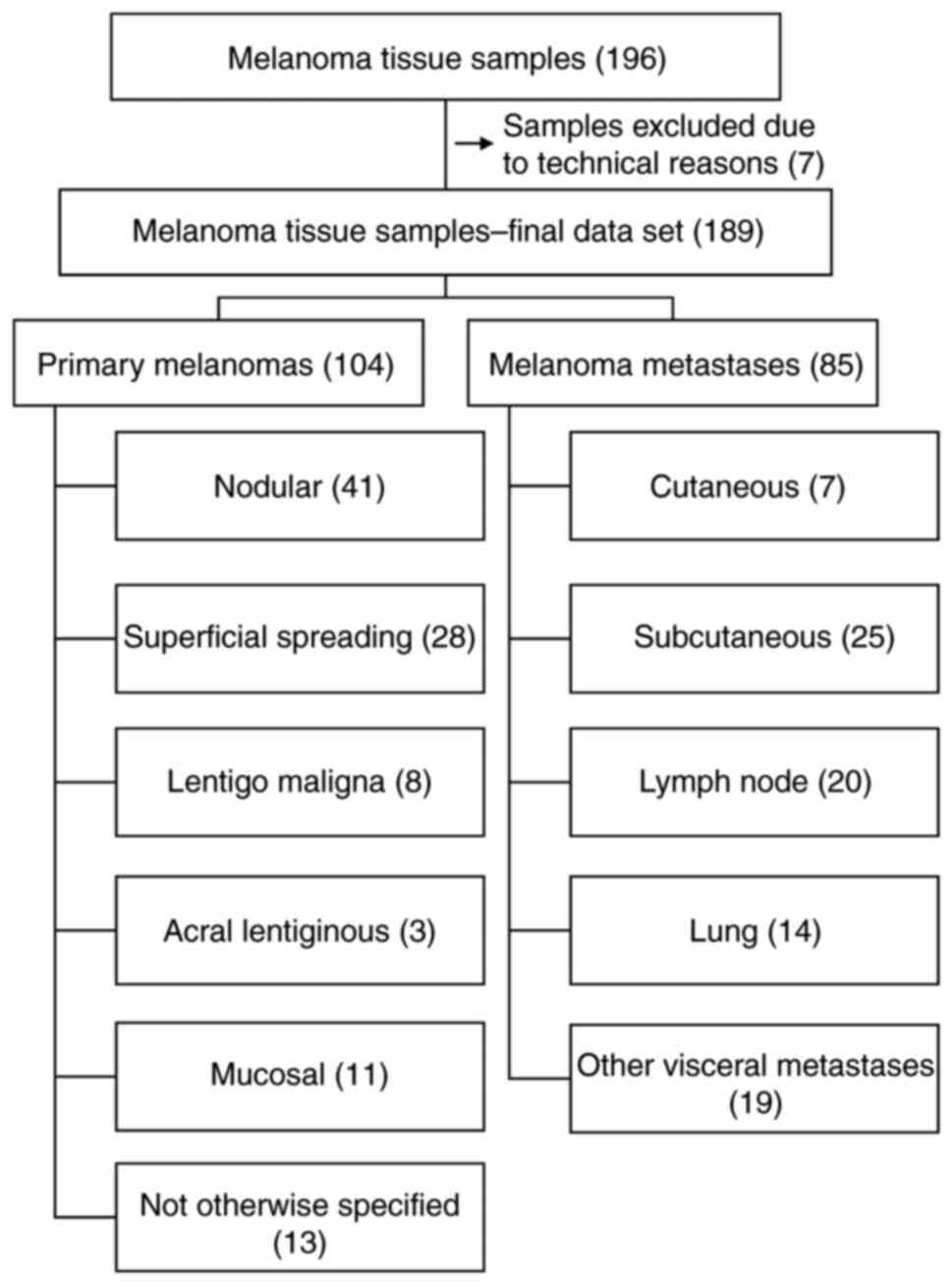
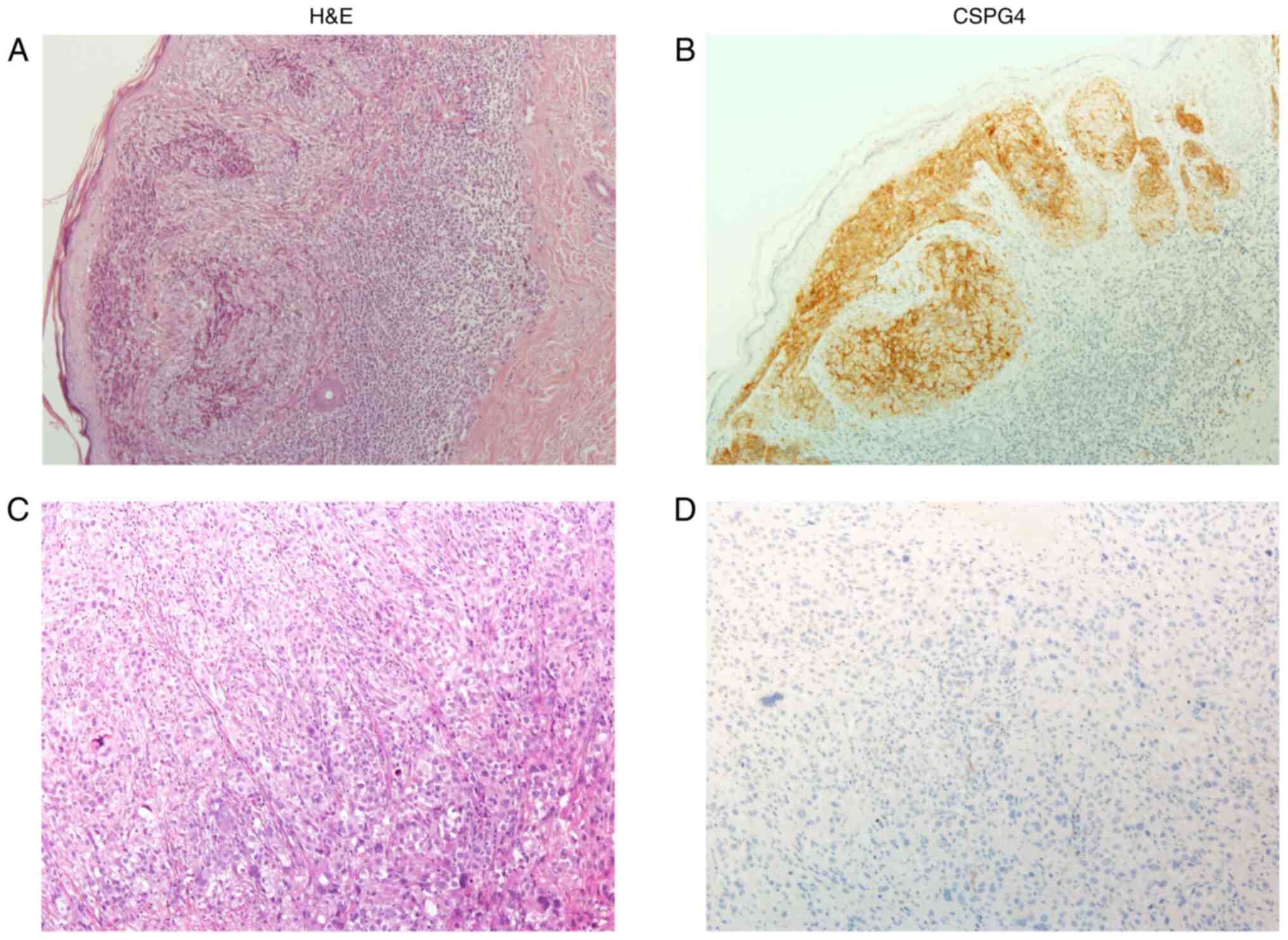
|
1
|
World Health Organization, International
Agency for Research on Cancer (IARC), . GLOBOCAN 2020: Estimated
incidence, mortality and prevalence rates in 2020, melanoma of
skin. Available from:. http://gco.iarc.fr/today/homeFeb 15–2022
|
|
2
|
Monshi B, Vujic M, Kivaranovic D, Sesti A,
Oberaigner W, Vujic I, Ortiz-Urda S, Posch C, Feichtinger H, Hackl
M and Rappersberger K: The burden of malignant melanoma-lessons to
be learned from Austria. Eur J Cancer. 56:45–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crocetti E, Mallone S, Robsahm TE, Gavin
A, Agius D, Ardanaz E, Lopez MC, Innos K, Minicozzi P, Borgognoni
L, et al: Survival of patients with skin melanoma in Europe
increases further: Results of the EUROCARE-5 study. Eur J Cancer.
51:2179–2190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duschek N, Skvara H, Kittler H, Delir G,
Fink A, Pinkowicz A and Waldhor T: Melanoma epidemiology of Austria
reveals gender-related differences. Eur J Dermatol. 23:872–878.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platz A, Egyhazi S, Ringborg U and Hansson
J: Human cutaneous melanoma; a review of NRAS and BRAF mutation
frequencies in relation to histogenetic subclass and body site. Mol
Oncol. 1:395–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larkin J, Ascierto PA, Dreno B, Atkinson
V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas
L, et al: Combined vemurafenib and cobimetinib in BRAF-mutated
melanoma. N Engl J Med. 371:1867–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Dabrafenib and trametinib versus dabrafenib and
placebo for Val600 BRAF-mutant melanoma: A multicentre,
double-blind, phase 3 randomised controlled trial. Lancet.
386:444–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: Adjuvant Nivolumab versus ipilimumab in
resected stage III or IV melanoma. N Engl J Med. 377:1824–1835.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribas A, Lawrence D, Atkinson V, Agarwal
S, Miller WH Jr, Carlino MS, Fisher R, Long GV, Hodi FS, Tsoi J, et
al: Combined BRAF and MEK inhibition with PD-1 blockade
immunotherapy in BRAF-mutant melanoma. Nat Med. 25:936–940. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Price MA, Wanshura LE, Yang J, Carlson J,
Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA and McCarthy JB:
CSPG4, a potential therapeutic target, facilitates malignant
progression of melanoma. Pigment Cell Melanoma Res. 24:1148–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bumol TF and Reisfeld RA: Unique
glycoprotein-proteoglycan complex defined by monoclonal antibody on
human melanoma cells. Proc Natl Acad Sci USA. 79:1245–1249. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pluschke G, Vanek M, Evans A, Dittmar T,
Schmid P, Itin P, Filardo EJ and Reisfeld RA: Molecular cloning of
a human melanoma-associated chondroitin sulfate proteoglycan. Proc
Natl Acad Sci USA. 93:9710–9715. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Price MA, Neudauer CL, Wilson C,
Ferrone S, Xia H, Iida J, Simpson MA and McCarthy JB: Melanoma
chondroitin sulfate proteoglycan enhances FAK and ERK activation by
distinct mechanisms. J Cell Biol. 165:881–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makagiansar IT, Williams S, Mustelin T and
Stallcup WB: Differential phosphorylation of NG2 proteoglycan by
ERK and PKCalpha helps balance cell proliferation and migration. J
Cell Biol. 178:155–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Price MA, Li GY, Bar-Eli M, Salgia
R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA and McCarthy
JB: Melanoma proteoglycan modifies gene expression to stimulate
tumor cell motility, growth, and epithelial-to-mesenchymal
transition. Cancer Res. 69:7538–7547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Natali PG, Giacomini P, Russo C, Steinbach
G, Fenoglio C and Ferrone S: Antigenic profile of human melanoma
cells. Analysis with monoclonal antibodies to histocompatibility
antigens and to melanoma-associated antigens. J Cutan Pathol.
10:225–237. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wang Y, Yu L, Sakakura K, Visus C,
Schwab JH, Ferrone CR, Favoino E, Koya Y, Campoli MR, et al: CSPG4
in cancer: Multiple roles. Curr Mol Med. 10:419–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fenton M, Whiteside TL, Ferrone S and
Boyiadzis M: Chondroitin sulfate proteoglycan-4 (CSPG4)-specific
monoclonal antibody 225.28 in detection of acute myeloid leukemia
blasts. Oncol Res. 22:117–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keleg S, Titov A, Heller A, Giese T,
Tjaden C, Ahmad SS, Gaida MM, Bauer AS, Werner J and Giese NA:
Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive
marker in pancreatic tumors. PLoS One. 9:e1001782014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu SC, Nadesan P, Puviindran V, Stallcup
WB, Kirsch DG and Alman BA: Effects of chondroitin sulfate
proteoglycan 4 (NG2/CSPG4) on soft-tissue sarcoma growth depend on
tumor developmental stage. J Biol Chem. 293:2466–2475. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egan CE, Stefanova D, Ahmed A, Raja VJ,
Thiesmeyer JW, Chen KJ, Greenberg JA, Zhang T, He B, Finnerty BM,
et al: CSPG4 is a potential therapeutic target in anaplastic
thyroid cancer. Thyroid. 31:1481–1493. 2021.PubMed/NCBI
|
|
27
|
Beard RE, Zheng Z, Lagisetty KH, Burns WR,
Tran E, Hewitt SM, Abate-Daga D, Rosati SF, Fine HA, Ferrone S, et
al: Multiple chimeric antigen receptors successfully target
chondroitin sulfate proteoglycan 4 in several different cancer
histologies and cancer stem cells. J Immunother Cancer. 2:252014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riccardo F, Tarone L, Iussich S, Giacobino
D, Arigoni M, Sammartano F, Morello E, Martano M, Gattino F, De
Maria R, et al: Identification of CSPG4 as a promising target for
translational combinatorial approaches in osteosarcoma. Ther Adv
Med Oncol. 11:17588359198554912019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Liao Q, Price M, Moriarity B, Wolf
N, Felices M, Miller JS, Geller MA, Bendzick L, Hopps R, et al:
Chondroitin sulfate proteoglycan 4, a targetable oncoantigen that
promotes ovarian cancer growth, invasion, cisplatin resistance and
spheroid formation. Transl Oncol. 16:1013182022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kageshita T, Nakamura T, Yamada M, Kuriya
N, Arao T and Ferrone S: Differential expression of melanoma
associated antigens in acral lentiginous melanoma and in nodular
melanoma lesions. Cancer Res. 51:1726–1732. 1991.PubMed/NCBI
|
|
31
|
Nishi H, Inoue Y, Kageshita T, Takata M
and Ihn H: The expression of human high molecular weight
melanoma-associated antigen in acral lentiginous melanoma. Biosci
Trends. 4:86–89. 2010.PubMed/NCBI
|
|
32
|
Kageshita T, Kimura T, Yoshi A, Hirai S,
Ono T and Ferrone S: Antigenic profile of mucosal melanoma lesions.
Int J Cancer. 56:370–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kageshita T, Kuriya N, Ono T, Horikoshi T,
Takahashi M, Wong GY and Ferrone S: Association of high molecular
weight melanoma-associated antigen expression in primary acral
lentiginous melanoma lesions with poor prognosis. Cancer Res.
53:2830–2833. 1993.PubMed/NCBI
|
|
34
|
Li Y, Madigan MC, Lai K, Conway RM,
Billson FA, Crouch R and Allen BJ: Human uveal melanoma expresses
NG2 immunoreactivity. Br J Ophthalmol. 87:629–632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hafner C, Breiteneder H, Ferrone S,
Thallinger C, Wagner S, Schmidt WM, Jasinska J, Kundi M, Wolff K,
Zielinski CC, et al: Suppression of human melanoma tumor growth in
SCID mice by a human high molecular weight-melanoma associated
antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer.
114:426–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Favoino E, Wang Y, Ma Y, Deng X and
Wang X: The CSPG4-specific monoclonal antibody enhances and
prolongs the effects of the BRAF inhibitor in melanoma cells.
Immunol Res. 50:294–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uranowska K, Samadaei M, Kalic T, Pinter
M, Breiteneder H and Hafner C: A chondroitin sulfate proteoglycan
4-specific monoclonal antibody inhibits melanoma cell invasion in a
spheroid model. Int J Oncol. 59:702021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroff RW, Woodhouse CS, Foon KA, Oldham
RK, Farrell MM, Klein RA and Morgan AC Jr: Intratumor localization
of monoclonal antibody in patients with melanoma treated with
antibody to a 250,000-dalton melanoma-associated antigen. J Natl
Cancer Inst. 74:299–306. 1985.PubMed/NCBI
|
|
39
|
Schroff RW, Morgan AC Jr, Woodhouse CS,
Abrams PG, Farrell MM, Carpenter BE, Oldham RK and Foon KA:
Monoclonal antibody therapy in malignant melanoma: Factors
effecting in vivo localization. J Biol Response Mod. 6:457–472.
1987.PubMed/NCBI
|
|
40
|
Oldham RK, Foon KA, Morgan AC, Woodhouse
CS, Schroff RW, Abrams PG, Fer M, Schoenberger CS, Farrell M and
Kimball E: Monoclonal antibody therapy of malignant melanoma: In
vivo localization in cutaneous metastasis after intravenous
administration. J Clin Oncol. 2:1235–1244. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wagner S, Hafner C, Allwardt D, Jasinska
J, Ferrone S, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H
and Breiteneder H: Vaccination with a human high molecular weight
melanoma-associated antigen mimotope induces a humoral response
inhibiting melanoma cell growth in vitro. J Immunol. 174:976–982.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wagner S, Krepler C, Allwardt D, Latzka J,
Strommer S, Scheiner O, Pehamberger H, Wiedermann U, Hafner C and
Breiteneder H: Reduction of human melanoma tumor growth in severe
combined immunodeficient mice by passive transfer of antibodies
induced by a high molecular weight melanoma-associated antigen
mimotope vaccine. Clin Cancer Res. 14:8178–8183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mittelman A, Chen ZJ, Yang H, Wong GY and
Ferrone S: Human high molecular weight melanoma-associated antigen
(HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody
MK2-23: Induction of humoral anti-HMW-MAA immunity and prolongation
of survival in patients with stage IV melanoma. Proc Natl Acad Sci
USA. 89:466–470. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Ko EC, Peng L, Gillies SD and
Ferrone S: Human high molecular weight melanoma-associated antigen
mimicry by mouse anti-idiotypic monoclonal antibody MK2-23:
Enhancement of immunogenicity of anti-idiotypic monoclonal antibody
MK2-23 by fusion with interleukin 2. Cancer Res. 65:6976–6983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mittelman A, Chen ZJ, Kageshita T, Yang H,
Yamada M, Baskind P, Goldberg N, Puccio C, Ahmed T and Arlin Z:
Active specific immunotherapy in patients with melanoma. A clinical
trial with mouse antiidiotypic monoclonal antibodies elicited with
syngeneic anti-high-molecular-weight-melanoma-associated antigen
monoclonal antibodies. J Clin Invest. 86:2136–2144. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Bruyn M, Rybczynska AA, Wei Y,
Schwenkert M, Fey GH, Dierckx RA, van Waarde A, Helfrich W and
Bremer E: Melanoma-associated chondroitin sulfate proteoglycan
(MCSP)-targeted delivery of soluble TRAIL potently inhibits
melanoma outgrowth in vitro and in vivo. Mol Cancer. 9:3012010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jordaan S, Chetty S, Mungra N, Koopmans I,
van Bommel PE, Helfrich W and Barth S: CSPG4: A target for
selective delivery of human cytolytic fusion proteins and TRAIL.
Biomedicines. 5:372017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schwenkert M, Birkholz K, Schwemmlein M,
Kellner C, Kugler M, Peipp M, Nettelbeck DM, Schuler-Thurner B,
Schaft N, Dörrie J, et al: A single chain immunotoxin, targeting
the melanoma-associated chondroitin sulfate proteoglycan, is a
potent inducer of apoptosis in cultured human melanoma cells.
Melanoma Res. 18:73–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geldres C, Savoldo B, Hoyos V, Caruana I,
Zhang M, Yvon E, Del Vecchio M, Creighton CJ, Ittmann M, Ferron S
and Dotti G: T lymphocytes redirected against the chondroitin
sulfate proteoglycan-4 control the growth of multiple solid tumors
both in vitro and in vivo. Clin Cancer Res. 20:962–971. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abken H, Hombach A, Heuser C and Reinhold
U: A novel strategy in the elimination of disseminated melanoma
cells: Chimeric receptors endow T cells with tumor specificity.
Recent Results Cancer Res. 158:249–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Burns WR, Zhao Y, Frankel TL, Hinrichs CS,
Zheng Z, Xu H, Feldman SA, Ferrone S, Rosenberg SA and Morgan RA: A
high molecular weight melanoma-associated antigen-specific chimeric
antigen receptor redirects lymphocytes to target human melanomas.
Cancer Res. 70:3027–3033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Geldres C, Ferrone S and Dotti G:
Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen
receptor-based T-cell immunotherapy of solid tumors. Expert Opin
Ther Targets. 19:1339–1350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Krug C, Birkholz K, Paulus A, Schwenkert
M, Schmidt P, Hoffmann N, Hombach A, Fey G, Abken H, Schuler G, et
al: Stability and activity of MCSP-specific chimeric antigen
receptors (CARs) depend on the scFv antigen-binding domain and the
protein backbone. Cancer Immunol Immunother. 64:1623–1635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wiesinger M, Marz J, Kummer M, Schuler G,
Dorrie J, Schuler-Thurner B and Schaft N: Clinical-scale production
of CAR-T cells for the treatment of melanoma patients by mRNA
transfection of a CSPG4-specific CAR under full GMP compliance.
Cancers (Basel). 11:11982019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Torisu-Itakura H, Schoellhammer HF, Sim
MS, Irie RF, Hausmann S, Raum T, Baeuerle PA and Morton DL:
Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific
BiTE antibody that engages patient-derived T cells. J Immunother.
34:597–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hoffmann RM, Crescioli S, Mele S, Sachouli
E, Cheung A, Chui CK, Andriollo P, Jackson PJM, Lacy KE, Spicer JF,
et al: A novel antibody-drug conjugate (ADC) delivering a DNA
mono-alkylating payload to chondroitin sulfate proteoglycan
(CSPG4)-expressing melanoma. Cancers (Basel). 12:10292020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Allen BJ, Singla AA, Rizvi SM, Graham P,
Bruchertseifer F, Apostolidis C and Morgenstern A: Analysis of
patient survival in a phase I trial of systemic targeted
alpha-therapy for metastatic melanoma. Immunotherapy. 3:1041–1050.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Allen BJ, Raja C, Rizvi S, Li Y, Tsui W,
Graham P, Thompson JF, Reisfeld RA and Kearsley J: Intralesional
targeted alpha therapy for metastatic melanoma. Cancer Biol Ther.
4:1318–1324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Raja C, Graham P, Rizvi SM, Song E,
Goldsmith H, Thompson J, Bosserhoff A, Morgenstern A, Apostolidis
C, Kearsley J, et al: Interim analysis of toxicity and response in
phase 1 trial of systemic targeted alpha therapy for metastatic
melanoma. Cancer Biol Ther. 6:846–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ilieva KM, Cheung A, Mele S, Chiaruttini
G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE,
et al: Chondroitin sulfate proteoglycan 4 and its potential as an
antibody immunotherapy target across different tumor types. Front
Immunol. 8:19112017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: evidence-based changes in the
American Joint Committee on Cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pucciarelli D, Lengger N, Takacova M,
Csaderova L, Bartosova M, Breiteneder H, Pastorekova S and Hafner
C: Anti-chondroitin sulfate proteoglycan 4-specific antibodies
modify the effects of vemurafenib on melanoma cells differentially
in normoxia and hypoxia. Int J Oncol. 47:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Zhao Y and Ma S: Racial
differences in six major subtypes of melanoma: Descriptive
epidemiology. BMC Cancer. 16:69112016. View Article : Google Scholar
|
|
64
|
Uranowska K, Kalic T, Valtsanidis V,
Kitzwögerer M, Breiteneder H and Hafner C: Expression of
chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma cells is
downregulated upon inhibition of BRAF. Oncol Rep. 45:142021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hochst B and Diehl L: Antigen shedding
into the circulation contributes to tumor immune escape.
Oncoimmunology. 1:1620–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|