
30–40%.
As the patient had an immunodeficiency, prophylactic
administration of clarithromycin (400 mg/day),
trimethoprim-sulfamethoxazole (TMP-SMZ) (1 g/day), and
valganciclovir (VGCV) (900 mg/day for 1 week followed by a resting
period of 2 weeks) were initiated to prevent opportunistic
infections during adjuvant chemotherapy. Initially, doxorubicin (60
mg/m2) and cyclophosphamide (600 mg/m2) were
administered. On day 14, the patient experienced febrile
neutropenia and cefepime (2 g every 12 h) was administered. On day
16, the white blood cell count decreased to 160/mm3
(normal range, 4,500-11,000/mm3), with a neutrophil
count of 0/mm3 (normal range,
2,500-7,000/mm3; grade 4 neutropenia). Consequently, the
next three cycles were administered in combination with
pegfilgrastim and levofloxacin prophylaxis. After four cycles, the
peripheral hemoglobin levels dropped to 7.4 g/dl (normal range,
11.6–15.0 g/dl; grade 3 anemia) and a red blood cell transfusion of
two units was performed. Subsequently, weekly paclitaxel (80
mg/m2) was administered. The first cycle of paclitaxel
was delayed because the peripheral blood count revealed a platelet
count of 27,000/µl (normal range, 150,000-450,000/µl; grade 3
thrombocytopenia) and a neutrophil count of 720/mm3
(grade 3 neutropenia). After a 3-week delay, paclitaxel was
administered at the patient's request, despite persistent grade 3
neutropenia. After three cycles of weekly paclitaxel, the treatment
was interrupted because a neutrophil count of
740–1,180/mm3 persisted for 3 weeks, eventually leading
to the discontinuation of adjuvant chemotherapy. At this time, bone
marrow testing to rule out other underlying hematological
malignancies such as myelodysplastic syndrome was considered,
although not performed, and replaced by careful follow-up of the
peripheral blood count, blood smear and reticulocyte counts.
Subsequently, the patient's hematopoiesis recovered to baseline,
diminishing the possibility of other hematological malignancies or
bone marrow failure. No evidence of relapse was observed during the
next 2-year and 2-month follow-up. No new complications occurred
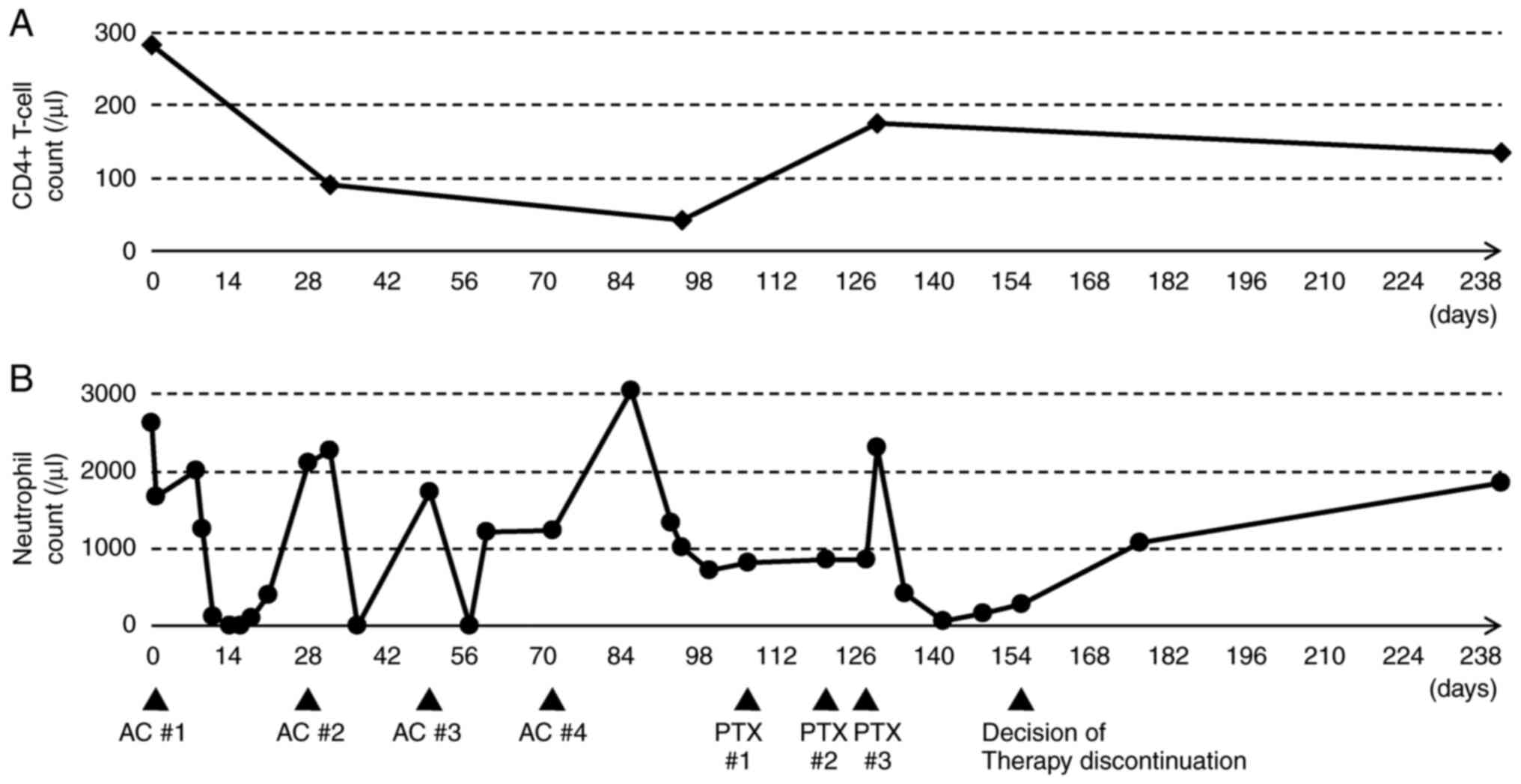
since the discontinuation of therapy. Fig. 3A and B represent a timeline graph
showing the chemotherapy cycles and blood counts during the course
of treatment in the present case.
Discussion
The present case report describes a patient with a
rare combination of ICL and breast cancer. To the best of our
knowledge, this is the first report describing the detailed
clinical course of this combination. The patient experienced severe
cytopenia throughout the course of adjuvant chemotherapy and was
forced to discontinue treatment.
Although the genetic etiologies are unclear in most
patients with ICL, certain studies have reported various germline
deficiencies in genes associated with T-cell development,
hematopoiesis and cytokine signaling (1,3,4).
Clinical features of this patient may suggest monoMAC syndrome, a
disorder sharing multiple presentations with ICL (6), but appeared unlikely due to the
absence of a family history, disorders in B cells and monocytes,
and absent hallmarks of underlying bone marrow failure.
Furthermore, normal presentations in bone marrow testing and no
abnormalities in follow-up blood smears lowered the possibility of
myelodysplastic syndrome. Detailed profiling of T cells and genetic
testing have not been performed in this patient due to the lack of
patient request, which may provide a clue to the pathogenesis of
ICL and cytopenia during chemotherapy. As the relationship between
the incidence of cytopenia during anticancer chemotherapy and ICL
remains unclear, further data accumulation is crucial.
Research on chemotherapy administration for
HIV-infected patients in the pre-antiretroviral therapy era
demonstrates a highly elevated risk of opportunistic infections in
patients with CD4+ T-cell deficiency, such as those treated with
liposomal doxorubicin (7),
paclitaxel (8), and a combination
of cyclophosphamide, doxorubicin and etoposide (9). Therefore, prophylactic drug
administration against opportunistic infections is crucial in the
management of CD4+ T cell-deficient patients (10). Among the prophylactic drugs used,
TMP-SMZ and VGCV commonly cause cytopenia (11). TMP-SMZ decreases blood counts by
inhibiting folate metabolism, consequently downregulating
granulopoiesis and erythropoiesis. Folate supplementation may have
reversed this side effect (11),
and pentamidine, dapsone and atovaquone may replace TMP-SMZ
(10), although this was not
attempted in the present case. VGCV is myelotoxic and a higher dose
(900 mg/day) is significantly associated with leucopenia (12). In transplant patients, a
meta-analysis revealed that a lower dose of VGCV (450 mg/day) had
equivalent effects on the prevention of CMV infection and a lower
risk of leukopenia than a higher dose (900 mg/day) (13), which may be considered in patients
with ICL. The present case had a history of CMV retinitis and
required secondary prophylaxis; therefore, a lower dose was not
administered.
Cytopenia during chemotherapy may be attributed to
CD4+ T-cell deficiency. Studies on patients with breast cancer and
HIV, a condition that also decreases the number of CD4+ T cells,
have reported elevated chemotherapy-related hematological toxicity
(14). Another study demonstrated a
lower relative dose intensity (RDI) in patients with breast cancer
with HIV infection, leading to worse outcomes (15). Multiple studies have reported
life-threatening cytopenia in HIV-infected patients receiving
anticancer chemotherapies (16–18).
The effects of antiretroviral therapies, commonly causing drug-drug
interactions, cannot be disregarded; however, similar complications
in patients with HIV and ICL receiving chemotherapy give rise to
the possibility that CD4+ T cell-deficient patients are prone to
develop cytopenia during chemotherapy.
The present study reported a rare case of breast
cancer occurring in a patient with ICL. The patient experienced
unusually severe cytopenia that led to treatment discontinuation.
The present study describes only a single case, which limits the
evidence regarding patients with ICL being more prone to developing
severe cytopenia and requiring particular attention to
complications such as opportunistic infections compared to those
without. In the future, cohort studies of patients with ICL
undergoing anticancer chemotherapy should be performed to support
this conclusion. Further investigations into the relationship
between ICL and cancer, the causes of cytopenia and the methodology
for maintaining the RDI in CD4+ T cell-deficient patients may
benefit patients with both ICL and HIV.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KM drafted the manuscript. AS revised the
manuscript. KM, AS, KW, HK, KN, DK and CS were involved in the
treatment of the patient. All authors read and approved the final
manuscript. KM and AS confirm the authenticity of the raw data.
Ethics approval and consent to
participate
This study was exempt from ethical approval by the
National Center for Global Health and Medicine Institutional Review
Board (Tokyo, Japan). Written informed consent for participation
was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images.
Competing interests
The authors have no competing interests to
declare.
Glossary
Abbreviations
Abbreviations:
|
ICL
|
idiopathic CD4+ lymphocytopenia
|
|
HIV
|
human immunodeficiency virus
|
|
RDI
|
relative dose intensity
|
|
CMV
|
cytomegalovirus
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor 2
|
|
TMP-SMZ
|
trimethoprim-sulfamethoxazole
|
|
VGCV
|
valganciclovir
|
|
AC
|
doxorubicin plus cyclophosphamide
|
|
PTX
|
paclitaxel
|
References
|
1
|
Walker UA and Warnatz K: Idiopathic CD4
lymphocytopenia. Curr Opin Rheumatol. 18:389–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmad DS, Esmadi M and Steinmann WC:
Idiopathic CD4 lymphocytopenia: Spectrum of opportunistic
infections, malignancies, and autoimmune diseases. Avicenna J Med.
3:37–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Régent A, Autran B, Carcelain G, Cheynier
R, Terrier B, Muylder BCD, Krivitzky A, Oksenhendler E,
Costedoat-Chalumeau N, Hubert P, et al: Idiopathic CD4
lymphocytopenia: Clinical and immunologic characteristics and
follow-up of 40 patients. Medicine (Baltimore). 93:61–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yarmohammadi H and Cunningham-Rundles C:
Idiopathic CD4 lymphocytopenia: Pathogenesis, etiologies, clinical
presentations and treatment strategies. Ann Allergy Asthma Immunol.
119:374–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiels MS, Cole SR, Kirk GD and Poole C: A
meta-analysis of the incidence of non-AIDS cancers in HIV-infected
individuals. J Acquir Immune Defic Syndr. 52:611–622. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu VH, Curry JL, Elghetany MT and Curry
CV: MonoMAC versus idiopathic CD4+ lymphocytopenia. Comment to
Haematologica. 2011.96((8)): 1221–5. Haematologica. 97:e9–e11.
2012.PubMed/NCBI
|
|
7
|
Hengge UR, Esser S, Rudel HP and Goos M:
Long-term chemotherapy of HIV-associated Kaposi's sarcoma with
liposomal doxorubicin. Eur J Cancer. 37:878–883. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saville MW, Lietzau J, Pluda JM,
Feuerstein I, Odom J, Wilson WH, Humphrey R, Feigel E, Steinberg S
and Broder S: Treatment of HIV-associated Kaposi's sarcoma with
paclitaxel. Lancet. 346:26–28. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sparano JA, Hu X, Wiernik PH, Sarta C,
Reddy DM, Hanau L and Henry DH: Opportunistic Infection and
immunologic function in patients with human immunodeficiency
virus-associated Non-Hodgkin's lymphoma treated with chemotherapy.
J Natl Cancer Inst. 89:301–307. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torres HA and Mulanovich V: Management of
HIV infection in patients with cancer receiving chemotherapy. Clin
Infect Dis. 59:106–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khalil MAM, Khalil MAU, Khan TFT and Tan
J: Drug-induced hematological cytopenia in kidney transplantation
and the challenges it poses for kidney transplant physicians. J
Transplant. 2018:94292652018.PubMed/NCBI
|
|
12
|
Brum S, Nolasco F, Sousa J, Ferreira A,
Possante M, Pinto J, Barroso E and Santos J: Leukopenia in kidney
transplant patients with the association of valganciclovir and
mycophenolate mofetil. Transplant Proc. 40:752–754. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalil AC, Mindru C and Florescu DF:
Effectiveness of valganciclovir 900 mg versus 450 mg for
cytomegalovirus prophylaxis in transplantation: Direct and indirect
treatment comparison meta-analysis. Clin Infect Dis. 52:313–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SN, Zhu Y, Chumsri S, Kesmodel S,
Gilliam BL and Riedel DJ: Outcomes and chemotherapy-related
toxicity in HIV-infected patients with breast cancer. Clin Breast
Cancer. 14:e53–e59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martei YM, Narasimhamurthy M, Setlhako DI,
Ayane G, Ralefala T, Chiyapo S, Gross R, Shulman LN, Grover S and
DeMichele A: Relative dose intensity and pathologic response rates
in patients with breast cancer and with and without HIV who
received neoadjuvant chemotherapy. JCO Glob Oncol. 8:e22000162022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ngidi S, Magula N, Sartorius B, Govender P
and Madiba TE: Incidence of chemotherapy-induced neutropenia in
HIV-infected and uninfected patients with breast cancer receiving
neoadjuvant chemotherapy. S Afr Med J. 107:595–601. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bundow D and Aboulafia DM: Potential drug
interaction with paclitaxel and highly active antiretroviral
therapy in two patients with AIDS-associated kaposi sarcoma. Am J
Clin Oncol. 27:81–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Rayes BF, Berenji K, Schuman P and
Philip PA: Breast cancer in women with human immunodeficiency virus
infection: Implications for diagnosis and therapy. Breast Cancer
Res Treat. 76:111–116. 2002. View Article : Google Scholar : PubMed/NCBI
|