Introduction
Breast cancer refers to a heterogeneous collection
of tumors characterized by distinct histological morphology,
genomic profiles, molecular behavior and treatment responses.
Invasive ductal carcinoma (IDC) is the most prevalent histological
type, comprising 72–80% of all invasive breast cancer cases. By
contrast, invasive lobular carcinoma (ILC) accounts for 5–15% of
all invasive breast cancer cases and is less common worldwide
(1). Bone, liver, lung and brain
are the most frequent sites of breast cancer metastasis, whereas
metastases to the gastrointestinal (GI) tract and thyroid gland are
less common. GI metastasis, which accounts for 5% of all recurrent
cases, is a rare occurrence (2).
Furthermore, cases of GI metastasis from breast cancer have been
estimated to range between 2 and 18% in some reports (3).
ILC is more likely to metastasize to the GI tract
than IDC (3,4). In particular, luminal-type breast
cancer [estrogen receptor (ER)-positive] or ILC tend to metastasize
to the stomach at a higher rate than other types of breast cancer
(5). Notably, in some studies, 97%
of GI metastases from breast cancer were revealed to be derived
from ILC (3,4). Typically, ductal tumors have a higher
tendency to metastasize to the liver, lung and brain (6), with intestinal metastases occurring in
some cases. The clinical presentation of gastric metastases is
nonspecific, and the endoscopic appearance of gastric metastases
varies, with solitary and submucosal lesions within the gastric
wall constituting the most prevalent findings (7,8).
Typically, 5–7 years pass between the initial diagnosis of breast
cancer and the development of stomach metastases (9).
The clinical presentation of metastases to the
stomach or thyroid is highly unspecific, and the endoscopic
appearance can be heterogeneous. In particular, the most prevalent
findings of gastric metastases are solitary and submucosal lesions
in the gastric wall, which can appear several years after the
initial breast cancer diagnosis (7,8).
Similarly, thyroid gland metastases are uncommon and can be easily
missed; they account for 1.4–3% of all patients who have surgery
for suspected thyroid cancer, with the most common primary cancer
types being soft tissue sarcoma, renal cell, colorectal, lung and
breast cancer (10). Metastatic
thyroid disease commonly results from cancer spreading via the
bloodstream, typically originating from a distant cancer or the
neoplastic process applying directly or through lymphatic channels
to the thyroid gland from nearby organs. Elderly patients,
particularly those in their 60s and 70s, are more likely to develop
metastatic thyroid tumors (8).
The present study describes a rare case of a young
patient diagnosed with human epidermal growth factor receptor 2
(HER2)-positive IDC who eventually developed stomach and thyroid
metastases following local and systemic treatment. This case
highlights the atypical metastatic patterns of breast cancer and
emphasizes the importance of considering distant metastases in
patients with a history of breast cancer. The present study
followed the CARE case report guidelines (11).
Case report
In November 2013, a 30-year-old woman was diagnosed
with IDC of the right breast and underwent a modified radical
mastectomy at Futian People's Hospital of Guangdong Medical College
(Shenzhen, China). Pathological examination confirmed IDC with
extensive high-grade ductal carcinoma in situ, non-special
type, grade III, without lymphovascular invasion.
Immunohistochemistry revealed ER (−), progesterone receptor (PR -)
and HER2 (++), with a Ki-67 index of 70%. Pathological TNM staging
was pT3N0M0 [T=12 cm; N (0/16)] (12). Medical imaging, including thoracic
and abdominal computed tomography (CT) scans and bone scans, showed
no distant metastasis. Fluorescence in situ hybridization
for HER2 was not determined at the time. Post-operative treatment
included six cycles (3 weeks per cycle) of adjuvant chemotherapy
with 5-fluorouracil, epirubicin and cyclophosphamide.
After surgery, the patient was followed up every 3
months for 2 years and every 6 months for 3–5 years. Tumor markers,
breast ultrasound, chest, abdominal and pelvic CT, and brain MRI
examinations were performed regularly, and positron emission
tomography (PET)/CT examinations were additionally performed when
necessary. In August 2015, 21 months after the surgery, the patient
developed an enlarged lymph node in the left side of their neck and
underwent lymph node biopsy. The immunohistochemical staining
revealed: ER (−), PR (−), HER2 (+++), Ki-67 (65%). Chest CT showed
bilateral hilar lymph nodes, bilateral supraclavicular lymph nodes
and axillary lymph nodes metastasis. The first-line regimen
included paclitaxel liposome and Nedaplatin combined with
trastuzumab for six cycles (3 weeks per cycle), and the best
efficacy observed was complete response PET/CT examination was
completed and no tumor signs were found. Trastuzumab maintenance
therapy was then followed; however, in March 2016, metastases were
detected in the chest wall and pleura, and a small amount of fluid
had accumulated in the right thoracic cavity.
Tumor markers (blood tests for CEA, CA125 and CA153)
were elevated and efficacy evaluation showed progressive disease.
Progression-free survival (PFS) represents the survival time of
patients without tumor progression; PFS1 represents PFS after
first-line treatment and PFS2 represents PFS after second-line
treatment. The PFS1 for the patient was 6.5 months. The second-line
treatment regimen included gemcitabine combined with trastuzumab
for six cycles (3 weeks per cycle), and the best efficacy observed
was stable disease. Maintenance therapy with capecitabine and
trastuzumab was then followed; however, the size of the tumor in
the chest wall progressed in December 2016, and PFS2 was 8.3
months. In the third-line treatment regimen, etoposide capsules
combined with trastuzumab and lapatinib were applied for 10 cycles
(3 weeks per cycle), and trastuzumab and lapatinib maintenance
therapy was continued. In August 2017, PET/CT showed metastatic
tumors in the right chest wall, metastasis in the left breast and
increased pleural effusion on the right side. Also in August 2017,
the patient was first admitted to Department of Medical Oncology,
National Cancer Center/National Clinical Research Center for
Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College (Shenzhen, China)
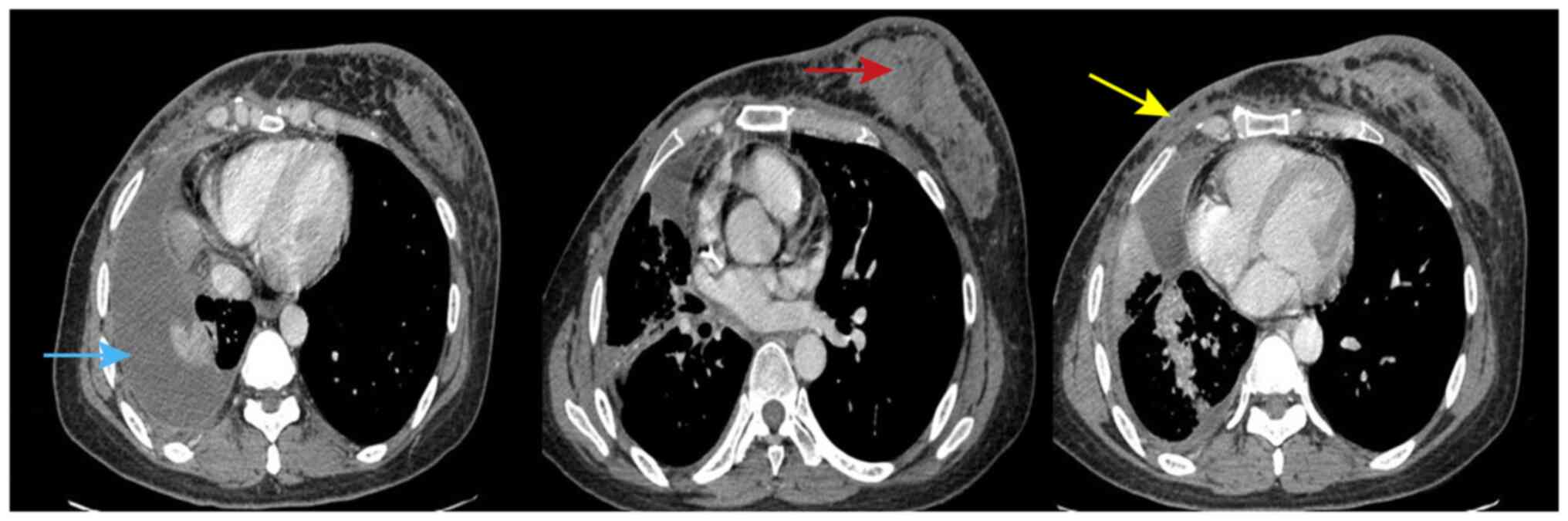
due to tightness in their chest. Chest CT indicated left breast
metastasis, metastasis in the right chest wall and right pleural
effusion (Fig. 1). For fourth-line
treatment, the patient received vinorelbine capsules combined with
trastuzumab and lapatinib for five cycles (3 weeks per cycle).
However, vinorelbine capsules and lapatinib were stopped for
economic reasons, and trastuzumab therapy was continued. The
patient gradually developed multi-site metastasis in November 2018,
including pleural, chest wall, lung lymphangitis carcinomatosa,
left breast, pancreas, adrenal and thyroid metastases (Fig. 1). In the present case, there were no
standard treatment plans in the guidelines for patients with such a
short PFS time and after multiline therapy.
To summarize, since the first recurrence and
metastasis, the patient was treated with various chemotherapy
drugs, including gemcitabine, capecitabine, vinorelbine, etoposide,
Herceptin and lapatinib, over a period of 2 years and 6 months.
Unfortunately, despite receiving multi-line chemotherapy and
targeted Herceptin-based therapy, their condition continued to
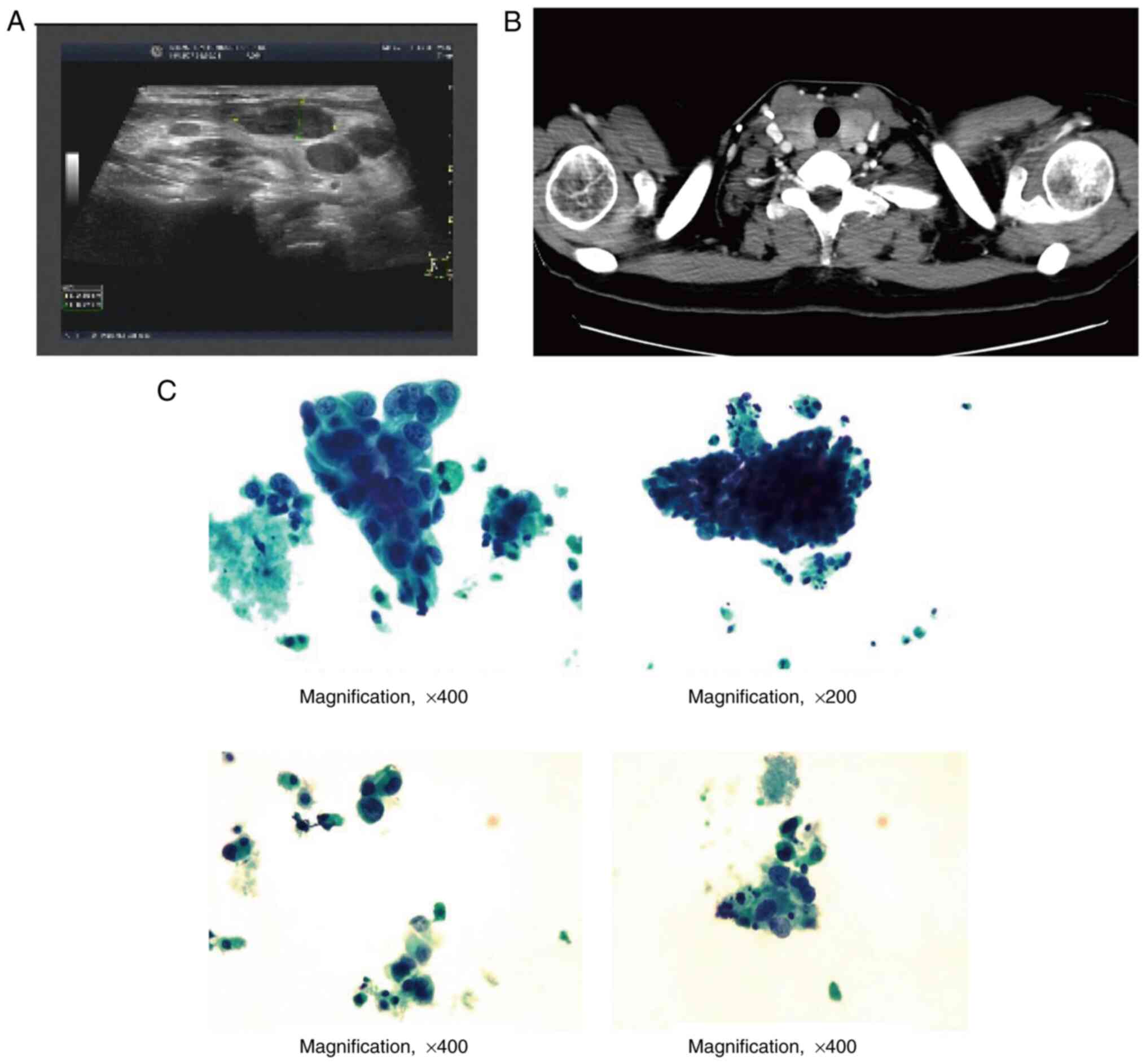
worsen. In December 2018, a mass developed in their left thyroid,
and a thyroid gland ultrasound revealed diffuse lesions with dense
microcalcification, raising concern for malignancy. Fine needle
biopsy was recommended for further evaluation (Fig. 2A). A neck CT also showed enlargement
of the thyroid gland and multiple low-density nodules in both lobes
(Fig. 2B). In January 2019, a core
needle biopsy on the thyroid was performed and cytological
examination of the mass in the left thyroid implied poorly
differentiated cancer cells, which were consistent with the history
of breast cancer metastasis (Fig.
2C). The serum thyroglobulin antibody level was found to be
576.2 IU/ml.
Apatinib, a medication that was recently authorized
by the National Medical Products Administration, may have
antiangiogenic and antitumor effects by inhibiting the
intracellular ATP-binding site of VEGFR-2 (13). Since October 2014, apatinib has been
authorized in China as a third-line therapy for people with
advanced gastric cancer. Recent research has demonstrated the
anticancer activity of apatinib in several solid malignancies,
including non-small cell lung and breast cancer. Since the previous
second-line treatment regimen resulted in the longest duration of
PFS, gemcitabine, trastuzumab plus apatinib was chosen for patient
treatment from October 2018, and was administered for six cycles (3
weeks per cycle). The best efficacy observed was stable disease
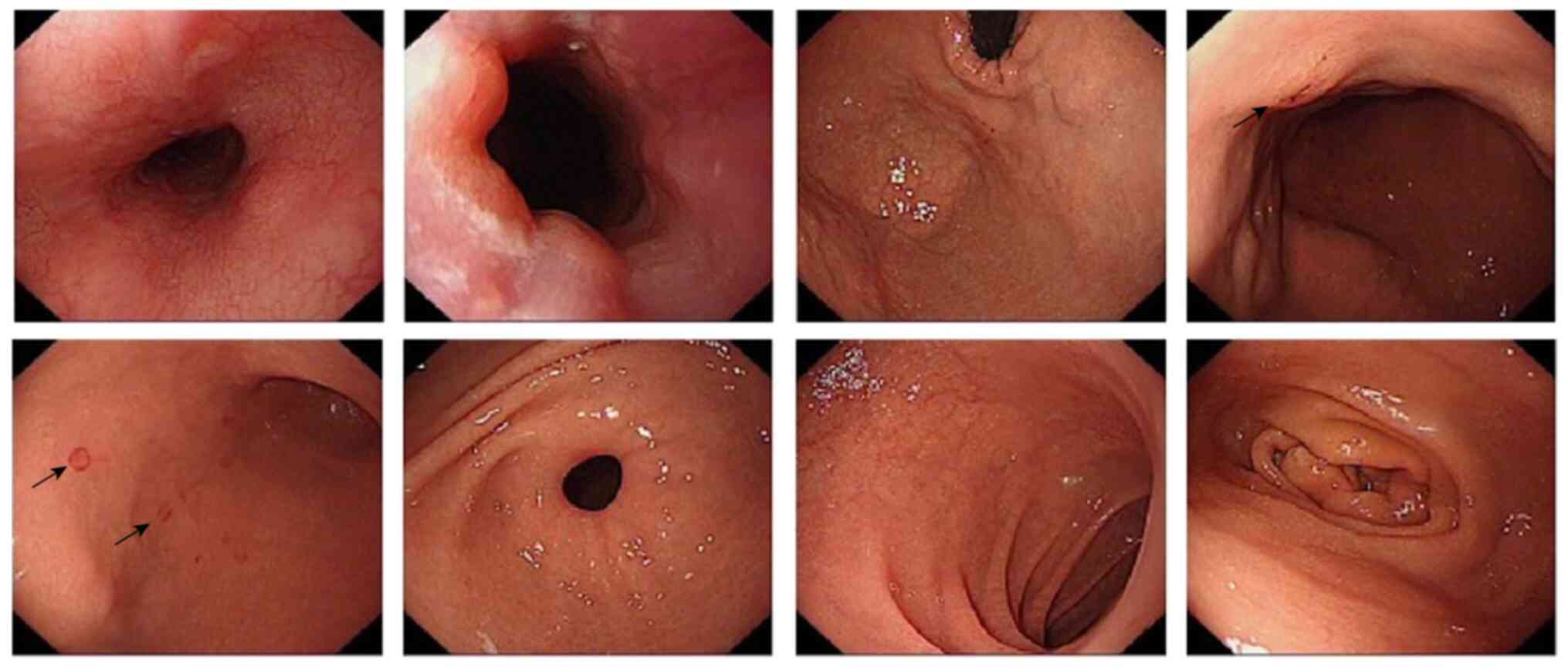
however, the patient eventually developed nausea and appetite loss,
and upper endoscopy showed gastric mucosal tissue indicative of
chronic gastritis with erosion (Fig.
3). A parallel gastric mucosa biopsy was taken in February
2019. After 7 days, the patient developed hematemesis and
hematochezia. Due to the adverse effect of apatinib, the patient
stopped taking apatinib, but no improvement was observed in gastric
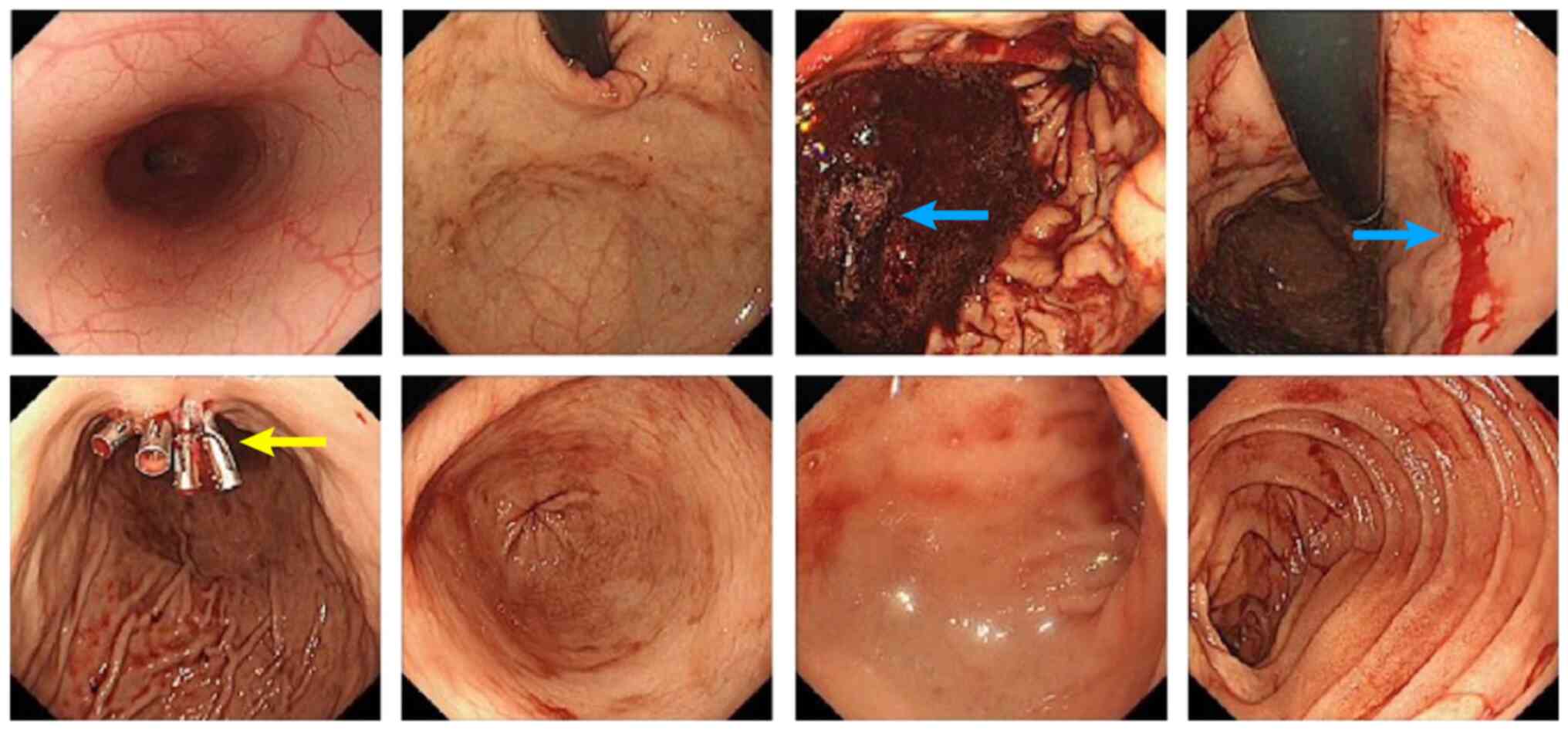
bleeding. Emergency gastroscopy showed active bleeding in the
lesser curvature of the stomach and gastric mucosa clamping was
performed to stop the bleeding (Fig.
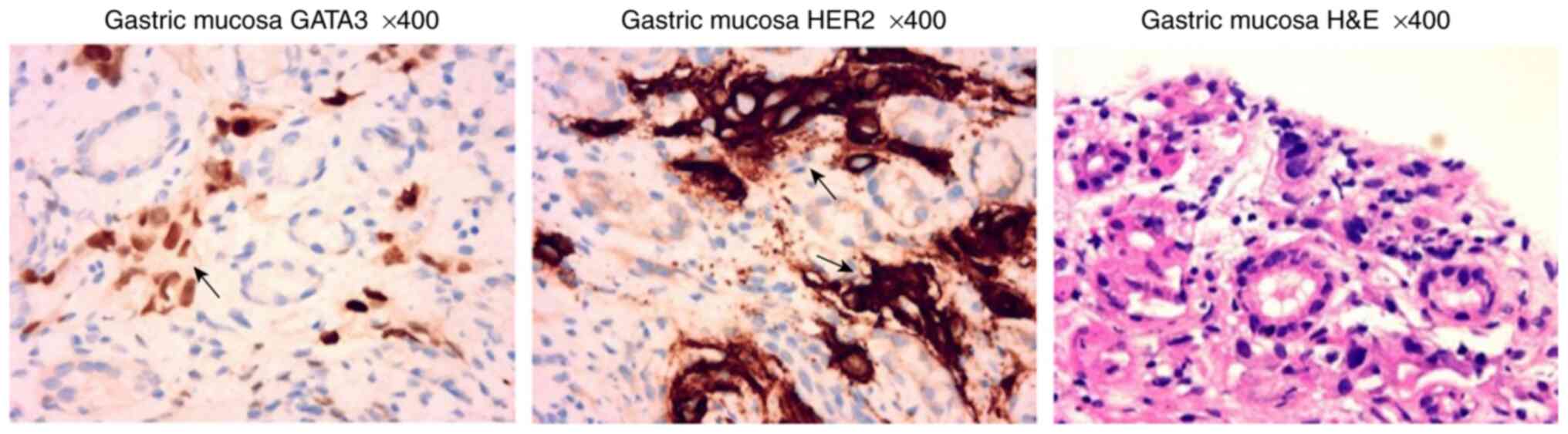
4). Pathological examination of the gastric mucosa showed
lamina propria visible in a variety of scattered nuclear layer
cells and degeneration, which, in combination with
immunohistochemical findings and medical history, suggested the
presence of breast cancer metastasis (14) (Fig.
5). Immunohistochemical results showed: GATA3 (++), AE1/AE3
(+++), HER2 (+++). Gene testing of the primary tumor performed in
March 2019 revealed that the breast tissue was negative for BRCA1/2
and had an ERBB2 (HER2) amplification mutation.
As the patient's disease progressed, palliative
chemotherapy and targeted therapy were continued. However, few
drugs were available for the patient, and they succumbed to heart
failure in December 2019.
Discussion
The present study describes a rare case of breast
cancer that spread to the stomach and thyroid gland following
surgery and systemic treatment. Gastric metastases of breast cancer
are infrequent and are difficult to distinguish from primary
gastric tumors. In the largest study to date, Taal et al
(15) reported that 51 cases of
metastatic breast cancer were diagnosed by stomach endoscopy,
including 36 ILC cases and 10 IDC cases, and the remaining five
with particular types. Another single-center retrospective study
found that in 97% (34/35) of patients, gastric metastases were
derived from lobular carcinoma, suggesting that ILC or luminal-type
breast cancer (ER-positive) are more likely to metastasize to the
stomach than IDC (4). The present
report describes a young patient with HER2-positive IDC with an
extensive intraductal component who developed gastric metastases 5
years after surgery, illustrating the possibility of gastric
metastases from breast IDC.
When diagnosing stomach metastasis of breast cancer,
imaging and endoscopic data are insufficient to distinguish between
primary and secondary GI tumors (16). Therefore, the diagnosis requires a
biopsy, pathology and endoscopy, and the histological result must
be compared to the initial breast cancer (17). Immunohistochemistry has a higher
accuracy in differentiating these entities than other methods, such
as imaging. With a 100% positivity rate for breast lobular
carcinoma and a 96% positivity rate for breast ductal carcinoma,
GATA3 has been widely classified as a marker of breast malignancies
(18). By contrast, GATA3 has been
reported to be stained positively in only 5% of malignant gastric
cancer cases (15). Therefore,
GATA3 serves as a hormonal marker similar to the function of ER and
PR in identifying metastatic cells of mammary origin.
Theoretically, Helicobacter pylori, inflammatory cells and
chemokines may create a favorable environment to attract tumor
cells, offering a promising diagnostic approach for gastric
metastasis of breast cancer (19).
However, some researchers do not agree that chemokines and
inflammatory events are involved in the metastatic process of
breast cancer (19). Treatment
options for GI breast cancer metastases are still subject to
debate. Systemic therapy is given high priority, and surgical
treatment is taken into account in cases of obstruction or
bleeding. In the present case, the patient received targeted
therapy combined with chemotherapy for treatment without any
further surgical intervention, and survival lasted 10 months, which
is similar to the median survival of gastric metastases (20).
Metastasis of breast cancer to the thyroid gland is
an uncommon occurrence in clinical practice. Despite the abundance
of blood vessels in the thyroid gland, metastatic disease at this
site remains rare; however, there have been reported cases of
metastatic disease affecting the thyroid gland (21). The most common initial tumor
locations for thyroid metastasis in a large series from the Mayo
Clinic (97 patients) were the kidney (22%), lung (22%), and head
and neck (12%) (22). To the best
of our knowledge, Egaña et al (23) reported the first case of a patient
with thyroid metastasis from breast cancer. In the present case, a
patient with thyroid metastasis from breast cancer had no
significant symptoms apart from diffuse lesions and elevated
thyroglobulin antibodies indicating autoimmune thyroid
inflammation. The expression of chemokine receptors could determine
the type of tumor that has metastasized, whereas the expression of
chemokine ligand can determine the spread site (24). Some chemokine receptors (such as
CXCR4) are overexpressed in some tumors and are involved in
directing tumor metastasis. Additional studies are required to
accurately determine the role of chemokine receptors in the process
of metastasis.
Although ILC metastasizes more often to the GI
tract, pelvic organs, peritoneum/retroperitoneum and urinary tract
than IDC, opportunities still exist for IDC to metastasize to the
GI tract. Therefore, it is necessary to consider the possibility of
gastric metastasis. An endoscopic examination should be considered
for patients with GI bleeding or other symptoms. Sometimes, it is
difficult to detect and diagnose gastric metastasis early due to
the absence of typical indicators of primary breast cancer coupled
with nonspecific GI symptoms.
In patients with a history of breast cancer, it is
essential to consider breast cancer metastasis if they have any
discomfort or physical abnormalities. Immunohistochemistry is
pivotal to the final diagnosis. For patients whose breast cancer
was well-treated and had no associated relapse or distant
metastasis, thyroidectomy can be an option for disease-free
survival for primary thyroid tumors. However, for most cases of
thyroid metastasis, it can respond well to chemotherapy; thus, no
thyroidectomy is required. Since metastatic thyroid illness
reflects the aggressiveness and advanced stage of the initial
disease, the prognosis is often poor (23).
Valuable experience has been gained from this case,
which highlights the significance of using a trastuzumab combined
chemotherapy treatment approach for advanced HER2-positive breast
cancer. However, there is no standardized treatment plan for
advanced HER2-positive breast cancer following multiline therapy.
Antiangiogenic drugs are targeted medications that inhibit the
supply of nutrients to tumor cells, thereby hindering their growth.
Apatinib is a small-molecule antiangiogenic drug that may be
regarded as a therapeutic choice based on its mechanism of action,
although its effectiveness requires further confirmation through
clinical trials (13) In the
present case, the inefficacy of posterior-line apatinib treatment
may be attributed to the poor prognosis of breast cancer metastasis
in the thyroid and stomach, and the patient's poor resilience
following multiline treatment.
In conclusion, this case report presents a patient
diagnosed with IDC with multiple metastases to the lung, pleura,
chest wall, various lymph nodes, thyroid, stomach and left adrenal
gland. The use of targeted therapy in combination with chemotherapy
is an important mode of treatment for patients with advanced breast
cancer, including those with gastric and thyroid metastases.
Further research should aim to elucidate the mechanism underlying
gastric and thyroid metastasis in HER2-positive breast cancer. The
prognosis of metastatic breast cancer in the stomach and thyroid
glands of young patients is relatively poor (17,22),
making systemic treatment more critical than local surgery.
Although ER positivity is uncommon in primary gastric cancer,
gastric tumors that are GATA3-positive or ER-positive were
previously thought to represent metastatic breast cancer (22). Although simultaneous thyroid and
stomach metastases from breast cancer are uncommon, they cannot be
ignored while assessing sites of metastasis, as overlooking them
could impede the diagnosis and treatment of advanced breast cancer
and adversely impact patient outcomes. Therefore, it is important
to distinguish between the primary tumor and metastatic tumors,
which can affect the treatment and prognosis of patients. In the
case of breast cancer metastasis to other organs, it can be treated
according to the treatment strategy for advanced breast cancer. In
the case of primary thyroid or stomach cancer, therapeutic
strategies should be selected according to the guidelines for
thyroid cancer and stomach cancer, and multidisciplinary
consultation should be considered as necessary.
Acknowledgments
Not applicable.
Funding
The National Natural Science Foundation of China (grant no.
81671750, 2016), the National Natural Science Foundation of
Guangdong Province (grant no. 2016A030312008) and the Shenzhen
Basic Research Program (grant no. JCYJ20180306171227129, 2018) all
provided funding in support of this study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XB, CBF and CWD designed the study and wrote the
manuscript. XB, BLL, JYH, XLC and XFX confirmed the authenticity of
all the raw data. SNH, WCD, ZZL and QYZ gathered medical pictures
and examined patient information. BLL, JYH, XLC, XFX, MDL, JYL,
JFG, LS, XFL and LPC contributed to the study's conceptualization,
general design and quality assurance. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Since the patient died, their husband provided
written informed consent for the publication of this case report
and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li CI, Uribe DJ and Daling JR: Clinical
characteristics of different histologic types of breast cancer. Br
J Cancer. 93:1046–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mukaiyama T: Analysis of metastatic and
causes of death in 100 autopsied patients with breast cancer.
Nyugan Rinsho. 4:121–126. 1989.
|
|
3
|
Ferri LE, Onerheim R and Emond C: Linitis
plastica as the first indication of metastatic lobular carcinoma of
the breast: Case report and literature review. Can J Surg.
42:466–469. 1999.PubMed/NCBI
|
|
4
|
Almubarak MM, Lae M, Cacheux W, de Cremoux
P, Pierga JY, Reyal F, Bennett SP, Falcou MC, Salmon RJ, Baranger B
and Mariani P: Gastric metastasis of breast cancer: A single centre
retrospective study. Dig Liver Dis. 43:823–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong J, Kim Y, Cho J, Lim SW, Park SE, Kim
HK, Lee H, Cho SY, Kim JY, Ahn JS, et al: Clinical features and
prognosis of breast cancer with gastric metastasis. Oncol Lett.
17:1833–1841. 2019.PubMed/NCBI
|
|
6
|
Pectasides D, Psyrri A, Pliarchopoulou K,
Floros T, Papaxoinis G, Skondra M, Papatsibas G, Macheras A,
Athanasas G, Arapantoni-Datioti P and Economopoulos T: Gastric
metastases originating from breast cancer: Report of 8 cases and
review of the literature. Anticancer Res. 29:4759–4763.
2009.PubMed/NCBI
|
|
7
|
Mazzaferri EL and Sipos J: Should all
patients with subcentimeter thyroid nodules undergo fine-needle
aspiration biopsy and preoperative neck ultrasonography to define
the extent of tumor invasion? Thyroid. 18:597–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson L: Neoplasms metastatic to the
thyroid gland. Ear Nose Throat J. 85:4804832006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DH, Son SM and Choi YJ: Gastric
metastasis from invasive lobular breast cancer, mimicking primary
gastric cancer: A case report. Medicine (Baltimore). 97:e02582018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung AY, Tran TB, Brumund KT, Weisman RA
and Bouvet M: Metastases to the thyroid: A review of the literature
from the last decade. Thyroid. 22:258–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riley DS, Barber MS, Kienle GS, Aronson
JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG,
Sox H, et al: CARE guidelines for case reports: Explanation and
elaboration document. J Clin Epidemiol. 89:218–235. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Arch.
472:697–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia H, Zhou C, Luo Z, Zhang P, Zhu L and
Gong Z: Apatinib-Induced Hand-foot skin reaction in chinese
patients with liver cancer. Front Oncol. 11:6243692021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hui Y, Wang Y, Nam G, Fanion J, Sturtevant
A, Lombardo KA and Resnick MB: Differentiating breast carcinoma
with signet ring features from gastrointestinal signet ring
carcinoma: Assessment of immunohistochemical markers. Hum Pathol.
77:11–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taal BG, Peterse H and Boot H: Clinical
presentation, endoscopic features, and treatment of gastric
metastases from breast carcinoma. Cancer. 89:2214–2221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciulla A, Castronovo G, Tomasello G,
Maiorana AM, Russo L, Daniele E and Genova G: Gastric metastases
originating from occult breast lobular carcinoma: diagnostic and
therapeutic problems. World J Surg Oncol. 6:782008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones GE, Strauss DC, Forshaw MJ, Deere H,
Mahedeva U and Mason RC: Breast cancer metastasis to the stomach
may mimic primary gastric cancer: Report of two cases and review of
literature. World J Surg Oncol. 5:752007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Shi J, Wilkerson ML and Lin F:
Immunohistochemical evaluation of GATA3 expression in tumors and
normal tissues: A useful immunomarker for breast and urothelial
carcinomas. Am J Clin Pathol. 138:57–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Villa Guzman JC, Espinosa J, Cervera R,
Delgado M, Paton R and Cordero Garcia JM: Gastric and colon
metastasis from breast cancer: Case report, review of the
literature, and possible underlying mechanisms. Breast Cancer (Dove
Med Press). 9:1–7. 2017.PubMed/NCBI
|
|
20
|
Xu L, Liang S, Yan N, Zhang L, Gu H, Fei
X, Xu Y and Zhang F: Metastatic gastric cancer from breast
carcinoma: A report of 78 cases. Oncol Lett. 14:4069–4077. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Magers MJ, Dueber JC, Lew M, Pang JC and
Davenport RD: Metastatic ductal carcinoma of the breast to the
thyroid gland diagnosed with fine needle aspiration: A case report
with emphasis on morphologic and immunophenotypic features. Diagn
Cytopathol. 44:530–534. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hegerova L, Griebeler ML, Reynolds JP,
Henry MR and Gharib H: Metastasis to the thyroid gland: Report of a
large series from the Mayo Clinic. Am J Clin Oncol. 38:338–342.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egaña N, Socias C, Matteucci T, Bilbao I
and Alvarez-Coca M: Thyroid metastasis of lobular breast carcinoma.
Endocrinol Nutr. 59:219–220. 2012.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DiNatale A, Castelli MS, Nash B, Meucci O
and Fatatis A: Regulation of tumor and metastasis initiation by
chemokine receptors. J Cancer. 13:3160–3176. 2022. View Article : Google Scholar : PubMed/NCBI
|