Head and neck cancers are a group of malignancies
that occur in various head and neck regions, including the oral
cavity, throat, voice box and nasal cavity. Head and neck cancers
account for ~4% of all cancer cases worldwide (1). The incidence varies globally, with
higher rates in certain regions, such as Southeast Asia, where
tobacco and betel nut use is prevalent (2). The primary risk factors for head and
neck cancers include tobacco use (including smoking and smokeless
forms) and alcohol consumption (3).
Human papillomavirus (HPV) infection, particularly HPV-16, is a
significant risk factor for oropharyngeal cancer (4). Men are more commonly affected by head
and neck cancers than women (5).
The incidence increases with age, with most cases diagnosed in
individuals >50 years (6). The
specific sites affected by head and neck cancers include the oral
cavity (including the tongue, gums and lips), pharynx (including
the oropharynx and hypopharynx), larynx, nasal cavity and paranasal
sinuses (7). Squamous cell
carcinoma (SCC) is the most common histological type, accounting
for most head and neck cancer cases. SCC accounts for 90–95% of all
head and neck cancer cases (8).
Other less common types include salivary gland tumors, lymphomas
and sarcomas (9).
The prognosis for head and neck cancers depends on
several factors, such as the stage of the disease at diagnosis, the
tumor's location and the patient's overall health. Early detection
and timely treatment significantly improve the chances of
successful outcomes (10).
Prevention and early detection through regular dental and medical
check-ups, lifestyle modifications (avoiding tobacco use and
excessive alcohol consumption) and vaccination against HPV (for
oropharyngeal cancers) are essential in reducing the burden of head
and neck cancers (11).
Head and neck cancer often presents a challenging
and complicated situation, with low survival rates for
advanced-stage patients and minor improvement in survival rate over
time (12). Clinical treatment
strategies include surgical procedures, chemotherapy, radiotherapy,
immunotherapy and specific combinatorial approaches (13). Immunotherapy has received an
increasing amount of attention and is considered the first line of
treatment for patients with head and neck cancer (14). Clinical preliminaries of immune
checkpoint inhibitors, monoclonal antibodies, adoptive T-cell
therapy and chimeric antigen receptor (CAR) T-cell therapy show
promising outcomes for head and neck cancer treatment (15). However, there is variation in
patients with head and neck cancer; thus, combining immunotherapy
with other treatment approaches such as surgery, chemotherapy and
radiotherapy presents a significant clinical advantage in treating
head and neck cancer (16). In
addition, numerous adverse effects remain to be eliminated to
optimize the clinical potentials of immunotherapy, including
incidental effects, patient choice, selection of known biomarkers
and the choice of novel immunotherapy (17). Immunotherapy has so far demonstrated
high efficacy for managing intermittent and metastatic cancer
(18). A better understanding of
the immune system, and its influence on the progression and spread
of head and neck cancer can lead to the discovery of new
biomarkers. These biomarkers can be used to categorize patients
into specific treatment plans, thereby conserving medical resources
and ensuring timely and optimal treatment for each individual
(19). Whilst the head and neck
region exhibits significant anatomical variations in comparison to
other parts of the body, there are several challenges in managing
head and neck cancer, since most confer a poor prognosis (20). Clinically, head and neck cancer are
challenging to treat with chemotherapy, radiotherapy and surgery,
since metastasis is common in a number of patients and this cancer
has a high chance of reoccurrence (21) (Fig.
1). Despite these challenges, immunotherapy shows significant
therapeutic potential for patients with cancer, since
immunotherapies can induce an immune response aiming to recognize
and eliminate cancer for a while (22). Utilizing immunotherapy,
chemotherapy, radiotherapy and surgery, alone or in combination,
brings high efficacy in patients with head and neck cancer.
Treatment efficacy in patients with head and neck cancer depends on
whether the tumor is benign or malignant (23).
Head and neck SCC (HNSCC) has comparable etiologies,
pathogenesis and therapeutic responses with other types of tumors
(24). Neoplasm growth is favorable
in organs lined with mucosa and in cells and tissues such as
neuroendocrine cells, lymphoid tissue, minor salivary gland tissues
and melanocytes. These cancers differ from the biology of HNSCC and
have a different natural history (25). HNSCC manifests as a persistent sore
throat, difficulty swallowing or a lump in the neck (26). By contrast, the symptoms of other
types of cancer, such as lung cancer, may include persistent cough,
shortness of breath or chest pain (27). Similarly, malignancies of the
thyroid and major salivary gland act differently to HNSCC.
Previously, head and neck cancer treatments were primarily limited
to surgeons and radiation oncologists (28,29).
However, advancements in medical knowledge and technology have
expanded the range of specialists involved in managing this
condition over time. Today, a multidisciplinary approach involves a
team of healthcare professionals such as surgeons, radiation
oncologists, medical oncologists, otolaryngologists, maxillofacial
surgeons, speech and swallowing therapists, nutritionists and
social workers (30). These
specialists collaborate to provide comprehensive care, tailoring
treatment plans to individual patient needs and improving outcomes
for those affected by head and neck cancer (31). There have been significant
advancements in surgery, radiotherapeutics, chemotherapy and
immunotherapy as treatment approaches. In radiotherapy, the
development of different fractionating schemes and
intensity-modulated radiotherapy has enhanced the delivery and
tolerance of radiation (32). Organ
function conservation is improved through the advancement in
conservative surgical procedures, including laryngeal prosthesis,
laser surgery and hemilaryngectomy (33).
Chemotherapy is a significant component in the
multimodality therapeutic strategies for advanced HNSCC, and the
United States Food and Drug Administration (FDA) approval of
various immunotherapies also brings hope to more patients with
HNSCC (34).
The multidisciplinary approach aiming to treat HNSCC
is unpredictable but is advancing. Several therapeutic approaches
are outlined in the current review, representing significant
achievements that can change the ideal treatment plan and results
in patients with HNSCC. Immunotherapy represents a chance to
improve the adequacy of conventional treatments. In fact,
immunotherapy has significantly enhanced the therapeutic scene for
patients with malignancy. Programmed cell death-ligand 1 (PD-L1)
and programmed cell death protein-1 (PD-1) checkpoint inhibitors
are the front lines of this clinical approach (35). The current review describes some new
improvements in HNSCC, highlighting the efficacy of the use of
immunotherapy combined with other therapies for improving the
prognosis of HNSCC. It also outlines the current challenges and
future perspectives for further research and clinical translation
aiming to improve overall survival.
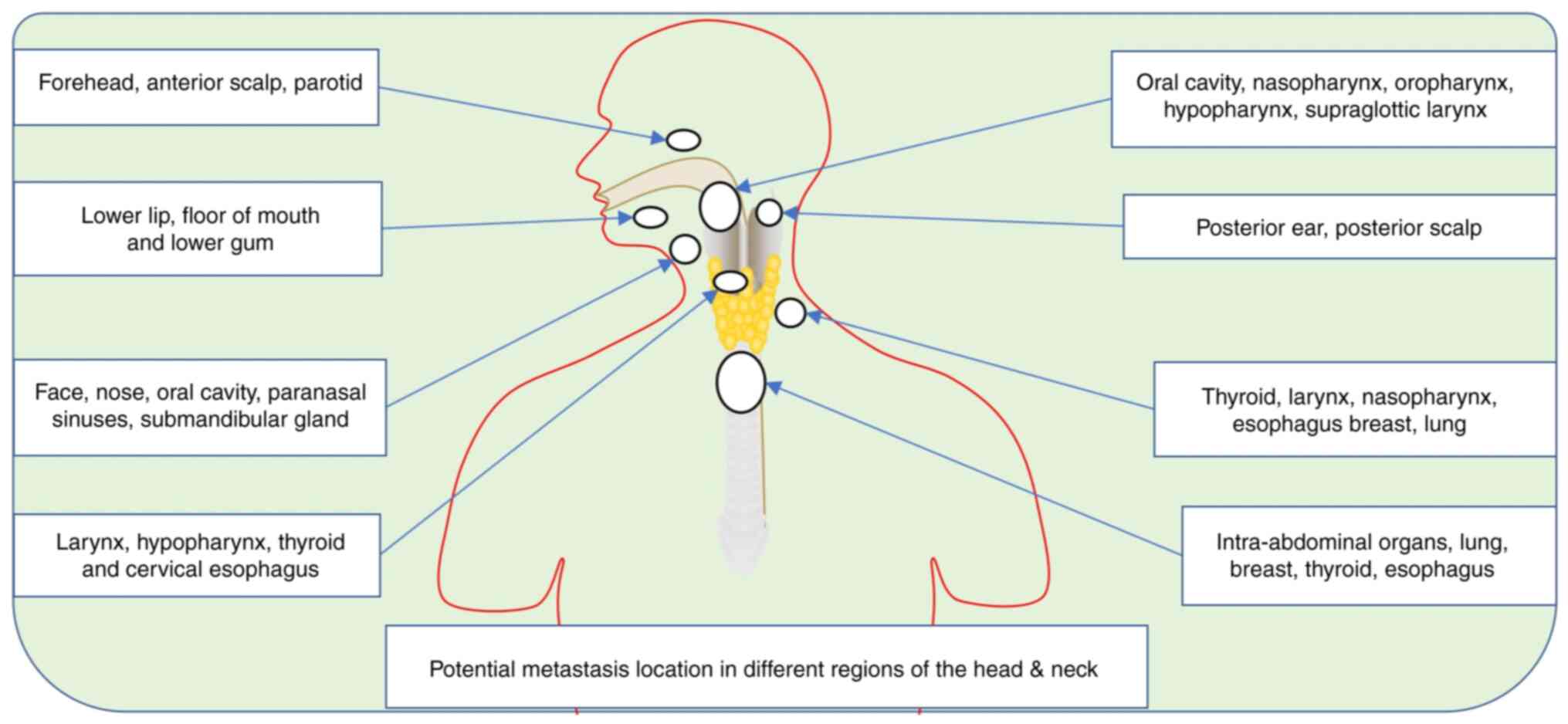
The head and neck are classified into differential
anatomical sections: Nasal-cavity, paranasal sinuses, oral cavity,
pharynx and larynx (36). The
pharynx comprises the nasopharynx, oropharynx and hypo-pharynx, as
the larynx comprises the supraglottic, glottic and subglottic
regions (37). Most patients
present with variable features based on the anatomical location of
the tumor (38). In most early
cases, the patients will present symptoms that are difficult to
diagnose just with a physical examination (Table I). Most HNSCC cases occur in
cigarette smokers and alcohol consumers. The rate and duration of
smoking and drinking increase the patient's chances of having oral
cavity cancer (HNSCC) (7,39). Geographical location is also an
influential factor for HNSCC; as frequently reported by the World
Health Organization, exposure to pollution and some viral agents
also increases the incidence of HNSCC (40). However, gene mutation and other
genetic factors are also contributing agents for HNSCC (41), which require further research, since
the mechanisms are not well understood.
HNSCC also affects a percentage of individuals
without the typical risk factors for these neoplasms. Subjects with
HNSCC who do not smoke and drink tend to be younger and have a
primary neoplasm in the lingual or palatine tonsils (42). Within this category of patients, HPV
is linked to HNSCC pathogenesis (5). HPV oncoproteins E6 and E7 inactivate
tumor suppressor genes within the host cells, enhancing cell cycle
control and suppressing programmed cell death based on the
hypothesized cancer mechanism (43). Different types of cancers exhibit
unique genetic and molecular attributes; at the same time, other
factors like the tumor microenvironment and interactions with the
immune system significantly contribute to cancer growth and
progression (44). The oral cavity
and the pharynx are the most common sites for HPV-related
malignancies, and although the larynx is not one of them (larynx is
primarily associated with other risk factors, such as tobacco and
alcohol use, and exposure to environmental carcinogens), 85–90% of
HPV-positive HNSCC is HPV-16 (45,46).
It is unknown whether HPV and cigarettes or alcohol have any
connection, and more research is required (47). Sexually transmitted diseases, such
as human immunodeficiency virus infection, commonly spread through
indiscriminate sexual partners and via oral and anal intercourse,
and have all been linked to HPV-positive HNSCC (48). Increased alterations of genes
previously implicated in the formation of HNSCC and exacerbated
HPV-mediated carcinogenesis are caused by abnormal DNA repair and
chromosomal destabilization, typical of this cancer (49). HNSCC with HPV appears to have a
better prognosis than HNSCC without HPV (5). HPV-positive cancers appear to be
highly radiosensitive, according to research (50). The lack of field cancerization and
concomitant diseases, such as chronic obstructive pulmonary disease
or cirrhosis, which influence the individual subject's overall
prognosis, are potentially responsible for superior results
(51). The discovery of HPV in
HNSCC has both epidemiological and therapeutic implications, as
individuals with HPV-positive malignancies are highly
radiosensitive, thus helping doctors to choose individual patients
for specific therapeutic approaches (50). The usage of HPV vaccines for
cervical tumors might potentially help to prevent HPV-positive
HNSCC (52). Poor oral health is
also associated with HPV and can modify the oral microbiota
(12).
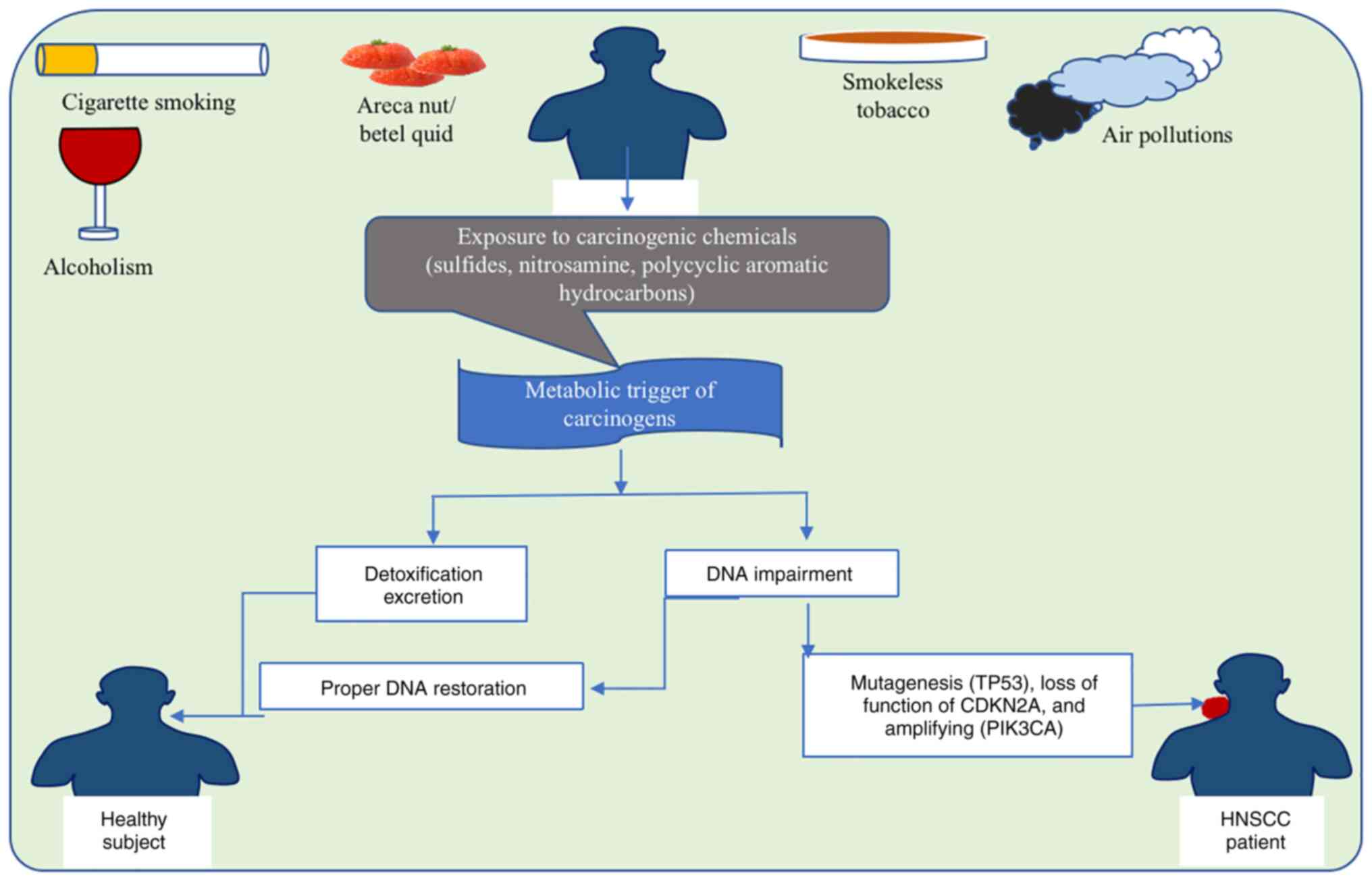
Several risks for HNSCC are geographically,
habitually and culturally prevalent, with smoking and alcoholism
scoring among the high-risk variables globally (53). It is worth noting that abusers of
both tobacco and alcohol have an up to 35 times higher increased
risk of HNSCC compared with non-tobacco and alcohol users (54). Tumor of the oral cavity has been
linked to areca nut chewing, specifically a variety of customized
combinations containing areca nut (Areca catechu; the
carcinogen source), betel leaf (Piper betle leaf), slaked
lime and tobacco, as well as spices commonly known as betel quid
according to local custom (55).
Oral cavity cancer is associated with the products of areca nut or
betel quid consumption in India (the 1st and 4th most frequent
neoplasm, respectively, in both sexes of the Indian population),
Taiwan and several regions in China mainland (56). The high male/female ratios with
HPV-negative HNSCC incidence indicate sex-specific patterning of
modifiable risk behaviors, such as tobacco, smokeless tobacco,
areca nut, betel quid and alcohol use (Fig. 2). The impact of electronic
cigarettes on the risk of HNSCC is unclear and will only become
apparent over the next few decades (57).
Carcinogenic air pollutants, such as organic and
inorganic compounds, are also risk factors for HNSCC, particularly
in developing nations/areas where air pollution is high, including
China and other Asian and African countries (58). Other risk factors include age,
improper dental hygiene and insufficient diet (59). Persistent HPV and EBV infections are
recognized HNSCC etiological risk factors from the oropharynx and
nasopharynx (60). The ratio of men
to women is typically higher for oropharyngeal cancer, which is the
most common site of HPV-associated HNSCC. Studies have reported a
male-to-female ratio ranging from 2:1 to 4:1 for HPV-positive
oropharyngeal cancer (61,62); this means that the incidence of
HPV-positive oropharyngeal cancer is generally higher in men than
in women (63). HPV infection
resulting in HNSCC is mainly spread through oral intercourse, and
the occurrence of HPV-positive HNSCC is on the rise, particularly
in individuals without the HPV vaccine before exposure to HPV; in
some cases, HNSCC is influenced by hereditary factors (64). Patients with Fanconi anemia, an
uncommon genetic genealogical condition characterized by poor DNA
repair (due to mutants in any of the 22 Fanconi anemia genes), have
a 500–700 times higher risk of HNSCC, primarily oral malignancies
(65). Although the reasons behind
individuals with Fanconi anemia's predisposition for HNSCC are
unknown, changes in Fanconi anemia pathway genes have a potential
role (66).
Polymorphisms in genes implicated in carcinogen
metabolism and immunity, such as interleukin-10 (IL-10,
1082A>G), cytotoxic T-lymphocyte associated protein 4 (rs231775
and rs4553808), cytochrome P450 1A1 (Ile462Val) and glutathione
S-transferase µ1 (null polymorphism), are linked to an elevated
risk, as demonstrated in a recent study (67). Thus, a weaker immune system and a
decreased ability of carcinogen digestion may play a role in HNSCC.
Carcinogens, such as tobacco smoke and alcohol, are known risk
factors for HNSCC (68). The
ability of the body to metabolize and detoxify these carcinogens
can impact the likelihood of developing cancer (69). If the body's digestion and
detoxification processes are impaired, carcinogens may accumulate
and cause damage to the cells of the head and neck region,
potentially leading to the development of HNSCC (70). Reduced cigarette usage, proper oral
care and universal HPV immunization could all contribute to
lowering the universal HNSCC occurrence (71).
The TME in HNSCC is a heterogeneous mixture of tumor
cells and stromal cells, which incorporate endothelial cells,
cancer-associated fibroblasts (CAFs) and immune cells (72). Tumor cells and CAFs promote the
production of growth factors, such as vascular endothelial growth
factor (VEGF), which binds on endothelial cells, invigorating
neo-vascularization and supplying oxygen and nutrients to the tumor
(73). Consequently, endothelial
cells release factors that help the endurance and
self-reestablishment of circulatory immune cells (74). CAFs are vital in HNSCC maturation
and are discriminated from typical fibroblasts by the abundant
expression of α-smooth muscle actin (αSMA). CAFs release EGF, VEGF
and hypoxia growth factor, interleukin 6 (IL-6), cytokines and
chemokines that advance tumor cell development, angiogenesis and
enrollment of immune defensive cells (75). Furthermore, CAFs are a significant
cellular component within the TME, engaged with the degradation and
regeneration of the extracellular matrix and the reinforcement of
EGFs, VEGF and TGF-β matrix-embedded growth, which leads to further
enhancement of tumor cell multiplication, angiogenesis and
immunosuppression (76). Elevated
αSMA levels in HNSCC tumors predict a poor prognosis; HNSCC tumors
contain newly formed adrenergic neurons whose presence boosts tumor
development (77). TP53 has diverse
functions in neurons, including neuronal development, DNA damage
response and neuroprotection (78).
In HNSCC, TP53 alterations contribute to tumor development,
progression and therapy resistance (79). Tumor-infiltrating lymphocytes (TILs)
such as T cells, B cells and natural killer (NK) cells, as well as
myeloid ancestry cells, including macrophages, neutrophils,
dendritic cells and myeloid-derived suppressor cells (MDSCs), are
the immune entities of HNSCC TME cells (80). Immune cells can invade HNSCC tumors
under exceptional circumstances, for example in response to an
inflammatory response, tumor antigen recognition, chemokine signals
and tumor-induced angiogenesis, despite the fact that the
infiltration composition depends on the tumor's anatomical location
and its causative agent (81).
The response to therapy by HNSCC results from a
specific immune phenotype; the most favorable prognosis of HNSCC is
achieved with an increase in TIL level, which is reliant upon the
availability of antitumor responses, vs. those with
immunosuppressive activities in the TIL population (82). The TME of most HNSCC tumors is
profoundly immunosuppressive, and antitumor immunity in the TME is
mediated mainly by T effector (T eff) cells and NK cells. By
contrast, immune suppression and tumor cell growth are mediated by
T regulatory (T reg) cells, MDSCs and macrophages (83). An increase in the survival of
patients is based on CD8+ T eff cells and NK cells in
the TME (84). Paradoxically, T reg
cells, MDSCs, neutrophils or macrophages increase and are
associated with late-stage HNSCC (85). Most patients with HNSCC present with
a variation in HPV-positive and HPV-negative tumors (86). HPV-positive tumors regularly have a
more prominent presence of TILs compared with HPV-negative tumors
(87). Patients with HPV-positive
tumors and a number of TILs strongly respond to immunotherapy
treatment. However, patients with HPV-positive tumors containing
low content of TILs show survival rates close to those with
HPV-negative HNSCC (47,88). The HNSCC TME milieu is rich in
immunosuppressive components and cytokines that advance the
enrolment or activity of MDSCs, T reg cells and macrophages, while
hindering the antitumor effect of T eff and NK cells, IL-6, IL-10,
VEGF and TGF-β (89). The HNSCC TME
is rich in IL-10 and TGF-β, elevating macrophage polarization to an
immunosuppressive phenotype (90).
Furthermore, HNSCC tumors in significantly advanced stage cancer
show upregulation of PD-L1, which weakens the cytolytic activity of
T cells (60).
HSCC is one of the most immunosuppressive
malignancies. Impaired immune-effector cell function, abnormal
cytokine expression and increased T reg cell frequency in tumors
and circulation characterizes HNSCC (91). In addition, the presence of T regs
in patients with HNSCC characterized by high expression levels of
immune checkpoint ligands such as cytotoxic T-lymphocyte-associated
protein 4 (CTLA-4) effectively downregulates the antitumor function
of cytotoxic T cells (92).
Cetuximab, an EGFR-specific monoclonal antibody used as an
immunological intervention for locally progressive HNSCC at
intermediate and high risk, shows promising results in patients
with HNSCC (93). Combining
radiation therapy for locally advanced HNSCCs and cytotoxic
chemotherapy for recurrent/metastatic HNSCs together with
immunotherapy improves survival (94). However, cetuximab, as a single
agent, has limited effectiveness in the treatment of clinically
treated advanced HNSCC and the response rate or clinical benefit
rate of cetuximab as a standalone therapy is <15% (95). Therefore, numerous studies aim to
explore combination treatment options to enhance the effectiveness
(96). An attempt to use cetuximab
plus radiation therapy in combination with ipilimumab, in a phase I
clinical trial of CTLA-4 monoclonal antibody for locally advanced
HNSCC (NCT01935921), showed impressive results. This study first
explored radiotherapy plus dual-target immunity (cetuximab,
ipilimumab) for locally progressive HNSCC (97). Regular cetuximab plus intensity
modulated radiation therapy was administered at 5, 8, 11 and 14
weeks of treatment with ipilimumab (1 mg/kg). The method is not
only safe but also curative; the 3-year disease-free survival and
overall survival (OS) rates reached 72% [90% confidence interval
(CI), 57–92] and 72% (90% CI, 56–93) respectively, with no
dose-limiting toxicity (98). Head
and neck malignancies are increasing, and >90% of them are SCCs
(99).
Recently, immune checkpoint inhibitors targeted PD-1
and CTLA-4 have gained rapid development in the field of cancer
therapy (100). The US FDA and the
European Medicines Agency approved palivizumab and nivolumab for
the first-line/second-line treatment of relapsed/metastatic HNSCC
(101). However, >60% of
patients with HNSCC are at stage III or IV; for locally advanced
patients, the prognosis is still poor under the current
multidisciplinary treatment model, with a 5-year OS rate of ~50%
and the risk of local recurrence or metastasis of ~40% (102). Using immunotherapy as a
neoadjuvant or perioperative treatment option for HNSCC to improve
survival in patients in the early and middle stages is an excellent
point for further research (103).
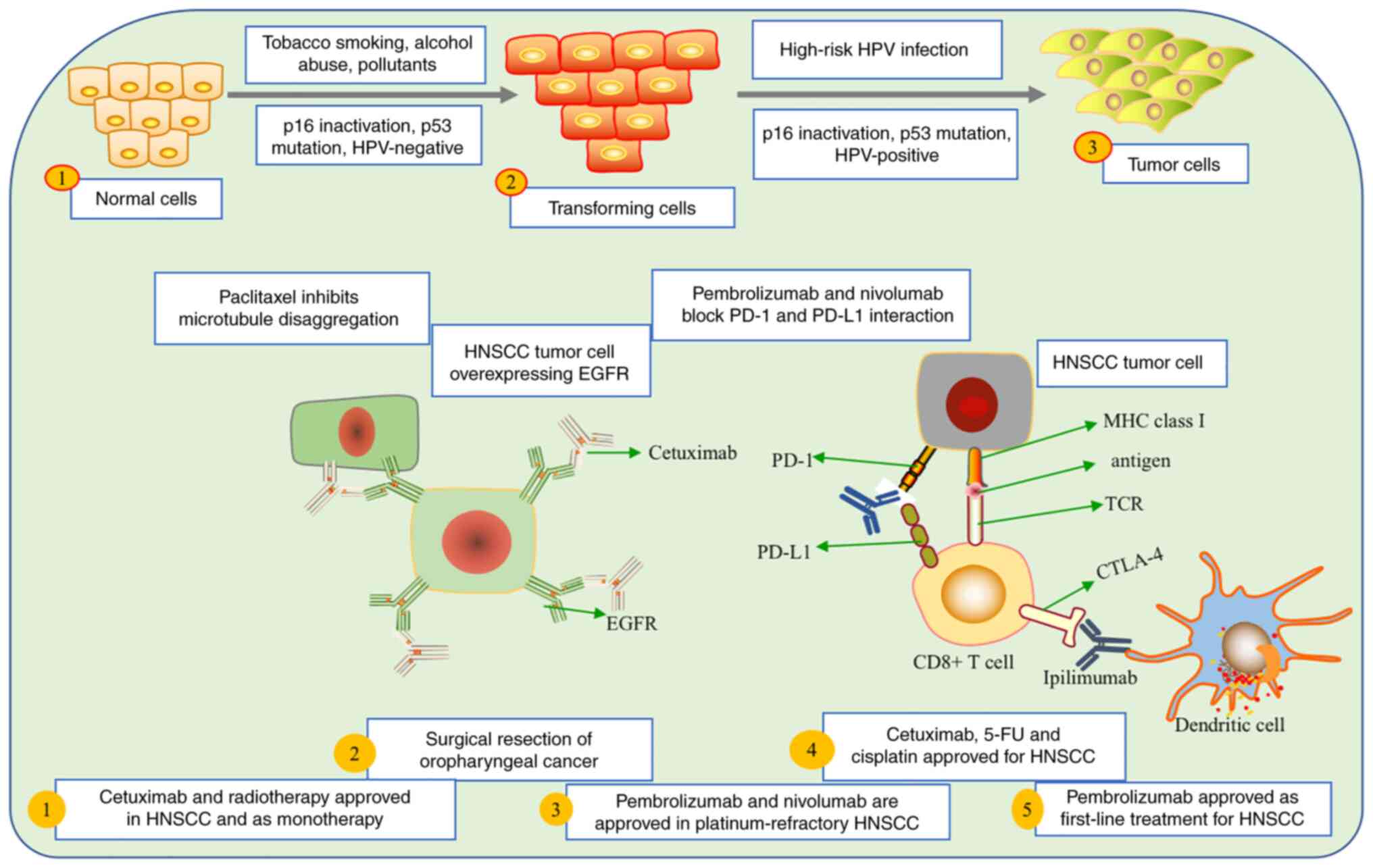
p16 and p53 are closely linked to HPV and its role in the
progression and prognosis of HNSCC (45). Various factors, such as tobacco
smoking, alcohol abuse and pollutants, can influence the
transformation of normal cells into tumor cells (104). Significant therapeutic
advancements have been made in the treatment of HNSCC. In 2006, the
FDA approved cetuximab as the first-line drug for recurrent and
metastatic HNSCC, marking a significant milestone (105). Surgical procedures remain the
primary option for removing non-metastasized oral cavity and
oropharyngeal cancer (106).
Furthermore, the FDA has approved nivolumab and
pembrolizumab for patients with platinum-refractory recurrent and
metastatic HNSCC. Cetuximab, 5-fluorouracil (5-FU) and cisplatin
have also received approval for treating patients with recurrent
and metastatic HNSCC (107)
(Fig. 3). Several therapeutic
agents exist for head and neck cancer with significant therapeutic
potential and efficacy; however, numerous patients cannot benefit
from these therapeutic approaches in full as they are at a more
advanced stage (108). When using
immunotherapy as a monotherapy or in combination with chemotherapy,
radiotherapy or surgery, a reasonable corrective improvement
outcome can be achieved, improving the patient's life and
prolonging the survival rate.
Immunotherapy has been one of the best therapeutic
achievements against HNSCC for almost 10 years. This achievement
can be attributed to immunosurveillance since the cancer cells need
to invade the TME and become clinically notable. Subsequently the
immune system fights against cancerous cells (109). The immune system patriates the
optimal protective role against the development and metastasis of
HNSCC. PD-1, which is primarily expressed on the surface of
activated T cells, particularly CD8+ cytotoxic T cells
and CD4+ helper T cells (110), acts as an immune checkpoint
receptor crucial in regulating T-cell responses and sends negative
feedback to abrogate the overactivation of T cells, thus preserving
homeostasis and preventing autoimmunity (111). However, tumors can take advantage
of this together with use of pre-existing inhibitory mechanisms
preventing destruction by the immune cells (112). Checkpoint blockade by monoclonal
antibodies senses the inhibitory signaling, which awakens T cells
to respond to the escaping tumors (113). Furthermore, some immune-related
adverse events can be caused by aberrantly activated autoreactive T
cells, among others, leading to inflammation in normal tissues
(62). In addition, the neo-antigen
peptides presented by the major histocompatibility complex activate
the immune system to recognize tumor cells (114). Tumor-mutational burden (TMB) is
connected with a mutation induced by smoking (signature 4
mutation-specific pattern of DNA damage caused by exposure to
tobacco smoke carcinogens), which can be a response to PD-1 pathway
blockade in both lung, and head and neck cancers (115). Nevertheless, some head and neck
carcinomas, such as oropharyngeal carcinomas, result from viral
agents such as HPV, leading to further comprehension of virally
triggered immune-oncology mechanisms, which may further support the
hypothesis that immunotherapy has the potential for optimal HNSCC
treatment (116). Nivolumab and
pembrolizumab have clinically demonstrated significant survival
benefits in a number of patients (94). In addition, patients accepting
anti-PD-1 agents appear not to show treatment-related adverse
events (117). Furthermore, a
phase I/II trial of durvalumab demonstrated a significant response
for an antibody against PD-L1 in patients with HNSCC (118). The importance of the immune system
in the progression and treatment of HNSCC has been appreciated for
the possibility of utilizing the immune system for memorizing and
eradicating cancer cells in preliminary clinical patients.
The immune phenotype of patients can be anticipated
based on the expression of biomarkers (71); clinically, combination therapy in
some specific patients induces an immunomodulatory response based
on the morphology of the HNSCC (119). The efficacy of cancer
immunotherapy depends on the immunological system's capacity to
detect tumor cells and develop a cancer-selective response, with
immunological memory possibly resulting in long-term cancer
management (120). Primary
resistance is a significant obstacle when the tumor appears,
causing a negligible response to immunotherapeutic agents or when
the tumor develops resistance, as well as when a tumor responds
initially but subsequently develops resistance, thus decreasing the
therapeutic efficacy of PD-L1-targeted treatment (121). An interaction exists between the
immune system and the tumor's development; the adaptive
immunological system takes advantage of both parties. The lack of
immunogenic antigen proteins or their delivery to immunological
systems are examples of T-cell-mediated resistance mechanisms
(122). Other inhibitory cells,
namely T reg cells, MDSCs and tumor-associated macrophages, can
hinder the function of cytotoxic T cells, and this inhibition is
observed in cases where PD-L1 expression is linked to cancer
development (123). Instead,
cancer cell-mediated, tissue-selective or acquired microenvironment
signal pathways might completely exclude T cells from the cancer
cells (124).
The current knowledge on prognostic and predictive
biomarkers must be improved in order to understand such a
complicated system (125). The
most frequently utilized technique to detect PD-L1 expression is
immunohistochemistry; the expression of PD-L1 is used as a
predictive marker for the response to specific treatments,
particularly immunotherapies targeting the PD-1/PD-L1 pathway
(35). An increased expression of
PD-L1 is associated with a higher response to PD-1/PD-L1 blockade
therapies (110). In addition,
tumors with higher levels of PD-L1 expression may have more
potential for interaction with PD-1 receptors on cytotoxic T cells,
leading to T-cell exhaustion and reduced antitumor immune response
(126). By blocking the
interaction between PD-L1 and PD-1, immunotherapies can restore
T-cell function and enhance antitumor activity as seen in some lung
tumors and HNSCC (127). Every
PD-L1-targeting antibody is accompanied by a diagnostic test
specifically designed for it, and these diagnostic tests possess
their own sensitivities and grading scales used to assess the level
of PD-L1 expression in tumor samples, thus highlighting the
importance of accurate diagnostic testing to determine the
suitability of PD-L1-targeted therapies for individual patients
(128). There is intra-tumoral
heterogeneity, and expression may depend entirely on which
metastatic location is affected and the changes in PD-L1 expression
levels over time (63). HNSCC can
metastasize to several region of the body, with higher levels of
expression in the liver and adrenal gland compared with those in
bone or brain metastases (129).
TMB is a new surrogate biomarker for immunotherapy response that is
still being investigated (130).
TMB is linked to responsiveness to checkpoint blockade in tumors
that have been shown to respond to immunotherapy, such as HNSCC,
melanoma and mismatch repair-deficient cancers (131). TMB measurement is dynamic and
changes depending on the platform used, similar to PD-L1 expression
(132). TMB evaluation is not yet
part of the clinical therapeutic strategy for lung tumors or HNSCC
(133). The need for more potent
prognostic and predictive biomarkers continues and will be critical
in improving patient selection for the expanding number of
treatments available (Fig. 4).
Patients diagnosed with metastatic or advanced-stage
HNSCC can benefit from a combined treatment approach involving
surgery and radiotherapy, which has demonstrated favorable
outcomes, particularly in cases of nasopharyngeal cancer (134). However, other patients prefer a
non-surgical approach; thus, the immune checkpoint inhibitor
pembrolizumab, an IgG4 humanized antibody to PD-1, is considered
the first-line treatment (135). A
stage III preliminary study investigated the therapeutic benefits
of pembrolizumab as a monotherapy or in combination with
platinum-based chemotherapy drugs (5-FU or cisplatin) and cetuximab
for patients with HNSCC, and demonstrated promising outcomes
(136). Chemotherapy, in addition
to pembrolizumab, further enhances efficacy, compared with
chemotherapy combined with cetuximab, causing less toxicity to
major organs (spleen, liver, heart, liver and kidney) (137). Pembrolizumab alone has low
efficacy compared with combined chemotherapy and cetuximab for
patients with HNSCC (62). Among
patients characterized by the expression of the biomarker PD-L1,
pembrolizumab monotherapy ensures a higher survival rate compared
with chemotherapy combined with cetuximab (138). Nevertheless, combining
chemotherapy with other cancer drugs shows a higher improvement
rate in patients with HNSCC compared with monotherapy.
Pembrolizumab and chemotherapy in patients with HNSCC demonstrate a
superior efficacy as compared with pembrolizumab as a monotherapy
(139). Hyper-progression is
described in HPV-negative patients with local or regional tumor
reoccurrence when immunotherapy is used without chemotherapy
(140). Even though progression is
related to a poor survival rate, most of the adverse events are
manageable before the administration of chemotherapy (141). It is crucial to promptly adjust
the treatment to enhance effectiveness while limiting
immune-related adverse effects, such as pneumonitis, colitis and
multiorgan injury (142). Patients
who cannot receive first-line immunotherapy treatment may receive
cetuximab combined with chemotherapy and platinum with 5-FU or
paclitaxel (143).
In patients with cisplatin-resistant conditions, it
is crucial to reconsider the expression of PD-1 in those with
multiple concurrent cancers since PD-1 inhibitors can be used to
overcome drug resistance in patients with HNSCC (144). When the prognosis for survival is
not optimal, PD-1 inhibition can be an option for patients
irrespective of the state of autoimmunity, which is worsened by
immune checkpoint inhibitors in patients with cisplatin-refractory
disease (145). PD-1 inhibition
can improve survival with optimal efficacy in patients with HNSCC
compared with the use of nivolumab or pembrolizumab monotherapy in
patients with cisplatin-refractory disease (146). An initial clinical study reported
on new immunotherapies for patients with metastatic or recurrent
HNSCC (147), and the
investigation of combined immuno-chemotherapy is ongoing (Fig. 3).
Surgery, radiotherapy and chemotherapy are
considered the best therapeutic options for HNSCC, with the
principal purpose being to free the patients from cancer and
prevent reoccurrence (84). In most
patients with oral cavity cancer, surgical procedures are most
likely to be the best option, whilst radiotherapy is considered the
best option for patients with pharyngeal and laryngeal cancers
(148). Advances in invasive
resection, such as transoral automated robotic surgery or laser
resection and larynx-saving partial laryngectomy, as well as
advanced reconstructive procedures, and improved knowledge of the
signs indicating the need for essential surgical management of
patients with head and neck cancer have been broadened (149). Unexpected metastases noticed in
draining cervical lymph nodes in some patients with small,
intrusive tumors demand the use of dissection to improve survival
(150). In case of failure of
therapy after the use of a single methodology, radiotherapy or
surgical procedure, the use of a different elective methodology
offers a higher probability of success (151). Postoperative radiotherapy or
chemoradiotherapy can ensure extended survival and minimize the
risk of tumor recurrence for advanced tumors or those that have
spread to nearby lymph nodes (152).
Immune checkpoint inhibitors are being examined in
preliminary studies in the therapeutic setting and in combination
with other treatment modalities (153). Radiotherapy can positively affect
cancerous cells and tissues by enhancing antitumor immune reactions
(98). When immunotherapy is
combined with radiotherapy, radiotherapy may improve the impact of
immunotherapy by advancing the release of cytokines and
tumor-associated antigens (154,155). Durvalumab in preclinical trials
after chemoradiotherapy in patients with stage III HNSCC increases
the survival rate when combined with chemoradiotherapy monotherapy
(156). Numerous stage I/II
preliminary studies on locoregionally progressed HNSCC are
exploring a combinatorial approach, adding anti-PD-l antibodies to
chemoradiotherapy (157). The
potential of combining immunotherapy and radiotherapy in HNSCC is
not well known, thus further investigation into the combination of
radiotherapy with immunotherapy approaches is required.
Numerous preliminary studies are currently assessing
combinatorial treatments, including immune checkpoint inhibitors,
therapeutic vaccines, co-stimulatory agonists and cytotoxic agents
(158). Combinations of
anti-CTLA-4 and anti-PD-1 antibodies show a synergistic effect in
patients with melanoma and are currently being tested in patients
with stage III HNSCC (156). The
anti-CTLA-4 antibody tremelimumab in combination with the
anti-PD-L1 antibody durvalumab in patients with metastatic HNSCC
demonstrated an increased efficacy compared with either
tremelimumab or durvalumab monotherapy (159). Numerous antibodies have
additionally been scrutinized in stage I/II preliminary studies for
patients with HNSCC (160).
Furthermore, laherparepvec, in combination with cisplatin and
radiotherapy, showed an increase in survival rate (161,162). A phase II study on the combination
of nivolumab with the synthetic long-peptide HPV-16 vaccine
demonstrated promising outcomes, with a superior response compared
with that of anti-PD-I treatment alone, thus supporting the need of
further investigation on the use of combinatorial immunotherapy for
higher clinical efficacy.
Combining immunochemotherapy and immunoradiotherapy
for head and neck cancer can improve prognosis and treatment
outcomes. This approach uses chemotherapy and radiotherapy to
enhance the immune response against cancer cells (163). Chemotherapy aims to destroy cancer
cells throughout the body and reduce the size of tumors; it can
also help sensitize the cancer cells to the effects of radiotherapy
(164). Radiotherapy targets
specific areas where the tumor is located, using high-energy
radiation to destroy cancer cells (165). Combining these treatments with
immunotherapy, which activates the body's immune system to
recognize and attack cancer cells, has a synergistic effect
(104). Immunotherapy helps in
enhancing the immune response, making it more effective in
recognizing and eliminating cancer cells (166). However, the prognosis of combining
immunochemotherapy and immunoradiotherapy for head and neck cancer
can vary depending on several factors, such as the stage and type
of cancer, the overall health of the patient and the individual
response to treatment (167).
Clinical trials and ongoing research are essential for evaluating
the effectiveness of these combined approaches and determining
their impact on long-term prognosis.
CAR T-cell therapy involves modifying a patient's
immune cells, particularly T cells, by introducing a synthetic
receptor called CAR onto their surface (168). This receptor empowers T cells to
recognize and bind to specific proteins known as antigens in cancer
cells, resulting in their destruction. CAR T-cell treatment offers
several advantages in addressing head and neck cancer (169). Firstly, these tumors often
overexpress specific antigens such as EGFR or HER2, which can be
targeted by CAR T cells (170). By
selectively attacking cancer cells and sparing normal cells, CAR T
cells reduce the risk of off-target effects. Secondly, CAR T-cell
therapy can provide long-term antitumor benefits (171). Once the modified CAR T cells are
introduced into the patient, they have the potential to persist and
continuously identify and eliminate cancer cells (172). This sustained response is
particularly beneficial for treating recurrent or metastatic head
and neck malignancies that are challenging to address using
conventional therapies (169). For
instance, a preclinical study has demonstrated the effectiveness of
CAR T cells targeting EGFRVIII, a type of EGFR widely expressed in
head and neck malignancies (173).
Furthermore, clinical studies focusing on CAR T-cell
treatment targeting HER2 have shown positive outcomes, with some
patients experiencing significant tumor shrinkage and extended
survival (170). However, it is
essential to note that there are still obstacles to overcome in CAR
T-cell therapy for head and neck cancer. The hostile tumor
microenvironment poses a significant barrier, hindering T-cell
activity and infiltration into the tumor (174). Combination therapies with immune
checkpoint inhibitors or cytokine injections are being explored to
enhance T-cell persistence and resistance to the immunosuppressive
tumor environment (168). Another
concern is the potential toxicity associated with CAR T-cell
treatment, such as cytokine release syndrome and neurotoxicity
(175). These systemic
inflammatory responses can range from mild symptoms to
life-threatening complications following CAR T-cell infusion
(176). Efforts are underway to
improve patient selection, dosing strategies and supportive care
measures to better understand and manage these toxicities (176). Ongoing research and clinical
trials provide valuable insights into optimizing the effectiveness
and safety of CAR T-cell therapy (177). With further advancements and
refinements, CAR T-cell therapy has the potential to become an
essential addition to the treatment options available for head and
neck cancer, offering new hope for patients facing this challenging
disease.
Several cancer therapies exist, the most recent
being cancer immunotherapy, which significantly improves cancer
treatment. Regardless of the achievements in cancer immunotherapy,
the response in patients is regularly limited and not long-lasting
(178). This is caused by multiple
tumor-mediated immune escape mechanisms. The head and neck
malignant growth rate changes across nations depending on hazardous
factors, including alcohol and tobacco utilization and the
comorbidity with HPV infection (179,180). Tumors positive for HPV overexpress
the viral E6 and E7 antigens, which can be recognized by the immune
system, thus stimulating an immune response (181). Current investigations suggest that
the T cells responsible for the reoccurrence in HPV-positive tumors
do not perceive these viral antigens but tumor neo-antigens or
germline antigens (182).
Promising antitumor viability in a murine HPV-16 E7
antigen-expressing tumor model, utilizing various combinations of
E7 peptide antibodies, has been reported (183). This restorative adequacy can
essentially be upgraded by combining PI3K-AKT pathway inhibitors
with PD-1, PD-L1 and CTLA-4, and tumor necrosis factor (TNF)
receptor superfamily member 4 (OX-40) and TNF receptor
family-related protein TNF receptor superfamily member 18 (GITR)
(184).
Combining various therapeutic approaches gives
patients with HPV-positive head and neck cancer a promising
outcome. Regardless of the clinical advantage of agents utilizing
the synergy between PD-1 and PD-L1, most patients do not benefit
from this treatment (185). T-cell
agonist antibodies targeting GITR and OX-40 have entered
preliminary clinical studies, and promising outcomes in preclinical
mouse models anticipate clinical utilization (123,186,187). A few clinical preliminary studies
are testing the efficacy of cancer immunotherapy in head and neck
tumors (126,188).
Whilst immunotherapy has shown promising results,
the high development, production and administration costs
contribute to its expensive price tag. The high cost of
immunotherapy can limit access for patients who may benefit from
this treatment (107). It is a
complex issue involving several factors, such as research and
development expenses, manufacturing costs and ongoing clinical
trials. Efforts are being made to address this issue (189). Some countries have implemented
healthcare policies to make immunotherapy more affordable and
accessible (190). Additionally,
ongoing research and advancements in medical technology may lead to
more cost-effective approaches to immunotherapy in the future.
Numerous researchers are examining the preclinical
adequacy of new combinations while interpreting the specific
mechanism of different treatments. There is promising potential in
consolidating various therapeutic agents, including immunotherapy,
chemotherapy and radiotherapy (191). Further understanding of the
correlations among multiple therapies is needed, and the current
review outlines how researchers can proceed in the future. It is
also essential to evaluate the adequacy of various therapies before
combining different immunotherapeutic agents (192). Finally, immunotherapy has shown a
high efficacy for aerodigestive malignancies and has paved the way
for an optimal methodology aiming at a novel therapeutic approach
(104). However, despite the
success, further efforts are needed to improve the clinical
efficacy in patients with challenging HNSCC.
In conclusion, enhancing immunochemotherapy and
immunoradiotherapy for head and neck cancer is an active research
and development area. Combining these treatment modalities aims to
improve the effectiveness of cancer treatment by utilizing the
body's immune system to target cancer cells. One approach involves
using immune checkpoint inhibitors, drugs that help unleash the
immune system to attack cancer cells. These inhibitors, such as
pembrolizumab or nivolumab, can be combined with chemotherapy or
radiation therapy to enhance the anticancer immune response.
Current research focuses on identifying novel immunotherapeutic
targets specific to head and neck cancer. By understanding the
molecular characteristics of tumors, researchers hope to develop
personalized treatment approaches that can stimulate the immune
system to recognize and destroy cancer cells more effectively. It
is important to note that specific treatment plans depend on
individual patients and should be discussed with a healthcare
professional. Clinical trials and advancements in this field
continue to evolve, offering potential improvements in
immunochemotherapy and immunoradiotherapy for patients with head
and neck cancer.
Not applicable.
The current review was supported by grants from the State
Project for Essential Drug Research and Development of the People,
Republic of China (grant no. 2018ZX09303014) and the Health and
Family Planning Commission of Sichuan Province (grant no.
8PJ194).
Not applicable.
CW was responsible for conceptualization, and
writing, RC and XL for conceptualization, writing and reviewing, QF
and MQ for conceptualization, figure generation and reviewing. SAUS
and TAM were responsible for writing, reviewing and editing. OJ was
responsible for the study concept and design, draft manuscript
preparation, and analysis and interpretation. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Shonka DC Jr, Ho A, Chintakuntlawar AV,
Geiger JL, Park JC, Seetharamu N, Jasim S, Abdelhamid Ahmed AH,
Bible KC, Brose MS, et al: American Head and Neck Society Endocrine
Surgery Section and International Thyroid Oncology Group consensus
statement on mutational testing in thyroid cancer: Defining
advanced thyroid cancer and its targeted treatment. Head Neck.
44:1277–1300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo Y: Therapy of head and neck cancer in
China: Introduction to the special issue. Head Neck. 44:2007–2008.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lahtinen S, Nurkkala J, Hannula S, Ohtonen
P, Koivunen P and Liisanantti JH: Perioperative risk factors for
one-year mortality in patients with free-flap reconstruction due to
cancer of the head and neck. J Oral Maxillofac Surg.
79:1384.e1–1384.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marziliano A, Teckie S and Diefenbach MA:
Alcohol-related head and neck cancer: Summary of the literature.
Head Neck. 42:732–738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ward G, Mehta V and Moore M: Morbidity,
mortality and cost from HPV-related oropharyngeal cancer: Impact of
2-, 4- and 9-valent vaccines. Hum Vaccin Immunother. 12:1343–1347.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mehanna H, Paleri V, West CM and Nutting
C: Head and neck cancer-Part 1: Epidemiology, presentation, and
prevention. BMJ. 341:c46842010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ravikumar S, Casellas NJ, Shah S and Rieth
K: Geographic disparities in head and neck cancer survival in
Upstate New York 2011–2019. Head Neck. 44:472–482. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andisheh-Tadbir A, Mehrabani D and Heydari
ST: Epidemiology of squamous cell carcinoma of the oral cavity in
Iran. J Craniofac Surg. 19:1699–1702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shehan JN, Alwani T, LeClair J, Mahoney
TF, Agarwal P, Chaudhry ST, Wang JJ, Noordzij JP, Tracy LF, Edwards
HA, et al: Social determinants of health and treatment decisions in
head and neck cancer. Head Neck. 44:372–381. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee T, Cho J, Baek CH, Son YI, Jeong HS,
Chung MK, Hong SD, Ahn YC, Oh DR, Noh JM, et al: Prevalence of NUT
carcinoma in head and neck: Analysis of 362 cases with literature
review. Head Neck. 42:924–938. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang TH, Xirasagar S, Cheng YF, Wu CS, Kao
YW, Shia BC and Lin HC: Association between pioglitazone use and
head and neck cancer: Population-based case-control study. Head
Neck. 42:653–659. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling Z, Cheng B and Tao X:
Epithelial-to-mesenchymal transition in oral squamous cell
carcinoma: Challenges and opportunities. Int J Cancer.
148:1548–1561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cramer JD, Burtness B, Le QT and Ferris
RL: The changing therapeutic landscape of head and neck cancer. Nat
Rev Clin Oncol. 16:669–683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saada-Bouzid E, Peyrade F and Guigay J:
Immunotherapy in recurrent and or metastatic squamous cell
carcinoma of the head and neck. Curr Opin Oncol. 31:146–151. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mei Z, Huang J, Qiao B and Lam AK: Immune
checkpoint pathways in immunotherapy for head and neck squamous
cell carcinoma. Int J Oral Sci. 12:162020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powell SF, Gold KA, Gitau MM, Sumey CJ,
Lohr MM, McGraw SC, Nowak RK, Jensen AW, Blanchard MJ, Fischer CD,
et al: Safety and efficacy of Pembrolizumab with chemoradiotherapy
in locally advanced head and neck squamous cell carcinoma: A Phase
IB study. J Clin Oncol. 38:2427–2437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qureshi HA and Lee SM: Immunotherapy
approaches beyond PD-1 inhibition: The future of cellular therapy
for head and neck squamous cell carcinoma. Curr Treat Options
Oncol. 20:312019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbott M and Ustoyev Y: Cancer and the
immune system: The history and background of immunotherapy. Semin
Oncol Nurs. 35:1509232019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar
S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, et al: Immune
landscape of Viral- and Carcinogen-driven head and neck cancer.
Immunity. 52:183–199.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cramer JD, Burtness B and Ferris RL:
Immunotherapy for head and neck cancer: Recent advances and future
directions. Oral Oncol. 99:1044602019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taberna M, Oliva M and Mesía R:
Cetuximab-Containing combinations in locally advanced and recurrent
or metastatic head and neck squamous cell carcinoma. Front Oncol.
9:3832019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elbers JBW, Al-Mamgani A, Tesseslaar MET,
van den Brekel MWM, Lange CAH, van der Wal JE, Verheij M, Zuur CL
and de Boer JP: Immuno-radiotherapy with cetuximab and avelumab for
advanced stage head and neck squamous cell carcinoma: Results from
a phase-I trial. Radiother Oncol. 142:79–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iovoli AJ, Hermann GM, Ma SJ, Platek AJ,
Farrugia MK, Yau E, Wooten KE, Arshad H, Gupta V, Kuriakose MA, et
al: Association of Nonsteroidal Anti-inflammatory drug use with
survival in patients with squamous cell carcinoma of the head and
neck treated with chemoradiation therapy. JAMA Netw Open.
3:e2071992020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei T, Leisegang M, Xia M, Kiyotani K, Li
N, Zeng C, Deng C, Jiang J, Harada M, Agrawal N, et al: Generation
of neoantigen-specific T cells for adoptive cell transfer for
treating head and neck squamous cell carcinoma. Oncoimmunology.
10:19297262021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson HL, D'Agostino RB Jr, Meegalla N,
Petro R, Commander S, Topaloglu U, Zhang W and Porosnicu M: The
prognostic and therapeutic value of the mutational profile of blood
and tumor tissue in head and neck squamous cell carcinoma.
Oncologist. 26:e279–e89. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen N, Fedewa S and Chen AY:
Epidemiology and demographics of the head and neck cancer
population. Oral Maxillofac Surg Clin North Am. 30:381–395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nooreldeen R and Bach H: Current and
future development in lung cancer diagnosis. Int J Mol Sci.
22:86612021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hommel DJ, Brown ML and Kinzie JJ:
Response to radiotherapy of head and neck tumors in AIDS patients.
Am J Surg. 154:443–446. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson RE: Surgical oncology. Cancer. 54
(Suppl 11):S2595–S2598. 1984. View Article : Google Scholar
|
|
30
|
Pagedar NA, Kendell N, Christensen AJ,
Thomsen TA, Gist M and Seaman AT: Head and neck cancer survivorship
from the patient perspective. Head Neck. 42:2431–2439. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vincent AG, Wang W, Shokri T and Ducic Y:
Treatment of oligometastatic disease in squamous cell carcinoma of
the head and neck. Laryngoscope. 131:E1476–E1480. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carron J, Torricelli C, Silva JK, Queiroz
GSR, Ortega MM, Lima CSP and Lourenço GJ: microRNAs deregulation in
head and neck squamous cell carcinoma. Head Neck. 43:645–667. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Philips R, Han C, Swendseid B, Curry J,
Argiris A, Luginbuhl A and Johnson J: Preoperative immunotherapy in
the multidisciplinary management of oral cavity cancer. Front
Oncol. 11:6820752021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Voortman J: Chemoradiotherapy plus a SMAC
mimetic for locally advanced squamous cell carcinoma of the head
and neck. Lancet Oncol. 21:1126–1128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Botticelli A, Cirillo A, Strigari L,
Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, Zizzari IG, Rocca
CD, D'Amati G, et al: Anti-PD-1 and Anti-PD-L1 in head and neck
cancer: A network meta-analysis. Front Immunol. 12:7050962021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deschuymer S, Nevens D, Duprez F, Daisne
JF, Dok R, Laenen A, Voordeckers M, De Neve W and Nuyts S:
Randomized clinical trial on reduction of radiotherapy dose to the
elective neck in head and neck squamous cell carcinoma; update of
the long-term tumor outcome. Radiother Oncol. 143:24–29. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Irfan M, Delgado RZR and Frias-Lopez J:
The oral microbiome and cancer. Front Immunol. 11:5910882020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang SH and O'Sullivan B: Overview of the
8th Edition TNM classification for head and neck cancer. Curr Treat
Options Oncol. 18:402017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou K, Li Y, Liao W, Zhang M, Bai L and
Li Q: Pembrolizumab alone or with chemotherapy for squamous cell
carcinoma of the head and neck: A cost-effectiveness analysis from
Chinese perspective. Oral Oncol. 107:1047542020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: A systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zilberg C, Lee MW, Kraitsek S, Ashford B,
Ranson M, Shannon K, Iyer NG, Ch'ng S, Low TH, Palme C, et al: Is
high-risk cutaneous squamous cell carcinoma of the head and neck a
suitable candidate for current targeted therapies? J Clin Pathol.
73:17–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Yang L, Han S, Zhu J, Li Y, Wang
Z, Fan YH, Lin E, Zhang R, Sahoo N, et al: Patterns of protein
expression in human head and neck cancer cell lines differ after
proton vs photon radiotherapy. Head Neck. 42:289–301. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akali NR, Buggaveeti R, Sukumaran SV,
Balasubramanian D, Iyer S and Thankappan K: Prior chemoradiotherapy
and pathological perineural invasion predict the survival outcomes
of salvage surgery in head and neck squamous cell carcinoma. Head
Neck. 43:874–883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ostuni R, Kratochvill F, Murray PJ and
Natoli G: Macrophages and cancer: From mechanisms to therapeutic
implications. Trends Immunol. 36:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ottria L, Candotto V, Cura F, Baggi L,
Arcuri C, Nardone M, Gaudio RM, Gatto R, Spadari F and Carinci F:
HPV acting on E-cadherin, p53 and p16: Literature review. J Biol
Regul Homeost Agents. 32 (2 Suppl 1):S73–S79. 2018.PubMed/NCBI
|
|
46
|
Gau M, Karabajakian A, Reverdy T,
Neidhardt EM and Fayette J: Induction chemotherapy in head and neck
cancers: Results and controversies. Oral Oncol. 95:164–169. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Zhong X, Jiang H, Jiang H, Xie T,
Tian Y, Li R, Wang B, Zhang J and Yuan Y: Comprehensive
characterization of the tumor microenvironment for assessing
immunotherapy outcome in patients with head and neck squamous cell
carcinoma. Aging (Albany NY). 12:22509–22526. 2020.PubMed/NCBI
|
|
48
|
Jeans C, Brown B, Ward EC, Vertigan AE,
Pigott AE, Nixon JL and Wratten C: Comparing the prevalence,
location, and severity of head and neck lymphedema after
postoperative radiotherapy for oral cavity cancers and definitive
chemoradiotherapy for oropharyngeal, laryngeal, and hypopharyngeal
cancers. Head Neck. 42:3364–3374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyauchi S, Kim SS, Pang J, Gold KA,
Gutkind JS, Califano JA, Mell LK, Cohen EEW and Sharabi AB: Immune
modulation of head and neck squamous cell carcinoma and the tumor
microenvironment by conventional therapeutics. Clin Cancer Res.
25:4211–4223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim DY, Wu HG, Kim JH, Lee JH, Ahn SH,
Chung EJ, Eom KY, Jung YH, Jeong WJ, Kwon TK, et al: Radiotherapy
versus surgery in early-stage HPV-positive oropharyngeal cancer.
Cancer Res Treat. 54:406–416. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Łasińska I, Kolenda T, Teresiak A,
Lamperska KM, Galus Ł and Mackiewicz J: Immunotherapy in patients
with recurrent and metastatic squamous cell carcinoma of the head
and neck. Anticancer Agents Med Chem. 19:290–303. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tovar JM, Bazaldua OV, Vargas L and Reile
E: Human papillomavirus, cervical cancer, and the vaccines.
Postgrad Med. 120:79–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Solomon B, Young RJ and Rischin D: Head
and neck squamous cell carcinoma: Genomics and emerging biomarkers
for immunomodulatory cancer treatments. Semin Cancer Biol.
52:228–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Ridder M, de Veij Mestdagh PD, Elbers
JBW, Navran A, Zuur CL, Smeele LE and Al-Mamgani A: Disease course
after the first recurrence of head and neck squamous cell carcinoma
following (chemo)radiation. Eur Arch Otorhinolaryngol. 277:261–268.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Singh P, Bennett B, Bailey T,
Taylor-Stokes G, Rajkovic I, Contente M, Curtis S and Curtis C:
Real-world study of the impact of recurrent/metastatic squamous
cell carcinoma of the head and neck (R/M SCCHN) on quality of life
and productivity in Europe. BMC Cancer. 21:8542021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferris RL, Licitra L, Fayette J, Even C,
Blumenschein G Jr, Harrington KJ, Guigay J, Vokes EE, Saba NF,
Haddad R, et al: Nivolumab in patients with recurrent or metastatic
squamous cell carcinoma of the head and neck: Efficacy and safety
in CheckMate 141 by prior cetuximab use. Clin Cancer Res.
25:5221–5230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Billard-Sandu C, Tao YG, Sablin MP,
Dumitrescu G, Billard D and Deutsch E: CDK4/6 inhibitors in
P16/HPV16-negative squamous cell carcinoma of the head and neck.
Eur Arch Otorhinolaryngol. 277:1273–1280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Paget-Bailly S, Cyr D and Luce D:
Occupational exposures and cancer of the larynx-systematic review
and meta-analysis. J Occup Environ Med. 54:71–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yousefi H, Lak E, Mohammadi MJ and
Shahriyari HA: Carcinogenic risk assessment among children and
adult due to exposure to toxic air pollutants. Environ Sci Pollut
Res Int. 29:23015–23025. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dok R, Bamps M, Glorieux M, Zhao P,
Sablina A and Nuyts S: Radiosensitization approaches for
HPV-positive and HPV-negative head and neck squamous carcinomas.
Int J Cancer. 146:1075–1085. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guo F, Chang M, Scholl M, McKinnon B and
Berenson AB: Trends in oropharyngeal cancer incidence among adult
men and women in the United States from 2001 to 2018. Front Oncol.
12:9265552022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zolkind P, Lee JJ, Jackson RS, Pipkorn P
and Massa ST: Untreated head and neck cancer: Natural history and
associated factors. Head Neck. 43:89–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Valero C, Ganly I and Shah JP: Head and
neck paragangliomas: 30-year experience. Head Neck. 42:2486–2495.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pike LRG, Royce TJ, Mahal AR, Kim DW,
Hwang WL, Mahal BA and Sanford NN: Outcomes of HPV-Associated
squamous cell carcinoma of the head and neck: Impact of race and
socioeconomic status. J Natl Compr Canc Netw. 18:177–184.
2020.PubMed/NCBI
|
|
65
|
Lach FP, Singh S, Rickman KA, Ruiz PD,
Noonan RJ, Hymes KB, DeLacure MD, Kennedy JA, Chandrasekharappa SC
and Smogorzewska A: Esophageal cancer as initial presentation of
Fanconi anemia in patients with a hypomorphic FANCA variant. Cold
Spring Harb Mol Case Stud. 6:a0055952020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Saksø M, Mortensen LS, Primdahl H,
Johansen J, Kallehauge J, Hansen CR and Overgaard J: Influence of
FAZA PET hypoxia and HPV-status for the outcome of head and neck
squamous cell carcinoma (HNSCC) treated with radiotherapy:
Long-term results from the DAHANCA 24 trial (NCT01017224).
Radiother Oncol. 151:126–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou C and Parsons JL: The radiobiology of
HPV-positive and HPV-negative head and neck squamous cell
carcinoma. Expert Rev Mol Med. 22:e32020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Larsen K, Rydz E and Peters CE:
Inequalities in environmental cancer risk and carcinogen exposures:
A scoping review. Int J Environ Res Public Health. 20:57182023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fishbein A, Hammock BD, Serhan CN and
Panigrahy D: Carcinogenesis: Failure of resolution of inflammation?
Pharmacol Ther. 218:1076702021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Boffetta P, Hecht S, Gray N, Gupta P and
Straif K: Smokeless tobacco and cancer. Lancet Oncol. 9:667–675.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hecht SS: Tobacco carcinogens, their
biomarkers and tobacco-induced cancer. Nat Rev Cancer. 3:733–744.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rumfield CS, Schlom J and Jochems C:
Combination therapies for HPV-associated malignancies. J Clin Cell
Immunol. 12:6082021.
|
|
73
|
Huang C and Zhan L: Network pharmacology
identifies therapeutic targets and the mechanisms of glutathione
action in ferroptosis occurring in oral cancer. Front Pharmacol.
13:8515402022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zandberg DP, Bhargava R, Badin S and
Cullen KJ: The role of human papillomavirus in nongenital cancers.
CA Cancer J Clin. 63:57–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang J, Sun H, Zeng Q, Guo XJ, Wang H, Liu
HH and Dong ZY: HPV-positive status associated with inflamed immune
microenvironment and improved response to anti-PD-1 therapy in head
and neck squamous cell carcinoma. Sci Rep. 9:134042019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Beddok A, Vela A, Calugaru V, Tessonnier
T, Kubes J, Dutheil P, Gerard A, Vidal M, Goudjil F, Florescu C, et
al: Proton therapy for head and neck squamous cell carcinomas: A
review of the physical and clinical challenges. Radiother Oncol.
147:30–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ding L, Ren J, Zhang D, Li Y, Huang X, Hu
Q, Wang H, Song Y, Ni Y and Hou Y: A novel stromal lncRNA signature
reprograms fibroblasts to promote the growth of oral squamous cell
carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis.
39:397–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nathan CA, Khandelwal AR, Wolf GT, Rodrigo
JP, Mäkitie AA, Saba NF, Forastiere AA, Bradford CR and Ferlito A:
TP53 mutations in head and neck cancer. Mol Carcinog. 61:385–391.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Renken S, Nakajima T, Magalhaes I,
Mattsson J, Lundqvist A, Arnér ESJ, Kiessling R and Wickström SL:
Targeting of Nrf2 improves antitumoral responses by human NK cells,
TIL and CAR T cells during oxidative stress. J Immunother Cancer.
10:e0044582022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen SMY, Krinsky AL, Woolaver RA, Wang X,
Chen Z and Wang JH: Tumor immune microenvironment in head and neck
cancers. Mol Carcinog. 59:766–774. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brand M, Laban S, Theodoraki MN, Doescher
J, Hoffmann TK, Schuler PJ and Brunner C: Characterization and
differentiation of the tumor microenvironment (TME) of orthotopic
and subcutaneously grown head and neck squamous cell carcinoma
(HNSCC) in immunocompetent mice. Int J Mol Sci. 22:2472020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang G, Zhang M, Cheng M, Wang X, Li K,
Chen J, Chen Z, Chen S, Chen J, Xiong G, et al: Tumor
microenvironment in head and neck squamous cell carcinoma:
Functions and regulatory mechanisms. Cancer Lett. 507:55–69. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Al-Assaf H, Erler D, Karam I, Lee JW,
Higgins K, Enepekides D, Zhang L, Eskander A and Poon I:
Stereotactic body radiotherapy for medically unfit patients with
cancers to the head and neck. Head Neck. 42:2050–2057. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Evrard D, Szturz P, Tijeras-Raballand A,
Astorgues-Xerri L, Abitbol C, Paradis V, Raymond E, Albert S, Barry
B and Faivre S: Macrophages in the microenvironment of head and
neck cancer: Potential targets for cancer therapy. Oral Oncol.
88:29–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Vengaloor Thomas T, Packianathan S, Bhanat
E, Albert A, Abraham A, Gordy X, Kanakamedala M, Mehta D and
Vijayakumar S: Oligometastatic head and neck cancer: Comprehensive
review. Head Neck. 42:2194–2201. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Raudenska M, Balvan J and Masarik M: Cell
death in head and neck cancer pathogenesis and treatment. Cell
Death Dis. 12:1922021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen YP, Wang YQ, Lv JW, Li YQ, Chua MLK,
Le QT, Lee N, Colevas AD, Seiwert T, Hayes DN, et al:
Identification and validation of novel microenvironment-based
immune molecular subgroups of head and neck squamous cell
carcinoma: Implications for immunotherapy. Ann Oncol. 30:68–75.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu P, Yuan G, Lu Z, Yang S, Zhu H, Zhou R,
Wilson Wai SH, Cai J and Raymond King YT: Extracranial/intracranial
vascular bypass to control carotid artery blowout in postirradiated
nasopharyngeal carcinoma patients. Lin Chung Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 35:448–452. 2021.(In Chinese). PubMed/NCBI
|
|
90
|
Pittet MJ, Michielin O and Migliorini D:
Clinical relevance of tumour-associated macrophages. Nat Rev Clin
Oncol. 19:402–421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
León X, Pujals G, Bulboa C, García J,
López M and Quer M: Head and neck squamous cell carcinoma in cigar
smokers. Distinctive epidemiological and prognostic
characteristics. Acta Otorrinolaringol Esp (Engl Ed). 72:222–229.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang Z, Wu B, Peng G, Xiao G, Huang J,
Ding Q, Yang C, Xiong X, Ma H, Shi L, et al: Neoadjuvant
chemoimmunotherapy for the treatment of locally advanced head and
neck squamous cell carcinoma: A Single-Arm phase 2 clinical trial.
Clin Cancer Res. 28:3268–3276. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Madhukar G and Subbarao N: Current and
future therapeutic targets: A review on treating head and neck
squamous cell carcinoma. Curr Cancer Drug Targets. 21:386–400.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Machiels JP, Tao Y, Burtness B, Tahara M,
Licitra L, Rischin D, Waldron J, Simon C, Gregoire V, Harrington K,
et al: Pembrolizumab given concomitantly with chemoradiation and as
maintenance therapy for locally advanced head and neck squamous
cell carcinoma: KEYNOTE-412. Future Oncol. 16:1235–1243. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hughes BGM, Munoz-Couselo E, Mortier L,
Bratland Å, Gutzmer R, Roshdy O, González Mendoza R, Schachter J,
Arance A, Grange F, et al: Pembrolizumab for locally advanced and
recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629
Study): An open-label, nonrandomized, multicenter, phase 2 trial.
Ann Oncol. 32:1276–1285. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wu J, Yuan Y and Tao XF: Targeted
molecular imaging of head and neck squamous cell carcinoma: A
window into precision medicine. Chin Med J (Engl). 133:1325–1336.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Rangabashyam MS, Lee SY, Tan SY, Mueller
S, Sultana R, Ho J, Skanthakumar T, Tan NC, Tan HK, Soo KC and Iyer
NG: Adherence of head and neck squamous cell carcinoma patients to
tumor board recommendations. Cancer Med. 9:5124–5133. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang JJ, Geduldig JE, Jacobs EB, Tai TYT,
Ahmad S, Chadha N, Buxton DF, Vinod K, Wirostko BM, Kang JH, et al:
Head and neck region dermatological Ultraviolet-Related cancers are
associated with exfoliation syndrome in a clinic-based population.
Ophthalmol Glaucoma. 5:663–671. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liang B, Tao Y and Wang T: Profiles of
immune cell infiltration in head and neck squamous carcinoma.
Biosci Rep. 40:BSR201927242020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kochanek SJ, Close DA, Camarco DP and
Johnston PA: Maximizing the value of cancer drug screening in
multicellular tumor spheroid cultures: A case study in five head
and neck squamous cell carcinoma cell lines. SLAS Discov.
25:329–349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kang KH, Lebow ES, Niemierko A, Bussière
MR, Dewyer NA, Daly J, McKenna MJ, Lee DJ, Loeffler JS, Busse PM
and Shih HA: Proton therapy for head and neck paragangliomas: A
single institutional experience. Head Neck. 42:670–677. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Guidi A, Codecà C and Ferrari D:
Chemotherapy and immunotherapy for recurrent and metastatic head
and neck cancer: A systematic review. Med Oncol. 35:372018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ferris RL, Moskovitz J, Kunning S, Ruffin
AT, Reeder C, Ohr J, Gooding WE, Kim S, Karlovits BJ, Vignali DAA,
et al: Phase I Trial of cetuximab, radiotherapy, and ipilimumab in
locally advanced head and neck cancer. Clin Cancer Res.
28:1335–1344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Faur CI, Falamas A, Chirila M, Roman RC,
Rotaru H, Moldovan MA, Albu S, Baciut M, Robu I and Hedesiu M:
Raman spectroscopy in oral cavity and oropharyngeal cancer: A
systematic review. Int J Oral Maxillofac Surg. 51:1373–1381. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kao HF and Lou PJ: Immune checkpoint
inhibitors for head and neck squamous cell carcinoma: Current
landscape and future directions. Head Neck. 41 (Suppl 1):4–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tao L, Zhou L, Zhang M, Wu H, Li X, Chen
X, Li C, Shi Y, Cheng L and Lin H: Postoperative prognostic risk
factors and treatment strategies for patients with T3-T4
hypopharyngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi. 35:400–404. 2021.(In Chinese). PubMed/NCBI
|
|
109
|
Okano S, Homma A, Kiyota N, Tahara M,
Hanai N, Asakage T, Matsuura K, Ogawa T, Saito Y, Sano D, et al:
Induction chemotherapy in locally advanced squamous cell carcinoma
of the head and neck. Jpn J Clin Oncol. 51:173–179. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang L, Ma Q, Yao R and Liu J: Current
status and development of anti-PD-1/PD-L1 immunotherapy for lung
cancer. Int Immunopharmacol. 79:1060882020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mingo KM, Derakhshan A, Abdullah N, Chute
DJ, Koyfman SA, Lamarre ED and Burkey BB: Characteristics and
outcomes in head and neck sarcomatoid squamous cell carcinoma: The
cleveland clinic experience. Ann Otol Rhinol Laryngol. 130:818–824.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu XC, Ma SR, Shi S, Zhao YF and Jia J:
Prognostic significance of lymph node ratio in patients with
squamous cell carcinoma of the floor of the mouth. Int J Oral
Maxillofac Surg. 51:307–313. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Varra V, Smile TD, Geiger JL and Koyfman
SA: Recent and emerging therapies for cutaneous squamous cell
carcinomas of the head and neck. Curr Treat Options Oncol.
21:372020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Highland J, Aylward A, Do O, Monroe M and
Buchmann L: Trust in physicians among patients with head and neck
cancer before and after treatment. Head Neck. 43:2580–2588. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tam S, Yao C, Amit M, Gajera M, Luo X,
Treistman R, Khanna A, Aashiq M, Nagarajan P, Bell D, et al:
Association of immunosuppression with outcomes of patients with
cutaneous squamous cell carcinoma of the head and neck. JAMA
Otolaryngol Head Neck Surg. 146:128–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang W, Green M, Choi JE, Gijon M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Valdes M, Villeda J, Mithoowani H, Pitre T
and Chasen M: Inflammatory markers as prognostic factors of
recurrence in advanced-stage squamous cell carcinoma of the head
and neck. Curr Oncol. 27:135–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cristina V, Herrera-Gómez RG, Szturz P,
Espeli V and Siano M: Immunotherapies and future combination
strategies for head and neck squamous cell carcinoma. Int J Mol
Sci. 20:53992019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chatterjee S, Kiyota N, Vaish R, Sharma A,
Tahara M, Noronha V, Prabhash K and D'Cruz A: Weekly versus
3-weekly cisplatin along with radiotherapy for locoregionally
advanced non-nasopharyngeal head and neck cancers: Is the equipoise
in literature addressed yet? Head Neck. 45:1594–1603. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang Y, Dong L, Bi Q, Li X, Wu D, Ge X,
Zhang X, Fu J, Zhang C, Wang C and Li S: Investigation of the
efficacy of a bevacizumab-cetuximab-cisplatin regimen in treating
head and neck squamous cell carcinoma in mice. Target Oncol.
5:237–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ham JC, van Meerten E, Fiets WE, Beerepoot
LV, Jeurissen FJF, Slingerland M, Jonker MA, Husson O, van der
Graaf WTA and van Herpen CML: Methotrexate plus or minus cetuximab
as first-line treatment in a recurrent or metastatic (R/M) squamous
cell carcinoma population of the head and neck (SCCHN), unfit for
cisplatin combination treatment, a phase Ib-randomized phase II
study Commence. Head Neck. 42:828–838. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kaboli PJ, Zhang L, Xiang S, Shen J, Li M,
Zhao Y, Wu X, Zhao Q, Zhang H, Lin L, et al: Molecular markers of
regulatory T cells in cancer immunotherapy with special focus on
acute myeloid leukemia (AML)-a systematic review. Curr Med Chem.
27:4673–4698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jia T, Ming SX, Cao QQ and Xu FL: Combined
treatment with acetazolamide and cisplatin enhances the
chemosensitivity of human head and neck squamous cell carcinoma
TU868 cells. Arch Oral Biol. 119:1049052020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Masarwy R, Kampel L, Horowitz G, Gutfeld O
and Muhanna N: Neoadjuvant PD-1/PD-L1 inhibitors for resectable
head and neck cancer: A systematic review and meta-analysis. JAMA
Otolaryngol Head Neck Surg. 147:871–878. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kimura H, Hamauchi S, Kawai S, Onozawa Y,
Yasui H, Yamashita A, Ogawa H, Onoe T, Kamijo T, Iida Y, et al:
Pretreatment predictive factors for feasibility of oral intake in
adjuvant concurrent chemoradiotherapy for patients with locally
advanced squamous cell carcinoma of the head and neck. Int J Clin
Oncol. 25:258–266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rotman J, den Otter LAS, Bleeker MCG,
Samuels SS, Heeren AM, Roemer MGM, Kenter GG, Zijlmans HJMAA, van
Trommel NE, de Gruijl TD and Jordanova ES: PD-L1 and PD-L2
expression in cervical cancer: Regulation and biomarker potential.
Front Immunol. 11:5968252020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Brody RM, Albergotti WG, Shimunov D,
Nicolli E, Patel UA, Harris BN and Bur AM: Changes in head and neck
oncologic practice during the COVID-19 pandemic. Head Neck.
42:1448–1453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jin Y and Qin X: Co-expression
network-based identification of biomarkers correlated with the
lymph node metastasis of patients with head and neck squamous cell
carcinoma. Biosci Rep. 40:BSR201940672020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Addeo A, Friedlaender A, Banna GL and
Weiss GJ: TMB or not TMB as a biomarker: That is the question. Crit
Rev Oncol Hematol. 163:1033742021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chan TA, Yarchoan M, Jaffee E, Swanton C,
Quezada SA, Stenzinger A and Peters S: Development of tumor
mutation burden as an immunotherapy biomarker: Utility for the
oncology clinic. Ann Oncol. 30:44–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Park R and Park JC: Current landscape of
immunotherapy trials in locally advanced and high-risk head and
neck cancer. Immunotherapy. 13:931–940. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10:5402019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Maio M, Blank C, Necchi A, Di Giacomo AM,
Ibrahim R, Lahn M, Fox BA, Bell RB, Tortora G and Eggermont AMM:
Neoadjuvant immunotherapy is reshaping cancer management across
multiple tumour types: The future is now! Eur J Cancer.
152:155–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hopkins-Rossabi T, Armeson KE, Zecker SG
and Martin-Harris B: Respiratory-swallow coordination and
swallowing impairment in head and neck cancer. Head Neck.
43:1398–1408. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ferrari M, Taboni S, Carobbio ALC,
Emanuelli E, Maroldi R, Bossi P and Nicolai P: Sinonasal squamous
cell carcinoma, a narrative reappraisal of the current evidence.
Cancers (Basel). 13:28352021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Long J, Wang D, Yang X, Wang A, Lin Y,
Zheng M, Zhang H, Sang X, Wang H, Hu K and Zhao H: Identification
of NOTCH4 mutation as a response biomarker for immune checkpoint
inhibitor therapy. BMC Med. 19:1542021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Kabzinski J, Maczynska M and Majsterek I:
MicroRNA as a novel biomarker in the diagnosis of head and neck
cancer. Biomolecules. 11:8442021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Suzuki A, Kashiwagi N, Doi H, Ishii K, Doi
K, Kitano M, Kozuka T, Hyodo T, Tsurusaki M, Yagyu Y and Nakanishi
K: Patterns of bone metastases from head and neck squamous cell
carcinoma. Auris Nasus Larynx. 47:262–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yu J, Smith J, Marwah R and Edkins O:
Return to work in patients with head and neck cancer: Systematic
review and meta-analysis. Head Neck. 44:2904–2924. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Patel JJ, Levy DA, Nguyen SA, Knochelmann
HM and Day TA: Impact of PD-L1 expression and human papillomavirus
status in anti-PD1/PDL1 immunotherapy for head and neck squamous
cell carcinoma-Systematic review and meta-analysis. Head Neck.
42:774–786. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
de Oliveira TB, Marta GN, de Castro Junior
G and Kowalski LP: Induction chemotherapy for advanced oral cavity
cancer. Curr Oncol Rep. 23:1292021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Campo F, Zocchi J, Moretto S, Mazzola F,
Petruzzi G, Donà MG, Benevolo M, Iocca O, De Virgilio A, Pichi B,
et al: Cell-free human papillomavirus-DNA for monitoring treatment
response of head and neck squamous cell carcinoma: Systematic
review and meta-analysis. Laryngoscope. 132:560–568. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lecocq M, Poncin A and Sautois B:
Immunotherapy for head and neck squamous cell carcinoma. Rev Med
Liege. 76:398–402. 2021.(In French). PubMed/NCBI
|
|
146
|
van de Goor R, van Hooren MRA, Henatsch D,
Kremer B and Kross KW: Detecting head and neck squamous carcinoma
using a portable handheld electronic nose. Head Neck. 42:2555–2559.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yan F, Lao WP, Nguyen SA, Sharma AK and
Day TA: Elective neck dissection in salivary gland malignancies:
Systematic review and meta-analysis. Head Neck. 44:505–517. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Qiang W, Dai Y, Xing X and Sun X:
Identification and validation of a prognostic signature and
combination drug therapy for immunotherapy of head and neck
squamous cell carcinoma. Comput Struct Biotechnol J. 19:1263–1276.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang Z, Goto Y, Allevato MM, Wu VH,
Saddawi-Konefka R, Gilardi M, Alvarado D, Yung BS, O'Farrell A,
Molinolo AA, et al: Disruption of the HER3-PI3K-mTOR oncogenic
signaling axis and PD-1 blockade as a multimodal precision
immunotherapy in head and neck cancer. Nat Commun. 12:23832021.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Eckel HE and Bradley PJ: Treatment options
for hypopharyngeal cancer. Adv Otorhinolaryngol. 83:47–53.
2019.PubMed/NCBI
|
|
151
|
Vasiliadou I, Breik O, Baker H, Leslie I,
Sim VR, Hegarty G, Michaelidou A, Nathan K, Hartley A, Good J, et
al: Safety and treatment outcomes of nivolumab for the treatment of
recurrent or metastatic head and neck squamous cell carcinoma:
Retrospective multicenter cohort study. Cancers (Basel).
13:14132021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Leidner R, Crittenden M, Young K, Xiao H,
Wu Y, Couey MA, Patel AA, Cheng AC, Watters AL, Bifulco C, et al:
Neoadjuvant immunoradiotherapy results in high rate of complete
pathological response and clinical to pathological downstaging in
locally advanced head and neck squamous cell carcinoma. J
Immunother Cancer. 9:e0024852021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Dua D, Kelly C, Kovarik J and Iqbal MS:
The role of combining immunotherapy with primary
(Chemo)radiotherapy in curative treatment settings of the head and
neck cancer. Asia Pac J Clin Oncol. 18:e3–e10. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Van Wigcheren GF, De Haas N, Mulder TA,
Horrevorts SK, Bloemendal M, Hins-Debree S, Mao Y, Kiessling R, van
Herpen CML, Flórez-Grau G, et al: Cisplatin inhibits frequency and
suppressive activity of monocytic myeloid-derived suppressor cells
in cancer patients. Oncoimmunology. 10:19355572021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Semrau S, Gostian AO, Traxdorf M, Eckstein
M, Rutzner S, von der Grün J, Illmer T, Hautmann M, Klautke G,
Laban S, et al: Implementation of double immune checkpoint blockade
increases response rate to induction chemotherapy in head and neck
cancer. Cancers (Basel). 13:19592021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li X, Fang Q, Du W, Zhang X, Dai L and
Qiao Y: Induction chemotherapy combined with immunotherapy in
locally advanced head and neck squamous cell carcinoma. BMC Cancer.
21:6222021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Niccoli Asabella A, Nappi AG, Trani O,
Sardaro A and Rubini G: Heterogeneous response to immunotherapy in
a patient with tonsillar squamous cell carcinoma assessed by
18F-FDG PET/CT. Diagnostics (Basel). 11:3482021.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Rothschild U, Muller L, Lechner A,
Schlösser HA, Beutner D, Läubli H, Zippelius A and Rothschild SI:
Immunotherapy in head and neck cancer-scientific rationale, current
treatment options and future directions. Swiss Med Wkly.
148:w146252018.PubMed/NCBI
|
|
159
|
Kim MS, Malik NH, Chen H, Poon I, Husain
Z, Eskander A, Boldt G, Louie AV and Karam I: Stereotactic
radiotherapy as planned boost after definitive radiotherapy for
head and neck cancers: Systematic review. Head Neck. 44:770–782.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Shibata H, Saito S and Uppaluri R:
Immunotherapy for head and neck cancer: A paradigm shift from
induction chemotherapy to neoadjuvant immunotherapy. Front Oncol.
11:7274332021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Koukourakis IM, Giakzidis AG, Kouroupi M,
Giatromanolaki A, Abatzoglou I, Karpouzis A and Koukourakis MI:
Cutaneous squamous-cell carcinoma of the head-neck area refractory
to chemo-radiotherapy: Benefit from anti-PD-1 immunotherapy. BJR
Case Rep. 7:202001702021.PubMed/NCBI
|
|
162
|
Hyytiäinen A, Wahbi W, Väyrynen O,
Saarilahti K, Karihtala P, Salo T and Al-Samadi A: Angiogenesis
inhibitors for head and neck squamous cell carcinoma treatment: Is
there still hope? Front Oncol. 11:6835702021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Jagadeeshan S, Prasad M, Ortiz-Cuaran S,
Gregoire V, Saintigny P and Elkabets M: Adaptive responses to
monotherapy in head and neck cancer: Interventions for
rationale-based therapeutic combinations. Trends Cancer. 5:365–390.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yang YM, Hong P, Xu WW, He QY and Li B:
Advances in targeted therapy for esophageal cancer. Signal
Transduct Target Ther. 5:2292020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Yu S, Wang Y, He P, Shao B, Liu F, Xiang
Z, Yang T, Zeng Y, He T, Ma J, et al: Effective combinations of
immunotherapy and radiotherapy for cancer treatment. Front Oncol.
12:8093042022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Huguet F, Durand B, Atallah S, Prébet C,
Richard S and Baujat B: Combination of radiation
therapy-immunotherapy for head and neck cancers: Promises kept?
Cancer Radiother. 25:811–815. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Biau J and Bourhis J: Combining
immunotherapy and radiotherapy in head and neck squamous cell
cancers: Which perspectives? Curr Opin Oncol. 32:196–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Wang HQ, Fu R, Man QW, Yang G, Liu B and
Bu LL: Advances in CAR-T cell therapy in head and neck squamous
cell carcinoma. J Clin Med. 12:21732023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Kosti P, Opzoomer JW, Larios-Martinez KI,
Henley-Smith R, Scudamore CL, Okesola M, Taher MYM, Davies DM,
Muliaditan T, Larcombe-Young D, et al: Hypoxia-sensing CAR T cells
provide safety and efficacy in treating solid tumors. Cell Rep Med.
2:1002272021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Huang J, Zheng M, Zhang Z, Tang X, Chen Y,
Peng A, Peng X, Tong A and Zhou L: Interleukin-7-loaded oncolytic
adenovirus improves CAR-T cell therapy for glioblastoma. Cancer
Immunol Immunother. 70:2453–2465. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mei Z, Zhang K, Lam AK, Huang J, Qiu F,
Qiao B and Zhang Y: MUC1 as a target for CAR-T therapy in head and
neck squamous cell carinoma. Cancer Med. 9:640–652. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Vedvyas Y, McCloskey JE, Yang Y, Min IM,
Fahey TJ, Zarnegar R, Hsu YS, Hsu JM, Van Besien K, Gaudet I, et
al: Manufacturing and preclinical validation of CAR T cells
targeting ICAM-1 for advanced thyroid cancer therapy. Sci Rep.
9:106342019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Soldierer M, Bister A, Haist C, Thivakaran
A, Cengiz SC, Sendker S, Bartels N, Thomitzek A, Smorra D, Hejazi
M, et al: Genetic engineering and enrichment of human NK cells for
CAR-enhanced immunotherapy of hematological malignancies. Front
Immunol. 13:8470082022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Sridhar P and Petrocca F: Regional
delivery of chimeric antigen receptor (CAR) T-cells for cancer
therapy. Cancers (Basel). 9:922017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Barata A, Hoogland AI, Kommalapati A,
Logue J, Welniak T, Hyland KA, Eisel SL, Small BJ, Jayani RV,
Booth-Jones M, et al: Change in Patients' perceived cognition
following chimeric antigen receptor T-cell therapy for lymphoma.
Transplant Cell Ther. 28:401.e72022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang G, Zhang Z, Zhong K, Wang Z, Yang N,
Tang X, Li H, Lu Q, Wu Z, Yuan B, et al: CXCL11-armed oncolytic
adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram
tumor microenvironment in glioblastoma. Mol Ther. 31:134–153. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Haist C, Poschinski Z, Bister A, Hoffmann
MJ, Grunewald CM, Hamacher A, Kassack M, Wiek C, Scheckenbach K and
Hanenberg H: Engineering a single-chain variable fragment of
cetuximab for CAR T-cell therapy against head and neck squamous
cell carcinomas. Oral Oncol. 129:1058672022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Plavc G and Strojan P: Combining
radiotherapy and immunotherapy in definitive treatment of head and
neck squamous cell carcinoma: Review of current clinical trials.
Radiol Oncol. 54:377–393. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Botticelli A, Pomati G, Cirillo A, Mammone
G, Ciurluini F, Cerbelli B, Sciattella P, Ralli M, Romeo U, De
Felice F, et al: Weekly chemotherapy as first line treatment in
frail head and neck cancer patients in the immunotherapy era. J
Transl Med. 19:3032021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Rajendra A, Noronha V, Joshi A, Patil VM,
Menon N and Prabhash K: Palliative chemotherapy in head and neck
cancer: Balancing between beneficial and adverse effects. Expert
Rev Anticancer Ther. 20:17–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Choi JS, Sansoni ER, Lovin BD, Lindquist
NR, Phan J, Mayo LL, Ferrarotto R and Su SY: Abscopal effect
following immunotherapy and combined stereotactic body radiation
therapy in recurrent metastatic head and neck squamous cell
carcinoma: A report of two cases and literature review. Ann Otol
Rhinol Laryngol. 129:517–522. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Davern M and Lysaght J: Cooperation
between chemotherapy and immunotherapy in gastroesophageal cancers.
Cancer Lett. 495:89–99. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhang W, Yan C, Gao X, Li X, Cao F, Zhao
G, Zhao J, Er P, Zhang T, Chen X, et al: Safety and feasibility of
radiotherapy plus camrelizumab for locally advanced esophageal
squamous cell carcinoma. Oncologist. 26:e1110–e1124. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Altay-Langguth A, Balermpas P, Brandts C,
Balster S, Ghanaati S, Winkelmann R, Burck I, Rödel F, Martin D,
Rödel C and von der Grün J: Re-irradiation with concurrent and
maintenance nivolumab in locally recurrent and inoperable squamous
cell carcinoma of the head and neck: A single-center cohort study.
Clin Transl Radiat Oncol. 28:71–78. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Patel B and Saba NF: Current aspects and
future considerations of EGFR inhibition in locally advanced and
recurrent metastatic squamous cell carcinoma of the head and neck.
Cancers (Basel). 13:35452021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Leu M, Patzer C, Guhlich M, Possiel J,
Pilavakis Y, Schirmer MA, Rieken S and Dröge LH: Postoperative
radiochemotherapy using modern radiotherapy techniques in elderly
patients with head and neck squamous cell carcinoma: The challenge
of weighing Up benefits and harms of treatment modalities in
clinical practice. Cancers (Basel). 13:33842021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Fang PQ, Gunther JR, Wu SY, Dabaja BS,
Nastoupil LJ, Ahmed S, Neelapu SS and Pinnix CC: Radiation and CAR
T-cell therapy in lymphoma: Future frontiers and potential
opportunities for synergy. Front Oncol. 11:6486552021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Wang H, Zhao Q, Zhang Y, Zhang Q, Zheng Z,
Liu S, Liu Z, Meng L, Xin Y and Jiang X: Immunotherapy advances in
locally advanced and recurrent/metastatic head and neck squamous
cell carcinoma and its relationship with human papillomavirus.
Front Immunol. 12:6520542021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Patin EC, Dillon MT, Nenclares P, Grove L,
Soliman H, Leslie I, Northcote D, Bozhanova G, Crespo-Rodriguez E,
Baldock H, et al: Harnessing radiotherapy-induced NK-cell activity
by combining DNA damage-response inhibition and immune checkpoint
blockade. J Immunother Cancer. 10:e0043062022. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Hecht M, Eckstein M, Rutzner S, von der
Grün J, Illmer T, Klautke G, Laban S, Hautmann MG, Brunner TB,
Tamaskovics B, et al: Induction chemoimmunotherapy followed by CD8+
immune cell-based patient selection for chemotherapy-free
radioimmunotherapy in locally advanced head and neck cancer. J
Immunother Cancer. 10:e0037472022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zeng S, Fu L, Zhou P and Ling H:
Identifying risk factors for the prognosis of head and neck
cutaneous squamous cell carcinoma: A systematic review and
meta-analysis. PLoS One. 15:e02395862020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Jin WJ, Erbe AK, Schwarz CN, Jaquish AA,
Anderson BR, Sriramaneni RN, Jagodinsky JC, Bates AM, Clark PA, Le
T, et al: Tumor-specific antibody, cetuximab, enhances the in situ
vaccine effect of radiation in immunologically cold head and neck
squamous cell carcinoma. Front Immunol. 11:5911392020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Cohen EEW, Bell RB, Bifulco CB, Burtness
B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL,
et al: The society for immunotherapy of cancer consensus statement
on immunotherapy for the treatment of squamous cell carcinoma of
the head and neck (HNSCC). J Immunother Cancer. 7:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kordbacheh F and Farah CS: Molecular
pathways and druggable targets in head and neck squamous cell
carcinoma. Cancers (Basel). 13:34532021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Myers JA and Miller JS: Exploring the NK
cell platform for cancer immunotherapy. Nat Rev Clin Oncol.
18:85–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Yokota T, Homma A, Kiyota N, Tahara M,
Hanai N, Asakage T, Matsuura K, Ogawa T, Saito Y, Sano D, et al:
Immunotherapy for squamous cell carcinoma of the head and neck. Jpn
J Clin Oncol. 50:1089–1096. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Boros A, Blanchard P, Dade A, Gorphe P,
Breuskin I, Even C, Nguyen F, Deutsch E, Bidault F, Janot F, et al:
Outcomes in N3 head and neck squamous cell carcinoma and role of
upfront neck dissection. Laryngoscope. 131:E846–E850. 2021.
View Article : Google Scholar : PubMed/NCBI
|