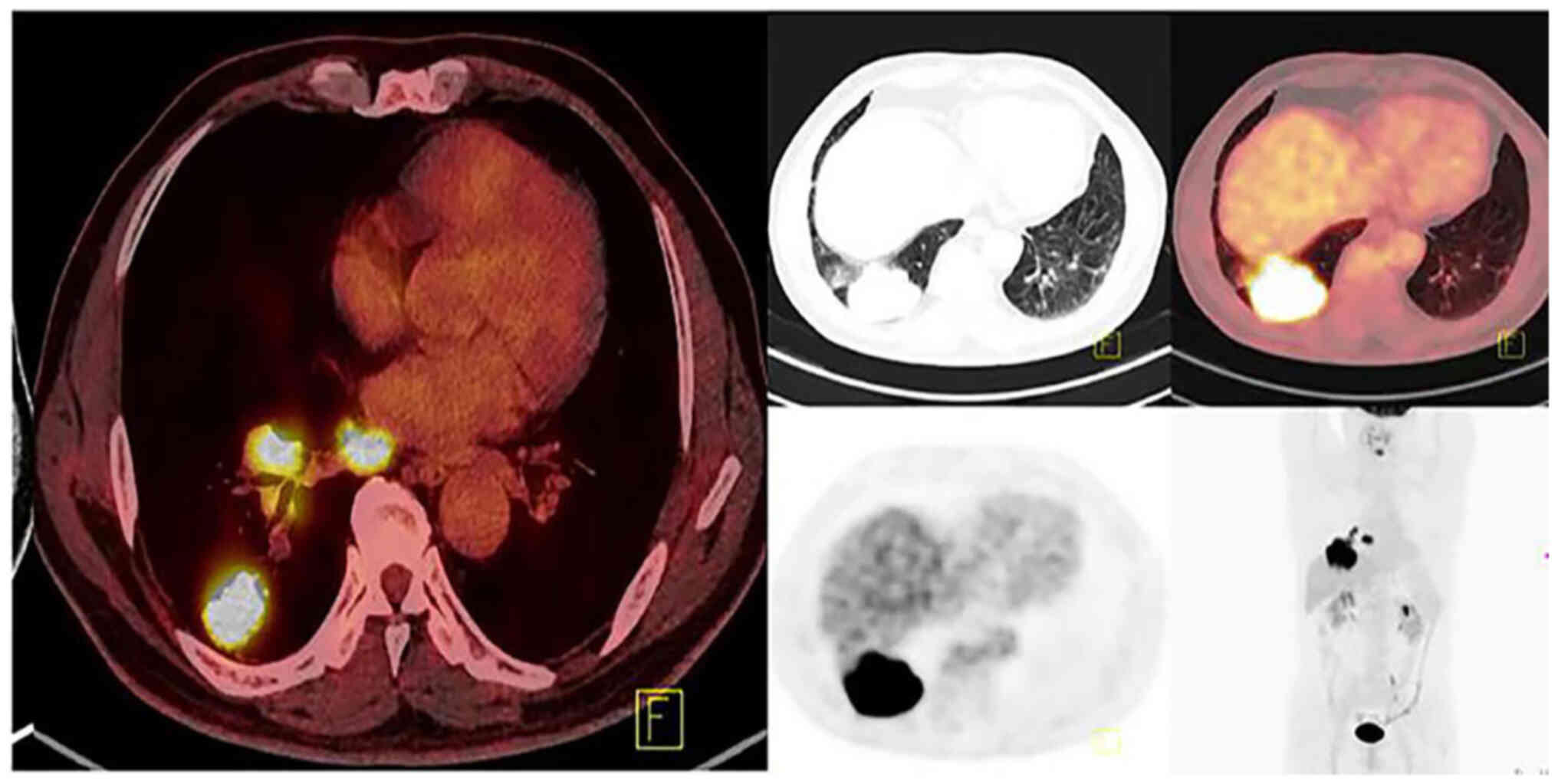
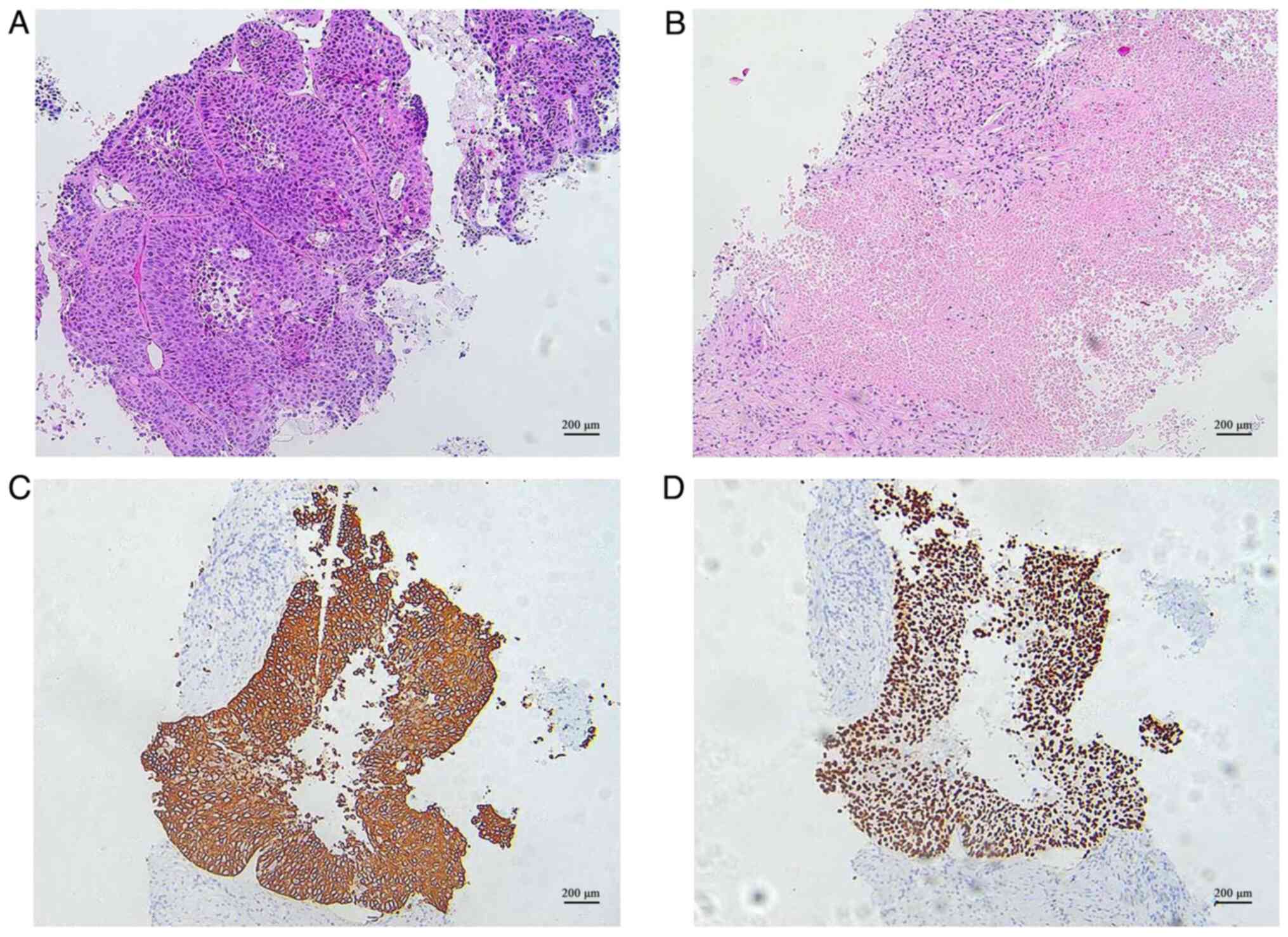
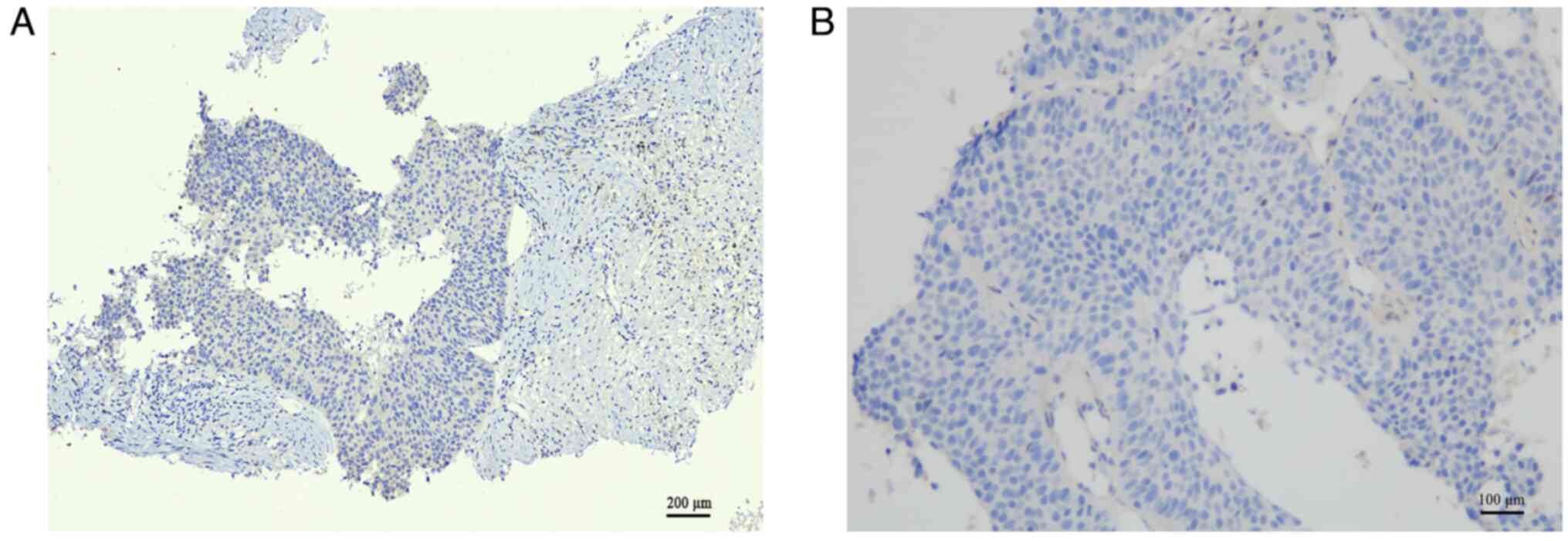
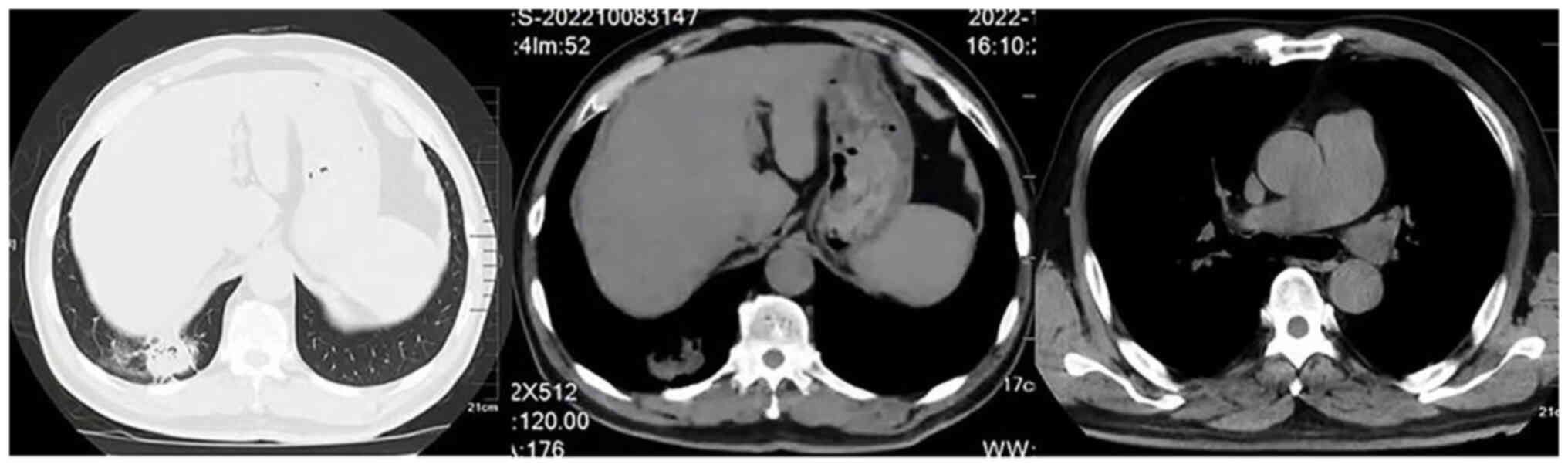
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NSCLC Meta-analyses Collaborative Group, .
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analysis Collaborative Group, :
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft
A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al:
Durvalumab with or without tremelimumab vs standard chemotherapy in
first-line treatment of metastatic non-small cell lung cancer: The
MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6:661–674.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eichhorn F, Klotz LV, Kriegsmann M,
Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U,
Heussel CP, Savai R, et al: Neoadjuvant anti-programmed death-1
immunotherapy by pembrolizumab in resectable non-small cell lung
cancer: First clinical experience. Lung Cancer. 153:150–157. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong BC, Gu L, Wang X, Wigle DA, Phillips
JD, Harpole DH Jr, Klapper JA, Sporn T, Ready NE and D'Amico TA:
Perioperative outcomes of pulmonary resection after neoadjuvant
pembrolizumab in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 163:427–436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuchert MJ, Normolle DP, Awais O,
Pennathur A, Wilson DO, Luketich JD and Landreneau RJ: Factors
influencing recurrence following anatomic lung resection for
clinical stage I non-small cell lung cancer. Lung Cancer.
128:145–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
William WN Jr, Pataer A, Kalhor N, Correa
AM, Rice DC, Wistuba II, Heymach J, Lee JJ, Kim ES, Munden R, et
al: Computed tomography RECIST assessment of histopathologic
response and prediction of survival in patients with resectable
non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 8:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong
W, Zhang Y, Zhou X, Wang Z, Wang Y, et al: The binding of an
anti-PD-1 antibody to FcγRI has a profound impact on its biological
functions. Cancer Immunol Immunother. 67:1079–1090. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee A and Keam SJ: Tislelizumab: First
approval. Drugs. 80:617–624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutob L and Schneider F: Lung cancer
staging. Surg Pathol Clin. 13:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng
K, Lu G and Zhou X: Detection of ALK protein expression in lung
squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer
Res. 33:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forbes SA, Bhamra G, Bamford S, Dawson E,
Kok C, Clements J, Menzies A, Teague JW, Futreal PA and Stratton
MR: The catalogue of somatic mutations in cancer (COSMIC). Curr
Protoc Hum Genet Chapter 10. Unit 10.11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei Y, Lei Y, Shi X and Wang J: EML4-ALK
fusion gene in non-small cell lung cancer. Oncol Lett. 24:2772022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chae YK, Hong F, Vaklavas C, Cheng HH,
Hammerman P, Mitchell EP, Zwiebel JA, Ivy SP, Gray RJ, Li S, et al:
Phase II study of AZD4547 in patients with tumors harboring
aberrations in the FGFR pathway: results from the NCI-MATCH trial
(EAY131) subprotocol W. J Clin Oncol. 38:2407–2417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunner AM, Costa DB, Heist RS, Garcia E,
Lindeman NI, Sholl LM, Oxnard GR, Johnson BE and Hammerman PS:
Treatment-related toxicities in a phase II trial of dasatinib in
patients with squamous cell carcinoma of the lung. J Thorac Oncol.
8:1434–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thatcher N, Hirsch FR, Luft AV, Szczesna
A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy
G, et al: Necitumumab plus gemcitabine and cisplatin versus
gemcitabine and cisplatin alone as first-line therapy in patients
with stage IV squamous non-small-cell lung cancer (SQUIRE): An
open-label, randomised, controlled phase 3 trial. Lancet Oncol.
16:763–774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367:eaax01822020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Broderick SR and Bott MJ: Neoadjuvant
immunotherapy in patients with resectable non-small cell lung
cancer. J Thorac Cardiovasc Surg. 158:1471–1474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lavin Y, Kobayashi S, Leader A, Amir ED,
Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH,
et al: Innate immune landscape in early lung adenocarcinoma by
paired single-cell analyses. Cell. 169:750–765.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williams PA, Zaidi SK and Sengupta R: AACR
report on the impact of COVID-19 on cancer research and patient
care. Clin Cancer Res. 28:609–610. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arriagada R, Dunant A, Pignon JP, Bergman
B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R,
Pinel MI, et al: Long-term results of the international adjuvant
lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy
in resected lung cancer. J Clin Oncol. 28:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Yu X, Lu S, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Phase III study of tislelizumab
plus chemotherapy vs chemotherapy alone as first-line (1L)
treatment for advanced squamous non-small cell lung cancer (sq
NSCLC). J Clin Oncol. 38 (Suppl):S95542020. View Article : Google Scholar
|
|
37
|
BeiGene, . Comparing the efficacy and
safety of a new additional treatment with tislelizumab in non-small
cell lung cancer (NSCLC). U.S. National Library of Medicine; 2023,
Available from:. https://clinicaltrials.gov/ct2/show/NCT04379635
|
|
38
|
Hu X, Hu C, Liu X, Ma F, Xie J, Zhong P,
Tang C, Fan D, Gao Y, Feng X, et al: Tumor regression rate, PD-L1
expression, pembrolizumab/nab-paclitaxel-based regimens, squamous
cell carcinoma, and comorbidities were independently associated
with efficacy of neoadjuvant chemoimmunotherapy in non-small cell
lung cancer. Front Oncol. 12:10576462023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
La Fleur L, Falk-Sörqvist E, Smeds P,
Berglund A, Sundström M, Mattsson JS, Brandén E, Koyi H, Isaksson
J, Brunnström H, et al: Mutation patterns in a population-based
non-small cell lung cancer cohort and prognostic impact of
concomitant mutations in KRAS and TP53 or STK11. Lung Cancer.
130:50–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Travis WD, Dacic S, Sholl LM and Wistuba
II: Pathologic assessment of lung squamous cell carcinoma after
neoadjuvant immunotherapy. J Thorac Oncol. 16:e9–e10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szeto GL and Finley SD: Integrative
approaches to cancer immunotherapy. Trends Cancer. 5:400–410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu J, Zhang L, Xia H, Yan Y, Zhu X, Sun F,
Sun L, Li S, Li D, Wang J, et al: Tumor microenvironment remodeling
after neoadjuvant immunotherapy in non-small cell lung cancer
revealed by single-cell RNA sequencing. Genome Med. 15:142023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bott MJ, Yang SC, Park BJ, Adusumilli PS,
Rusch VW, Isbell JM, Downey RJ, Brahmer JR, Battafarano R, Bush E,
et al: Initial results of pulmonary resection after neoadjuvant
nivolumab in patients with resectable non-small cell lung cancer. J
Thorac Cardiovasc Surg. 158:269–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee J, Chaft J, Nicholas A, Patterson G,
Waqar S, Toloza E, Haura E, Raz D, Reckamp K, Merritt R, et al:
P2.04–88 surgical outcomes of a multicenter phase II trial of
neoadjuvant atezolizumab in resectable stages Ib-IIIb NSCLC: Update
on LCMC3 clinical trial. J Thorac Oncol. 14 (Suppl):S7442019.
View Article : Google Scholar
|
|
46
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun C, Liu Y, Zhang P, Wang X, Xu Y, Lin
X, Ma X, Guo Y, Qiu S, Shao G, et al: Interim analysis of the
efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab)
combined with chemotherapy (nab-paclitaxel and carboplatin) in
potentially resectable stage IIIA/IIIB non-small cell lung cancer:
A single-arm, phase 2 trial. J Cancer Res Clin Oncol. 149:819–831.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Provencio-Pulla M, Nadal-Alforja E, Cobo
M, Insa A, Costa Rivas M, Majem M, Rodriguez-Abreu D, Lopez-Vivanco
G, Domine M, Del Barco Morillo E, et al: Neoadjuvant
chemo/immunotherapy for the treatment of stages IIIA resectable
non-small cell lung cancer (NSCLC): A phase II multicenter
exploratory study-NADIM study-SLCG. J Clin Oncol. 36
(Suppl):S85212018. View Article : Google Scholar
|
|
49
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|