Introduction
Gastric cancer, including gastro-esophageal junction
cancer (GC/GEJC), is the fourth leading cause of cancer-related
death worldwide (1). Patients with
newly diagnosed advanced GC/GEJC often have a poor prognosis, with
a life expectancy of ~1 year (2).
For advanced GC/GEJC, chemotherapy with platinum and
fluoro-pyrimidine is currently the standard first-line therapy
(3,4). However, initial chemotherapy is
frequently unsuccessful, and the majority of patients will
experience a relapse with a tendency of systemic metastasis,
necessitating second-line treatments (2). Second-line treatment options for
advanced or metastatic GC/GEJC patients include docetaxel,
paclitaxel, or irinotecan, and the anti-vascular endothelial growth
factor receptor 2 antibody ramucirumab as either monotherapy or in
combination with paclitaxel (5–7).
Although chemotherapy regimens for these patients have recently
developed, the prognosis of advanced GC/GEJC is still
disappointing. Thus, novel treatment options for patients with
advanced gastric or gastroesophageal junction cancer are urgently
required.
Immune checkpoint inhibitors (ICIs) such as
programmed cell death 1 (PD-1) and programmed cell death ligand 1
(PD-L1) inhibitors have been recommended as treatments for GC/GEJC,
which overexpress immune checkpoint ligands (8–10). A
previous meta-analysis investigated the efficacy and safety of
anti-PD-1/PD-L1 agents vs. chemotherapy in patients with GC/GEJC
(11). Additionally, Zheng et
al (12) also pooled data on
the efficacy and safety of PD-1/PD-L1 inhibitor combined with
chemotherapy in patients with advanced GC/GEJC. However, the
patients examined in these two previous meta-analyses included both
PD-L1-positive and negative patients. Notably, PD-1/PD-L1
inhibitors inhibit T cell activation by blocking the binding of the
PD-1 receptor, an immune checkpoint protein expressed on tumor
cells, to PD-1 on the surface of T cells, leading to tumor immune
escape (13). Therefore, the
present systematic review and meta-analysis focused on comparing
the efficacy and safety of PD-1/PD-L1 inhibitors or PD-1/PD-L1
inhibitors combined with chemotherapy vs. chemotherapy alone in
PD-L1 positive GC/GEJC patients, defined as patients whose tumors
had a PD-L1 combined positive score (CPS) of ≥1, aiming to provide
more precise guidance for the clinical treatment of GC/GEJC
patients.
Materials and methods
Literature search
The inclusion criteria were: Study object,
patients with advanced GC/GEJC; intervention measures, PD-1/PD-L1
inhibitors, PD-1/PD-L1 inhibitors combined with chemotherapy, or
chemotherapy alone. All included studies were randomized controlled
trials (RCTs) limited to English. Outcome indicators included were:
Overall survival (OS), progression-free survival (PFS), objective
response rate (ORR), stable disease (SD) rate, progressive disease
(PD) rate, and incidence of adverse events.
The exclusion criteria were: Studies where full text
could not be obtained, lacking information, studies on which data
could not be extracted, studies using animal experiments, reviews,
and systematic reviews.
Search strategy
PubMed, Embase, and Cochrane Library databases were
searched from the establishment of the database till June 2022,
with the following search terms: ‘Stomach Neoplasm’, ‘gastric
cancer’, ‘stomach cancer’, ‘gastroesophageal cancer’ AND
‘Nivolumab’, ‘Pembrolizumab’, ‘Durvalumab’, ‘Tremelimumab’,
‘Avelumab’, ‘Atezolizumab’, ‘PD-1’, ‘PD-L1’ AND ‘Chemotherapy’,
‘Chemotherapeutics’.
Literature screening and data
extraction
Relevant studies were independently identified by
two researchers, with disagreements being resolved through
discussion with a third investigator. Information extracted from
relevant studies included the experiment name, study design, sample
size of all patients, sex, age, Eastern Cooperative Oncology Group
ECOG performance status, the sample size of patients that were
PD-L1 positive, interventions, and outcome indicators including OS,
PFS, ORR, SD rate, PD rate, and incidence of adverse events.
Literature quality assessment
RevMan 5.3 (The Cochrane Collaboration) risk
assessment tool was used by two investigators to independently
assess study quality based upon the Cochrane risk assessment scale
(14), which assesses study quality
based on random sequence generation, allocation concealment,
blinding method, whether research results were evaluated in a
blinded manner, and the completeness of reported data. Studies were
also examined for potential selective reporting, sex bias, and
other biases. This meta-analysis was executed in compliance with
the PRISMA statement (15).
Data synthesis and statistical
analysis
All data were analyzed using STATA (version 15.1,
Stata Corporation) (16). OS and
PFS were evaluated based on the hazard ratio (HR) with 95%
confidence intervals (CIs), while ORR, SD rate, PD rate, and
incidence of adverse events were assessed based on risk ratio (RR)
and 95% CI. Heterogeneity was evaluated based on the I2
statistic, with fixed effects models being used to analyze normally
distributed data (P≥0.1 and I2≤50%), whereas a
random-effects model or descriptive statistics, were used in cases
where the data were not normally distributed (P<0.1,
I2>50%) and the sources of such heterogeneity could
not be determined through sensitivity analyses. Since the
literature included in the indicators evaluated in this study were
all <3, the publication bias of the literature was not
evaluated.
Results
Results of the literature search
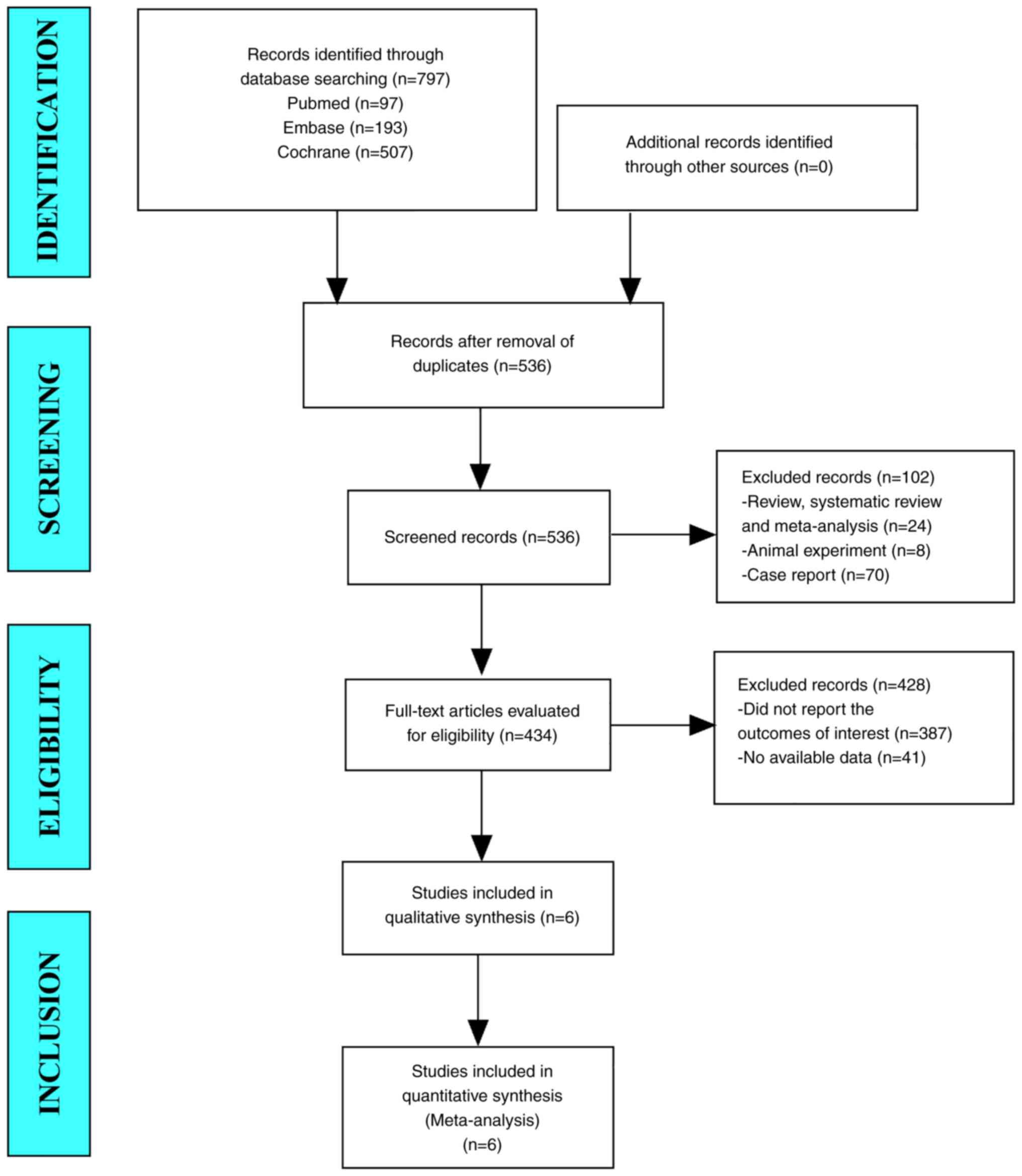
In this meta-analysis, a total of 797 studies from
PubMed, Embase, and Cochrane Library were obtained. After
eliminating duplicate studies, 536 studies remained. After reading
the titles and abstracts, 434 studies were retained. After browsing
the full text, 6 studies were obtained and excluded any that did
not report the outcomes of interest and other ablation methods.
Finally, 6 studies were included in the final meta-analysis
(Fig. 1).
Baseline characteristics and quality
assessment of the included studies
Baseline characteristics of the included
studies
In total, 6 RCTs (16–21)
were included in this meta-analysis. The patient sample size
totaled 5,030, including 2,518 in the experimental group and 2,512
in the control group. The median age of the patients ranged from
59–64. The sample size of patients with PD-L1 positive cancer
totaled 3,286, including 1,640 in the experimental group and 1,646
in the control group. Interventions include PD-1/PD-L1 inhibitors
or PD-1/PD-L1 inhibitors combined with chemotherapy (Table I).
 | Table I.Baseline features of the included
randomized controlled trials. |
Table I.
Baseline features of the included
randomized controlled trials.
|
|
| Sample sizes | Sex, n,
male/female | Age, year | Eastern Cooperative
Oncology Group performance status | Sample size of
patients with PD-L1 positive cancer | Interventions |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Study name | Experimental
group | Control group | Experimental
group | Control group | Experimental
group | Control group | Experimental
group | Control group | Experimental
group | Control group | Experimental
group | Control group | (Refs.) |
|---|
| Fuchs et al,
2022 | KEYNOTE-061 | 296 | 296 | 202/94 | 208/88 | 62.5 (27–87) | 60.0 (20–86) |
0/1/2:127/169/0 |
0/1/2:137/158/1 | 196 | 199 | Pembrolizumab | Paclitaxel | (16) |
| Shitara et
al, 2020 | KEYNOTE-062 | 256 | 250 | 180/76 | 179/71 | 61.0 (20–83) | 62.5 (23–87) | 125 (1) | 135 (1) | 256 | 250 | Pembrolizumab | Cisplatin and
fluorouracil | (17) |
| Shitara et
al, 2020 | KEYNOTE-062 | 257 | 250 | 195/62 | 179/71 | 62.0 (22–83) | 62.5 (23–87) | 138 (1) | 138 (1) | 257 | 250 | Pembrolizumab plus
cisplatin and fluorouracil | Cisplatin and
fluorouracil | (17) |
| Bang et al,
2018 | JAVELIN Gastric
300 | 185 | 186 | 140/45 | 127/59 | 59.0 (29–86) | 61.0 (18–82) | 0/1:66/169 | 0/1:62/124 | 46 | 39 | Avelumab | Paclitaxel | (18) |
| Janjigian et
al, 2021 | CheckMate 649 | 789 | 792 | 331/142 | 349/133 | 62.0 (54–69) | 61.0 (53–68) |
0/1/2:194/279/0 |
0/1/2:203/278/0 | 641 | 655 | Nivolumab plus
XELOX or FOLFOX | XELOX or
FOLFOX | (19) |
| Kang et al,
2022 | ATTRACTION-4 | 362 | 362 | 253/109 | 270/92 | 64.0 (25–86) | 65.0 (27–89) | 0/1:195/167 | 0/1:194/168 | 58 | 56 | Nivolumab plus SOX
or CAPOX | SOX or CAPOX | (20) |
| Sun et al,
2021 | KEYNOTE590 | 373 | 376 | 306/67 | 319/57 | 64.0 (28–94) | 62.0 (27–89) |
0/1/2:149/223/1 |
0/1/2:150/225/1 | 186 | 197 | Pembrolizumab plus
5-fluorouracil and cisplatin | Fluorouracil and
cisplatin | (21) |
Quality assessment of the included
studies
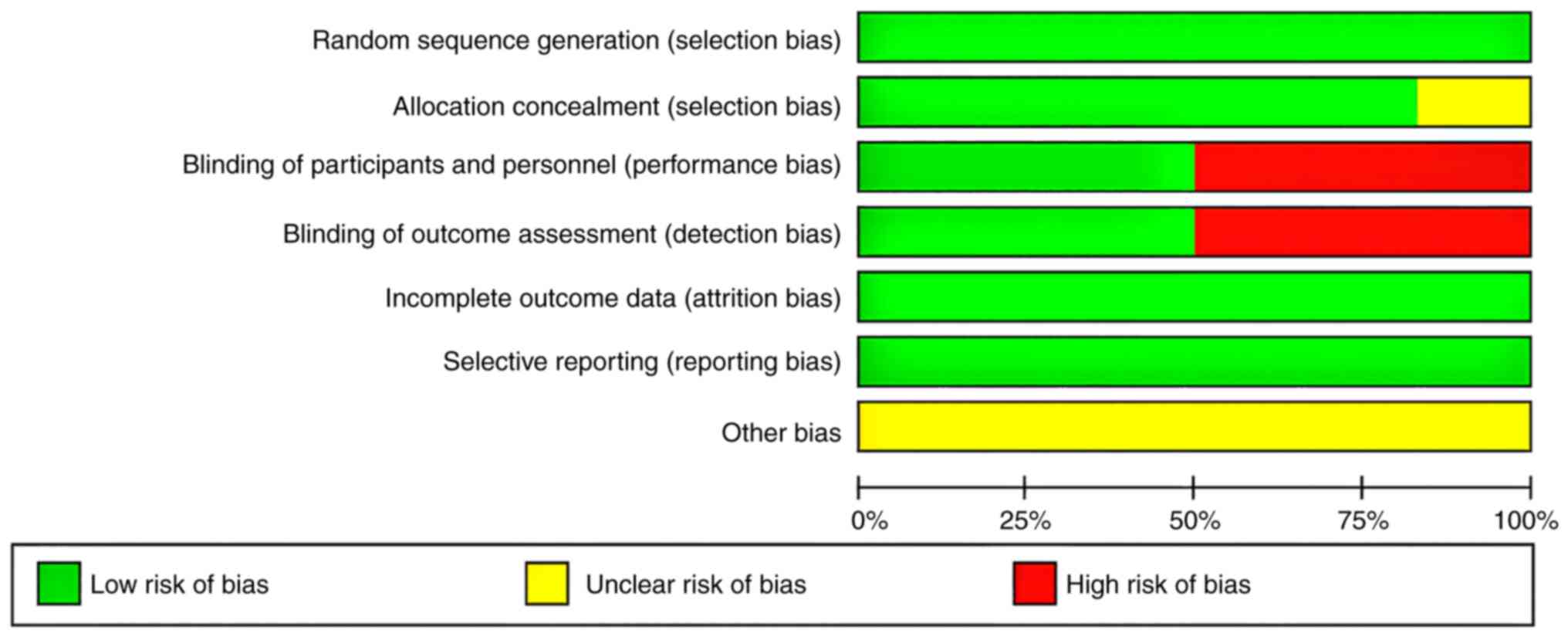
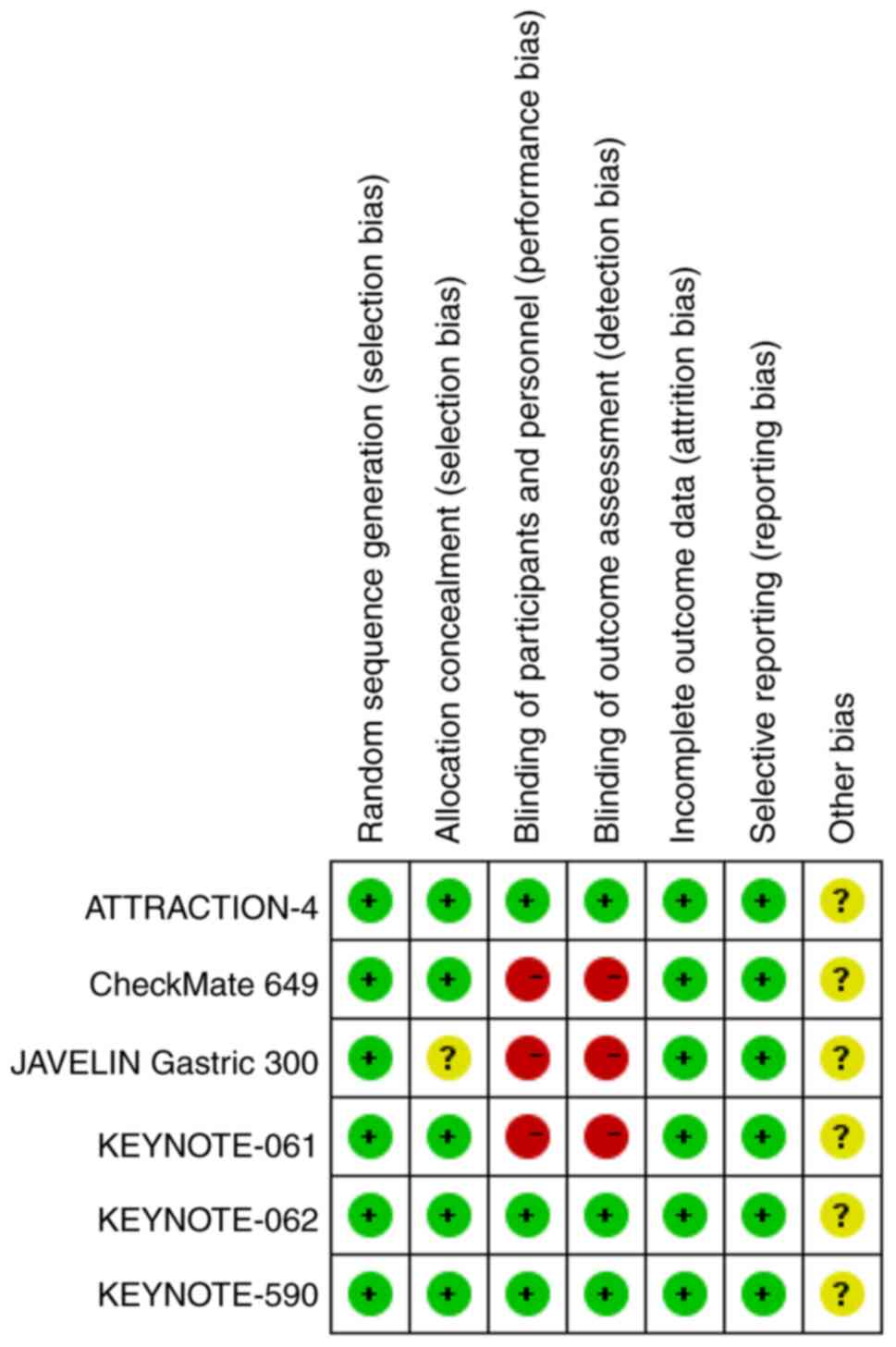
All the studies included in this meta-analysis
described their random sequence generation strategies; three
studies were double-blinded, whereas the other three did not use
any blinding methods (Figs. 2 and
3). Additionally, allocation
concealment was performed in 5 studies. Overall, the quality of the
included studies was relatively high.
Efficacy for all patients
OS
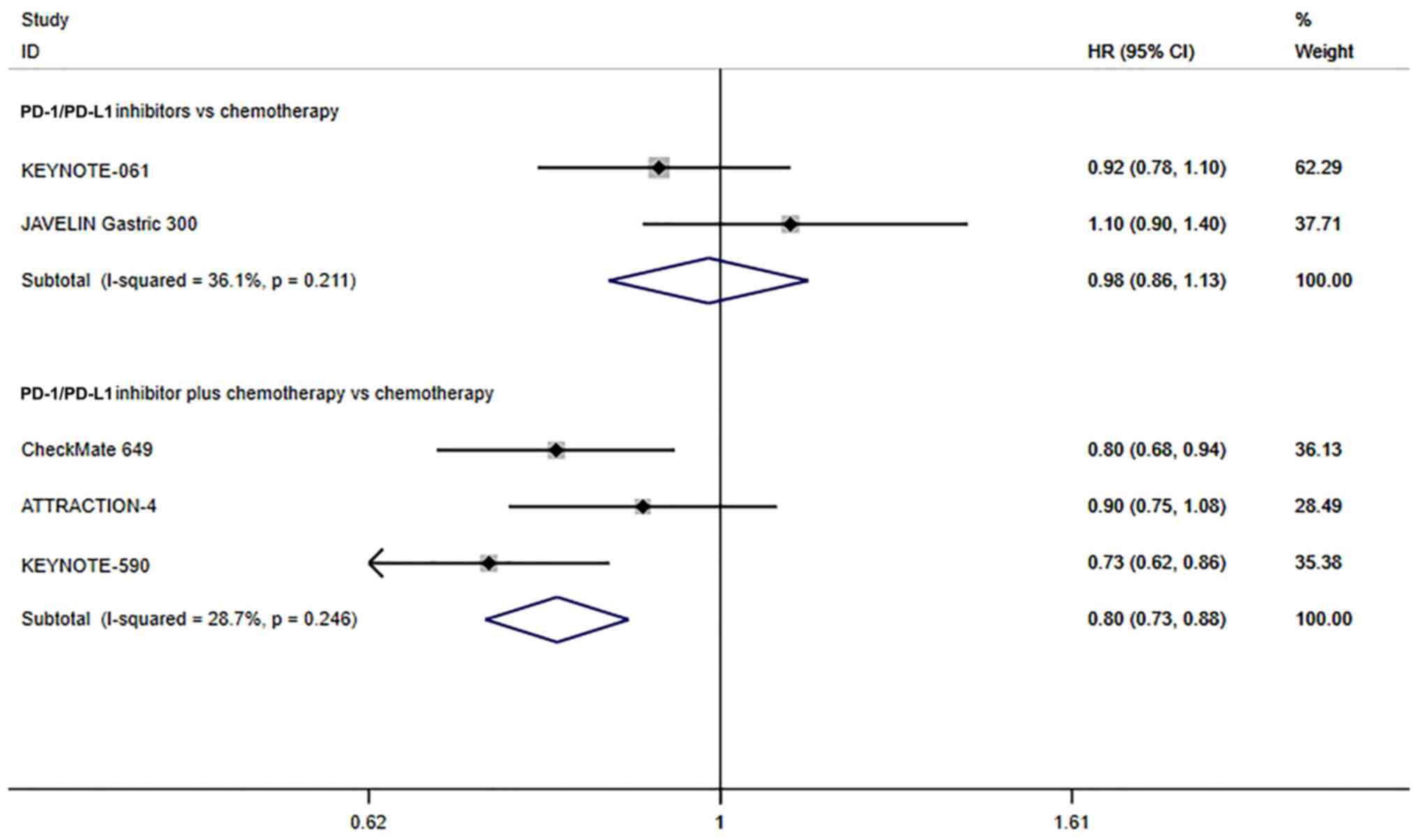
Only 2 studies reported on the OS of all patients
that underwent PD-1/PD-L1 inhibitors vs. chemotherapy. Since there
was no significant heterogeneity (I2=36.1%, P=0.211), a
fixed effects model was used. The pooled results indicated that the
PD-1/PD-L1 inhibitors did not improve the OS compared with patients
that underwent chemotherapy alone (HR=0.98; 95% CI, 0.86–1.13;
P=0.817; Fig. 4). In addition, 3
studies reported on the OS of all patients that underwent
chemotherapeutic treatment alone or combined with PD-1/PD-L1
inhibitors. Since there was no significant heterogeneity
(I2=28.7%, P=0.246), the fixed effects model was used.
The OS of PD-1/PD-L1 inhibitors combined with chemotherapy for
patients with advanced GC/GEJC was significantly longer than that
of chemotherapy alone (HR=0.80; 95% CI, 0.73–0.88; P≤0.001;
Fig. 4). These results suggest that
chemotherapy combined with immunotherapy prolongs OS in patients
with advanced GC/GEJC.
PFS
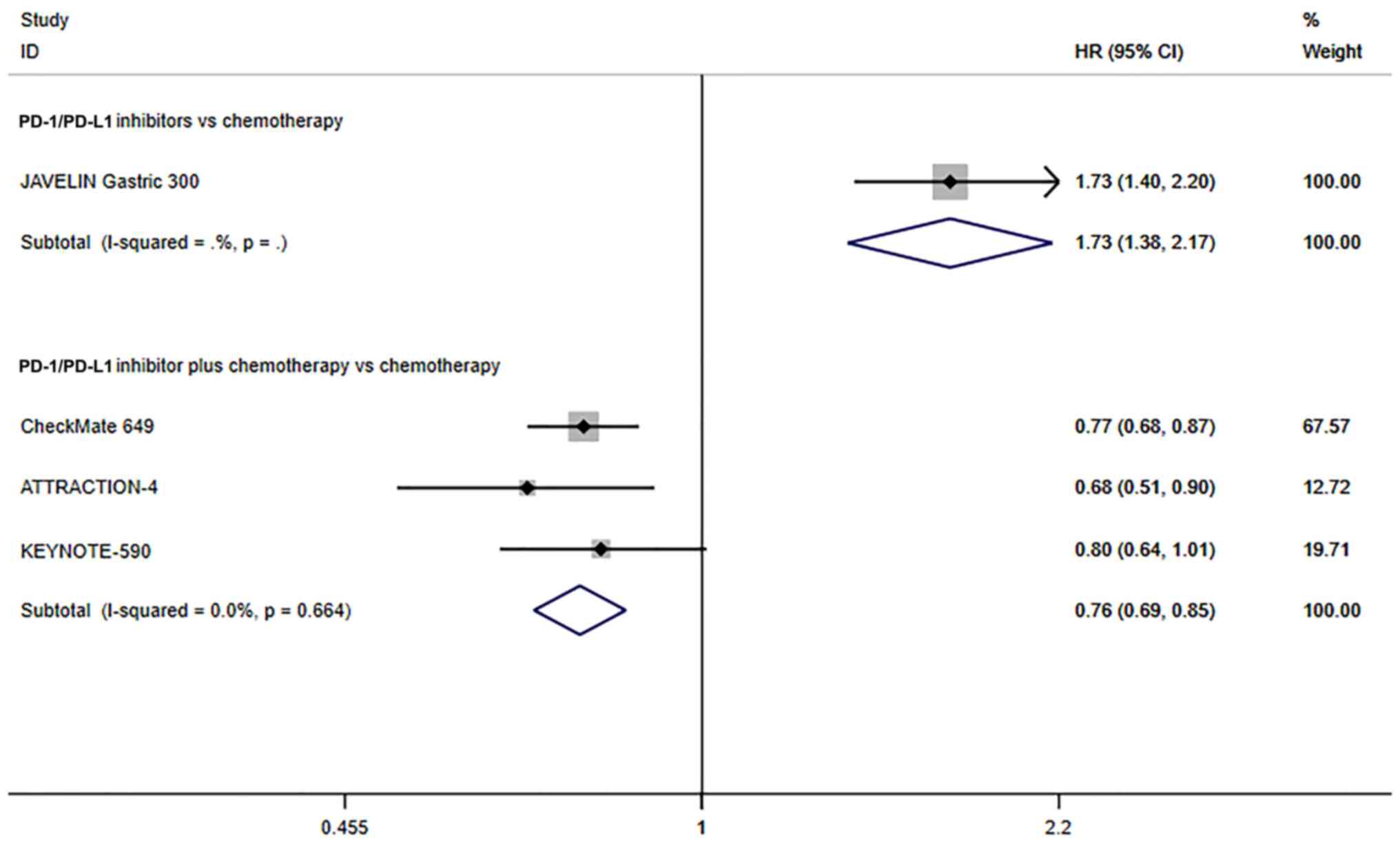
The JAVELIN Gastric 300 study (19) reported that the PFS of patients with
advanced GC/GEJC treated with PD-1/PD-L1 inhibitors was
significantly lower than that of patients treated with chemotherapy
alone (HR=1.73, 95% CI, 1.38–2.17; P≤0.001; Fig. 5). In addition, 3 studies reported on
the PFS of all patients that underwent chemotherapeutic treatment
alone or combined with PD-1/PD-L1 inhibitors. Since there was no
significant heterogeneity (I2=0.0%, P=0.246), a fixed
effects model was used. The PFS of PD-1/PD-L1 inhibitors combined
with chemotherapy for advanced GC/GEJC was significantly longer
than that of chemotherapy alone (HR=0.80, 95% CI, 0.73–0.88;
P≤0.001; Fig. 5). The above results
showed that for advanced GC/GEJC, the PFS with PD-1/PD-L1
inhibitors alone or in combination with chemotherapy was improved
compared with chemotherapy.
Efficacy for patients with PD-L1
positive cancer
ORR
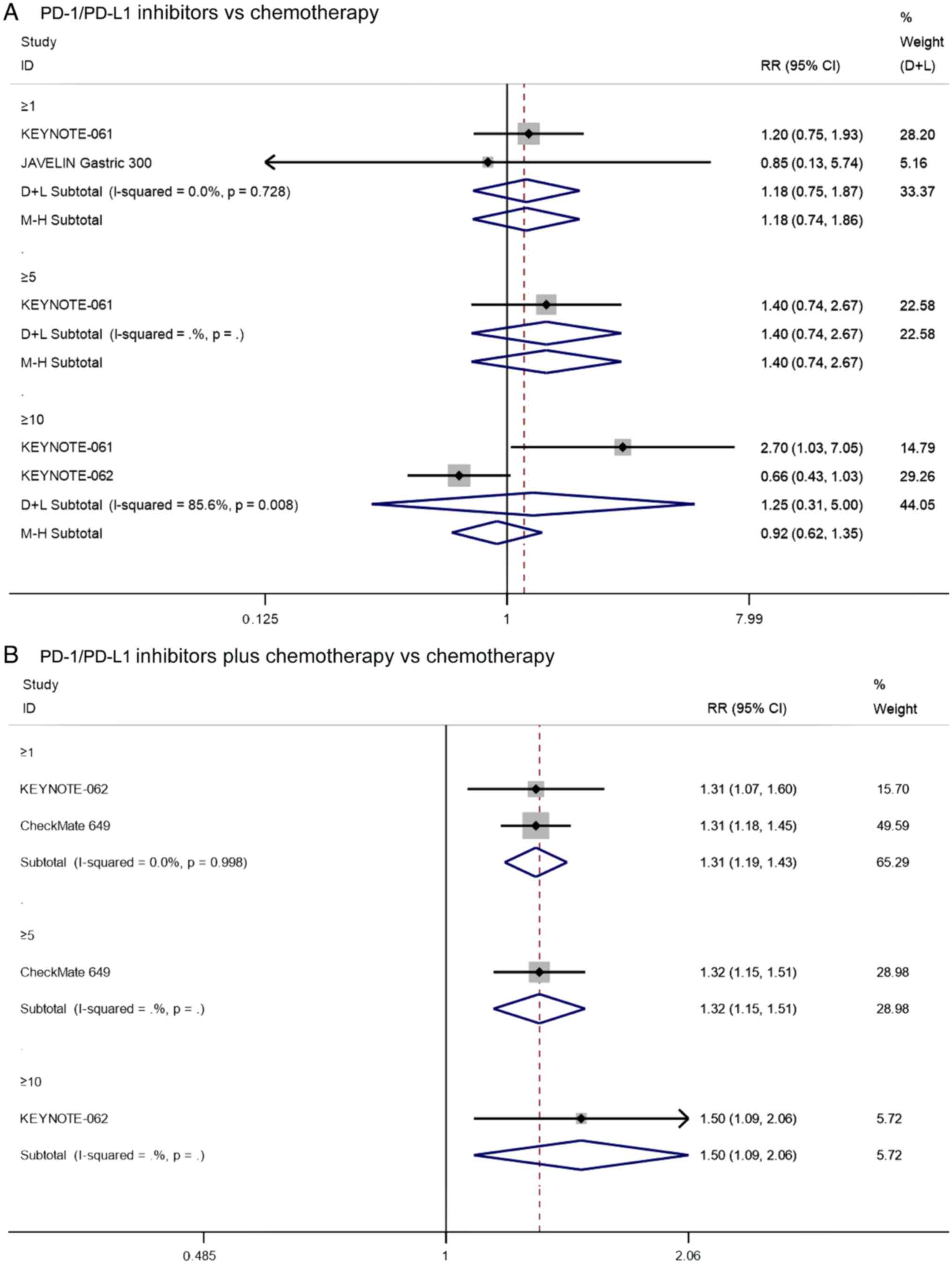
First, the ORR of PD1/PD-L1 inhibitors vs.
chemotherapy was compared for patients with PD-L1 CPS≥1, and three
studies reported on the ORR of patients with PD-L1 CPS≥1 that
underwent PD-1/PD-L1 inhibitors vs. chemotherapy. Since there was
significant heterogeneity (I2=85.8%, P=0.001) (Fig. S1), sensitivity analysis was
performed, and it was found that the KEYNOTE-62 had a notable
impact on the results of this study. Heterogeneity was
significantly reduced after excluding this study
(I2=0.0%, P=0.728). The pooled results indicated that
the PD-1/PD-L1 inhibitors did not improve the ORR in patients with
PD-L1 CPS≥1 that underwent chemotherapy (RR=1.18, 95% CI:
0.74–1.86, P=0.481; Fig. 6A).
Additionally, the KEYNOTE-61 study (17) reported that there was no significant
difference in the ORR between PD1/PD-L1 inhibitors and chemotherapy
for patients with PD-L1 CPS≥5 (RR=1.40, 95% CI: 0.74–2.67, P=0.306;
Fig. 6A). There were also two
studies that reported on the ORR of patients with PD-L1 CPS≥10 that
underwent PD-1/PD-L1 inhibitors vs. chemotherapy. Since there was
significant heterogeneity (I2=85.6%, P=0.008), the
analysis was performed using the random effects model. The pooled
results showed that there was no significant difference in ORR
between PD1/PD-L1 inhibitors and chemotherapy for patients with
PD-L1 CPS≥10 (RR=0.92, 95% CI: 0.62–1.35, P=0.751; Fig. 6A). These results suggest that
PD1/PD-L1 inhibitors do not significantly increase ORR in
PD-L1-positive patients compared with chemotherapy.
Two studies reported on the ORR of patients with
PD-L1 CPS≥1 that underwent PD-1/PD-L1 inhibitors combined with
chemotherapy vs. chemotherapy. Since there was no significant
heterogeneity (I2=0.0%, P=0.998), the analysis was
performed using a fixed effects model. The pooled results showed
that the ORR of PD-1/PD-L1 inhibitors combined with chemotherapy in
patients with PD-L1 CPS≥1 was significantly higher than that of
chemotherapy alone (RR=1.31, 95% CI: 1.19–1.43, P≤0.001; Fig. 6B). In addition, the results of
CheckMate 649 (20) and KEYNOTE-062
(18) showed that PD-1/PD-L1
inhibitor combined with chemotherapy significantly increased ORR
compared with chemotherapy alone in patients with PD-L1 CPS≥5
(RR=1.32, 95% CI: 1.15–1.51, P≤0.001; Fig. 6B) and PD-L1 CPS≥10 (RR=1.50, 95% CI:
1.09–2.06, P=0.014; Fig. 6B),
respectively. The above results suggest that for PD-L1-positive
patients, the ORR of PD1/PD-L1 inhibitors combined with
chemotherapy was significantly higher than that of
chemotherapy.
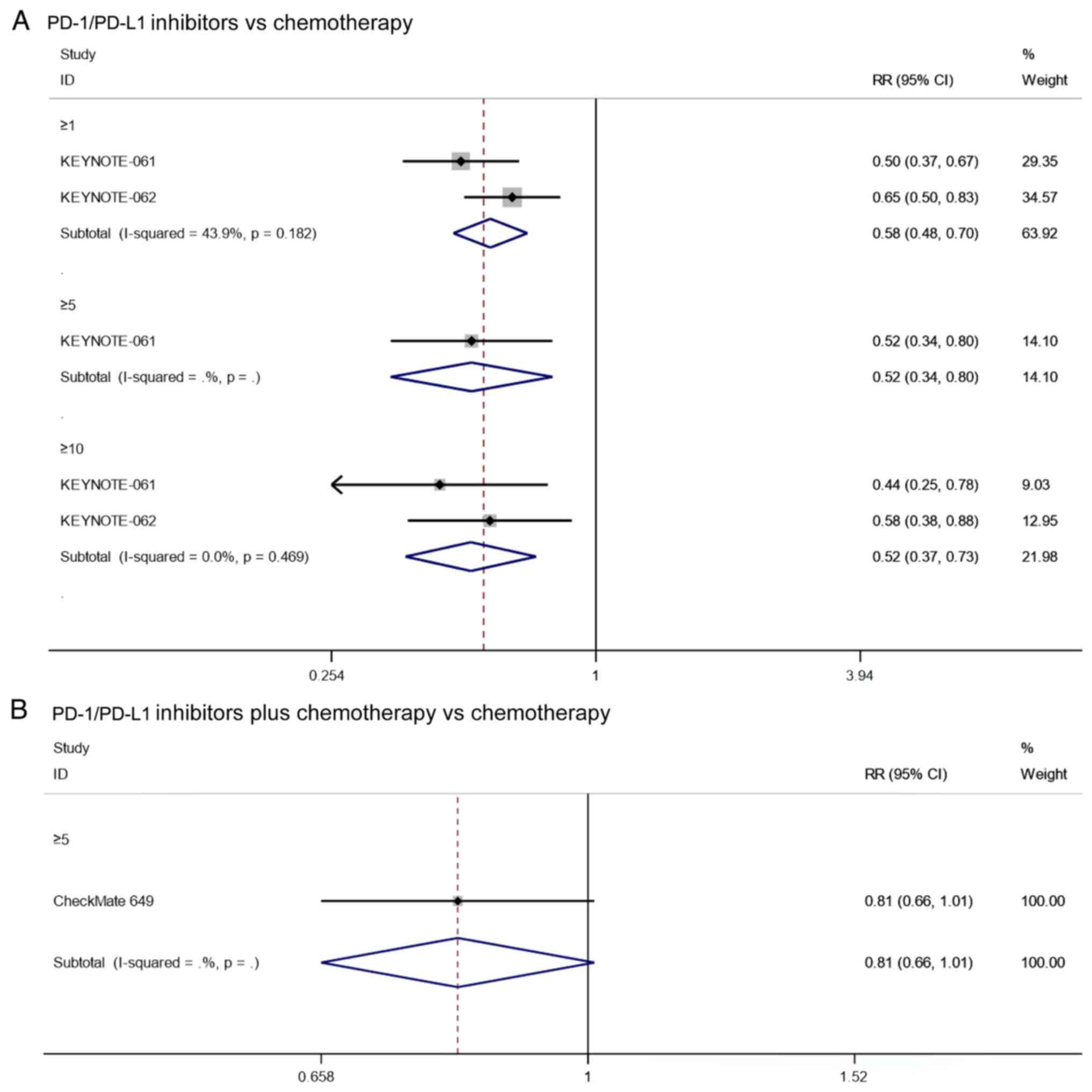
SD rate
Two studies reported on the SD rate of patients with
PD-L1 CPS≥1 that underwent PD-1/PD-L1 inhibitors vs. chemotherapy.
Since there was no significant heterogeneity (I2=43.9%,
P=0.182), the analysis was performed using a fixed effects model.
The pooled results showed that the SD rate of PD-1/PD-L1 inhibitors
in patients with PD-L1 CPS≥1 was significantly lower than that of
chemotherapy (RR=0.58, 95% CI: 0.48–0.70, P≤0.001; Fig. 7A). Notably, the results of
KEYNOTE-061 (17) showed that
PD-1/PD-L1 inhibitor had a significantly lower SD rate than
chemotherapy in patients with PD-L1 CPS≥5 (RR=0.52, 95% CI:
0.37–0.73, P=0.003; Fig. 7A).
Furthermore, the pooled results also showed that the SD rate of
PD-1/PD-L1 inhibitors in patients with PD-L1 CPS≥10 was
significantly lower than that of chemotherapy (RR=0.52, 95% CI:
0.37–0.73, P≤0.001; Fig. 7A). In
summary, PD-1/PD-L1 inhibitors have higher SD rates compared to
chemotherapy in PD-L1-positive patients.
As for the SD rate of patients with PD-L1 positive
cancer who underwent PD1/PD-L1 inhibitor combined with chemotherapy
vs. chemotherapy, the results of CheckMate 649 (20) showed that there was no significant
difference in the SD rate of patients with PD-L1 CPS≥5 between
PD-1/PD-L1 inhibitor combined with chemotherapy and chemotherapy
alone (RR=0.81, 95% CI: 0.66–1.01, P=0.062; Fig. 7B).
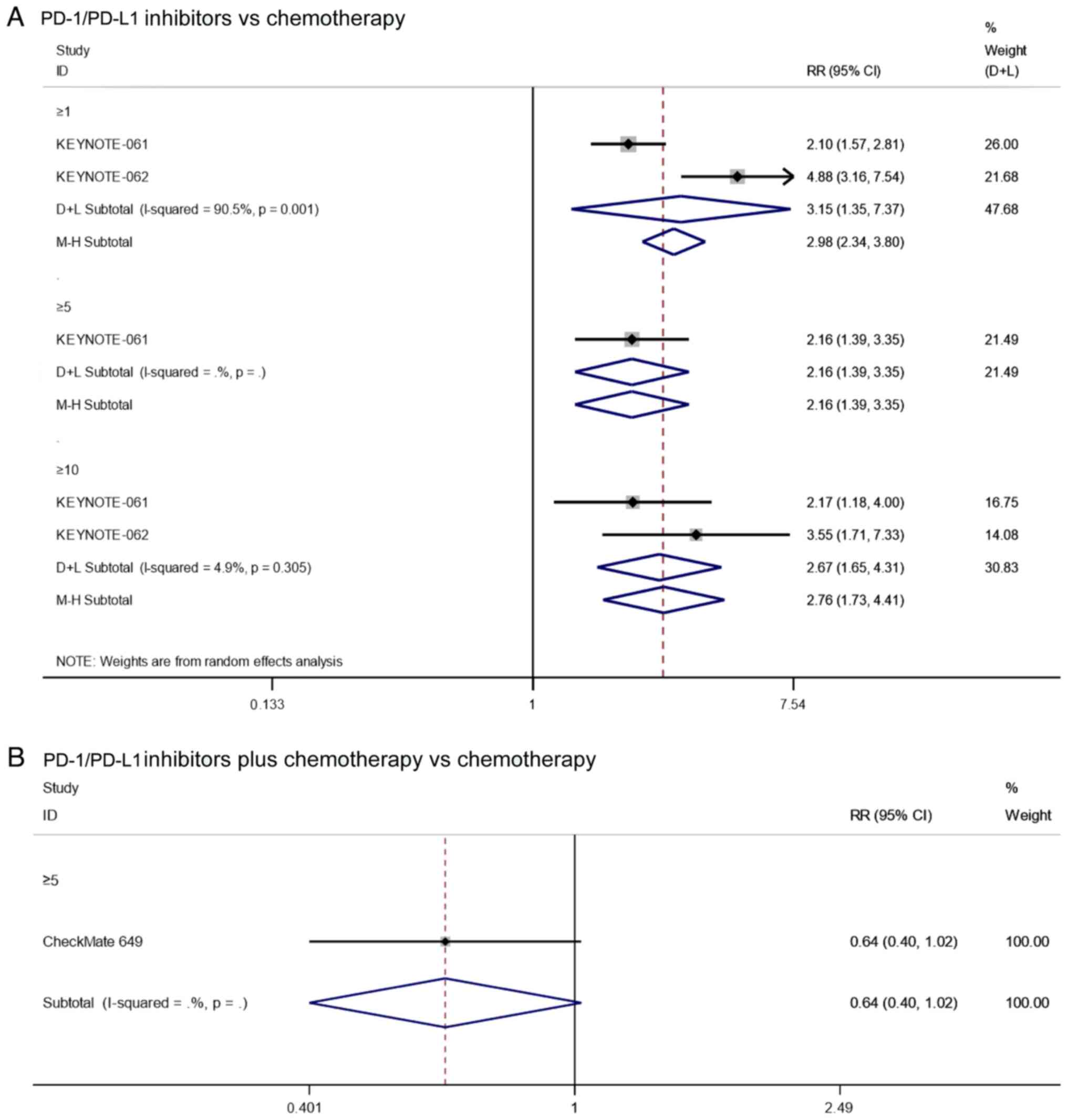
PD rate
Two studies reported on the PD rate of patients with
PD-L1 CPS≥1 that underwent treatment with PD-1/PD-L1 inhibitors vs.
chemotherapy. Since there was significant heterogeneity
(I2=90.5%, P=0.001), the analysis was performed using a
random effects model. The pooled results showed that the PD rate of
PD-1/PD-L1 inhibitors in patients with PD-L1 CPS≥1 was
significantly higher than that of chemotherapy (RR=3.15, 95% CI:
1.35–7.37, P=0.008; Fig. 8A). In
addition, the results of KEYNOTE-061 (17) showed that PD-1/PD-L1 inhibitors
significantly increased the PD rate compared with chemotherapy in
patients with PD-L1 CPS≥5 (RR=2.16, 95% CI: 1.39–3.35, P=0.001;
Fig. 8A). Furthermore, the pooled
results also showed that the PD rate of PD-1/PD-L1 inhibitors in
patients with PD-L1 CPS≥10 was significantly higher than that of
chemotherapy (RR=2.78, 95% CI: 1.73–4.41, P≤0.001; Fig. 8A). In summary, PD-1/PD-L1 inhibitors
had higher PD rates compared to chemotherapy in PD-L1-positive
patients.
As for the PD rates of patients with PD-L1 positive
cancer undergoing PD1/PD-L1 inhibitor combined with chemotherapy
vs. chemotherapy alone, the results of CheckMate 649 (20) showed that there was no significant
difference in the PD rate of patients with PD-L1 CPS≥5 when treated
with PD-1/PD-L1 inhibitors combined with chemotherapy vs.
chemotherapy alone (RR=0.64, 95% CI: 0.40–1.02, P=0.062; Fig. 8B).
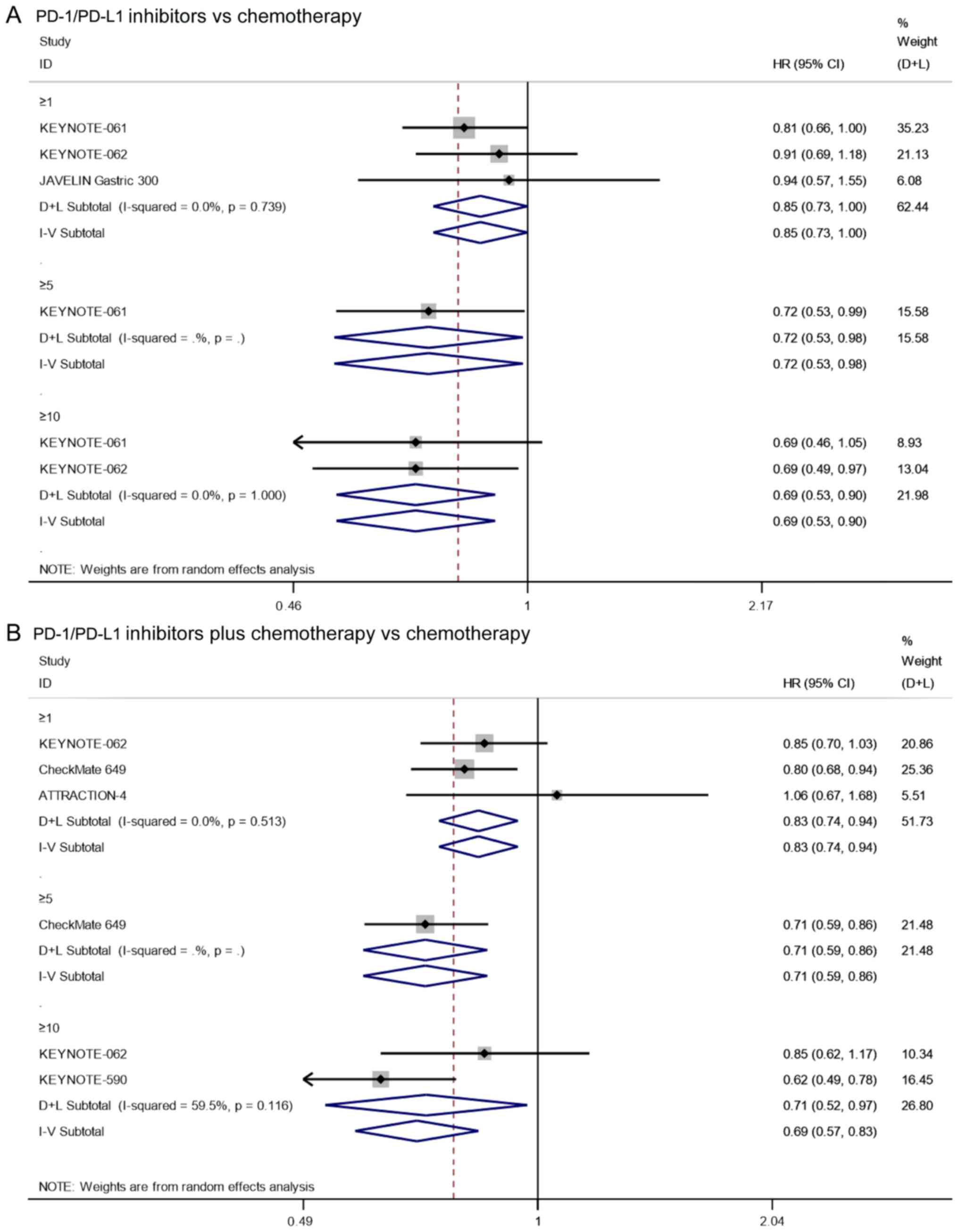
OS
Three studies reported on the OS of patients with
PD-L1 CPS≥1 that underwent treatment with PD-1/PD-L1 inhibitors vs.
chemotherapy. Since there was no significant heterogeneity
(I2=0.0%, P=0.739), the analysis was performed using a
fixed effects model. The pooled results showed that the OS of
PD-1/PD-L1 inhibitors in patients with PD-L1 CPS≥1 was
significantly longer than that of chemotherapy (HR=0.85, 95% CI:
0.73–1.00, P=0.049; Fig. 9A). In
addition, the results of KEYNOTE-061 (17) showed that PD-1/PD-L1
inhibitor-treated group had a significantly longer OS than
chemotherapy in patients with PD-L1 CPS≥5 (HR=0.72, 95% CI:
0.53–0.98, P=0.039; Fig. 9A). As
for the OS of patients with PD-L1 CPS≥10, the pooled results also
showed that the OS of PD-1/PD-L1 inhibitors in patients with PD-L1
CPS≥10 was significantly longer than that of chemotherapy (HR=0.69,
95% CI: 0.53–0.90, P=0.006; I2=0.0%, P=1.000; Fig. 9A). Altogether, in PD-L1-positive
patients, PD-1/PD-L1 inhibitors increased OS compared with
chemotherapy.
Three studies reported on the OS of patients with
PD-L1 CPS≥1 that underwent treatment with PD-1/PD-L1 inhibitors
combined with chemotherapy vs. chemotherapy alone. Since there was
no significant heterogeneity (I2=0.0%, P=0.513), the
analysis was performed using a fixed effects model. The pooled
results showed that the OS of PD-1/PD-L1 inhibitors combined with
chemotherapy in patients with PD-L1 CPS≥1 was significantly longer
than that of chemotherapy alone (HR=0.83, 95% CI: 0.74–0.94,
P=0.003; Fig. 9B). In addition, the
results of CheckMate 649 (20)
showed that PD-1/PD-L1 inhibitor-treated group combined with
chemotherapy had a significantly longer OS than chemotherapy alone
in patients with PD-L1 CPS≥5 (HR=0.71, 95% CI: 0.59–0.86, P≤0.001;
Fig. 9B). As for the OS of patients
with PD-L1 CPS≥10, the pooled results also showed that the OS of
the PD-1/PD-L1 inhibitors combined with chemotherapy in patients
with PD-L1 CPS≥10 was significantly longer than that of
chemotherapy alone (HR=0.71, 95% CI: 0.52–0.97, P=0.030;
I2=59.5%, P=0.116; Fig.
9B). In conclusion, in PD-L1-positive patients, PD-1/PD-L1
inhibitors combined with chemotherapy also prolonged OS compared
with chemotherapy alone.
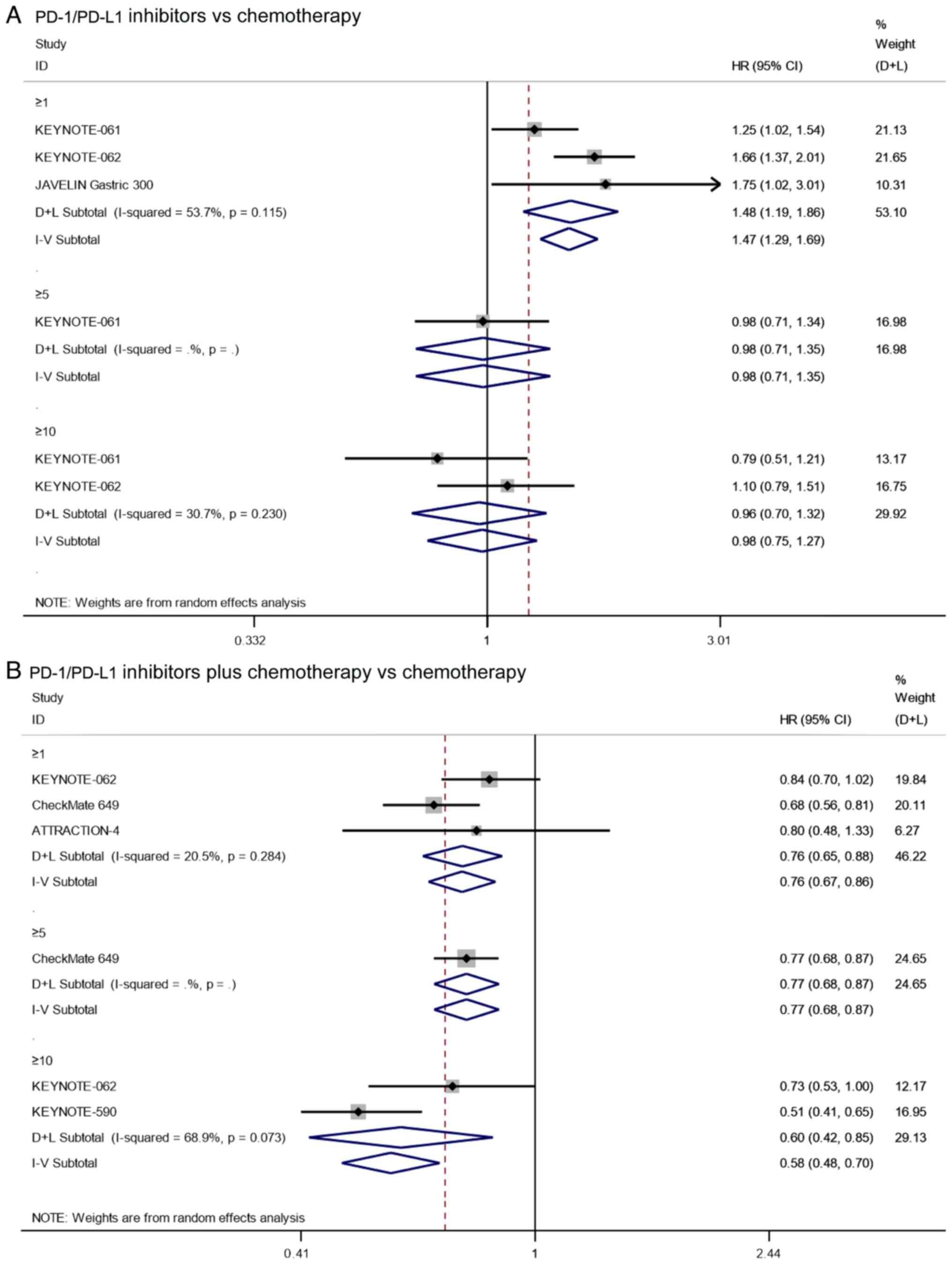
PFS
Three studies reported on the PFS of patients with
PD-L1 CPS≥1 that underwent treatment with PD-1/PD-L1 inhibitors vs.
chemotherapy alone. Since there was significant heterogeneity
(I2=53.7%, P=0.116), the analysis was performed using a
random effects model. The pooled results showed that the PFS of
PD-1/PD-L1 inhibitors in patients with PD-L1 CPS≥1 was
significantly shorter than that of chemotherapy (HR=1.48, 95% CI:
1.19–1.86, P=0.001; Fig. 10A).
However, the results of KEYNOTE-061 (17) showed that there was no significant
difference between PD-1/PD-L1 inhibitors and chemotherapy in
patients with PD-L1 CPS≥5 (HR=0.98, 95% CI: 0.71–1.35, P=0.901;
Fig. 10A). As for the PFS of
patients with PD-L1 CPS≥10, the pooled results also showed that
there was no significant difference between treatment with
PD-1/PD-L1 inhibitors and chemotherapy in patients with PD-L1
CPS≥10 (HR=0.98, 95% CI: 0.75–1.27, P=0.857; I2=30.7%,
P=0.230; Fig. 10A). The results
suggest that PD-1/PD-L1 inhibitors can prolong PFS in patients with
PD-L1 CPS≥1 compared with chemotherapy, but this conclusion does
not hold in patients with PD-L1 CPS≥5 or 10, indicating that there
was a potential correlation between the CPS and drug efficacy.
The pooled results showed that the PFS of PD-1/PD-L1
inhibitors combined with chemotherapy in patients with PD-L1 CPS≥1
was significantly longer than that of chemotherapy alone (HR=0.76,
95% CI: 0.67–0.86, P≤0.001; I2=20.5%, P=0.284; based on
three studies; Fig. 10B).
Additionally, the results of CheckMate 649 (20) showed that the PFS of PD-1/PD-L1
inhibitors combined with chemotherapy in patients with PD-L1 CPS≥5
was significantly longer than that of chemotherapy alone (HR=0.77,
95% CI: 0.68–0.87, P≤0.001; Fig.
10B). As for the PFS of patients with PD-L1 CPS≥10, the pooled
results also showed that the PFS of PD-1/PD-L1 inhibitors combined
with chemotherapy was significantly longer than that of
chemotherapy alone (HR=0.60, 95% CI: 0.42–0.85, P=0.004;
I2=68.9%, P=0.073; Fig.
10B). These results suggest that PD1/PD-L1 inhibitors combined
with chemotherapy can prolong PFS regardless of the CPS.
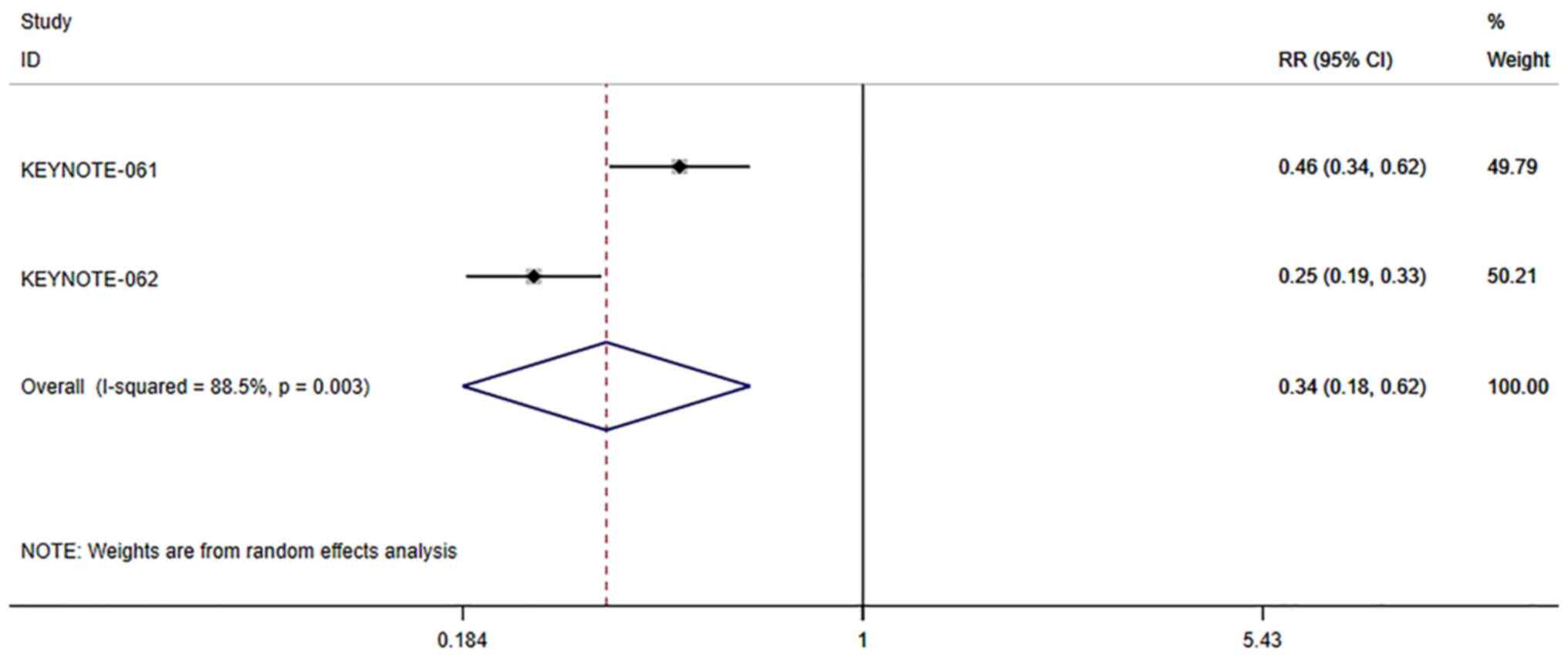
Adverse events for patients with PD-L1
positive cancer
The incidence of adverse events of PD-1/PD-L1
inhibitors vs. chemotherapy in patients with PD-L1 positive cancer
was also analyzed. Since there was significant heterogeneity
(I2=53.7%, P=0.116), the analysis was performed using a
random effects model. The pooled results showed that the incidence
of adverse events of PD-1/PD-L1 inhibitors in patients with PD-L1
positive cancer was significantly lower than that of chemotherapy
(RR=0.34, 95% CI: 0.18–0.62, P≤0.001; Fig. 11).
Sensitivity analysis
To assess whether any individual study included in
this meta-analysis had an undue impact on the overall results,
sensitivity analysis was performed wherein individual studies were
sequentially excluded from the summary analysis. This approach
indicated that none of the included studies biased the overall
results of the meta-analysis, as evidenced by the stability of the
results, further emphasizing the reliability of these findings
(Fig. S2, Fig. S3, Fig.
S4, Fig. S5, Fig. S6).
Discussion
Patients diagnosed with advanced GC/GEJC often have
poor outcomes despite the use of standard treatments, such as
chemotherapy and biologic agents. Previous studies have focused on
evaluating combinations of ICIs, standard chemotherapy, and
biologic agents as well as novel biomarkers to prolong survival and
improve quality of life. However, most chemotherapy therapeutic
approaches fail to provide substantial efficacy benefits (22). The present systematic review and
meta-analysis included 6 studies consisting of 3,286 PD-L1 positive
patients, comparing the OS, PFS, ORR, SD, PD, and incidence of
adverse events of PD-1/PD-L1 inhibitor or PD-1/PD-L1 inhibitor
combined with chemotherapy vs. chemotherapy in PD-L1-positive
GC/GEJC patients, aiming to provide more precise guidance for the
clinical treatment of GC/GEJC patients.
The present study first analyzed the OS and PFS
rates of PD1/PD-L1 inhibitors or PD1/PD-L1 inhibitors combined with
chemotherapy vs. chemotherapy alone for advanced gastric or
gastro-esophageal junction cancer. The pooled results showed that
single-agent PD-1/PD-L1 inhibitor did not result in a relative
improvement in OS compared with chemotherapy in the treatment of
patients with advanced GC/GEJC, which was consistent with the
conclusions of Wang et al (11). Conversely, in the analysis of PFS,
the JAVELIN Gastric 300 study (18)
reported that the PFS of PD-1/PD-L1 inhibitors for advanced GC/GEJC
was significantly lower than that of chemotherapy. Notably, Wang
et al (11) also reported
that GC/GEJC patients treated with PD-1/PD-L1 inhibitors had an
improved PFS compared with chemotherapy. These data suggest that
PD-1/PD-L1 antagonists may not be superior to chemotherapy in the
treatment of patients with advanced GC/GEJC. However, insufficient
clinical trials analyzing PFS made it impossible to obtain
objective evidence-based medical evidence. In addition, the results
showed that the OS and PFS of PD-1/PD-L1 inhibitors combined with
chemotherapy were both significantly longer than that of
chemotherapy alone.
Next, the analysis of OS, PFS, ORR, SD, and PD, as
well as the incidence of adverse events of PD-1/PD-L1 inhibitors or
PD-1/PD-L1 inhibitors combined with chemotherapy vs. chemotherapy
alone in PD-L1 positive GC/GEJC patients were considered. Pooled
results suggested that single-agent PD1/PD-L1 inhibitor did not
improve the ORR of patients with PD-L1 positive cancer compared to
chemotherapy. However, single-agent PD1/PD-L1 inhibitor did lower
the SD rate and increase the PD rate, regardless of CPS level. This
showed that the single use of PD1/PD-L1 inhibitors had no advantage
in the short-term efficacy of the tumor; that is, in the short
term, the disappearance or regression of the tumor, compared with
chemotherapy did not notably differ. Encouragingly, in the
evaluation of long-term efficacy, the findings found that the OS of
PD-1/PD-L1 inhibitors in patients with PD-L1 CPS≥1, 5, and 10 were
all significantly longer than that of chemotherapy. Additionally,
the HRs of patients with PD-L1 CPS≥1, 5, and 10 were 0.85, 0.72,
and 0.69, respectively. Despite the lack of statistical evidence
for differential analysis, it is hypothesized that OS may be
prolonged with increased PD-L1 expression. Additionally, the pooled
results showed that the incidence of adverse events of PD-1/PD-L1
inhibitors in patients with PD-L1 positive was significantly lower
than that of chemotherapy. Therefore, it can be suggested that
anti-PD1/PD-L1 treatment was safer than chemotherapy.
As for the comparison of PD-1/PD-L1 inhibitor
combined with chemotherapy vs. chemotherapy alone in PD-L1 positive
GC/GEJC patients, the pooled results first showed that the ORR of
PD-1/PD-L1 inhibitors combined with chemotherapy in patients with
PD-L1 CPS≥1 was significantly higher than that of chemotherapy
alone. In addition, the results of CheckMate 649 (20) and KEYNOTE-062 (18) showed that patients treated with
PD-1/PD-L1 inhibitor combined with chemotherapy had a significantly
higher ORR than chemotherapy alone in patients with PD-L1 CPS≥5 and
PD-L1 CPS≥10. The results of CheckMate 649 (20) showed that there was no significant
difference in the SD and PD rates of patients with PD-L1 CPS≥5
between the patients treated with PD-1/PD-L1 inhibitor combined
with chemotherapy vs. chemotherapy alone. Although the lack of more
clinical trial results challenges the objectivity of the SD and PD
rate analysis, the pooled results still suggest that PD-1/PD-L1
inhibitor combined with chemotherapy had better short-term efficacy
than chemotherapy alone in patients with PD-L1 positive cancer.
Furthermore, the findings suggest that the OS and PFS of PD-1/PD-L1
inhibitors combined with chemotherapy in patients with PD-L1 CPS≥1,
5, and 10 were all significantly longer than that of chemotherapy
alone. It can also be speculated that with the increase in PD-L1
expression, the survival advantage of combination therapy over
chemotherapy alone may become more apparent.
The present study has some limitations. First, there
was heterogeneity in the research in some of the indicators in this
study; due to the small number of included studies, it was not
possible to identify the source of heterogeneity. Second, due to
the lack of relevant clinical trials, some of the results can only
be systematically reviewed and not meta-analyzed to obtain more
objective evidence-based medical evidence. Third, the lack of
clinical trials makes it impossible to assess the publication bias
of this study. Future studies need to supplement the assessment of
publication bias based on the inclusion of more clinical
trials.
In conclusion, single agent of PD-1/PD-L1 inhibitor
alone or in combination with chemotherapy significantly prolonged
the survival of patients compared with chemotherapy alone with
fewer adverse effects. However, the degree of CPS may affect
efficacy, but further investigation is needed to verify this.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Joint Funds for the innovation
of Science and Technology, Fujian province (grant no.
2019QH1120).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQS wrote the manuscript and analyzed the data. SQS
confirms the authenticity of the raw data and has read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC/GEJC
|
gastric or gastro-esophageal junction
cancer patients
|
|
RCT
|
randomized controlled trial
|
|
ICI
|
immune checkpoint inhibitor
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
CPS
|
combined positive score
|
|
OS
|
overall survival
|
|
PFS
|
progression free-survival
|
|
ORR
|
objective response rate
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
CI
|
confidence interval
|
|
RR
|
risk ratio
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Digklia A and Wagner AD: Advanced gastric
cancer: Current treatment landscape and future perspectives. World
J Gastroenterol. 22:2403–2414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Laethem JL, Carneiro F, Ducreux M,
Messman H, Lordick F, Ilson DH, Allum WH, Haustermans K, Lepage C,
Matysiak-Budnik T, et al: The multidisciplinary management of
gastro-oesophageal junction tumours: European Society of Digestive
Oncology (ESDO): Expert discussion and report from the 16th ESMO
World Congress on Gastrointestinal Cancer, Barcelona. Dig Liver
Dis. 48:1283–1289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee, : Gastric
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, et al: Docetaxel versus active symptom control for
refractory oesophagogastric adenocarcinoma (COUGAR-02): An
open-label, phase 3 randomised controlled trial. Lancet Oncol.
15:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thuss-Patience PC, Kretzschmar A, Bichev
D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer-a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amatatsu M, Arigami T, Uenosono Y,
Yanagita S, Uchikado Y, Kijima Y, Kurahara H, Kita Y, Mori S,
Sasaki K, et al: Programmed death-ligand 1 is a promising blood
marker for predicting tumor progression and prognosis in patients
with gastric cancer. Cancer Sci. 109:814–820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawazoe A, Kuwata T, Kuboki Y, Shitara K,
Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A and Ochiai A:
Clinicopathological features of programmed death ligand 1
expression with tumor-infiltrating lymphocyte, mismatch repair, and
Epstein-Barr virus status in a large cohort of gastric cancer
patients. Gastric Cancer. 20:407–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan J, Zhang J, Zhu Y, Li N, Tian T, Li
Y, Li Y, Li Z, Lai Y, Gao J and Shen L: Programmed death-ligand-1
expression in advanced gastric cancer detected with RNA in situ
hybridization and its clinical significance. Oncotarget.
7:39671–39679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang BC, Zhang ZJ, Fu C and Wang C:
Efficacy and safety of anti-PD-1/PD-L1 agents vs chemotherapy in
patients with gastric or gastroesophageal junction cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
98:e180542019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Z, Guo Y and Zou CP: Oncological
outcomes of addition of anti-PD1/PD-L1 to chemotherapy in the
therapy of patients with advanced gastric or gastro-oesophageal
junction cancer: A meta-analysis. Medicine (Baltimore).
99:e183322020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and Group P: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Wang J and Lin Y: Comparison of
the efficacy and safety of repeated hepatectomy and radiofrequency
ablation in the treatment of primary recurrent liver cancer: A
meta-analysis. World J Surg Oncol. 20:1822022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuchs CS, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated PD-L1-positive advanced gastric or gastroesophageal
junction cancer: 2-year update of the randomized phase 3
KEYNOTE-061 trial. Gastric Cancer. 25:197–206. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang YJ, Ruiz EY, Van Cutsem E, Lee KW,
Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, et
al: Taieb, Phase III, randomised trial of avelumab versus
physician's choice of chemotherapy as third-line treatment of
patients with advanced gastric or gastro-oesophageal junction
cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol.
29:2052–2060. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miceli R, Tomasello G, Bregni G, Di
Bartolomeo M and Pietrantonio F: Adjuvant chemotherapy for gastric
cancer: Current evidence and future challenges. World J
Gastroenterol. 20:4516–4525. 2014. View Article : Google Scholar : PubMed/NCBI
|